Abstract
Springs supply water for drinking and livelihood for a vast majority of rural population in Nepal. Haphazard development activities are affecting springs adversely in hills and mountains of Nepal. This study aims to map springs, characterize their status from visual inspection and local/indigenous knowledge, characterize water quality, and then evaluate their suitability for drinking and irrigation in the Thuligaad watershed, western Nepal. Analysis of 160 springs mapped within four Wards (Ward is the smallest (fourth level) Administrative Unit in Nepal) in the Jorayal Rural Municipality in the Thuligaad watershed indicated that most of the springs are perennial (95%), located in less than 1,200 or within 1400–1600 m above the sea level elevation (70.6%), and have discharge less than five liters per minutes (80% during pre-monsoon season). Multiple tools such as Gibb’s diagram, Piper’s diagram, and principal component analysis were used to characterize water quality. Forty springs selected for spring water quality analysis indicated that it is dominated by Ca2+ > Mg2+ > Na+ > K+ and HCO3− > Cl− > SO42− water types, thus revealing rock–water interaction as dominant process controlling the spring water quality. Furthermore, water chemistry is dominantly Ca–HCO3 (52% in pre-monsoon) and Mixed Ca–Mg–Cl (28% in pre-monsoon) types. Finally, suitability analysis revealed that spring water quality is of excellent quality for drinking and concentration of various parameters comply with national and World Health Organization’s standard for drinking water quality. Similarly, the water is sage against various hazards for use in irrigation. Results of this first study in the study area provided valuable baseline information for spring water source protection and management.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Water is a key input for human survival, livelihood support, and socio-economic development (Petraccia et al. 2006). However, contaminated drinking water is also a primary cause of about 80 percentage of disease in the world and one-third of deaths in the developing countries (WHO 2004). Securing quality, quantity and timing of freshwater to meet various needs of the growing population and ecosystem are the global challenges in this century (Pant et al. 2018). Springs are the form of groundwater that play an important role for ensuring reliable supply of surface water in the watershed. Mineral compositions in the spring water are considered good for health (Nguyet and Goldscheider 2006). The spring water travels through a network of cracks and fissures and appears on the surface of the earth at appropriate locations in the form of natural springs (Nguyet and Goldscheider 2006).
Springs are perennial or short-lived. As per Sharma et al. (2016), perennial springs flow continuously and fed from a level below groundwater table whereas short-lived springs are fed by increased groundwater level after the monsoon season. Rate and volume of spring discharge and quality of water are determined by and hydro-geological and geochemical characteristics of spring-shed, the area that drains to the spring. The mineral composition of natural spring water or groundwater depends on the type of geological formation (Oyem et al. 2014) as well as anthropogenic influences (Bhat and Jeelani 2016). Therefore, each spring can be unique in terms of discharge, water quality, and potential to supply water for a long term, which all are important to assess before we use the spring as a source of water for planning water and sanitation systems in an area.
Due to population growth and various forms of human interventions leading to climatic and non-climatic changes in the watershed, water resources are impacted in terms of quantity, quality, and timing of flow (Pant et al. 2018). As springs are the reliable source of water for a vast majority of rural population in Nepal for various uses, it is important to assess situation of spring sources, reliability of water availability, and quality of the spring water to develop strategies for providing safe drinking water and sanitation for the population and conserving ecosystem. These efforts will then contribute to realize the Sustainable Development Goals (SDGs) and associated targets. The spring water is highly valuable for water supply, sanitation, and livelihood, more specifically for rural communities; however, springs are under increasing stress due to human interventions in various forms including haphazard road constructions. Conservation of springs is one of the major challenges that we are facing today (Kaur et al. 2017).
Assessment of urban springs and groundwater has been a topic of research over the years (e.g., Pandey et al. 2010; Mofor et al. 2017; Poudel and Duex 2017; Batool et al. 2018); however, springs in rural areas have not get adequate attention. As most of the people living in the rural areas depend on spring water for household use and economic activities, it is important to assess rural springs from those aspects. Spring in rural areas of Nepal are gradually getting affected due to development activities like road construction, sealing of land surface with housing and associated developments, abandonment of agricultural lands, and deforestation. Furthermore, a number of processes can affect water quality, which include atmospheric inputs, contamination of soil covers that control the infiltration process, interaction of water with soil and rock, and inputs of chemical derived from anthropogenic activities (e.g., industrial, agriculture, urbanization, etc.), among others (Simeonov et al. 2003). As ensuring good quality water is directly linked to human and environmental health, assessment of spring water quality has been highlighted in many studies across the globe, such as in Camroon (Mofor et al. 2017); China (Zhang et al. 2017), India (Chauhan et al. 2020); Italy (Gaglioti et al. 2017), Jordan (Al-Khashman 2008), Pakistan (Batool et al. 2018), Pakistan (Memon et al. 2016) and Nepal (Bhusal and Gyawali 2015). Such studies not only provide a baseline in the areas where no studies are conducted before, but also provide valuable insights on potential interventions required for conserving spring-shed to improve quality, quantity and timing of spring water.
Most of the spring-related research starts with locating the springs and evaluating their status using technical and questionnaire instruments with adequate interactions with local people (Simiyu et al. 2009). For measuring flow rate of open springs, parameters like distance (2 m in general), time taken to travel the distance, and cross-sectional area of the flow path (i.e., breadth and depth) are measured. Whereas for the closed spring, spring flow is collected in a closed area of known volume and time required to fill that area is measured (Ilmonen et al. 2012). For assessing quality of spring water and evaluating suitability for various uses, hydro-chemical approach is widely used (Tyagi et al. 2013; Batabyal and Chakraborty 2015; Gaglioti et al. 2017). Various process are likely to contribute in variation of hydro-chemical composition of spring water, which can be evaluated using multivariate statistical methods such as principal component analysis (PCA) and factor analysis (Gibbs 1970; Siddiqui et al. 2005; Ako et al. 2012). Delineating flow paths of springs are also equally important as that may indicate potential sources of contamination as well as gain and loss of water in the springs. It requires analysis of major ions as natural tracers can be a very effective method (Chung et al. 2015). Furthermore, flow pattern and origin of chemical history of groundwater can be assessed through analysis of hydro-geochemical facies (Chung et al. 2015). Generally, the approach is to divide the samples into hydro-chemical facies using piper trilinear diagram (Piper 1944). The spatial analysis can reveal heterogeneity and connectivity as well as the physical and chemical processes controlling the water chemistry (Ako et al. 2012). Ultimately, springs are used for various uses such as drinking and irrigation. Suitability for those uses are basically determined based on the concentration and composition of dissolved ions which are generally governed by lithology of surfaces, velocity and quantity of spring water flow, nature of geochemical reactions, solubility of salts and anthropogenic activities (Kaur et al. 2017). Those concentrations are then compared with various guiding thresholds developed for various uses.
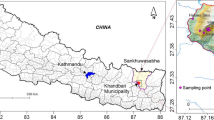
The objectives of this study are to map and characterize major springs, assess spring water quality and seasonality, and evaluate their suitability for drinking and irrigation uses in the Thuligaad watershed, located in Sudurpaschim Province, western Nepal (Fig. 1).
Materials and methods
Study area
Thuligaad watershed, covering an area of 850 km2 spread over Kailali and Doti districts, is a tributary of the Karnali river in western Nepal (Fig. 1). This watershed stretches across the Jorayal and Baddikedar rural municipalities (in Doti district) and the Chure and Mohanyal rural municipalities (in Kailali district) in the south-western Nepal. Located in Sudurpaschim Province, the Thuligaad watershed extends between 29°12′30″ N latitude and 80°35′0″ E longitude. This watershed has four freshwater lakes (i.e., Brahm, Jwalaban, Rakxes and Chhatiwan), which are important habitat for aquatic species in this region. Thuligaad watershed is sub-tropical, rich in forest crops and agricultural biodiversity. It covers climatic zones like lower tropical, upper tropical, sub-tropical, temperature and sub-alpine. Average annual temperature in the Thuligaad watershed, based on the data at the nearest station, is 23 °C, with the mean maximum and minimum monthly temperature ranging from 5 to 24 °C in winter to 21 and 40 °C. In terms of rainfall, there is a clear monsoonal pattern with the highest rainfall from June to September. The mean annual rainfall in the watershed is 1122 mm, of which more than 80% falls during monsoon (USAID 2018). The highest and lowest monthly rainfall occur in the months of July and November, respectively.
This study focuses within four wards of the Jorayal Municipality located in the headwater of the watershed (Fig. 1) excluding two wards of this municipality as they do not feed on Thuligaad watershed. The four wards are Gaira, Budar, Saraswati Nagar and Laxmi Nagar, and they cover an area of 262 km2. The topography of the four wards vary widely, with elevation ranging from 801 to 2716 m above the mean sea level (masl). The total population of four wards of Jorayal Rural Municipality is 21,296 (CBS 2011). The population density is 100 persons/km2. In terms of cast and ethnicity, major groups in the area include Brahmin, Chhetri, Dalit, and a wide range of Janajatis, including Magar, Badi, Majhi, Lama, Bhote, Gurung and Newar (CBS 2015). The major crops are rice, wheat, maize, barley, millet, buckwheat, and soybeans. The study area is also known for fruits, vegetables and cereal crops. Livestock are also an important source of income, particularly pigs, sheep, buffalo and cattle, among others. Beyond agriculture, wage labor, seasonal labor migration, and gravel mining work are other important sources of livelihoods for local households. This watershed and its tributaries are the available sources of water for drinking, domestic and agricultural uses; however, local springs play important role to enhance reliability of surface sources as well as supply directly for drinking, domestic, and irrigation purposes (USAID 2018).
The geological formation of this watershed is granites, basic rocks, dolomites, limestone, quartzite and carbonates (Fuchs and Frank 1970). The upper reaches of the watershed of the Thuligad Khola on its left bank catchment is composed of carbonate rocks—dolomite, limestone and calcareous shale. Extension of Baitadi Carbonates in Budar–Joryal area is thickly bedded limestone, dolomites, and intercalated quartzite and phyllite (Dhital 2015).
Spring sources mapping and status assessment
The locations of major water resources were identified through a questionnaire survey with the local people, thus utilizing local and indigenous knowledge. All identified springs, either in use or not in use or dried up, were mapped with information such as location; discharge and its seasonality; land use/cover around the spring; physical characteristics of the spring site; number of households using the water source; use of spring; and perception on water quality, ownership and management system. A global positioning system (GPS) was used to collect the information related to location and altitude. Other information such as current discharge and its historical trends (increasing/decreasing) were also gathered through field study and questionnaire survey as did in (Simiyu et al. 2009). Finally, a map with location of springs and their characteristics was prepared and spatial distribution was analyzed and discussed.
A questionnaire was designed and survey was implemented with local people to characterize the status of spring sources compared with the past 20 years. The questionnaire includes status of spring sources, its trend (increasing, decreasing, no change, or dried up), purpose/use of spring sources, water availability, accessibility and management or conservation effort. The discharge rates of closed and open springs were also analyzed to assess the actual discharge rate and seasonally. Discharge of identified springs were also measured while mapping. For free-flowing springs, outflow from the spring was allowed to travel through a channel of fixed distance and defined cross section and time required for the travel or to collect certain volume of water was noted. Spring discharge was calculated by dividing volume of water collected by time. In case of stagnant springs, time required to fill certain volume of water in the closed area was noted and discharge was calculated by dividing the filled volume by time. The time required to fill up the closed area was also confirmed through questionnaire survey with local people as did in Ilmonen et al. (2012).
Spring water sampling and quality analysis
From among the mapped springs, a total of 80 samples, 40 in post-monsoon (November, 2018) and 40 in pre-monsoon (April, 2019) seasons were collected using purposive sampling method. The water samples were collected according to priority and accessibility of the springs, identified based on discussion with local people, with full utilization of local and indigenous knowledge. The onsite observation was carried out within 24 h of sample collection using a probe for temperature, pH, electric conductivity (EC) and total dissolved solids (TDS). A model H18314 portable pH meter (Hanna instruments, manufactured with the accuracy of ± 0.01 at 20 °C) was used for measurement of temperature and pH. Conductivity meter (model 4150 with accuracy of a 0.5% ± 2 digits) was used for measurement of EC and TDS. Titration method was carried out within 24 h of sample collection in the field for parameters such as chlorine, alkalinity, calcium, magnesium and free CO2 (APHA 1998).
The samples analyzed for the parameters ammonia, nitrate, phosphate, sulfate, iron, sodium and potassium were not filtered and analyzed directly for dissolved elements but not for the particulates. The method used for the analysis of ammonia, nitrate, sulfate and iron in water samples are phenate method, phenol disulfonic acid, turdibimetric, and phenanthroline methods, respectively. The parameters (ammonia, nitrate, phosphate, sulfate and iron) were then analyzed in the laboratory using spectrophotometer (model UV–VIS Spectrophotometer SSI 2101) with the wavelength of 635 nanometers (nm), 410, 690, 420 and 510 nm, respectively. For analysis of iron, the samples were acidified with nitric acid for preservation. Those acidified samples were preserved in the refrigerator at 4 °C in the laboratory. Similarly, sodium and potassium concentration were analyzed by flame photometer (model ESICO-1382/1386). For laboratory analysis, samples were collected and preserved in pre-cleaned and well-dried sample bottles. Distilled water was used for preparing the solutions and blank throughout the analysis. All mathematical calculations like maximum, minimum and average were calculated for physico-chemical parameters using Excel 2007.
The strength of linear relationship between various parameters was evaluated based on Spearman correlation coefficient calculated using R studio. The PCA technique was used to identify the governing principle chemical composition of spring water using SPSS. Gibb’s method was applied to illustrate three important natural mechanisms controlling the major iron chemistry of the spring water using scattered method (Gibbs 1970). Hydro-chemical facies was applied for presenting concentration of dominant cations and anions to help in understanding the sources of the dissolved constituents of water using Golden Grapher (version 15.2.1).
Evaluation of spring water quality
The quality of spring water for drinking and irrigation uses were further evaluated as described in the following sub-sections.
Evaluation for drinking purpose
Water Quality Index (WQI) represents the overall water quality of a location and time and can be computed based on physic-chemical (Tiwari and Mishra 1985) using Eq. 1;
where qn and Wn are the quality rating and weights to each quality parameters, respectively.
Fourteen parameters were chosen for computing WQI in this study, as did in other studies (e.g., Chatterji and Razuiddin 2002; Sahu and Sikdar 2008, Yogendra and Puttaiah 2008). The parameters were pH, EC, TDS, sodium, potassium, chloride, bicarbonate, total hardness, calcium, magnesium, ammonia, nitrate, sulfate and iron. The weightage for each parameter were assigned in a range of 1–5; 5 indicating the highest importance and 1 indicating the lowest in overall WQI. The maximum weight of 5 was assigned to the parameters pH, potassium and nitrate; 4 to parameters EC, TDS, sulfate and iron; 3 to chloride, total alkalinity and ammonia; and 2 to total hardness, calcium and magnesium. Finally, water quality status of each spring was categorized in a scale of “unsuitable” to “excellent” based on range of WQI values as shown in Table 1.
Furthermore, the suitability of spring water for drinking was also evaluated based on concentration of physico-chemical parameters against the Nepal Drinking Water Quality Standard Guidelines (NDWQS 2005).
Evaluation for irrigation purpose
To assess suitability of spring water for irrigation, following parameters were evaluated—Sodium Adsorption Ratio (SAR), Sodium percentage (Na %), Alkalinity hazard, Magnesium ratio and Corrosivity ratio (Tripathi et al. 2012).
(i) Alkalinity Hazard/Sodium Adsorption Ratio (SAR): SAR is an irrigation water quality parameters which measures suitability of water for agricultural use (Subramani et al., 2005). The SAR values for each water sample were calculated using Eq. 2 (Richards 1954).
where concentrations are expressed in milli-equivalent per liters (meq/L).
(ii) Sodium percentage (Na %): Sodium (Na) reacts with soil, and reduces its permeability due to absorbing Na by clay particles and displacing Ca2+ and Mg2+ from the soil. This will eventually result in limited air and water circulation during and reduces soil permeability (Kaur et al. 2017). The Na% in can be calculated using Eq. 3 and water quality can be classified based on Na%.
where all ionic concentrations are expressed in meq/L.
(iii) Integrated effect of EC and SAR (Alkalinity Hazard): Alkalinity hazard is identified based on a diagram of SAR against EC designated by US Salinity Laboratory in 1954.
(iv) Magnesium ratio (MR): Based on the magnesium ratio (Eq. 4), water can be classified as suitable for irrigation purpose, if the MR ratio is greater than 50% (Paliwal and Singh 1967):
(v) Corrosivity Ratio (CR): Corrosion can change pH and hardness of water. The groundwater with corrosivity ratio < 1 is considered to be safe for transport of water in any type of pipes, whereas > 1 indicates corrosive nature, and hence not to be transported through metal pipes (Raman 1983). It is expressed as in Eq. 5:
Results and discussion
Status and usage of springs
One-hundred and sixty (160) spring sources in Jorayal Rural Municipality (JRM) (Fig. 2) were located, mapped and documented. The elevation of the mapped springs ranges from 956 to 1855 masl; however, 47.5% of the springs are located in the elevation lower than 1,200 masl. About 88.1% (or 141 number) of springs in the JRM are perennial and rest are of seasonal nature. As per local knowledge gained through questionnaire survey, springs with discharge increasing, no change, decreasing, and completely dried up are 0.6% (1 spring), 67.5% (108 springs), 27.5% (44 springs), and 4.4% (7 springs), respectively (Table 2). The drying-up of springs was attributed to the rapidly increasing haphazard construction of rural roads, deforestation for farming purpose, and no proper management efforts for the spring source conservation and management. Similarly, likely reasons for the decreasing rate of discharge could be water channel diversion, improper management, deforestation, construction of road and other infrastructures that block the passage of water discharge.
Rate of flow (or discharge) from the springs during both pre-monsoon and post-monsoon seasons was analyzed by classifying into three classes, which are: below 5 L-per-minute (lpm), 5–10 lpm, and 10–50 lpm. Results as tabulated in Table 2 showed that relative proportions of springs under each category for both pre- and post-monsoon seasons are comparable. In both the seasons, a vast majority of the springs are within the category of below 5 lpm discharge and other two category have same proportion of springs. Discharge in the post-monsoon are higher than in the pre-monsoon (or dry season), which is because of saturation of soil as well as subsequent infiltration of water during monsoon season and subsequent re-appearance of the same in the springs right after the monsoon season. No data were recorded for 2 springs in the post-monsoon season due to inaccessibility.
These results indicate relatively more water scarcity in the pre-monsoon season than in the post-monsoon season in the study area. The results were further validated with information collected from the local people during interactions. For example, during pre-monsoon season, household having piped water also have to collect water from the nearest springs located far from their settlements. In the study area, though most of the household have access to piped water supply system, almost half of the household are deprived of adequate and reliable supply of drinking water. Those households are using and will continue to use spring water. Springs are being used for many purposes like drinking/domestic (73%), and irrigation (15%) purposes; however, 12% of the mapped springs are not in use except for meeting natural ecological needs. The reasons for not using those springs were located relatively far from the settlements, nearby households have access to piped water supply, some of the unused springs were at the locations where waste were disposed long years back, and there was algal formation inside the springs. However, no management and conservation effort initiated from the local and national levels so far.
Characterization of spring water quality
Forty out of 160 spring sources (Fig. 1) were selected for water assessing water quality and suitability for various uses. Physio-chemical and hydro-chemical properties of spring water were analyzed for pre- and post-monsoon seasons. Maximum, minimum and, average values were calculated for these two seasons to characterize water quality between the two seasons and the results are reported in Table 3. Details water quality of all forty springs for pre- and post-monsoon seasons are provided in Appendix 1 and Appendix 2, respectively.
Seasonal variations of physico-chemical characteristics
The seasonal variations of physico-chemical parameters were evaluated for pre- and post-monsoon seasons (Table 3). pH values were found alkaline in nature. Even though pH has no effect on human health, it is closely related to other chemical constituents of water (Ako et al. 2012). No significant variation in two seasons was observed. WHO has suggested that the drinking water should have pH in the range of 6.5–8.5. The average pH value was within the standard guideline values set up by Nepal Drinking Water Quality and WHO. pH of water is relatively neutral as the sources are at the forest, vegetation area and geology and decaying of vegetation in the soil could produce humic acid (Mesner and Geiger 2005).
TDS vary from 22 to 186 mg/L in pre-monsoon and from 24 to177 mg/L in post-monsoon season, thus indicating similar ranges in both the seasons. TDS depends on the number of organic and inorganic salts are found dissolved in natural. A low level of TDS contents of the spring water allows the water for drinking and other domestic uses, whereas higher concentration causes salty water and distress to domestic animals (Chatterji and Razuiddin 2002).
EC ranges are also comparable in both seasons, with value ranging from 54 to 372 μS/cm in pre-monsoon and from 50 to 354 μS/cm in the post-monsoon season. The variation in the values might be due to total dissolves salts in water and its organic load (Ako et al. 2012). Also, variations in EC and TDS values are primarily attributed to geochemical process like ion exchange, evaporation, precipitation, dilution, discharge, local geological settings, rock–water interaction, sediment dissolution, rainwater infiltration, and anthropogenic sources (Ehya and Marbouti 2016). The high conductivity is some water samples could be due to the presence of calcium and magnesium ions contributing to the increase conductivity in some samples (Deshwal et. Al 2016).
Bicarbonate ranged from 12 to 128 mg/L in both seasons, it is caused by HCO3− ion only. It suggested that dissolution has been occurred due to interaction between soil and rainwater, giving groundwater alkaline character (Singh et al. 2012). The carbonate and bicarbonate concentration in spring water is derived from carbonate weathering, as well as dissolution of carbonic acid in the aquifers (Kumar et al. 2009).
Calcium concentration ranges from 3.2 to 60 mg/L in pre-monsoon season and 3.2 to 144 mg/L in post-monsoon season. The calcium concentration of 144 mg/L is within the NDWQS (2005) limit for drinking water quality; however, slightly higher than WHO (2004) limit. The likely reason for higher concentration could be geological formation of the region, which consists of calcium-rich minerals, limestone, carbonates, dolomites and basic rocks (Fuchs and Frank 1970; Dhital 2015). Another potential reason for the higher concentration of calcium in the post-monsoon season could be due to impact of soil in the agricultural area and forest area. As less precipitation occur during this time, its concentration might get accumulated.
Magnesium concentration ranges from 0.4 to 60.5 mg/L in pre-monsoon season and 0.5 to 60.5 mg/L in post-monsoon season. Sulfate values ranges from 0.2 to 6.8 mg/L and 0.3 to 5.7 mg/L in pre- and post-monsoon, respectively. High sulfate content may be due to breakdown of organic substances of weathered soil and anthropogenic activities (Miller 1979) but the study area contains quite less sulfate which indicates less amount of sulfur compounds, gypsum and iron sulfide. Similar variations show in post-monsoon as well.
Na+ and K+ concentrations in spring water range from 1.2 to 7.9 mg/L in pre-monsoon season and 0.5 to 8 mg/L in post-monsoon season, and 0.3 to 2.7 mg/L in pre-monsoon season and 0.3 to 2.8 mg/L in post-monsoon season respectively (Table 3). So, there is similar values in both seasons. Aghazadeh et al. (2017) reported that high concentrations of Na+ in the ground water are attributed to cation exchange among minerals and anthropogenic activities (agricultural activities) and poor drainage conditions. But in this study, values are much lower which indicates that there are low anthropogenic activity and cations exchange among minerals. Bicarbonates and calcium are the dominant anion and cation in the study area. Thakur et al. (2016) also showed that the bicarbonate and calcium as dominate anion and cation in the study area. The area was geologically carbonate dominated so that the concentration of bicarbonate was much higher than the other anions. There are no much seasonal variations in physico-chemical parameters in spring water of the study area. The abundance of the major ions in spring sources in both seasons was in following order:
Ca2+> Mg2+ > Na+ > K+ and HCO3− > Cl− > SO42−
The low concentration of nitrate and phosphate is due to less anthropogenic activity, minimum use of chemical fertilizer and disposal of waste products (Alemu et.al 2015). The value of nitrate ranges from 0.3 to 20.9 mg/L in pre-monsoon season and 0.3 to 19.5 mg/L in post-monsoon season.
The average concentration of phosphate ranged from 0.3 to 0.6 mg/L in pre-monsoon and 0.3 to 0.7 mg/L in post-monsoon season. The iron content is very minimum as well, i.e., ranged from 0.24 to 0.25 mg/L in pre-monsoon season and 0.2 to 0.3 mg/L in post-monsoon season but found high in lower elevation in Punjab, India (Kaur et al. 2017). Thus, the result showed that there are not seasonality variations among concentrations of parameters.
Hydro-chemical characteristics
Hydro-chemical characteristics of the spring sources were analyzed for both the seasons and the results are depicted in Fig. 3 and tabulated in Table 4. Water quality data of both the seasons are plotted in Piper trilinear diagram (Fig. 3), which is a graphical representation of the chemistry of water samples, to understand sources of the dissolved constituents of water (Piper 1944). It reveals similarities and difference among the water samples because those with similar qualities will tend to plot together as groups. It is expressed in mill-equivalent percentage (meq %). It contains two triangular faces and one intervening diamond-shaped field. The results plotted in the central diamond field show the overall characteristics of the spring water chemistry, i.e., the dominance of the alkaline earth metals (Ca2+ and Mg2+) over the alkalis (Na+ + K+) and the weak acids (HCO3−) over the strong acids (Cl− and SO42−). In general, the classification of the sample points in the piper diagram is done into six fields. They are: (i) Ca–HCO3, (ii) Na–Cl, (iii) Ca–Mg–Cl, (iv) Ca–Na–HCO3, (v) Ca–Cl, and (vi) Na–HCO3. The percentage reacting values at the three cation groups, i.e., Ca2+, Mg2+ and (Na+ + K+) are plotted in the left triangular field. Similarly, the percentage reacting values at the three anion groups, i.e., (HCO3− + CO32−), SO42− and Cl− in the right triangular field.
In pre-monsoon and post-monsoon seasons, on the cation plot, most of the samples lie in the lower left corner, indicating the dominance of the calcium type and few samples display that at the sodium and potassium ions indicating the influence of Na+ and K+ (Fig. 3). The anion diagram shows all the samples lie at the bottom which is bicarbonate and dominated type. It showed 52% of the water samples belong to Ca–HCO3 water type, which indicates that carbonate hardness exceeds 50%. Similarly, for the post-monsoon season, 55% of the water samples belong to Ca–HCO3 type which indicates that carbonate hardness exceeds 50%. The calcium bicarbonate water resulted mainly from dissolution of limestone and calcium–sodium bicarbonate resulted from dissolution of limestone in the catchment area (Nguyet and Goldscheider 2006). Similar information was also provided by Sahu and Sikdar (2008) that water belonging to Ca–HCO3 might be due to the carbonate dissolution process and wide contact with limestone and that might represent permanent hardness of the groundwater. Thus, the above results indicated no substantial change in hydro-chemical characteristics of spring water during pre- and post-monsoon seasons which clearly indicates that most of the major ions are of natural origin.
Mechanism controlling spring water quality
Chemical composition of spring water is the result of the rock–water interaction and chemical processes (Sreedhar and Nagaraju 2017). Gibb’s diagram (Gibbs 1970) is plotted as scatter diagram to illustrate dominance of the three important natural mechanisms controlling the major ion chemistry of the spring water, which includes rock–water interaction, evaporation and atmospheric precipitation (Fig. 4). The diagram includes TDS vs Na/(Na + Ca) for cations against TDS vs Cl/(Cl + HCO3) for anion. The locations of the samples illustrate the dominancy of geogenic factors in both seasons. The dominant mechanism that control spring water quality in the study area is the rock–water interaction followed by precipitation as most of the samples are plotted in the rock-dominance area followed by precipitation-dominance area (Fig. 4). The rock–water interaction ultimately controls the chemical composition of water (Raju et al. 2015). The samples falling in evaporation dominant area signifying the role of evaporation on groundwater/spring water chemistry (Kaur et al. 2017) but in this study, the role of evaporation was non-existence.
The results obtained are in good agreement with those obtained in alluvial aquifer region (Dhakate and VV S 2015). The Gibb’s plot, thus, indicates that the geology/lithology plays a significant role in controlling the overall quality of groundwater/spring water apart from various other local environmental conditions in the study area. The diagram includes TDS vs Na/(Na + Ca) for cations against TDS vs Cl/(Cl + HCO3) for anions. For the identification of major sources of ions, there are three conditions in Gibbs plot: (i) low TDS (0.5 to near about 1), indicating at lower right corner and reflecting the influence of precipitation; (ii) medium TDS (70–300 mg/L) and low Na+ vs (Na+ + Ca2+) ratio (300 mg/L); and (iii) high Na+ vs (Na+ + Ca2+) ratio (> 0.5 to near about 1) locating on the upper right corner and reflecting the influence of evaporate dissolution (Gibbs 1970). So, the minimum value that validate the parameter as belonging to the main component in the Gibbs method is < 0.5. As TDS value ranges from 22 to 186 mg/L in pre-monsoon season and from 24 to 177 mg/L in post-monsoon seasons, the mechanism that controls the spring water samples has a predominance of rock weathering including some precipitation dominance as well. It showed consistent distribution of anions in both the seasons and rock weathering was the main process controlling the hydro-chemical types of ground water in the study area. There is very low seasonal variation in controlling spring water hydrochemistry which might be due to similar kind of rainfall pattern and low dilution rate but, however, can be variation can be seen as compared with rainy season. In particular, the groundwater recharge from rainfall resulted in the dilution of chemical compositions in groundwater, and this promoted the similarity of chemical types at different locations.
Association among the hydro-chemical attributes
The PCA and correlation coefficient matrix were used to understand association among the different hydro-chemical attributes. PCA is a powerful technique applied to the geochemical dataset to reduce the dimensionality consisting of large number of interrelated variables (Hundal 2011). It identifies the governing principle of chemical composition of spring water (Pant et al. 2018). Grouping of parameters in the loadings suggests their significant mutual positive relationship. Factor loading plot of the PCA is shown in Fig. 5 and variables associated with each component and the percentage of variance that the component explains in Table 5. Three principle components are identified (Fig. 5), which can explain 87.95% of variance in the hydro-chemical characteristics of the spring water. The PC1, PC2, and PC3, account for 48.96, 24.36, and 14.63% of the variance in hydro-chemical characteristics with eigen values of 6.365, 3.167 and 1.092, respectively. The PC1 has strong loadings on sulfate, sodium, nitrate, magnesium and iron, which are mainly responsible for breakdown of organic substance, anthropogenic activities (agricultural activities), and cation exchange among minerals in groundwater, (Miller 1979; Aghazadeh et al. 2017; Sahu and Sikdar 2017). The PC2 has strong loading on EC, TDS, chloride and bicarbonate, which might due to total dissolved salts and its organic concentration and dissolution of carbonate weathering (Kumar et al. 2009; Ako et al. 2012). The PC3 has loading on Ca++ and TH, which might indicate carbonate weathering and calcium-rich minerals, limestone and carbonates (Pant et al. 2018; Kumar et al. 2009; Dhital 2015). There is no significant difference between pre- and post-monsoon seasons in this analysis which might be due to low dilution rate and same geological condition.
We further attempted to analyze whether few numbers of variable can sufficiently predict other variables, using correlation matrix between two variables at 95% confidence interval (Table 6). Correlation matrix is a widely used statistical tool to establish the relationship between two hydro-chemical attributes for predicting the dependency of one variable to another (Pant et al. 2018). Since most of the chemical parameters were not normally distributed, correlations between major ions were carried out using Spearman’s correlation analysis. Strong positive correlation was observed between EC and HCO3, Ca and EC, Ca and HCO3, Mg and HCO3, NO3− free CO2, and Ca and Mg. It represents a common origin of these ions which might be weathering of carbonate rocks and higher influence on spring water quality rather than other physical parameters. Strong negative correlation was observed between PO4 and Cl, which indicate no potential anthropogenic interferences with the natural water quality. It is because the presence of phosphate in the groundwater is due to the presence of natural minerals or introduction of pollution from fertilizer sewage and industrial pollutants in the nearby area (Alemu et. al. 2015). However, there is very low application of fertilizer in the study area as farmers use organic fertilizer produced from agricultural residue, manure of domestic animals, slurry or any other decomposable materials. People have less access to inorganic or chemical fertilizer. There is no correlation between Cl and SO4, and NO3 is not correlated with any of the parameter as there is no any source for nitrate formation due to less influence of anthropogenic activity. Thus, we can conclude that some parameters are interrelated with one another due to some common origin of geological activity, some show less or no correlation between parameters and has no influence upon one another Table 7.
Spring water’s suitability for drinking
The maximum, minimum and average were calculated for all physico-chemical and hydro-chemical parameters for both pre- and post-monsoon seasons (Table 3) and they were evaluated against drinking water quality standard set by the Government of Nepal and WHO (2004). The parameters include pH, EC, TDS, turbidity, chloride, bicarbonate, free CO2, total hardness, calcium, magnesium, ammonia, nitrate, phosphate, sulfate, iron, sodium and potassium. The concentration of major ions in the spring water samples and their comparisons with the NDWQS (2005) and WHO (2004) is shown in (Table 3). In general, the concentration of major ions of all springs water was within the NDWQS (2005) and WHO (2004). However, some samples, i.e., 90% of the sample in pre-monsoon and 95% of the samples in post-monsoon season showed pH concentration above the NDWQS (2005) permissible limit and 93% of the sample in pre-monsoon and 95% of the sample in post-monsoon showed pH concentration above WHO (2004). This, based on field observation, is due to algal formation, waste disposal and not in use since long time. Also, seasonal variation of water quality parameters was found relatively lower. 90% of the samples in pre-monsoon and 97% of the samples in post-monsoon season were within the WHO permissible limit; whereas, 100% of the samples were in the guideline limit. 95% of the samples in pre- and post-monsoon seasons were within the WHO (2004) guideline limit. The results showed that all the parameters in both the seasons comply with national drinking water quality standard and, therefore, are suitable for drinking (Table 3).
The water quality was also evaluated by computing water quality index (WQI). The results showed WQI values range from 16.08 to 62.51 in the pre-monsoon season. Results indicate 87% (35 nos) of spring water samples are having excellent water, remaining 13% (5 nos.) have good water, and none has bad water quality. The excellent water requires no further treatment and good water requires some basic treatment and only bad water requires special treatment and suggested for unsuitable water for drinking purposes. Similarly, in the post-monsoon season, the WQI values ranges from 13.00 to 70.23. Some 82% (33 nos) of the samples have the excellent water quality, 18% (7 nos.) have good water quality, and none have bad water quality. Most of the samples are of good quality in the study area because there is still very low human disturbance, low expose to toxic inorganic substances, heavy metals, bacterial and other pathogens increased nitrogen concentration and other trace elements and micronutrients in drinking water resources.
Spring water’s suitability for irrigation
Spring water suitability for irrigation in this study was assessed from the perspective of alkalinity hazard, salinity hazard, sodium percentage (Na+%), magnesium ratio, and corrosivity ratio as suggested in (Tripathi et al. 2012).
Alkalinity hazard
The sodium absorption ration (SAR) was used as an indicator of Alkalinity hazard. SAR values range from 0.05 to 1.26 in pre-monsoon and from 0.02 to 1.09 in the post-monsoon seasons (Table 8). It indicates 100% of the samples in both the seasons have excellent water quality for irrigation from alkalinity perspective. A high SAR content in irrigation water has a potential to limit the growth of crops and plants due to lowering of permeability subsequent lack of soil moisture (Pant et al. 2018 and Compton 2011). SAR greater than 12 was considered sodic and threatens the survival of vegetation by increasing soil dispersion and reducing soil permeability (Kuipers et al. 2004).
Salinity hazard
Electrical conductivity (EC) is a good measurement of salinity hazard to crops as it reflects the TDS in groundwater. The primary effect of high EC is that it reduces the osmotic activity of plants and, thus, interferes with the absorption of water and nutrients from the soil (Ako et al. 2012). Each parameter may vary across the seasons and therefore required to evaluate in different seasons (Ouyang et al. 2006). A graphical representation of irrigated water (US salinity Laboratory 1954) and the integrated effect of EC and SAR concluded that the spring water of the study area possess high salinity with low sodium. In this study, all the water samples are falling under high specific conductance and low sodium C1S1 and C2S1, as per US Salinity Laboratory (1954), in both the seasons. In the Fig. 6, blue triangles are water samples of pre-monsoon and orange circles are water samples of post-monsoon season. The x-axis on the graph represents electrical conductance and y-axis represents sodium percent ratio (SAR). According to the USSL diagram (Fig. 6), all water samples falling under very high specific conductance and low sodium (C1S4 and C2S4) are excellent quality and suitable for irrigation purpose. The water did not produce any undesirable effects of changing soil properties, does not reduce soil permeability and does not compact (Kelley 1951). Thus, spring water in the study area are in excellent condition, with high salinity and low sodium, for irrigation purpose.
Quality of spring water in relation to electrical conductivity (EC) and Sodium Adsorption Ratio (after US Salinity Laboratory 1954). Blue triangles and orange circles are water samples of pre-monsoon and post-monsoon seasons, respectively. The x-axis represents the electrical conductance and y-axis represents sodium
Sodium percentage
Wilcox (1955) adopted EC against sodium for classification of spring water for irrigation suitability. In the Fig. 7, blue asterisks are pre-monsoon samples and orange squares are post-monsoon samples. The different indicators are given to understand the result of water samples of pre- and post-monsoon season. (EC). The analytical data plotted on Wilcox diagram explain that 90% (30 nos.) of the total samples fall in excellent and good category and no sample falls in unsuitable category (Fig. 7). Similar results were obtained in both seasons. The Na+ plays a very major role in the suitability of the water for irrigation purpose, because higher amount destroys the plants and crops, damage soil structure and dispersion of clay particles (Pant et al. 2018). The role of sodium for irrigation was emphasized because of the fact that sodium reacts with soil and as a result clogging of particles, thereby reducing the permeability (Siddiqui et al. 2005). Thus, all samples of spring water were found to be suitable for irrigation purpose in both seasons.
Magnesium ratio (MR)
Generally, Ca++ and Mg++ are present in equilibrium in most of the waters. The quality of soil is affected adversely when magnesium content is high in water, resulting in alkaline nature of the soil and thereby reducing the crop yield (Kumar et al. 2007). Based on MR of the spring water in this study (Table 8), 35 nos. of the samples were unsuitable and 5 nos. of samples were suitable for irrigation in pre-monsoon season whereas 30 nos. of samples were unsuitable and 10 nos. of samples were suitable for irrigation in post-monsoon season based on magnesium ratio (Table 8).
Corrosivity ratio (CR)
The water is considered safe for the transport by pipes if corrosivity ratio is less than 1, while the value greater than 1 indicates corrosive nature of water (Tripathi et al. 2012). At least 85% of the spring water samples in this study have CR of less than 1, indicating that the spring water is safe for irrigation (Table 8). Thus, except magnesium hazard, the spring water is safe against all other four forms of hazards, and therefore, suitable for irrigation purpose.
Conclusions
The study assessed status and characteristics of spring sources at Jorayal Rural Municipality in the Thuligaad watershed, western Nepal, and evaluated their suitability for drinking and irrigation purposes. 160 springs located at elevation range of 956–1893 masl were mapped and their status were assessed. Out of them, 40 were selected for detailed analysis of water quality and evaluating for different uses. Key conclusions are listed hereunder;
-
Overall status of springs: most of the springs (95%) are perennial; located at the elevation of less than 1200 masl (47.5%) or at the elevation range of 1400–1600 masl (23.1%); have discharge less than 5 L per minutes (lpm) (80% of springs in pre-monsoon season); and have no trend (67.5%) or decreasing trend (27.5%) in discharge. Most of the springs (73%) are being used for drinking or domestic purposes.
-
Water quality characteristics of the springs: calcium and bicarbonate are the dominant anion and cation in the study area. The abundance of major cations and anions in the spring water of the study area during both the seasons are Ca2+ > Mg2+ > Na+ > K+ and HCO3− > Cl− > SO42−, respectively. The water chemistry is dominantly Ca-HCO3 (52% in pre-monsoon and 55% in post-monsoon) and Mixed Ca–Mg–Cl (28% in pre-monsoon and 25% in post-monsoon) types. The dominant mechanism that controls spring water quality in the study area is the rock–water interactions, followed by precipitation. There is dominance of geogenic factors in the water quality in both the seasons. Three principal components (PCs) are identified that account for 87.95% of total variance in spring water quality–PC1 accounts for 48.96% and is related to geo-chemistry or dissolution process of minerals; PC2 accounts for 24.36% of variance and is related to dominance of atmospheric inputs, carbonate weathering, and anthropogenic activities; and PC3 accounts for 14.63% of variance and is related to natural environmental hydro-geochemical dominance. Correlation analysis revealed that natural process such as mineral dissolution, precipitation and cations exchange are dominant factors influencing the water chemistry.
-
Suitability of spring water for drinking purpose: more than one-fifth (87% in pre-monsoon and 82% in post-monsoon seasons) water samples are of excellent water quality as indicated by water quality index (WQI); and most of the water quality parameters in both the seasons are within the permissible limits for drinking water defined by Nepal Drinking Water Quality Standard (NDWQS) and the World Health Organization (WHO). Therefore, the spring water is suitable for drinking purpose.
-
Suitability of spring water for irrigation purpose: except magnesium hazard, the spring water is safe against all other four forms of hazards, and therefore, suitable for irrigation purpose.
The results of this study provided valuable information on spring water characteristics which can potentially be used for developing strategies for spring-shed protection, spring water conservation, and management of spring water sources in the study area. This study could also help characterize spring water quality in the areas having similar geographical structure and conditions. This study certainly provides a useful baseline on quality and potential usability of spring water in the area, however, continuous monitoring is required for sustainable utilization of the water sources for various purposes in a long run.
References
CBS (2015).Central Bureau of Statistics. Kathmandu. Statistical Year Book Nepal. Kathmandu: Government of Nepal. Accessed Jan 11, 2018. (http://cbs.gov.np/image/data/2017/Statistical%20Year%20Book%202015.pdf).
Aghazadeh N, Chitsazan M, Golestan Y (2017) Hydrochemistry and quality assessment of groundwater in the Ardabil area Iran. Appl Water Sci 7(7):3599–3616
Ako AA, Shimada J, Hosono T, Kagabu M, Ayuk AR, Nkeng GE, Eyong GET, Takounjou ALF (2012) Spring water quality and usability in the Mount Cameroon area revealed by hydrogeochemistry. Environ Geochem Health 34(5):615–639
Alemu ZA, Teklu KT, Alemayehu TA, Balcha KH, Mengesha SD (2015) Physicochemical quality of drinking water sources in Ethiopia and its health impact: a retrospective study. Environ Syst Res 4:22
Al-Khashman OA (2008) Assessment of the spring water quality in The Shoubak area Jordan. Environmentalist 28(3):203–215
APHA (2005) Standard methods for examination of water and wastewater, 21st edn. American Public Health Association, Washington, D.C
Batabyal AK, Chakraborty S (2015) Hydrogeochemistry and water quality index in the assessment of groundwater quality for drinking uses. Water Environ Res 87(7):607–617
Batool A, Samad N, Kazmi SS, Ghufran MA, Imad S, Shafqat M, Mahmood T (2018) Spring water quality and human health: an assessment of natural springs of margalla hills Islamabad zone—III. Int J Hydrol 2:41–46
Bhat NA, Jeelani G (2016) Hydrogeochemical assessment of water resources of Bringi Watershed, Kashmir Himalayas, India; implication on drinking and irrigation water quality. Int J Plant Animal Environ Sci 6(1):215–227
Bhusal J, Gyawali P (2015) Water quality of springs in Badigad Catchment, Western Nepal. Bull Dep Geol 18:67–74
CBS (2011) Central Bureau of Statistics. Kathmandu, Nepal. https://www.npc.gov.np/en/page/99/department/central_bureau_of_statistics_cbs_. Accessed 11 Jan 2018
Chapagain PS, Ghimire M, Shrestha S (2019) Status of natural springs in the Melamchi region of the Nepal Himalayas in the context of climate change. Environ Dev Sustain 21(1):263–280
Chatterji C, Raziuddin M (2002) Determination of water quality index (WI) of a degraded river in Asnol Industrial area, Ranigunj, Burdwan, West Bengal. Nat Environ Pollut Technol 1(2):181–189
Chauhan JS, Badwal T, Badola N (2020) Assessment of potability of spring water and its health implication in a hilly village of Uttarakhand India. Appl Water Sci 10(2):73
Chung S, Venkatramanan S, Kim T, Kim D, Ramkumar T (2015) Influence of hydrogeochemical processes and assessment of suitability for groundwater uses in Busan City, Korea. Environ Dev Sustain 17(3):423–441
Collins R, Jenkins A (1996) The impact of agricultural land use onstream chemistry in the Middle Hills of the Himalayas. Nepal J Hydrol 185(1):71–86
Compton A (2011) A review of rationale for EC and SAR standards; water quality planning bureau, water quality standards section; montana department of environmental quality. Helena, MT, USA
Deshwal JP, Saharan JP, Deshwal BR (2016) A study on water quality parameters of drinking water of rural areas of Narwana, Haryana (India). Int J Curr Res 8:39329–39333
DhakateVV RSG (2015) Hydrochemical assessment of groundwater in alluvial aquifer region, Jalandhar District, Punjab India. Environ Earth Sci 73(12):8145–8153
Dhital MR (2015) Geology of the Nepal Himalaya: regional perspective of the classic collided orogen. Springer, London, UK. ISBN: 978-3-319-02496-7
Ehya F, Marbouti Z (2016) Hydrochemistry and contamination of groundwater resources in the Behbahan plain SW Iran. Environ Earth Sci 75(6):455
Fuchs G, Frank W (1970) The geology of West Nepal between the rivers kali gandaki and thulo bheri. Wien Publishing
Gaglioti S, Infusino E, Caloiero T, Callegari G (2017) Hydrochemical and qualitative assessment of natural water spring in southern Italy. Euro Water 57:399–405
Gibbs RJ (1970) Mechanisms controlling world water chemistry. Science 170(3962):1088–1090
Glover ET, Akiti TT, Osae S (2012) Major ion chemistry and identification of hydrogeochemical processes of groundwater in the Accra Plains. Geoscience 50:10279–10288
NDWQS (2005) National drinking water quality standards. http://climate.mohp.gov.np/downloads/National_Drinking_Water_Quality_Standards_2005.pdf. Accessed 11 Jan 2018
Hundal H (2011) Geochemistry and assessment of hydrogeochemical processes in groundwater in the southern part of Bathinda district of Punjab, northwest India. Environ Earth Sci 64(7):1823–1833
Ilmonen J, Mykrä H, Virtanen R, Paasivirta L, Muotka T (2012) Responses of spring macroinvertebrate and bryophyte communities to habitat modification: community composition, species richness, and red-listed species. Freshw Sci 31(2):657–667
Kaur T, Bhardwaj R, Arora S (2017) Assessment of groundwater quality for drinking and irrigation purposes using hydrochemical studies in Malwa region, southwestern part of Punjab India. Appl Water Sci 7(6):3301–3316
Kelley WP (1951) Alkali soils; their formation, properties, and reclamation. Reinhold Publishing, New York
Kuipers JR, MacHardy K, Merschat W and Myers T (2004) Coal bed methane-produced water: management options for sustainable development, In Proceedings. 11th International Petroleum Environmental Conference. Albuquerque, NM, USA, pp 12–15
Kumar M, Kumari K, Ramanathan A, Saxena R (2007) A comparative evaluation of groundwater suitability for irrigation and drinking purposes in two intensively cultivated districts of Punjab India. Environ Geol 53(3):553–574
Kumar H, Saini V, Kumar D, Chaudhary RS (2009) Influence of trisodium phosphate (TSP) anti-salant on the corrosion of carbon steel in cooling water systems Indian. J Chem Technol 16:401–410
Memon AH, Ghanghro AB, Jahangir TM, Lund GM (2016) Arsenic contamination in drinking water of district Jamshoro, Sindh Pakistan. Biomed Lett 2(1):31–37
Mihale MT (2015) Nitrogen and phosphorous dynamics in the waters of the great Ruaha River, Tanzania. J Water Res Ocean Sci 4:59–71
Mofor NA, Tamungang EBN, Mvondo-Zé AD, Kome GK, Mbene K (2017) Assessment of physico-chemical and heavy metals properties of some agricultural soils of Awing, North West, Cameroon. Arch Agricult Environ Sci 2(4):277–286
Nguyet VTM, Goldscheider N (2006) Tracer tests, hydrochemical and microbiological investigations as a basis for groundwater protection in a remote tropical mountainous karst area Vietnam. Hydrogeol J 14(7):1147–1159
Ouyang Y, Nkedi-Kizza P, Wu Q, Shinde D, Huang C (2006) Assessment of seasonal variations in surface water quality. Water Res 40(20):3800–3810
Oyem HH, Oyem IM, Ezeweali D (2014) Temperature, pH, electrical conductivity, total dissolved solids and chemical oxygen demand of groundwater in Boji-BojiAgbor/Owa Area and immediate suburbs. Res J Environ Sci 8:444–450. https://doi.org/10.3923/rjes.2014.444.450
Paliwal K, Singh S (1967) Effect of gypsum application on the quality of irrigation waters. Madras Agric J 59:646–647
Pandey R, Venkatesharaju K, Somashekar RK (2010) Major ion chemistry and hydrochemical studies of groundwater of Bangalore South Taluk India. Environ Monit Assess 163(1–4):643–653
Pant RR, Zhang F, Rehman FU, Wang G, Ye M, Zeng C, Tang H (2018) Spatiotemporal variations of hydrogeochemistry and its controlling factors in the Gandaki River Basin, Central Himalaya Nepal. Sci Total Environ 622:770–782
Petraccia L, Liberati G, Masciullo SG, Grassi M, Fraioli A (2006) Water, mineral waters and health. Clin Nutr 25(3):377–385
Piper AM (1944) A graphic procedure in the geochemical interpretation of water-analyses. EOS Trans Am Geophys Union 25(6):914–928
Poudel DD, Duex TW (2017) Vanishing springs in nepalese mountains: assessment of water sources, farmer’s perceptions, and climate change adaptation. Mt Res Dev 37(1):35–46
Raju NJ, Patel P, Gurung D, Ram P, Gossel W, Wycisk P (2015) Geochemical assessment of groundwater quality in the Dun valley of central Nepal using chemometric method and geochemical modeling. Groundw Sustain Dev 1(1–2):135–145
Raman V (1983) Impact of corrosion in the conveyance and distribution of water. J Indian Water Works Assoc 15(1):115–121
Richards L (1954) Diagnosis and improvement of saline and alkali soils. Handbook 60. https://www.ars.usda.gov/ARSUserFiles/20360500/hb60_pdf/hb60complete.pdf. Accessed 15 April 2018
Sahu P, Sikdar P (2008) Hydrochemical framework of the aquifer in and around East Kolkata Wetlands, West Bengal India. Environ Geol 55(4):823–835
Sharma B, Nepal S, Gyawali D, Pokharel G, Wahid S, Mukherji A, Acharya S, Shrestha A (2016) Springs, storage towers, and water conservation in the midhills of Nepal. International Centre for Integrated Mountain Development (ICIMOD), Kathmandu, Nepal
Siddiqui A, Naseem S, Jalil T (2005) Groundwater quality assessment in and around Kalu Khuhar, super highway, Sindh Pakistan. J Appl Sci 5(7):1260–1265
Simeonov V, Stratis J, Samara C, Zachariadis G, Voutsa D, Anthemidis A, Sofoniou M, Kouimtzis T (2003) Assessment of the surface water quality in Northern Greece. Water Res 37(17):4119–4124
Simiyu G, Ngetich J, Esipila T (2009) Assessment of spring water quality and quantity, and health implications in Tongaren division, Nzoia River catchment, Kenya. Afr J Ecol 47:99–104
Singh AK, Mondal GC, Singh TB, Singh S, Tewary BK, Sinha A (2012) Hydrogeochemical processes and quality assessment of groundwater in Dumka and Jamtara districts, Jharkhand, India. Environ Earth Sci 67:2175–2191
Sreedhar Y, Nagaraju A (2017) Groundwater quality around Tummalapalle area, Cuddapah District, Andhra Pradesh India. Appl Water Sci 7(7):4077–4089
Subramani T, Elango L, Damodarasamy S (2005) Groundwater quality and its suitability for drinking and agricultural use in Chithar River Basin, Tamil Nadu India. Environ Geol 47(8):1099–1110
Thakur T, Rishi MS, Naik PK, Sharma P (2016) Elucidating hydrochemical properties of groundwater for drinking and agriculture in parts of Punjab India. Environ Earth Sci 75(6):467
Tiwari T, Mishra M (1985) A preliminary assignment of water quality index of major Indian rivers Indian. J Environ Prot 5(4):276–279
Tripathi A, Mishra U, Mishra A, Tiwari S, Dubey P (2012) Studies of hydrogeochemical in groundwater quality around Chakghat area, Rewa District, Madhya Pradesh, India. Int J Mod Eng Res Technol 2(6):4051–4059
Tyagi S, Sharma B, Singh P, Dobhal R (2013) Water quality assessment in terms of water quality index. Am J Water Res 1(3):34–38
USAID (2018) Thuligaad watershed health report. https://pdf.usaid.gov/pdf_docs/PA00TRTZ.pdf. Accessed 29 Feb 2018
USSL (1954) Diagnosis and improvement of saline and alkaline soils, US Dept. of Agriculture, Hand Book, 60, 160
Walker JCG, Hays PB, Kasting JF (1981) A negative feedback mechanism for the long-term stabilization of Earth’s surface temperature. J Geophys Res 86(C10):9776–9782
WHO (2004) Guideline for drinking water quality, 3rd edn. Recommendations. World Health Organization, Geneva, 34. https://www.who.int/water_sanitation_health/dwq/GDWQ2004web.pdf. Accessed 20 Aug 2018
WHO (2011) Guidelines for drinking water quality, 4th (edn), WHO, Geneva. https://www.who.int/water_sanitation_health/publicatio ns/2011/dwq_guidelines/en/. Accessed 20 Aug 2018
Wilcox LV (1955) Classification and use of irrigation waters. U.S. Department of Agriculture, Washington, DC, p 696
Yilmaz E, Koç C (2014) Physically and chemically evaluation for the water quality criteria in a farm on Akcay. J Water Resour Prot 6:63–67. https://doi.org/10.4236/jwarp.2014.62010
Yogendra K, Puttaiah E (2008) Determination of water quality index and suitability of an urban waterbody in Shimoga Town, Karnataka. In: Proceedings of Taal2007: the 12th world lake conference. pp 342–346
Zhang Q, Liang X, Xiao C (2017) The hydrogeochemical characteristic of mineral water associated with water-rock interaction in Jingyu County, China. Proc Earth Planet Sci 17:726–729
Acknowledgements
We would like to acknowledge Central Department of Environmental Science (CDES), Tribhuvan University, Kathmandu, Nepal for their technical guidance, knowledge and support and necessary facilities to carry out this research. We would like to heartily acknowledge Youth Alliance for Environment (YAE) and Ministry of Industry, Tourism, Forest and Environment (MOITFE), Sudurpaschim province for providing such a great opportunity for conducting the research. We are also very much thankful to the reviewers for their critical and technical review and comments that helped in improving the quality of the manuscript. This research would not have been possible without support from my colleagues, local people and citizen scientists who helped in the field and research. We would like to thank everyone who helped directly and indirectly in this research process.
Funding
Not applicable.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Authors declare no conflict of interests.
Ethical approval
Not applicable, as it is not related to life sciences study.
Data availability
Yes, and available upon request.
Code availability
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Dumaru, B., Kayastha, S.P. & Pandey, V.P. Spring water assessment for quality and suitability for various uses: the case of Thuligaad watershed, western Nepal. Environ Earth Sci 80, 586 (2021). https://doi.org/10.1007/s12665-021-09826-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12665-021-09826-w