Abstract
The study was carried out in the Khandbari Municipality, Sankhuwasabha District, Eastern Nepal to document the spring location and assess the water quality of the spring water for drinking and irrigation purposes. A total of 85 springs were mapped, which are located from 274 to 2176 m in altitude. Spring water samples were collected from 33 springs in the pre-monsoon (November, 2021) and 31 springs in the post-monsoon (March, 2022). Correlation matrices, t-test, principal component analysis (PCA), Piper diagram, Gibbs diagram, water quality index (WQI), United States Salinity Laboratory (USSL) diagram, and Wilcox diagram were applied for evaluating the spring water. All the physicochemical parameters were within the Nepalese National Drinking Water Quality Standard (NDWQS) and drinking water quality guidelines of the World Health Organization (WHO) except for pH in the pre-monsoon and iron in the post-monsoon season. The main contributors to the groundwater are Na+, Ca2+, Cl-, total dissolved solids (TDS), and total hardness, which exhibit significant correlations with electrical conductivity (EC) similar to TDS, suggesting their common source of origin. Based on the WQI, spring water is excellent in the post-monsoon and excellent and good in the pre-monsoon season. Furthermore, the spring water is excellent for irrigation purposes except for the percent sodium in the post-monsoon and the magnesium ratio in the pre-monsoon season. Gibbs diagram illustrates that spring water is mainly governed by rock and precipitation dominance in some springs. The PCA indicates that anthropogenic activities (mixing of human waste and agricultural run-off in the spring water) are the main causes of contamination. Piper trilinear diagram demonstrates carbonate dissolution and silicate weathering as major processes for controlling the spring water chemistry. The study reveals that 62.5% of spring water was contaminated with microbes. For benthic macroinvertebrates, 18 springs were sampled, where nine orders and 17 families were recorded in the pre-monsoon and six orders and ten families in the post-monsoon season. The main influencing variables for macroinvertebrate assemblages are elevation, discharge, NO3-, and NH3.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Asia is one of the largest and most populated continents of the world, resulting serious water shortages and contamination due to anthropogenic activities (Shan et al. 2020). Springs are the important source of water mainly in the Himalayas to meet domestic and daily livelihood of the people (Verma and Jamwal 2022).
The groundwater in the mountains and hills is presented in the form of naturally occurring discharge as spring water (Khadka et al. 2019). The spring water is groundwater-fed and mainly accumulates during the monsoon season in the aquifers. Springs can be perennial or short-lived; perennial springs provide water throughout the year from a level below the groundwater table, and short-lived springs provide water for a short period just after the monsoon. Spring water is considered a lifeline in the mid-hills of Nepal (Sharma et al. 2016; Shrestha et al. 2016), as it is the only source of water (Chapagain et al. 2019; Pandit et al. 2019).
Groundwater quality in the world is a sensitive issue that can be evaluated by analyzing the physical, chemical, and biological parameters of water (Tyagi et al. 2020). A crucial factor for the development and regulation of the springs is believed to be spring discharge. A high discharge rate is considered valuable as it helps to reduce the spring water depletion problem (Taloor et al. 2020). However, the climate change and high demand of water have shown reduction of spring discharge in the Himalayan regions (Mahamuni and Kulkarni 2012). The parameters of spring water quality are critical to evaluating the water for human consumption, irrigation, or industrial use and are usually influenced by factors such as climate, types of sub-soil in which water flows, and groundwater utilization (Ragno 2007). However, it is not only affected by the characteristics of groundwater but also by the local environment. So, the quality of spring water is determined by the whole water system and its constituents, the hydrogeological unit, and the aquifers (Guo et al. 2019).
The analysis of the biological parameters of spring water is equally important. Macroinvertebrates are the biological indicators of water. Understanding the habitat preferences and having adequate knowledge of macroinvertebrates and factors influencing their assemblage help in the conservation of springs (Ilmonen et al. 2009; Ilmonen and Paasivirta 2005). Water chemistry has a direct effect on macroinvertebrate assemblages due to the osmoregulation process (Olson 2012). In Nepal, research on macroinvertebrates in rivers and lakes is common, but research on springs is limited.
Water pollution due to microbes is one of the most serious problems in the environment and has greatly affected human health (Amin et al. 2019). Escherichia coli is an indicator of fecal pollution in drinking water. Its contamination in water is commonly from the direct disposal of sewage in the drainage channel (Ghanem et al. 2021) and varies seasonally, mainly in the rainy season (WHO 2017).
In the Hindu-Kush Himalayan Region, spring water is the prime source of water for domestic purposes, which are on the verge of depletion (Negi and Joshi 2002). The settlement pattern is also usually influenced by the availability of spring water sources. However, springs are drying up and becoming seasonal, indicating the depletion of groundwater, which is a serious issue for society and the environment (Pandit et al. 2019). So proper mapping of spring water sources and its status is important today. However, the springs in Nepal are poorly understood and mapped (Sharma et al. 2016). In order to address the problem of springs, it is necessary to identify the spring sources and understand how they function for further water resource conservation and management strategy in the mid-hills. Therefore, this study provides a critical analysis of spring water quality, microbial analysis, and analysis of benthic macroinvertebrates in the Khandbari Municipality, Sankhuwasabha District, Eastern Nepal.
The study area
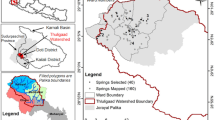
The Khandbari Municipality covers an area of 91.03 km2, which is located in Sankhuwasabha District of Koshi Province. This municipality is the district headquarter of Sankhuwasabha District, which consists of 11 wards (Fig. 1), and the study focuses on all the 11 wards that lie within the study area.
It extends between 27.368–27.382 N and 87.194–87.213 E with altitudes ranging from 274 to 2176 m. The greatest trans-Himalayan river, Arun, flows through Sankhuwasabha District, creating one of the deepest valleys in the world, Arun Valley. The Khandbari Municipality has a total population of 35,565 in 9309 households (NSO 2021). The major drinking water source in the Khandbari Municipality is spring water. A road connects Khandbari directly south to Biratnagar through Dhankuta, Dharan, and Itahari municipalities. It is the trading point for the people of Sankhuwasabha and some northeastern parts of Bhojpur District as well. A study area is also the main gateway to the 5th highest mountain in the world, Makalu. The study area comprises of two lithological formulations namely st and kgn. The st consists of grey to greenish grey phyllites, gritty, and quartzite with minor conglomeratic layer. Some basic intrusions are also noted with in st Formation. The kgn is the augen gneiss that is considered to be derived from the sediments of upper horizons of the Lesser Himalaya and is considered metamorphosed during the thrust movement (Amatya and Jnawali 1994).
Materials and methods
At the outset of the study, the mapping of the springs was conducted. Pre-monsoon (March 2022) and post-monsoon (November 2021) spring water samples were collected for the assessment of physicochemical parameters. A total of 33 springs in the post-monsoon and 31 in the pre-monsoon were assessed. The benthic macroinvertebrates were collected from 18 springs in the both seasons.
Spring mapping
Spring mapping was carried out in the Khandbari Municipality of Sankhuwasabha District. For the mapping purposes of the spring sources, both flowing and stagnant types were located using the global positioning system (GPS). The locals’ assistance was used to map all the springs, whether they were active or dried up. Using GIS (ArcMap 10.8) software, the GPS data was used to generate an inventory map.
Physicochemical parameters and discharge rate of the springs
The water sample collected was based on the importance and accessibility of the springs. It was collected for the physical and chemical analysis in a polyethylene bottle capable of holding at least 1 L. Before collecting the sample, proper care was taken to make the container neat and clean in order to avoid impurities. For handling, preservation, and analysis of the water sample, the standard procedure of APHA-AWWA-WEF (2017) was followed to ensure quality and consistency of the data. In the field, temperature, pH, electrical conductivity (EC), and total dissolved solids (TDS) were measured using a Hanna Combo Hi98129 (USA) multimeter probe, and dissolved oxygen (DO) was measured using an Oxy 70 Vio dissolved oxygen meter (China). Alkalinity, total hardness (TH), calcium hardness, magnesium hardness, free CO2, and chloride (Cl-) were analyzed using the titrimetric method following the APHA-AWWA-WEF (2017). Nitrate (NO3-), ammonia (NH3), and phosphate (PO43-) were measured by using a Lovibond field photometer (MD 610) (Germany) in the field. The water samples were preserved in an ice box that maintained a temperature of 4 °C for laboratory analysis before being transferred to the laboratory of the Central Department of Environmental Science, Tribhuvan University. Turbidity was measured using a 2100 AN IS turbidimeter (USA). The iron and sulphate were analyzed in the laboratory following the turbidimetric and phenanthroline methods using a spectrophotometer (SSI 2101, China), respectively, as per APHA-AWWA-WEF (2017). Similarly, potassium and sodium were analyzed by the flame photometric method using a flame photometer (ESICO 1382, China) as per APHA-AWWA-WEF (2017). The cation anion balance equation is computed using the Eq. (1):
Spring discharge was measured using a bucket and stopwatch method. This method is used to determine the discharge rate of springs, which is carried out only in the flowing springs. It is calculated in liters per minute (lpm) using Eq. (2):
Evaluation of spring water quality for drinking and irrigation purposes
The water quality index (WQI) is one of the best tools for the assessment of the quality of water and helps concerned citizens, authorities, and policymakers to communicate about the condition of water (Dohare et al. 2014). The WQI represents the complete water quality, which is computed using Eq. (3). It has been used in various studies for the analysis of water quality (Batabyal and Chakraborty 2015; Taloor et al. 2020). The weighted arithmetic water quality index (WAWQI) method classified water quality into various classes, following Brown et al. (1970) (Table 1).
where,
Qi = Quality rating scale
Wi = Unit weightage
For Qi,
where,
V i = estimated concentration of analyzed water
V 0 = ideal value of parameters in pure water (for pH = 7)
Si = Standard value of parameters (NDWQS)
For unit weight Wi,
where,
k=Proportionality constant and is calculated using Eq. (6):
In order to evaluate the irrigation water quality of the study area, SAR, %Na, the United States Salinity Laboratory (USSL) diagram, salinity hazards, magnesium ratios, and corrosivity ratio were selected. All the samples from both seasons were analyzed to understand the suitability of spring water for irrigation purposes. Evaluation of spring water quality for irrigation purposes is an important part of the study, as the study area is highly dominated by agricultural practices.
-
i.
Sodium adsorption ratio (SAR)
SAR is a measurement of a water’s suitability for irrigation of agricultural land. The water is less acceptable for irrigation when the sodium adsorption ratio is higher. The SAR values were determined using Eq. (7) (Richards 1954):
where concentrations are expressed in milliequivalent per liter (mEq/L).
-
ii.
Integrated effect of EC and SAR (salinity hazard)
SAR is plotted against the EC. This diagram was designed by the US Salinity Laboratory in 1954 and gives information about groundwater salinity and sodium ratio.
-
iii.
Sodium percentage
Clay particles absorb water with a high sodium content, which causes an exchange of Na+ ions in water and the displacement of Ca2+ and Mg2+ in soil. This Na+, Ca2+, and Mg2+ ion exchange mechanism lowers the permeability and finally leads to soil with inadequate internal drainage. The %Na is calculated using Eq. (8) (Todd and Mays 2005):
-
iv.
Magnesium ratio
Magnesium concentration is calculated using the Eq. (9):
-
v.
Corrosivity ratio
The susceptibility of groundwater to corrosion is measured by the corrosivity ratio (CR). The CR ratio less than 1 is regarded as safe for irrigation activities, while CR ratio greater than 1 is unsafe (Raju et al. 2011). It is calculated using Eq. (10):
Macroinvertebrates sampling and its identification
Benthic macroinvertebrate samples were collected using a net of 500-μm-mesh size from both flowing and stagnant springs (Ilmonen and Paasivirta 2005). In stagnant springs, by sweeping submerged or pressing mosses or muddy substrates, benthic macroinvertebrate samples were collected. For flowing springs, sampling time was assigned based on the spring area, viz., 2, 5, 10, 20, and 30 min for 1 m2, 2 m2, 3 m2, 4 m2, and 5 m2 of the spring area, respectively (Ilmonen et al. 2012). The samples were sorted in the field to reduce the amount of collected samples and preserved in 70% ethanol in the vial. In the laboratory, further identification was performed using a high-quality light microscope. Identification was done up to family level with the help of an identification key (Shah et al. 2020).
Microbial analysis
Fecal contamination in drinking water is a primary concern in terms of health because it might cause various diseases and poses a risk to the public health (Barakat et al. 2018). The presence/absence (P/A) vial was developed by the Environment and Public Health Organization (ENPHO), Kathmandu, Nepal, in 2001 and was employed to determine fecal contamination in the drinking water. It is a simple and low-cost method that has been used in drinking water quality analysis in different studies (Atreya et al. 2006). The water sample was taken in a P/A vial upright inside at warm temperature between 22 °C and 44 °C for 48 h. The change in color was observed after 48 h in the vials. The detection of a black color indicates fecal pollution, and the water is unfit for drinking.
Data analysis
The correlation analysis, t-test, and principal component analysis (PCA) were performed using IBM SPSS version 27. The Spearman correlation is a nonparametric method for determining how closely two independent variables are correlated (Gauthier 2001). In order to determine the correlations between the major ions, Spearman’s correlation analysis was performed, since the majority of the chemical parameters were not normally distributed. To compare the significant differences in physicochemical parameters between the seasons, the independent t-test was performed. The PCA helps to extract variables giving information on controlling water chemistry (Lourenco et al. 2010). The Piper diagram helps to identify the effect of chemical processes between the minerals in the water bodies (Selvam and Venkatramanan 2020). The Piper diagram and Gibbs diagram were generated by using Origin (2018) software. The Gibbs diagram can be used for assessment of the major ion concentrations in water samples. The USSL diagram and Wilcox diagram was prepared to determine the suitability of water for irrigation by using Diagramme software version 6.77.
Benthic macroinvertebrates data were examined using the Vegan package in R Studio. Canonical correspondence analysis (CCA) was plotted to evaluate the relationships between macroinvertebrates and influencing variables.
Results and discussion
Spring usage and discharge rate
A total of 85 springs were mapped in the Khandbari Municipality, which are perennial in nature (Fig. 2) and are located at elevations between 399 and 1584 m. Chapagain et al. (2019), Thapa et al. (2020), and Tiwari et al. (2020) observed a large number of springs at altitude below 1500 m, which is similar to this study.
Geology of the study area (st, grey to greenish grey phyllites, gritty and quartzite with minor conglomeratic layer and basic intrusions; kgn, augen gneiss). Adapted from: Amatya and Jnawali (1994)
In the study area, the majority of the springs (86%) are used for multiple purposes like drinking, washing, and irrigation. However, few springs are used for a single purpose, such as drinking (1%), washing (6%), irrigation (4%), and religious use (1%). Two percent of the springs are not currently in use (Fig. 3). A study conducted in the mid-hills of Nepal has a similar result, where the maximum percentage of people use spring water for multiple purposes (Chapagain et al. 2019). People in the study area have very limited access to the water distributed by water suppliers. Therefore, they are completely dependent on the spring water for multiple uses. The majority of the spring sources in the study area were well protected by the concrete wall and fence.
The spring discharge rate in the Khandbari Municipality decreased in the pre-monsoon in contrast to the post-monsoon (Table 2). Fifty-eight percent of spring discharge in the post-monsoon was in between 10 and 50 lpm and 42% between 5 and 10 lpm in the pre-monsoon. This result is similar to the study carried out in Dailekh District, Nepal, by Khadka et al. (2019), where the increment in spring discharge during the post-monsoon season might be due to the monsoonal precipitation. Therefore, the springs with a high discharge rate are valuable because of their high utility (Taloor et al. 2020).
Seasonal variation of physicochemical parameters of the spring water
The independent t-test depicts significant differences (p < 0.05) in two seasons for temperature, pH, alkalinity, HCO3-, TH, Mg2+, free CO2, turbidity, and SO42- (Table 3). There has been a decrease in temperature, pH, Mg2+, free CO2, and turbidity from the pre-monsoon to the post-monsoon. In contrast, alkalinity, HCO3-, TH, and SO42- have increased during this transition. This could be related to the effect of monsoon rain in the spring water.
Table 4 exhibits the monitored physicochemical parameters in the pre- and post-monsoon seasons. All the samples are within the permissible limits of both NDWQS (2005) and the drinking water quality guideline value of WHO (2017) in both seasons, except for pH in the pre-monsoon in 11 sites and iron in one site in the post-monsoon. The pH in the pre-monsoon ranging from 7.1 to 9.7, with a mean of 8.5, is deemed to be slightly alkaline (Szczucińska 2016). The pH beyond the range of 6.5 to 8.5 can have an adverse effect on the taste and appearance of the water (Ngari et al. 2013), while it has no direct effect on the consumer’s health but can corrode the distribution of water pipes, contaminating the drinking water (WHO 2017; Kim et al. 2011). The fluctuation in the pH of the spring water might be due to the low rate of decomposition of organic matter and high amount of calcium carbonate and magnesium carbonate in the study area (Sheikh et al. 2013). The pre-monsoon EC ranged from 21 to 559 μS/cm (mean = 128 μS/cm). The EC ranged from 20 to 582 μS/cm (mean = 143 μS/cm) in the post-monsoon. The enrichment of EC in the spring water is attributed to monsoonal rain which drains the surrounding soils and causes the water to infiltrate into the springs (Sorensen et al. 2015). The concentration of Cl- varied from 11 to 115 mg/L in the pre-monsoon (mean = 33.8 mg/L). In the post-monsoon, Cl- concentration ranged from 10 to 99 mg/L (mean = 30.6 mg/L). The presence of considerable levels Cl- in the spring water is linked with the influence of human activity related to increased pollution due to sewage and domestic waste (Mirza et al. 2007). The NO3- concentration in the pre- and post-monsoon ranged from 0.1 to 21.2 mg/L (mean = 3.7 mg/L) and 0.1 to 20.0 mg/L (mean = 2.9 mg/L), respectively. The notable amount of NO3- in the spring water manifests the contamination of the springs by anthropogenic activities, viz., agriculture, and domestic wastewater discharge (Rivett et al. 2008; Yu et al. 2020; Weber and Kubiniok 2022). The concentration of PO43- varied from 0.05 to 1.62 mg/L in pre-monsoon (mean = 0.40 mg/L), while in post-monsoon, the range varied from 0.05 to 0.71 mg/L (mean = 0.30 mg/L). The high level of PO43- could be related to anthropogenic activities as well (Nisa and Umar 2023). The elevated levels of iron in the spring water are due to the rock-water interaction (Sorlini et al. 2013). It degrades the taste of the water rather than its health effect and promote the growth of iron bacteria as pH of most samples are alkaline in nature (Jayana et al. 2009; Metzger 2005). The concentration of Na+, K+, Ca2+, and Mg2+ varied significantly within the inter-spring samples and Ca2+ being dominant in the both seasons.
Based on mean values during both seasons, Ca2+ > Mg2+ > Na+ > K+ and HCO3- > Cl- > SO42- are the cationic and anionic dominant species, respectively. The cation anion balance of measured physicochemical parameters in the spring water samples were less than 5%.
Association among hydrochemical attribute
A correlation analysis and PCA were performed to establish the relationships among the hydrochemical attributes of the spring water. There are strong positive correlations between EC and TDS, EC and Cl-, EC and TH, EC and Ca2+, TDS and Cl-, TDS and TH, TDS and Ca2+, TH and Ca2+ and Mg2+, and TH and Na+ and K+ (Table 5). In contrast, TH and pH exhibit a negative correlation (r = – 0.503, p < 0.01). The main contributors to the groundwater are Na+, Ca2+, Cl-, TDS, and TH, which show significant correlations with EC similar to TDS, suggesting their common source of origin. The strong correlation between EC and TDS (r = 0.982, p < 0.01) implies that the conductivity increases with the increment of the concentration of dissolved ions (Batabyal and Chakraborty 2015). The EC, TH, Ca2+, and Mg2+ exhibit strong positive correlations, which indicate the dissolution of limestone with water sources (Thakur et al. 2020) and demonstrate the complexity of the groundwater, where the natural water always contains dissolved and suspended substances (Elkrail and Obied 2013). A correlation between Cl- and Na+ (r = 0.619, p < 0.01) suggests that there is wastewater infiltration or the use of fertilizers in the study area (Al-Khashman 2008). The TH is also positively correlated with Ca2+ and Mg2+, since TH is the measurement of Ca2+ and Mg2+. The positive correlations between TDS with Cl- and Mg2+ reveal that these ions are the main contributors to TDS. The negative correlations of pH with other chemical parameters suggest that low pH has a remarkable effect on the dissolution of underlying rocks in the study area (Shihab and Baqi 2010).
The factor loading plot of the PCA is applied to 64 water samples from 37 sites within 12 chemical variables. The reliability of PCA was examined using the Kaiser-Meyer-Olkin (KMO) and Bartlett’s tests, with indicative values of 0.76 and 1061.9, respectively. Three major principal components explain the chemical composition of the data (Fig. 4), responsible for the geochemical evolution of the spring water.
The PC1, PC2, and PC3 account for 42.5 %, 20.1 %, and 12.6 % of variance with 5.1, 2.4, and 1.5 eigen values, respectively (Table 6). In particular, EC, TDS, Cl-, TH, and Na+ are correlated with the high loading factor (> 0.75) in PC1. The correlations between these variables are attributed to rainwater or anthropogenic activities (Ansari et al. 2019). In addition, the correlation of Cl- and Na+ in this component could be related to contamination of the spring water sources by human waste. Likewise, the correlation of EC, TDS, and TH in the component depicts that the dissolved solids and ions are mostly contributed by TH of the spring water. Similarly, PC2 has a high loading factor of HCO3- and SO42- and moderate loading factor (> 0.6) of NH3. The correlation of SO42- and NH3 in the component might be resulted from the use of sulphate-bearing fertilizer, e.g., ammonium sulphate ((NH4)2SO4) (Sun et al. 2010). The fertilizers are commonly used in agricultural areas, which are often in proximity to the springs. Additionally, only HCO3- has been grouped in this principal component as natural source, suggesting that carbonate or silicate weathering as the controlling source of the spring water (Ansari et al. 2019; Pant et al. 2018). Based on the geology of the study area as indicated by Amatya and Jnawali (1994), silicate weathering could be considered as one of the major contributors of HCO3- in the spring water.
PC3 has a high loading of NO3- and moderate loading factor of PO43- which visualizes the pollution caused by domestic and agricultural effluents, such as the use of nitrate containing fertilizers (Al-Khashman 2008; Ansari et al. 2019; Lourenco et al. 2010; Tashtoush and Al-Subh 2015; Weber and Kubiniok 2022). The PCA indicates that a single process is not controlling the chemistry of the spring water, but is controlled by anthropogenic activities (contamination of spring water by human waste and agricultural run-off), carbonate dissolution, and silicate weathering.
Hydrochemical facies
The evaluation of the hydrogeochemical facies and water types is simplified by the Piper diagram. The general properties of the spring water chemistry are expressed by the milli-equivalent percentage of the principal ions. The cation plot that manifests most of the monitored springs in the pre- and post-monsoon lies in the lower right corner. The Piper diagram’s diamond-shaped field explains the spring water’s general features. The diagram shows six sub-fields: Ca-HCO3, Na-Cl, mixed Ca-Na-HCO3, mixed Ca-Mg-Cl, Ca-Cl, and Na-HCO3. Figure 5 depicts that 43% of the water samples in the pre-monsoon belong to the Ca-HCO3 type and 58% in the post-monsoon season to the mixed Ca-Na-HCO3 type.
In this study, alkalis (Na+ and K+) exceed alkaline earth metals (Ca2+ and Mg2+), and weak acids (HCO3- and CO32-) exceed strong acids (SO42- and Cl-). The common minerals that contribute to Na+ are silicates, which indicate that the spring waters are salinized to some extent (Thakur et al. 2020). Despite the predominance of weak acids over strong acids, Cl- concentrations in some spring water are notably high. The elevated levels of Cl- and Na+ suggest that the springs may be contaminated with human waste. The HCO3- rich minerals are associated with calcareous shales and carbonate rocks (Caron et al. 2008), where weathering of carbonate minerals is a key factor controlling the groundwater chemistry. Furthermore, the enrichment of HCO3- in the springs indicates that carbonate rocks and silicate rocks are the dominant geological formations in comparison to gypsum minerals. This suggests that the weathering of carbonate and silicate minerals is the primary source of HCO3- in the spring water. This is supported by the fact that carbonate and silicate weathering processes consume CO2 and generate bicarbonate ions. The dominant class type in pre-monsoon (43%) is Ca-HCO3 water type, which indicates the presence of carbonate hardness. Similarly, 58% of samples in the post-monsoon period belong to mixed Ca-Na-HCO3 type. The majority of the samples from both seasons displayed in the right corner of the cation triangle show that sodium containing minerals are dissolved and undergone ion exchange, which raises the concentration of Na+ in the spring water. As a result, changes in Ca2+ and Mg2+ concentrations in groundwater alter the chemistry of the water, which was observed by Li et al. (2013) in the groundwater quality of Pengyang County, China, and exhibited a similar outcome.
Mechanism controlling quality of spring water
The mechanisms governing the hydrogeochemistry of water are explained by the Gibbs diagram. Gibbs diagram shows the three conditions in the plot: dominance of rock, evaporation, and precipitation’s interaction with water. The influence of precipitation can be indicated at low TDS (< 10 mg/L) with a high ratio of Cl-/(Cl- + HCO3-) and Na+/(Na+ + Ca2+) (0.5–1). The influence of rock weathering is indicated at medium TDS (70–300 mg/L), low ratio of Cl-/(Cl- + HCO3-), and Na+/(Na+ + Ca2+) (< 0.5). The high TDS (> 300 mg/L), a high ratio of Cl-/(Cl- + HCO3-), and Na+/(Na+ + Ca2+) (0.5–1) reflect the influence of evaporation (Gibbs 1970). Figure 6 illustrates the dominance of rock-water weathering and precipitation as the main processes in the study area that regulate the spring water. It shows dissolution of carbonate minerals while moving from the recharge area to the aquifer (Raju et al. 2011; Taloor et al. 2020). The relationship between TDS and Cl-/(Cl- + HCO3-) ratio demonstrates the prevalence of precipitation in many springs, indicating the dissolution of a moderate quantity of chloride. The relationship between TDS and Na+/(Na+ + Ca2+ ) ratio indicates that rock weathering processes are dominant in many springs, leading to an increased concentration of sodium. So, the source of the Na+ and Cl- could be related to rock-water interaction as well. Chemical weathering of rock forming minerals, dissolution of carbonates, and ion exchange between water and clay minerals are the forms of water-rock interaction, and the main process of rock water interaction in the study area is attributed to interaction between groundwater and surrounding minerals in the alluvium plane (Raju et al. 2011). The monitored springs do not fall into the evaporation dominance group, indicating that there is no contact between water and evaporation.
Water quality index, suitability of water usage, and hazards
The water quality in the study area is classified as excellent and good (Table 7). In the pre-monsoon season, the water is classified as excellent in 55.0% and good in 45.2% of the monitored springs. However, in the post-monsoon season, all the springs are classified as excellent. The high value of WQI in the pre-monsoon might be related to elevated concentrations of chloride. The primary cause of elevated chloride levels in the spring water is the disposal of human waste in the surrounding area of the spring water sources (Mirza et al. 2007). The decrease in chloride concentrations during the post-monsoon can be attributed to the dilution effect caused by rainfall during the monsoon season in the spring water sources. The spring water quality remains consistently excellent and good in the both seasons. Excellent water quality can be used directly for drinking, whereas good water quality requires prior treatment such as filtration or boiling for drinking purposes (Ameen 2019).
Salinity hazard
The classification of spring water samples and their distribution in the study area with respect to salinity hazards ranges from excellent to good in both seasons (Table 8). Approximately 87% of the springs in the pre-monsoon season are excellent for irrigation. Similarly, about 81% of the springs in the post-monsoon are excellent for irrigation. Therefore, the spring water has no effect on the growth of plants in the study area. A similar result was noted in Almora District, Uttarkhand, India (Bhandari and Joshi 2013).
Sodium adsorption ratio
SAR classification and its distribution are listed in Table 8 and classified based on Richards (1954). The SAR value ranged from 0.07 to 4.30 in the pre-monsoon and from 0.03 to 5.43 in the post-monsoon. Therefore, all the springs are in excellent condition in both seasons. Salinity causes adverse effects on environment of irrigation land as crop production decreases (Raju et al. 2011). The USSL plot (SAR against EC) is applied to categorize the suitability of spring water for irrigation activities. The spring samples lie within the fields of C1, S1 and C2, and S1 (low and medium salinity to low sodium), which is suitable for irrigation and has no adverse effect on crops (Fig. 7). Thus, spring water in the study area is in excellent condition for irrigation purposes (Banoeng-Yakubo et al. 2009; Dumaru et al. 2021).
Sodium percentage
Sodium is one of the common elements found in water, and its evaluation is very important for the analysis of irrigation suitability (Taloor et al. 2020). Excess sodium salts cause an osmotic effect on soil and water, resulting in low agricultural yields. High sodium content has an effect on soil permeability and the infiltration of water, where clay particles absorb sodium and displace magnesium and calcium (Bhandari and Joshi 2013). Sodium combines with carbonate to form alkaline soils, and chloride forms saline soils, where both types of soils do not support the growth of plants (Raju et al. 2011). The %Na is classified based on Todd and Mays (2005). All the pre-monsoon water samples were plotted within the excellent class, but 36% of the post-monsoon samples belong to the doubtful to unsuitable class based on %Na (Table 8).
The percent sodium and EC of the spring water were plotted within the Wilcox diagram (Fig. 8) to determine the irrigation suitability of the spring water. The diagram reveals that all the pre-monsoon samples fall into the excellent category, but very few (three) post-monsoon samples fall into the permissible to doubtful category based on high %Na.
Magnesium ratio
A magnesium ratio greater than 50% affects crop yield as it makes the soil alkaline. The magnesium ratio in 26 spring water in the pre-monsoon season was unsuitable, and five were suitable for irrigation purposes. On the contrary, 26 spring water in the post-monsoon samples were suitable, and seven were unsuitable for irrigation (Table 8). Therefore, the 80% monitored springs in the pre-monsoon season exceed the ratio of 50 and considered unsuitable and harmful for irrigation (Fig. 9). The high magnesium ratio in the pre-monsoon could be related to enrichment of Mg2+ in comparison of Ca2+ during pre-monsoon. There is typically lower water availability and reduced groundwater recharge in dry season. This results in a lower dilution effect and higher concentration of dissolved minerals, including magnesium. In contrast, calcium, being more abundant in the environment and having a higher solubility, may be more evenly distributed throughout the year.
Corrosivity ratio
A corrosivity ratio greater than 1 is unsafe. Four springs in the pre-monsoon and one in the post-monsoon have CR values greater than 1. The majority of pre- and post-monsoon samples are safe for irrigation (Table 8). However, out of 31 in the pre-monsoon and 33 in the post-monsoon, four and one springs are corrosive, respectively (Fig. 9). Therefore, noncorrosive pipes should be used for the distribution of such water (Raju et al. 2011).
Microbial analysis
The presence or absence of fecal contamination was determined in the study area. Approximately 62.5% of spring water was contaminated with the microbes (Fig. 10).
The fecal contamination is attributed to a lack of water treatment, contamination at the source, unplanned urbanization, a poor or no sewerage system, and poor sanitation (Rai et al. 2009). A similar result was observed in the study of animal herds in Soreq Catchment in Israel, where manure piles and wastewater from the sewerage system near the spring outlet are the main reasons for fecal contamination (Jebreen and Ghanem 2015). Most of the springs in the study area are located near residential areas and agricultural land, which could be related to the fecal contamination of the many springs. The unmanaged sewerage system in the residential areas and the use of animal dung on agricultural land as fertilizer might be one of the reasons for the fecal contamination in that area. A study conducted in the drinking water of Sankhuwasabha District also observed the presence of coliform (Rai et al. 2009).
Relationship of benthic macroinvertebrates with environmental variables
Eighteen springs were sampled for benthic macroinvertebrates in the Khandbari Municipality. Eighty-nine individuals were recorded in the pre-monsoon and 50 in the post-monsoon.
Altogether, nine orders and 17 families were recorded in the pre-monsoon season and six orders and ten families in the post-monsoon season (Table 9). Chironomidae were the most widely spread species in both seasons.
The CCA plot shows the association between the macroinvertebrates and environmental variables (Fig. 11). Discharge, temperature, turbidity, DO, pH, and EC are the major physicochemical parameters indicating variation with the macroinvertebrate assemblage (Hahn 2000; Sharifinia et al. 2012). The influencing variables in the study area include elevation (Ele), DO, EC, discharge (Dis), Na+, NO3-, NH3, and SO42-. The eigenvalues of the first two canonical axes were 0.6842 and 0.5629, respectively. A study in Jhimruk Watershed, Nepal, also evidenced the macroinvertebrate assemblage with discharge rate, elevation , EC, and NO3- (Thapa et al. 2020). The variables with a long arrow in the CCA plot are more strongly correlated with the ordination axes than the shorter ones (Gower et al. 1994; Ter Braak 1987). The sites such as MM1 and MM2, which are spread along CCA1 have high discharge, and Hydropsychidae are associated with increased discharge (Gower et al. 1994). Elevated spring discharge is regarded as the disturbance that affects the macroinvertebrates assemblage (Fumetti and Nagel 2012). The elevated EC in the water restricts the macroinvertebrate assemblage (Olson 2012). Similarly, communities at these sites were negatively correlated with NH3. A significant correlation of nitrogen suggests that nutrients have a link with macroinvertebrate assemblages (Wang et al. 2007). Sources of nitrogen are anthropogenic activities, organic matter, and natural sources. Higher nitrate content increases the decomposition rate, which alters the organic matter and makes the habitat unsuitable for the macroinvertebrates. Several taxa of macroinvertebrates, e.g., Limoniidae and Tipulidae, are tolerant to NH3. On the other side, Hydropsychidae and Scirtidae decrease with increasing NH3. Chironomidae are indicator of species that are highly tolerant of pollution. Therefore, sites KS3, S5, MM1, and MM4 are dominant in Chironomidae taxa. Site S8 is at the highest elevation (Fig. 11), represented by the arrow aligned to elevation. The site MM2 had the highest discharge, and the macroinvertebrates associated with discharge are Hydropsychidae, Perlidae, Tipulidae, and Limoniidae, which oppose the discharge in the plot.
Conclusion
The study mapped the spring locations and assessed the physicochemical characteristics of the spring water, its suitability for different purposes, and microbial and biological analysis in the Khandbari Municipality, Sankhuwasabha District, Eastern Nepal. In spite of the large number of springs in the study area, there is a lack of proper management and a scarcity of water in the dry season. This study concludes that the spring water is within the drinking water guidelines and standards and is therefore suitable for drinking purposes. However, from the microbial study, the majority of the samples were contaminated with fecal pollution, suggesting the need for prior treatment. The WQI categorizes the spring water as excellent in the post-monsoon season. In the pre-monsoon, the spring water falls within the range of excellent to good. The spring water is suitable for irrigation purposes except for the magnesium hazard. The major mineral contributing to the spring water quality are carbonate rocks and silicates. One of the main controlling factors in the quality of spring water in the study area is anthropogenic activities, primarily attributed to human waste and agricultural run-off. Benthic macroinvertebrates were mainly linked with influencing variables like discharge, altitude, NO3-, and NH3. To ensure the conservation of water quality for both human consumption and biodiversity, it is imperative to develop a comprehensive conservation and management plan in the study area.
Data availability
All the data used for the present study appear in the journal manuscript. The raw data can be provided upon reasonable requests.
References
Al-Khashman OA (2008) Assessment of the spring water quality in the Shoubak area, Jordan. Environmentalist 28:203–215. https://doi.org/10.1007/s10669-007-9129-1
Amatya KM, Jnawali BM (1994) Geological map of Nepal. Department of Mines and Geology, Government of Nepal, Kathmandu
Ameen HA (2019) Spring water quality assessment using water quality index in villages of Barwari Bala, Duhok, Kurdistan Region, Iraq. Appl Water Sci 9(8):176. https://doi.org/10.1007/s13201-019-1080-z
Amin R, Ali SS, Anwar Z, Khattak JZK (2019) Microbial analysis of drinking water and water distribution system of urban Lahore. Nurture 13(1):1–7
Ansari A, Deodhar A, Kumar US (2019) Modeling of geochemical processes and multivariate statistical analysis for hydrochemical assessment of spring water of the Outer Himalaya. India. Environ Earth Sci 78:665. https://doi.org/10.1007/s12665-019-8682-5
APHA-AWWA-WEF (2017) Standard methods for examination of water and wastewater, 23rd edn. American Public Health Association, Washington DC
Atreya K, Panthee S, Sharma P (2006) Bacterial contamination of drinking water and the economic burden of illnesses for the Nepalese households. Int J Environ Health Res 16(5):385–390. https://doi.org/10.1080/09603120600869448
Banoeng-Yakubo B, Yidana SM, Nti E (2009) Hydrochemical analysis of groundwater using multivariate statistical methods - The Volta region, Ghana. KSCE J Civ Eng 13:55–63. https://doi.org/10.1007/s12205-009-0055-2
Barakat A, Meddah R, Afdali M, Touhami F (2018) Physicochemical and microbial assessment of spring water quality for drinking supply in Piedmont of Béni-Mellal Atlas (Morocco). Phys Chem Earth 104:39–46. https://doi.org/10.1016/j.pce.2018.01.006
Batabyal AK, Chakraborty S (2015) Hydrogeochemistry and water quality index in the assessment of groundwater quality for drinking uses. Water Environ Res 87(7):607–617. https://doi.org/10.2175/106143015×14212658613956
Bhandari NS, Joshi HK (2013) Quality of spring water used for irrigation in the Almora District of Uttarakhand, India. Chin J Geochem 32:130–136. https://doi.org/10.1007/s11631-013-0615-5
Brown RM, McClelland NI, Deininger RA, Tozer RG (1970) A water quality index-do we dare. Water Sewage Works 117(10):339–343
Caron ME, Grasby SE, Cathryn Ryan M (2008) Spring water trace element geochemistry: a tool for resource assessment and reconnaissance mineral exploration. Appl Geochem 23(12):3561–3578. https://doi.org/10.1016/j.apgeochem.2008.07.020
Chapagain PS, Ghimire M, Shrestha S (2019) Status of natural springs in the Melamchi region of the Nepal Himalayas in the context of climate change. Environ Dev Sustain 21:263–280. https://doi.org/10.1007/s10668-017-0036-4
Dohare D, Deshpande S, Kotiya A (2014) Analysis of ground water quality parameters: a review. Res J Eng Sci 3(5):26–31
Dumaru B, Kayastha SP, Pandey VP (2021) Spring water assessment for quality and suitability for various uses: the case of Thuligaad watershed, western Nepal. Environ Earth Sci 80(17):586. https://doi.org/10.1007/s12665-021-09826-w
Elkrail AB, Obied BA (2013) Hydrochemical characterization and groundwater quality in Delta Tokar alluvial plain, Red Sea coast-Sudan. Arab J Geosci 6:3133–3138. https://doi.org/10.1007/s12517-012-0594-6
Fumetti VS, Nagel P (2012) Discharge variability and its effect on faunistic assemblages in springs. Freshwater Sci 31(2):647–656. https://doi.org/10.1899/10-159.1
Gauthier TD (2001) Detecting trends using Spearman’s rank correlation coefficient. Environ Forensic 2(4):359–362. https://doi.org/10.1006/enfo.2001.0061
Ghanem M, Ahmad W, Keilani Y, Sawaftah F, Schelter L, Schuettrumpf H (2021) Spring water quality in the central West Bank, Palestine. J Asian Earth Sci X:100052. https://doi.org/10.1016/j.jaesx.2021.100052
Gibbs RJ (1970) Mechanisms controlling world water chemistry. Science 170(80):1088–1090
Gower AM, Myers G, Kent M, Foulkes ME (1994) Relationships between macroinvertebrate communities and environmental variables in metal-contaminated streams in south-west England. Freshw Biol 32(1):199–221. https://doi.org/10.1111/j.1365-2427.1994.tb00877.x
Guo F, Jiang G, Zhao H, Polk J, Liu S (2019) Physicochemical parameters and phytoplankton as indicators of the aquatic environment in karstic springs of South China. Sci Total Environ 659:74–83. https://doi.org/10.1016/j.scitotenv.2018.12.329
Hahn HJ (2000) Studies on classifying of undisturbed springs in Southwestern Germany by macrobenthic communities. Limnologica 30(3):247–259. https://doi.org/10.1016/S0075-9511(00)80055-9
Ilmonen J, Mykra H, Virtanen R, Paasivirta L, Muotka T (2012) Responses of spring macroinvertebrate and bryophyte communities to habitat modification: community composition, species richness, and red-listed species. Freshwater Sci 31(2):657–667. https://doi.org/10.1899/10-060.1
Ilmonen J, Paasivirta L (2005) Benthic macrocrustacean and insect assemblages in relation to spring habitat characteristics: Patterns in abundance and diversity. Hydrobiologia. 533:99–113. https://doi.org/10.1007/s10750-004-2399-4
Ilmonen J, Paasivirta L, Virtanen R, Muotka T (2009) Regional and local drivers of macroinvertebrate assemblages in boreal springs. J Biogeogr 36(5):822–834. https://doi.org/10.1111/j.1365-2699.2008.02045.x
Jayana BL, Prasai T, Singh A, Yami KD (2009) Assessment of drinking water quality of Madhyapur-Thimi and study of antibiotic sensitivity against bacterial isolates. Nepal J Sci Technol 10:167–172. https://doi.org/10.3126/njst.v10i0.2955
Jebreen H, Ghanem M (2015) Spring water qualitative assessment in mountainous areas, case study: Soreq Catchment/Ramallah/West Bank. J Water Res Protect 7(11):851–859. https://doi.org/10.4236/jwarp.2015.711069
Khadka K, Pokhrel G, Dhakal M, Desai J, Shrestha RB (2019) Springshed management: an approach to revive drying springs in the Himalayas. In: Proceedings of the seminar on “Leaving no one behind”. Nepal Academy of Science and Technology, Lalitpur, pp 10–22
Kim EJ, Herrera JE, Huggins D, Braam J, Koshowski S (2011) Effect of pH on the concentrations of lead and trace contaminants in drinking water: a combined batch, pipe loop and sentinel home study. Water Res 45(9):2763–2774
Li P, Wu J, Qian H (2013) Assessment of groundwater quality for irrigation purposes and identification of hydrogeochemical evolution mechanisms in Pengyang County, China. Environ Earth Sci 69:2211–2225. https://doi.org/10.1007/s12665-012-2049-5
Lourenco C, Ribeiro L, Cruz J (2010) Classification of natural mineral and spring bottled waters of Portugal using principal component analysis. J Geochem Explor 107(3):362–372. https://doi.org/10.1016/j.gexplo.2010.08.001
Mahamuni K, Kulkarni H (2012) Groundwater resources and spring hydrogeology in South Sikkim, with special reference to climate change. In: Arrawatia ML, Tambe S (eds) Climate change in Sikkim-patterns, impacts and initiatives. Information and Public Relations Department, Government of Sikkim, Gangtok, pp 261–274
Metzger M (2005) The relationhip between iron and pH. National Testing Laboratories Ltd., Cleveland
Mirza MA, Khuhawar MY, Arain R (2007) Quality of spring water in the catchment areas of the Indus River. Asian J Chem 19(7):5279–5304
NDWQS (2005) National drinking water quality standards and directives. Ministry of Physical Planning and Works, Government of Nepal, Kathmandu
Negi GCS, Joshi V (2002) Drinking water issues and development of spring sanctuaries in a mountain watershed in the Indian Himalaya. Mt Res Dev 22(1):29–31. https://doi.org/10.1659/0276-4741(2002)022[0029:DWIADO]2.0.CO;2
Ngari MS, Wangui WT, Ngoci NS, Mavura JW (2013) Physico-chemical properties of spring water in Kabare and Baragwi locations, Gichugu Division Kirinyaga County of Kenya. Int J Sci Res 2(7):280–285
Nisa FU, Umar R (2023) Evaluation of physicochemical and microbiological parameters, and their correlation in Himalayan spring water systems: a case study of District Kulgam of Kashmir Valley, India. Western Himalaya. Environ Monit Assess 195(4):41. https://doi.org/10.1007/s10661-023-11025-y
NSO (2021) Nepal census 2021. National Satistical Office, Government of Nepal, Kathmandu
Olson JR (2012) The influence of geology and other environmental factors on stream water chemistry and benthic invertebrate assemblages. Dissertation, Utah State University
Pandit S, Shakya N, Shrestha SR (2019) Distribution and classification of springs in Bansbari area of Melamchi municipality, Sindhupalchowk, Nepal. J Nepal Geol Soc 59:49–58. https://doi.org/10.3126/jngs.v59i0.24985
Pant RR, Zhang F, Rehman FU, Wang G, Ye M, Zeng C, Tang H (2018) Spatiotemporal variations of hydrogeochemistry and its controlling factors in the Gandaki River Basin, Central Himalaya Nepal. Sci Total Environ 622–623:770–782. https://doi.org/10.1016/j.scitotenv.2017.12.063
Ragno G, De Luca M, Ioele G (2007) An application of cluster analysis and multivariate classification methods to spring water monitoring data. Microchem J 87(2):119–127. https://doi.org/10.1016/j.microc.2007.06.003
Rai SK, Ono K, Yanagida JI, Kurokawa M, Rai CK (2009) Status of drinking water contamination in Mountain Region, Nepal. Nepal Med Coll J 11(4):281–283
Raju NJ, Shukla UK, Ram P (2011) Hydrogeochemistry for the assessment of groundwater quality in Varanasi: a fast-urbanizing center in Uttar Pradesh, India. Environ Monit Assess 173:279–300. https://doi.org/10.1007/s10661-010-1387-6
Richards LA (1954) Diagnosis and improvement of saline and alkali soils. Agriculture Handbook No. 60. United States Department of Agriculture, Washington DC
Rivett MO, Buss SR, Morgan P, Smith JW, Bemment CD (2008) Nitrate attenuation in groundwater: a review of biogeochemical controlling processes. Water Res 42(16):4215–4232. https://doi.org/10.1016/j.watres.2008.07.020
Selvam S, Venkatramanan S (2020) Groundwater geochemistry and credentials of hydrogeochemical processes in a Tuticorin Coastal region, Southern Tamil Nadu, India. In: Pradhan AMS, Ganesh NK, Upadhyaya B, Khanal A, Khatiwada M (eds) Bulletin of Nepal Hydrogeological Association, vol 5. Nepal Hydrogeological Association, Kathmandu, pp 47–60
Shah RDT, Shah DN, Sharma S (2020) Rivers Handbook-a guide to the health of rivers in the Hindu-Kush Himalaya. Aquatic Ecology Centre, School of Science, Kathmandu University, Dhulikhel
Shan V, Singh SK, Haritash AK (2020) Water Crisis in the Asian countries: status and future trends. In: Kumar M, Munoz-Arriola F, Furumai H, Chaminda T (eds) Resilience, response, and risk in water systems: shifting management and natural forcings paradigms. Springer, Singapore, pp 173–194
Sharifinia M, Javid NI, Amin BM (2012) Benthic macroinvertabrate distribution in Tajan river using canonical correspondence analysis. Casp J Environ Sci 10(2):181–194
Sharma B, Nepal S, Gyawali D, Pokharel GS, Wahid S, Mukherji A, Acharya S, Shrestha AB (2016) Springs, storage towers, and water conservation in the midhills of Nepal (ICIMOD Working Paper). Nepal Water Conservation Foundation and International Center for Integrated Mountain Development (ICIMOD), Kathmandu
Sheikh MA, Dar IY, Yaseen S, Pal A, Pandit AK (2013) A study of physico-chemical characteristics of three fresh water springs of Kashmir Himalaya, India. Int J Water Res Environ Eng 5(6):328–331
Shihab A, Baqi Y (2010) Multivariate analysis of ground water quality of Makhmor Plain/North Iraq. Damascus Univ J 1(26):19–26
Shrestha BG, Pandey BR, Shrestha E, Ghimire S, Bhattarai S (2016) Characterization of microogranisms for production of bioethanol from agriculture wastes and using it as a biofuel. In: Bhuju DR, McLaughlin K, Sijapati J, Devkota BD, Shrestha N, Ghimire GP, Neupane PK (eds) Building knowledge for climate resilience in Nepal: Research Brief. Nepal Academy of Science and Technology, Lalitpur, pp 99–102
Sorensen JPR, Lapworth DJ, Nkhuwa DCW, Stuart ME, Gooddy DC, Bell RA, Chirwa M, Kabika J, Liemisa M, Chibesa M, Pedley S (2015) Emerging contaminants in urban groundwater sources in Africa. Water Res 72:51–63. https://doi.org/10.1016/j.watres.2014.08.002
Sorlini S, Palazzini D, Sieliechi JM, Ngassoum MB (2013) Assessment of physical-chemical drinking water quality in the Logone Valley (Chad-Cameroon). Sustainability 5(7):3060–3076
Sun H, Han J, Li D, Zhang S, Lu X (2010) Chemical weathering inferred from riverine water chemistry in the lower Xijiang basin, South China. Sci Total Environ 408(20):4749–4760
Szczucińska A (2016) Spring water chemistry in a formerly glaciated area of western Poland: the contribution of natural and anthropogenic factors. Environ Earth Sci 75:1–15. https://doi.org/10.1007/s12665-016-5548-y
Taloor AK, Pir RA, Adimalla N, Ali S, Manhas DS, Roy S, Singh AK (2020) Spring water quality and discharge assessment in the Basantar watershed of Jammu Himalaya using geographic information system (GIS) and water quality Index(WQI). Groundw Sustain Dev 10:100364. https://doi.org/10.1016/j.gsd.2020.100364
Tashtoush SM, Al-Subh SA (2015) Interpretation of groundwater quality parameters for springs in Tafileh area in south of Jordan using principal components analysis. Environ Sci 3:31–44. https://doi.org/10.12988/es.2015.523
Ter Braak CJF (1987) The analysis of vegetation-environment relationships by canonical correspondence analysis. Vegetatio 69:69–77. https://doi.org/10.1007/BF00038688
Thakur N, Rishi M, Keesari T, Sharma AD (2020) Suitability of spring water from the Upper Beas River Basin in Kullu Valley (Western Himalaya, India) for drinking and irrigation purposes. Arab J Geosci 13:1–14. https://doi.org/10.1007/s12517-020-06143-7
Thapa B, Pant RR, Thakuri S, Pond G (2020) Assessment of spring water quality in Jhimruk River Watershed, Lesser Himalaya, Nepal. Environ Earth Sci 79:504. https://doi.org/10.1007/s12665-020-09252-4
Tiwari S, Chamlagain D, Atwood A, Sayami M (2020) Quality assessment and status of spring water in Helambu area, Sindhupalchok district, central Nepal. J Nepal Geol Soc 60:59–74. https://doi.org/10.3126/jngs.v60i0.31274
Todd DK, Mays LW (2005) Groundwater hydrology, 3rd edn. John Wiley & Sons, Hoboken
Tyagi S, Sharma B, Singh P, Dobhal R (2020) Water quality assessment in terms of water quality index. Am J Water Resour 1(3):34–38. https://doi.org/10.12691/ajwr-1-3-3
Verma R, Jamwal P (2022) Sustenance of Himalayan springs in an emerging water crisis. Environ Monit Assess 194(2):87
Wang L, Robertson DM, Garrison PJ (2007) Linkages between nutrients and assemblages of macroinvertebrates and fish in wadeable streams: implication to nutrient criteria development. Environ Manag 39:194–212. https://doi.org/10.1007/s00267-006-0135-8
Weber G, Kubiniok J (2022) Spring waters as an indicator of nitrate and pesticide pollution of rural watercourses from nonpoint sources: results of repeated monitoring campaigns since the early 2000s in the low mountain landscape of Saarland, Germany. Environ Sci Eur 34(1):53. https://doi.org/10.1186/s12302-022-00632-0
WHO (2017) Guidelines for drinking water quality: fourth edition incorporating the first addendum. World Health Organization, Geneva
Yu G, Wang J, Liu L, Li Y, Zhang Y, Wang S (2020) The analysis of groundwater nitrate pollution and health risk assessment in rural areas of Yantai. China BMC Public Health 20:437
Acknowledgements
We would like to acknowledge Central Department of Environmental Science, Tribhuvan University, Kathmandu, Nepal, for their technical guidance, knowledge, and support and providing necessary facilities to carry out this research. We would like to heartily acknowledge Water Resources Research and Development Centre (WRRDC), Ministry of Energy, Water Resources and Irrigation, Government of Nepal, Lalitpur, Nepal, for providing an opportunity for conducting the research.
Author information
Authors and Affiliations
Contributions
Alina Shrestha was involved in the study design, data collection, data analysis, and manuscript preparation. Suman Man Shrestha contributed in the supervision, writing review, manuscript preparation, and editing. Ananta Man Singh contributed in the supervision, spatial analysis, and reviewing.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Consent to participate and publish
All authors have agreed to participate and publish the manuscript.
Conflict of interest
The authors declare no competing interests.
Additional information
Responsible Editor: Xianliang Yi
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shrestha, A., Shrestha, S.M. & Pradhan, A.M.S. Assessment of spring water quality of Khandbari Municipality in Sankhuwasabha District, Eastern Nepal. Environ Sci Pollut Res 30, 98452–98469 (2023). https://doi.org/10.1007/s11356-023-29138-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-023-29138-9