Abstract
Sediments are considered as suitable matrices to study the contamination levels of aquatic environment since they represent a sink for multiple contaminant sources. In this study, the influence of sediment characteristics on the distribution of polycyclic aromatic hydrocarbons (PAHs) and its potential risk in euryhaline, freshwater and humic aquatic bodies of Douglas/Stubbs creek, Ikpa River and Eniong River, respectively, were investigated. The level of PAHs in sediment was quantified using GC–MS, while sediment properties including total organic carbon (TOC) content and grain size were determined by the wet oxidation and hydrometer methods, respectively. The results revealed that the total levels of PAHs in sediment varied significantly between the euryhaline, freshwater and humic freshwater ecosystems. In Ikpa River freshwater ecosystem, a total PAHs load of 1055.2 ng/g was recorded with the suites concentration ranging from 13.0 ng/g (for acenaphthylene) to 161 ng/g (for pyrene). The humic ecosystem of Eniong River had a total PAH load of 11.06 ng/g, while the suites level recorded ranged from 0.04 ng/g for acenaphthene to 2.65 ng/g for chrysene. The total level of PAHs detected in the euryhaline Douglas/Stubbs creek was 14.47 ng/g, and suite concentrations varied between 4.27 ng/g for naphthalene and 5.13 ng/g for acenaphthylene. This shows variation in quantity and quality of PAH contaminants with the nature of ecosystems. It implies complex and diverse contamination sources as well as different capabilities to recover from PAH contamination. Correlation analysis has shown that sediment particle and TOC content influenced PAHs burden in bottom sediments, but the effects varied with the molecular weight of PAHs and the nature of the ecosystems. The TOC was the most significant determinant of PAHs load and distribution in sediment of the freshwater Ikpa River and euryhaline Douglas/Stubbs but had little or no influence in the humic sediment of Eniong River, while the influence of particle size was generally indefinite but slightly associated with PAHs accumulation in the euryhaline sediment. Generally, the total PAH levels (11.0–1055.2 ng/g) recorded were low and below the allowable limit for aquatic sediments. The ecological risk assessment revealed that these levels were lower than the effects range low and effects range medium values. This indicates no acute adverse biological effect although the accumulation of PAHs in freshwater ecosystem of Ikpa River may pose ecological risks as most of the carcinogenic PAH suites had relatively high pollution indices compared to other ecosystem types studied.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Polycyclic aromatic hydrocarbons (PAHs) are a large set of organic pollutants consisting of multiple fused aromatic rings in their chemical structure. Most PAHs are known to be carcinogenic, genotoxic and ubiquitous in the environment, and their metabolites show mutagenic activity towards humans and animals (Skupinska et al. 2004; Lang et al. 2012; Perera et al. 1992). PAHs have also been reported to possess endocrine disrupting properties (Ratola et al. 2006). In 1997, the US Environmental Protection Agency defined 16 PAHs to be highly toxic and recommended the analysis of their concentrations in the environment (Skupinska et al. 2004). Sources of PAHs are generally discriminated into three major types: petrogenic, pyrogenic and biogenic. PAHs that are derived from petrogenic origin are related to petroleum and its refined products. Significant introduction of PAHs into aquatic ecosystems may be through wastewater effluents, aerial fallout, surface run-offs, among others (Cardellicchio et al. 2007). Due to their high hydrophobicity, PAHs entering aquatic environments preferentially adsorb onto particulates and accumulate in bottom sediments (Barakat et al. 2011). PAHs in water and sediments may be transferred into the food chain through aquatic organisms and thus pose some health implications to humans (Kannam et al. 2005; Zuloaga et al. 2009).
Water resources within the floodplain Niger Delta ecosystem are very unique and varied with their physicochemical attributes. Much of it is marine (with salinity of usually 35 ppt and above), but there are remarkable bodies of brackish (with salinity in the range of 0.5–29 ppt) euryhaline (with slight intrusion of salts) and freshwater ecosystems. There is also the “black water” or humic ecosystem characterised by intense colouration due to humic substance and possibly soluble iron (Udofia et al. 2015). The ecosystems also exhibit diverse ecological status and rate of biogeochemical cycling due to variation in physiochemistry and biodiversity. Some organisms are euryhaline because their life cycle involves migration between freshwater and marine environment, while others are stenohaline and can only survive with narrow range of salinities. Salt levels in water may influence oxidative phosphorylation in biological cells creating extreme environmental conditions in aquatic ecosystems (Kelley et al. 2016). Several studies have also shown that humic substance have a modulatory effect on microorganisms as well as increase growth rate of many forms of beneficial microorganisms in part by stimulating enzyme activities and assist in their nutrition by complexing and delivering trace elements like iron to microbial cell surfaces (Pouneva 2005; Burkowska and Donderski 2007). The biological activities affect the organic carbon flux and probably the fate of organic pollutants in aquatic sediments. In addition, information on sediment composition and characteristics is useful for modelling of feasible remediation methods. These sediment characteristics, especially those relating to natural organic matter (NOM) content and salinity gradients, are mostly dependent on geochemical and environmental conditions of the watershed (Kim et al. 2006).
The uptake and accumulation of organic chemicals in aquatic systems depend on the exchange and partitioning of the compounds between the sediment and water phases (Gobas and Maclean 2003). The partition as well as distribution of the compounds is in turn influenced by the sediment characteristics (El Nemr et al. 2007). The fate and biogeochemical cycling of organic compounds in natural sediments are also governed by several other factors more than the carbon-normalised partitioning model (focKoc) which deems all organic matter as equally sorptive (Lohmann et al. 2005). These factors help sequester the compounds or limit their accessibility or accumulation (Accardi-Dey and Gschwend 2002). There have been reports that some sediment characteristics (e.g. organic carbon content) affect the sorption and hydrophobic interaction of organic contaminants in aquatic sediments (You et al. 2010). However, the dynamics of these parameters may differ significantly between ecosystems (Gobas and Maclean 2003; Liu et al. 2013). For these reasons, many researchers have studied and reported the influence of sediment characteristics on the distribution of some persistent organic pollutants (POPs) in different aquatic ecosystems (Dahle et al. 2003; Rockne et al. 2002; Sprovieri et al. 2007; King et al. 2004; Warren et al. 2003; Maskaoui et al. 2002; Dominquez et al. 2010; Zuloaga et al. 2013).
Over the years, the tropical water ecosystems in the Niger Delta region of Nigeria have been heavily impacted from oil and gas exploration and production activities. PAHs contamination has continued to be of major concerns due to its persistence nature even after remediation of polluted sites. However, many studies conducted in the region focus on contaminant quantification and effects prediction based on observed levels (Anyakora et al. 2005; Sojinu et al. 2010; Ekpo et al. 2012) but less on the factors that influence the fate of organic pollutants and their potential risks in aquatic sediment. There are few reports that present the distribution of PAHs in sediments of different water ecosystems.
In this study, the influence of some sediment characteristics on the occurrence and distribution of polycyclic aromatic hydrocarbons in diverse aquatic systems within the Niger Delta Region of Nigeria was investigated to establish its potential risk to biota. The sediment characteristics considered were organic matter and particle size. The organic matter (OM) content in sediments, particle size of sediments and the levels of PAHs in the sediment samples collected from the study areas were determined thereafter, and the data were subjected to correlation models to determine the variability or association of OM, particle size and PAHs. This will support in sound management of contaminated sites in the Niger Delta region as suitability of remediation methods would be based on specific characteristics of the different ecosystems in the area.
Materials and methods
Description of the study area
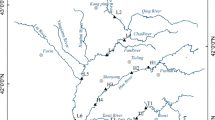
The study area (Fig. 1) comprises (a) Ikpa River Basin (A), a river bifurcate of the middle course of Cross River (Udosen et al. 2004). It is a freshwater system that drains a flood-prone catchment area of 516.5 km2, 14.8% (76.5 km2). The river basin has a main channel with total length of 53.5 km and lies between latitude 5°2′N–5°16′N and longitude 7°53′E–8°07′E. (b) Eniong River (B) is a “blackwater” aquatic system that flows as a tributary of the middle course of the Cross River. The river lies between latitude 5°12′N–5°22′N and longitude 7°54′E–8°2′E. It is characterised by intense colouration due to humic substance and possibly soluble irons. The river serves as a source of water, means of transport and fishing for the communities within its catchment. The river also harbours diverse species of aquatic animals including the highly endangered aquatic mammal: manatee (Udofia et al. 2015) and (c) Douglas/Stubbs creek (C) which lies between latitude 4°30′ to 4°45′N and longitude 7°30′ to 8°45′E is a small left bank perennial rainforest euryhaline ecosystem, a tributary of the Qua Iboe Estuary (Udosen et al. 2007).
Sample collection
Bottom sediment samples were collected using a Shipek grab sampler and thoroughly mixed to give a composite sample (Tao et al. 2004). At Ikpa River Basin, samples were collected between April and July 2014 at seven strategic sites distributed across the urban/suburban area of the river basin, covering about seventy percentage of its total length (Fig. 1). Ten sediment samples were collected upstream, midstream and downstream of the Eniong River, while seven samples were obtained from the Douglas/Stubbs creek between August 2015 and February 2016. Three sub-samples were collected at each sampling site into one-litre amber glass bottles, stored in an ice box and transported to the laboratory for analysis.
Analysis of polycyclic aromatic hydrocarbons
The method for sample preparation followed Soxhlet extraction and then by silica gel/alumina (1:1) using dichloromethane/n-hexane as solvent (7:3 v/v) and quantification by gas chromatography (Agilent 6890N) interfaced with mass spectrometer detector (Agilent 5973N) (GC–MS). Quantification was done using a five-point calibration curve established using dichloromethane-based internal standards for each individual PAH built in the range of 1–5 μl. The correlation coefficient (R2) values of the PAH calibration curves were all greater than 0.97, and the recovery of standards ranged from 54% for benzo(ghi)perylene to 94% for anthracene. This range is acceptable since the recoveries obtained for most of the target compounds were above 70% (USEPA 2007). Further experimental details for quality control and assurance have been described previously (Inam et al. 2016). Instrumental conditions for the GC–MS analysis are summarised in Table 1, while the targeted 16 US EPA priority PAH compounds are listed in Table 2 alongside some of their properties.
Determination of total organic carbon (TOC) of sediments
Homogenised samples for TOC analysis were acidified with dilute hydrochloric acid (HCl) before analysis to remove carbonates (Barhoumi et al. 2014; Ma et al. 2014). Total organic carbon analysis was carried out using wet oxidation method (Jones 2001; Yu et al. 2014). Here, potassium dichromate (K2Cr2O7) is used with external heat and back-titration to measure the amount of unreacted dichromate. The percentage organic carbon was calculated using the formula given below:
where C is the concentration of FAS solution, V1 is the titre value of blank, V2 is the titre value of sample, and W is the weight of the sample.
Determination of sediment particle sizes
Samples were air-dried and sieved through a mechanical sieve to remove shells, debris, etc. Dried samples were subjected to size fraction analysis following the procedure of Wentworth (1992). One hundred grams of the each sample was taken and sieved through 2-mm mesh-sized screen for 10 min in a mechanical sieve shaker to homogenise samples. The sample that remained in 0.063-mm mesh after sieving was weighed and treated as sand. The samples which passed through the 0.063-mm sieve were the silt and clay. The silt and clay fractions were then separated by means of hydrometer method described elsewhere (Brady and Weil 1996; Udo et al. 2009).
Statistical analysis of data
Descriptive statistics was performed using Excel 2007 spreadsheet, while multivariate analyses were done using the SPSS statistical software package version 20.0 with the level of significance set at p < 0.05. Data derived from the various analyses were subjected to regression correlation (Johnson et al. 2001; Wang et al. 2010) to establish the relationship between the distribution of PAHs concentration, particle size and TOC.
Results and discussion
Levels and distribution of PAHs
The levels of PAHs in sediments from the ecosystem studied are presented in Table 3. The levels of total PAH burden in the three different ecosystems differed significantly. Sediment from the freshwater system of Ikpa River was the most contaminated with 1055.2 ng/g of PAH, followed by the euryhaline Douglas/Stubbs creek with 14.47 ng/g of PAH and the humic Eniong River system with 11.06 ng/g of PAH. The Ikpa River Basin tends to accumulate higher levels of PAHs compared to other ecosystems. This may be because the river is in a more urbanised area and as such would receive inputs from run-offs from nearby landfills, atmospheric fallouts from exhausts fumes from high traffic areas and run-offs from coal tar-based road pavements. Previous report by Schindler et al. (1995) has shown that high concentrations of PAH in sediment samples from freshwater ecosystem may be ascribed to high proportion of inputs of the contaminants from its terrestrial catchments. Accumulation of the PAHs in sediment can vary significantly from one point to another as a result of variability in the sources, composition of particulates, particulate deposition/erosion process as well as origins of microbial inocula (Sandoli et al. 1996; Mitra et al. 1999b). Furthermore, sedimentary organic matter may undergo some changes during burial and diagenesis and as such would result in differences in contaminant interaction in time or depth even within the same ecosystem (Mitra et al. 1999a).
The concentration of PAH in sediment also varied with the different PAH suite (Fig. 2). In Ikpa River freshwater ecosystem, the PAH levels ranged from 13.0 ng/g for Ace to 161 ng/g for Pyr. In the humic ecosystem of Eniong River, the levels recorded ranged from 0.04 ng/g for Acy to 2.65 ng/g for Chry, while the values recorded for euryhaline Douglas/Stubbs creek varied between 4.27 ng/g for Naph and 5.13 ng/g for Ace. Eight (8) PAH suites were below detectable limits in the Eniong River and thirteen (13) in the Douglas/Stubbs creek, while Ikpa River had all the suites detected. This shows variation in quantity and quality of PAH contaminants with the nature of ecosystem. This implies diverse contamination sources as well as different capabilities to recover from PAH contamination (Essien et al. 2011, 2012).
The concentration of low molecular weight (LMW) PAHs encountered in the humic ecosystem of Eniong River was low compared to the freshwater and euryhaline ecosystems. The LMW-PAHs detected in the humic ecosystem were acenaphthylene, acenaphthene, fluoranthene and pyrene. Also encountered but in low concentrations in the humic water body were the high molecular weight (HMW) carcinogenic PAHs such as chrysene, benzo[k]fluoranthene, benzo[a]pyrene and a naturally occurring PAH compound, perylene. Researchers have reported higher organic degradation in humic ecosystem because of its higher modulating influence on autochthonous degraders (Anyakora et al. 2005). Therefore, the low content of the more soluble low molecular weight PAHs in the humic ecosystem could be as a result of their faster degradation compared to high molecular weight compounds (Mac Rae and Hall 1998; Yun et al. 2003). Their preferential loss during particulate lateral and vertical transport throughout the water column would lead to sedimentation of particles depleted in low molecular weight PAHs.
Influence of other sediment characteristics on PAH loads
Previous studies have shown that the distribution and concentration of PAHs in sediments are influenced by various factors including total organic carbon (TOC) content, sediment particle size, depth and current (He et al. 2014; Froehner et al. 2010). The highest TOC was found in humic ecosystem of Eniong River, followed by freshwater system of Ikpa River and lastly the euryhaline Douglas/Stubbs creek (Table 4). Generally, the sediments from the three ecosystems were predominantly sandy with highest percentage recorded for Eniong River. For silt content, lowest percentage content was recorded for Eniong River, followed by Ikpa River, while the highest percentage was recorded for Douglas/Stubbs creek. Again, Douglas/Stubbs creek recorded the highest percentage content of clay, followed by Ikpa River and the least at Eniong River. The results reveal that the sediment of the euryhaline ecosystem of Douglas/Stubbs creek were the finest of all the three ecosystems studied.
Sediments with high TOC have strong affinity for hydrophobic compounds like PAHs compared to sediments with lower TOC (Rahmanpoor et al. 2014). Also, PAHs are mainly adsorbed to small particles (clay), which exhibit higher surface area and consequently possessing greater capacity for adsorption (He et al. 2014; Rahmanpoor et al. 2014). In this study, correlation analysis was carried out to investigate the relationship between PAH concentration and the percentage TOC, clay, silt and sand in the sediment samples of the three ecosystems. The analysis was done in a site-specific pattern (each sampling point against its corresponding average total PAHs concentrations). The TOC and particle size properties of the three ecosystems are presented in Table 4. It shows that the PAHs accumulation in sediments of the euryhaline ecosystem of Douglas/Stubbs creek could be more influenced by its finer particulate content when compared to coarse or more sandy sediments of Eniong River. This result is in agreement with the findings of Amellal et al. (2001) on the effect of soil structure on the bioavailability of PAHs. Li et al. (2010) also opined that low molecular weight (LMW) PAHs in mangrove sediments degrade faster than high molecular weight (HMW) PAHs under low oxygen conditions. Degradation of LMW-PAHs is more influenced by sorption with soil (or sediment) organic matter, whereas degradation of the HMW-PAHs fraction is more influenced by microbial action (Yang et al. 2011). These findings suggest that petroleum-derived LMW-PAHs may be more easily degraded in sandy sediment of humic ecosystem than the near anoxic sediment of the euryhaline ecosystem. Similar findings have been reported by Essien et al. (2012). The same authors reported that the degradation of HMW-PAHs is most likely controlled by microbial activities in the anoxic sediments of the tropical mangrove swamp. Although Knaebel et al. (1996) reported that some humic substances could retard the bioavailability of PAHs to microbial attack under acidic conditions, although recent studies have shown that humic substances generally enhanced hydrocarbons degradation in aquatic systems. Degradation may be enhanced by the presence of electron acceptors such as Fe(III), nitrates and sulphates (Li et al. 2010) common in humic systems.
In the freshwater Ikpa River, total PAH concentrations showed no significant correlation with total organic carbon, clay content, silt content and sand content (Fig. 3). The result suggests that the PAH concentration distribution in the Ikpa River Basin may not solely depend on these chosen parameters, but on others variables like current and depths. Nevertheless, a slight variation may be observed in the correlation coefficients (R2) of the parameters. He et al. (2014) observed results for sediments of the Hormuz strait (Persian Gulf), where the total PAH concentration did not correlate with clay content, TOC and depths. However, positive correlation between PAH concentrations and TOC was observed in a study by Witt (1995) and Froehner et al. (2010).
In Eniong River (Fig. 4) there was no significant correlation between TOC and total PAHs, indicating that the high TOC in the humic ecosystem had little or no influence on the accumulation of PAHs in the sediments. This finding is in contradiction to some other studies which reported that the sorption of hydrocarbons is highly related to the organic matter content of sediments (Ouyang et al. 2006; Wu et al. 2012). Nam et al. (2008) also reported that, in an environment where there is continuous input of fresh PAH contamination, a lack of correlation should be expected, at least until equilibrium is reached. Another explanation could be the dissolution of PAHs into the high organic matrix followed by an uneven mineralisation assisted by the humic substances in the ecosystem. However, a positive correlation was observed between TOC and total PAHs in the Douglas/Stubbs creek (Fig. 5), suggesting that TOC can be the most important parameter, among others considered, influencing PAH occurrence in the euryhaline ecosystem. There was no correlation with grain sizes in Douglas/Stubbs creek.
Maximum PAH concentration is always observed in smallest grain size fraction comprising of clay and silt. In this study, a poor correlation was observed between particle sizes and PAH concentrations as the sediment textural class at all sampling points were sandy with little of loamy sand (mixture of sand, silt and clay with organic matter), while a weak linear correlation was observed between particle sizes which were sandy loam and PAH concentrations at Douglas/Stubbs creek (Fig. 5). This study reveals that sediments characteristics such as TOC and grain sizes are more important in euryhaline ecosystem than in freshwater rivers.
Furthermore, between Ikpa River Basin and Eniong River, there was no significant difference in sand, silt and total PAH (p > 0.05) (Table 4). Clay was not significantly different between Ikpa River Basin and Douglas/Stubbs creek (p > 0.05). This low organic matter content in Douglas/Stubbs creek may be attributed to the continuous tide which reduces the organic matter content in estuarine environments. Also, Eniong River being a humic ecosystem tends to have more organic matter built up due to the species of plants in the ecosystem which fall off rapidly as compared to the mangrove swamp species in Douglas/Stubbs creek. In general, the sediment samples collected from the study areas were characteristically more coarse than fine, implying that they were predominantly sandy. These results are expected given the relatively fast tidal current that tends to winnow the finer-grained sediments.
The multiple regression analysis showing factors that influence total PAH in the studied ecosystems are presented in Table 5. Result shows adjusted coefficient of determination of 0.506. This implies that 50.6% of the variation in total PAH was accounted for by TOC, sand, silt and clay. The F calculated of 4.835 with p value of 0.017 was obtained which means that these variables jointly predict total PAH (p < 0. 05). Result also reveals that among these independent variables, sand (p = 0.032, p < 0.05) and clay (p = 0.043, p < 0.05) were the only variable that significantly influence total PAH in the study areas. The most important variable that influence total PAH was sand.
Ecological risks of PAHs in the three ecosystems
Using the effects range low (ERL) and the effects range medium (ERM) values proposed by Long et al. (1995), the concentrations of individual PAHs recorded in the study ranged from BDL to 161.3 ng/g which were much lower than the ERL and ERM values with none exceeding the ERM values. As shown in Table 6, the levels of PAHs in Eniong River, Douglas/Stubbs creek and Ikpa River were far below the ERL values except for Acy and Flu in Ikpa River which had PAH levels between the ERL and ERM values. Generally, the concentration of total PAH levels was far below the ERL and ERM values. This indicates that there is no frequent acute adverse biological effect in the ecosystems under study.
Conclusion
Polycyclic aromatic hydrocarbon loads in the studied ecosystems varied greatly across the river stretch, implying possible differences in contamination sources. The total PAHs contamination range of 11.0–1055.2 ng/g recorded was generally low and below the allowable limit for aquatic sediment. Correlation analysis has shown that sediment particle and TOC content influenced PAHs burden in bottom sediments, but the effects varied with the molecular weight of PAHs and the nature of the system. TOC was the most significant determinant of PAHs load and distribution in sediment of the freshwater Ikpa River and euryhaline Douglas/Stubbs but had little or no influence in the humic sediment of Eniong River, while the influence of particle size was generally indefinite but slightly associated with PAHs accumulation in the euryhaline sediment. It implies that PAH levels in freshwater ecosystem of Ikpa River tend to be high due to limited sorption into organic matter and subsequent mineralisation by microbial action. The ecological risk assessment of PAHs accumulation revealed that the concentration of individual PAHs recorded in the study area ranged from BDL to 161.3 ng/g which was much lower than the ERL and ERM values. This indicates no frequent acute adverse biological effect although the accumulation of PAHs in freshwater ecosystem of Ikpa River may pose ecological risks as most of the carcinogenic PAHs suites recorded comparatively high pollution indices. In the light of the present findings, remedial actions required for cleanup of polluted ecosystems in the Niger Delta should be site-specific.
References
Accardi-Dey A, Gschwend PM (2002) Assessing the combined roles of natural organic matter and black carbon as sorbents in sediments. Environ Sci Technol 36(1):21–29
Amellal N, Portal J, Berthelin J (2001) Effect of soil structure on the bioavailability of polycyclic aromatic hydrocarbons within aggregates of a contaminated soil. Appl Geochem 16:1611–1619
Anyakora C, Ogbeche A, Palmer P, Coker H, Ukpo G, Ogah C (2005) GC/MS analysis of polynuclear aromatic hydrocarbons in sediment samples from the Niger Delta region. Chemosphere 60:990–997
Barakat AO, Mostafa A, Wade TL, Sweet ST, El Sayed NB (2011) Distribution and characteristics of PAHs in sediments from the Mediterranean coastal environment of Egypt. Mar Pollut Bull 62:1969–1978
Barhoumi B, LeMenach K, Devier MH, Ameur WB, Etcheber H, Budzinski H, Cachot J, Driss MR (2014) Polycyclic aromatic hydrocarbons (PAHs) in surface sediments from the Bizerte Lagoon, Tunisia: levels, sources, and toxicological significance. Environ Monit Assess 186:2653–2669
Brady N, Weil R (1996) The nature and property of soils, 11th edn. Prentice Hall Inc., Upper Saddle River, p 103
Burkowska A, Donderski W (2007) Impact of humic substances on bacterioplankton in eutrophic lake. Polish Journal of Ecology 55(1):155–160
Cardellicchio N, Buccolieri A, Giandomenico S, Lopez L, Pizzulli F, Spada L (2007) Organic pollutants (PAHs, PCBs) in sediments from the Mar Piccolo in Taranto (Ionian Sea, Southern Italy). Mar Pollut Bull 55:451–458
Dahle S, Savinov VM, Matishov GG, Evenset A, Næs K (2003) Polycyclic aromatic hydrocarbons (PAHs) in bottom sediments of the Kara Sea shelf, Gulf of Ob and Yenisei Bay. Sci Total Environ 306:57–71
Dominquez C, Sarkar S, Bhattacharya A, Chatterjee M, Bhattacharaya BD, Jover E, Albaiges J, Bayona JM, Alam MdA, Satpathy KK (2010) Quantification and source identification of polycyclic aromatic hydrocarbons in core sediments from Sundarbans mangrove wetland, India. Arch Environ Contam Toxicol 59(1):49–61
Ekpo BO, Oyo-Ita OE, Oros DR, Simoneit BRT (2012) Distributions and sources of polycyclic aromatic hydrocarbons in surface sediments from Cross River estuary, SE Niger Delta, Nigeria. Environ Monit Assess 184:1037–1047
El Nemr A, Said TO, Khaled A, El-Sikaily A, Abd-Allah AMA (2007) The distribution and sources of polycyclic aromatic hydrocarbons in surface sediments along the Egyptian Mediterranean coast. Environ Monit Assess 124:343–359
Essien JP, Eduok SI, Olajire AA (2011) Distribution and ecotoxicological significance of polycyclic aromatic hydrocarbons in sediments from Iko River estuary mangrove ecosystem. Environ Monit Assess 176:99–107
Essien JP, Ebong GA, Asuquo JE, Olajire AA (2012) Hydrocarbons contamination and microbial degradation in mangrove sediments of the Niger Delta region (Nigeria). Chem Ecol 28(5):421–434
Froehner S, Maceno M, Luz ECD, Souza DB, Mechado KS (2010) Distribution of polycyclic aromatic hydrocarbons in marine sediments and their potential toxic effects. Environ Monit Assess 168:205–213
Gobas FAPC, Maclean LG (2003) Sediment-water distribution of organic contaminants in aquatic ecosystems: the role of organic carbon mineralization. Environ Sci Technol 37:735–741
He X, Pang Y, Song X, Chen B, Feng Z, Ma Y (2014) Distribution, sources and ecological Risk assessment of PAHs in surface sediments from Guan River Estuary, China. Mar Pollut Bull 80:52–58
Inam E, Offiong N, Essien J, Kang S, Kang S, Antia B (2016) Polycyclic aromatic hydrocarbons loads and potential risks in freshwater ecosystem of the Ikpa River Basin, Niger Delta—Nigeria. Environ Monit Assess 188(1):49. https://doi.org/10.1007/s10661-015-5038-9
Johnson M, Huang W, Weber W (2001) A disturbed reactivity model for sorption by soils and sediments. 13. Simulated diagenesis of natural sediment organic matter and its impact on sorption/desorption equilibria. Environ Sci Technol 35:1680–1687
Jones B (2001) Laboratory guide for conducting soil tests and plant analysis. CRC Press, Boca Raton, pp 139–148
Kannam K, Johnsom-Restrepo B, Yohn SS, Giesy JP, Long DT (2005) Spatial and temporal distribution of polycyclic aromatic hydrocarbons in sediments from Michigan inland lakes. Environ Sci Technol 39:4700–4706
Kelley J, Arias-Rodriguez L, Martin D, Yee M, Bustamante C, Tobler M (2016) Mechanisms underlying adaptation to life in hydrogen sulfide-rich environments. Mol Biol Evol 33(6):1419–1434. https://doi.org/10.1093/molbev/msw020
Kim HC, Yu MJ, Han I (2006) Multi-method study of the characteristic chemical nature of aquatic humic substances isolated from the Han River, Korea. Appl Geochem 21(7):1226–1239
King AJ, Readman JW, Zhou JL (2004) Dynamic behaviour of polycyclic aromatic hydrocarbons in Brighton Marina, UK. Mar Pollut Bull 48:229–239
Knaebel DB, Vestal JR, Federle TW, McAvoy DC (1996) Microbial mineralization of organic compounds in an acidic agricultural soil: effects of preadsorption to various soil constituents. Environ Toxicol Chem 15(11):1865–1875
Lang Y, Wang N, Gao H, Bai J (2012) Distribution and risk assessment of polycyclic aromatic hydrocarbons (PAHs) from Liaohe estuarine wetland soils. Environ Monit Assess 184:5545–5552
Li CH, Wong YS, Tam NFY (2010) Anaerobic biodegradation of polycyclic aromatic hydrocarbons with amendment of iron(III) in mangrove sediment slurry. Biores Technol 101(21):8083–8092
Liu Y, Beckingham B, Ruegner H, Li Z, Ma L, Schwientek M, Xie H, Zhao J, Grathwohl P (2013) Comparison of sedimentary PAHs in the rivers of Ammer (Germany) and Liangtan (China): differences between early- and newly- industrialized countries. Environ Sci Technol 47:701–709
Lohmann R, MacFarlane JK, Gschwend PM (2005) Importance of black carbon to sorption of native PAHs, PCBs, and PCDDs in Boston and New York Harbor sediments. Environ Sci Technol 39(1):141–148
Long ER, MacDonald DD, Smith SL, Calder FD (1995) Incidence of adverse biological effects within ranges of chemical concentrations in marine and estuary sediments. Environ Manage 19:81–97
Ma C, Ye S, Lin T, Ding X, Yuan H, Guo Z (2014) Source apportionment of polycyclic aromatic hydrocarbons in soils of wetlands in the Liao River Delta, Northeast China. Mar Pollut Bull 80:160–167
Mac Rae J, Hall K (1998) Biodegradation of polycyclic aromatic hydrocarbons (PAHs) in marine sediment under denitrifying conditions. Water Sci Technol 38:177–185
Manoli E, Samara C (1999) Polycyclic aromatic hydrocarbons in natural waters: sources, occurrence and analysis. Trends Anal Chem 18(6):417–427
Maskaoui K, Zhou JL, Hong HS, Zhang ZL (2002) Contamination by polycyclic aromatic hydrocarbons in the Jiulong River Estuary and Western Xiamen Sea, China. Environ Pollut 118:109–122
Mitra S, Dellapenna T, Dickhut R (1999a) Polycyclic aromatic hydrocarbon distribution within lower hudson river estuarine sediments: physical mixing vs sediment geochemistry. Estuar Coast Shelf Sci 49(3):311–326
Mitra S, Dickhut RM, Kuehl SA, Kimbrough KL (1999b) Polycyclic aromatic hydrocarbon (PAH) source, sediment deposition patterns, and particle geochemistry as factors influencing PAH distribution coefficients in sediments of the Elizabeth River, VA, USA. Mar Chem 66:113–127
Nam J, Thomas G, Jaward F, Steinnes E, Gustafsson O, Jones K (2008) PAHs in background soils from Western Europe: influence of atmospheric deposition and soil organic matter. Chemosphere 70:1596–1602
Ouyang Y, Zhang J, Ou L (2006) Temporal and spatial distributions of sediment total organic carbon in an estuary river. J Environ Qual 35(1):93–100
Perera FP, Hemminki K, Gryzbowska E, Motykiewicz G, Michalska J, Santella RM, Young T, Dickey C, Brandt-Rauf P, DeVivo I, Blaner W, Tsai W, Chorazy M (1992) Molecular and genetic damage in humans from environmental pollution in Poland. Nature 360:256–258
Pouneva ID (2005) Effect of humic substances on the growth of microalgal cultures. Russ J Plant Physiol 52:410. https://doi.org/10.1007/s11183-005-0060-3
Rahmanpoor S, Ghafourian H, Hashtroudi SM, Bastani KD (2014) Distribution and sources of polycyclic aromatic hydrocarbons in surface sediments of the Hormuz Strait, Persian Gulf. Mar Pollut Bull 78:224–229
Ratola N, Lacorte S, Alves A, Barcelo D (2006) Analysis of polycyclic aromatic hydrocarbons in pine needles by gas chromatography-mass spectrometry: comparison of different extraction and clean-up procedures. J Chromatogr A 1114:198–204
Rockne KJ, Shor LM, Young LY, Taghon GL, Kosson DS (2002) Distributed sequestration and release of PAHs in weathered sediment: the role of sediment structure and organic carbon properties. Environ Sci Technol 36:2636–2644
Sandoli RL, Ghiorse WC, Madsen EL (1996) Regulation of microbial phenanthrene mineralization in sediment samples by sorbent-sorbate contact time, inocula and gamma irradiation-induced sterilization artifacts. Environ Toxicol Chem 15(11):1901–1907
Schindler DW, Kidd KA, Muir DCG, Lockhart WL (1995) The effects of ecosystem characteristics on contaminant distribution in northern freshwater lakes. Sci Total Environ 160:1–17
Skupinska K, Misiewicz I, Kasprzycka-Guttman T (2004) Polycyclic aromatic hydrocarbons: physicochemical properties, environmental appearance and impact in living organisms. Acta Pol Pharmacuetica Drugs Res 61:233–240
Sojinu OS, Wang J, Sonibare OO, Zeng EY (2010) Polycyclic aromatic hydrocarbons in sediments and soils from oil exploration areas of the Niger Delta, Nigeria. J Hazard Mater 174:641–647
Sprovieri M, Feo ML, Prevedello L, Manta DS, Sammartino S, Tamburrino S, Marsella E (2007) Heavy metals, polycyclic aromatic hydrocarbons and polychlorinated biphenyls in surface sediments of the Naples harbour (southern Italy). Chemosphere 67:998–1009
Tao S, Cao H, Liu W, Li B, Cao J, Xu F, Wang X, Coveney R, Shen W, Qin B, Sun R (2004) Fate modeling of phenanthrene with regional variation in Tianjin, China. Environ Sci Technol 37:2453–2459
Udo EJ, Ibia TO, Ogunwale JA, Ano AO, Esu IE (2009) Manual of soil, plant and water analyses. Sibon Books Ltd, Lagos, pp 11–13
Udofia E, Inam E, Abraham N, Asamudo N, Essien J (2015) Crude oil and PAH degrading bacteria isolated from humic freshwater ecosystem of Eniong River—Nigeria. Paper presented at the 1st UNIUYO & GIST Joint Programme Workshop held in Abuja, Nigeria. 15–18 June
Udosen ED, Udoh AP, Ekong CI (2004) Variations in trace metal levels in sediments from Ikpa River in Itu Area of Akwa Ibom State, Nigeria. J Appl Sci 7:4229–4240
Udosen E, Benson N, Essien J (2007) Trends in heavy metals and total hydrocarbon burdens in Stubbs Creek A tributary of Qua Iboe River Estuary, Nigeria. Trends Appl Sci Res 2(4):312–319
United States Environmental Protection Agency (US EPA) (2007) US EPA Method 8270D –Semivolatile organic compounds by gas chromatography/mass spectrometry (GC/MS). U.S.EPA, Washington, pp 15–67
Wang R, Liu G, Chou C, Liu J, Zhang J (2010) Environmental assessment of PAHs in soils around the Anhui Coal District, China. Arch Environ Contam Toxicol 59(1):62–70
Warren N, Allan IJ, Carter JE, House WA, Parker A (2003) Pesticides and other micro-organic contaminants in freshwater sedimentary environments—a review. Appl Geochem 18:159–194
Wentworth CK (1992) A scale of grade and class terms for clastic sediments. J Geol 30:377–392
Witt G (1995) Polycyclic aromatic hydrocarbons in water and sediment of the Baltic Sea. Mar Pollut Bull 31:237–248
Wu F, Xu L, Sun Y, Liao H, Zhao X, Guo J (2012) Exploring the relationship between polycyclic aromatic hydrocarbons and sedimentary organic carbon in three Chinese lakes. J Soils Sediments 12:774–783
Yang Y, Zhang N, Xue M, Lu ST, Tao S (2011) Effects of soil organic matter on the development of the microbial polycyclic aromatic hydrocarbons (PAHs) degradation potentials. Environ Pollut 159(2):591–595
You C, Jia C, Pan G (2010) Effect of salinity and sediment characteristics on the sorption and desorption of perfluorooctane sulfonate at sediment-water interface. Environ Pollut 158:1343–1347
Yu H, Xiao H, Wang D (2014) Effects of soil properties and biosurfactant on the behavior of PAHs in soil-water systems. Environ Syst Res 3(6):1–11
Yun T, Tianling Z, Xinhong W (2003) PAH contamination and PAH-degrading bacteria in Xiamen Western Sea. Chem Speciat Bioavailab 14:25–34
Zuloaga O, Prieto A, Usobiaga A, Sarkar SK, Chatterjee M, Bhattacharya BD, Bhattacharya A, Alam MA, Satpathy KK (2009) Polycyclic aromatic hydrocarbons in intertidal marine bivalves of Sunderban mangrove wetland, India: an approach to bioindicator species. Water Air Soil Pollut 201:305–318
Zuloaga O, Prieto A, Ahmed K, Sarkar SK, Bhattacharya AK, Chatterjee M, Bhattacharaya BD, Satpathy KK (2013) Distribution of polycyclic aromatic hydrocarbons in recent sediments of Sundarban mangrove wetland of India and Bangladesh: a comparative approach. Environ Earth Sci 68:355–367
Acknowledgements
This study was supported by the Ministry of Science and Technology in South Korea through the Institute of Science and Technology for sustainability (UNU & GIST joint programme) in 2014 and 2016. We thank the two anonymous reviewers whose comments improved the quality of the original manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Inam, E., Etuk, I., Offiong, NA. et al. Distribution and ecological risks of polycyclic aromatic hydrocarbons (PAHs) in sediments of different tropical water ecosystems in Niger Delta, Nigeria. Environ Earth Sci 77, 216 (2018). https://doi.org/10.1007/s12665-018-7396-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12665-018-7396-4