Abstract
The present work is the first attempt to compare the data of a comprehensive study of the origin and distribution of 16 priority pollutant polycyclic aromatic hydrocarbons (PAHs) in surface sediments (<63 μm) from 18 sampling stations, 9 from Sundarban of Bangladesh and 9 from Indian counterpart. Σ19PAHs concentration in sediments showed wide variations from 208.3 to 12,993.1 ng g−1 dry weight in Indian Sundarban, whereas 208.4 to 4,687.9 ng g−1 in the case of Bangladesh. Fluoranthene, pyrene, benzo(b)fluoranthene, benzo(a)pyrene and dibenzo(a,h)anthracene were predominant species for both the countries. The PAH diagnostic ratios indicated that the PAHs in sediments from both the countries were of mixed source of hydrocarbons of both petrogenic and pyrolytic origin. According to the numerical effect-based sediment quality guidelines, the levels of PAHs in the Sundarban wetland of Bangladesh and India should not exert adverse biological effects. The TEQ values calculated for samples from the Bangladesh and Indian Sundarban varied from 13.68 to 1,014.75 and 1.31 to 2,451 ng g−1 d.w. with an average of 221.02 and 358.63 ng g−1, respectively. The overall contamination status of PAH was higher in India than Bangladesh.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Polycyclic aromatic hydrocarbons (PAHs) are by-products of incomplete combustion of organic materials and have raised severe environmental concerns, owing to their carcinogenic properties (Wang et al. 2010). Anthropogenic inputs of PAHs from oil spills, ship traffic, urban runoff, waste discharge, as well as atmospheric fallout of vehicle exhaust and industrial stack emission have caused significant accumulation of PAHs in the marine environment, which is generally considered as the ultimate pool (Simpson et al. 1996; Seruto et al. 2005). PAHs, most of which are categorized as persistent organic pollutants (POPs), are of environmental significance due to their widespread distribution in the environment and potential toxicity to organisms. These contaminants are often hydrophobic and easily accumulated in organisms through food chains and present potential threat to aquatic ecosystems and also to human health (Colborn et al. 1993). PAHs are hydrophobic compounds; therefore, sediments represent one of the most important reservoirs of PAHs in the marine environment (Perra et al. 2011). The sorptive properties of PAHs are largely controlled by their hydrophobicity and by the particle organic carbon content. In the water column, PAH tend to associate with suspended particulate matter and are eventually deposited in sediments (Santschi et al. 2001). Since the deposition of sediments continues over time, sediments can act as geochronometers of the contaminant deposition in the environment as well as of general environmental change over time (Hites et al. 1977; Yamashita et al. 2000; Luo et al. 2004; Kannan et al. 2005; Van Metre and Mahler 2005). Studies concerning PAHs in water column and sediments can lead to better understanding of PAH cycles in top-to-bottom processes and their historical trend. Recent studies on the pollution status of Indian Sundarban wetland have revealed the presence of high concentrations of heavy metals (Zn and Cu), as well as persistent organic pollutants in sediments (Chatterjee et al. 2007; Sarkar et al. 2007; Sharif et al. 1991, 1993a, b). These findings have initiated our present collaborative work to evaluate the occurrence of PAHs in surface sediment samples from the Sundarban wetland belonging to both India and Bangladesh. The present paper aims to identify and quantify the potential sources of the PAH contamination in sediments to get a coherent picture of the two neighboring countries. Through the comparison of levels and distributions for PAHs, this paper aims at studying the coherence of results issued from Sundarban wetland of two neighboring countries structured by the same objectives and, therefore, by the same type of sampling strategies.
Methodology
Geographical setting
The funneled mouth of the Ganga–Brahmaputra–Meghna Rivers stretches for an east–west length of more than 380 km at the Bay of Bengal sea face, of which the Hugli estuary, the westernmost distributory of the Ganges occurs in the westernmost part of the Teri River, a distributory system of the Meghna river occurs in the easternmost direction. Despite a huge amount of sediment supply by the former two major rivers (~500 × 106 tons/year by the Ganges and ~700 × 106 tons/year by the Brahmaputra), the delta has not shown any appreciable accretion since the last 250 years due to subsidence along the north–northwest axis of the present river mouths (Eisma 1998). In this huge deltaic complex, the southerly flowing rivers are quite active in the eastern (Bangladesh) part, whereas tidal flows dominate over riverine flows in the western part (India). In the Indian part, the tidal flows surpass the fluvial fluxes for about 8 months of the year and river Ganges receives freshwater only during the four monsoon months of the year (Goodbred 2003) together with some surface runoff from the reclaimed catchment areas. Tides at the major river and estuary mouths are macrotidal (4.2–6 m) and semi-diurnal. The tidal amplitude may reach as high as 7 m or above during monsoon. In the southernmost portion of the delta, i.e., complex, the tidal creeks and large channels are generally closed by embankments except Khulna district of Bangladesh and the adjacent areas in India. The impact of reclamation with partly or fully cut mangroves is however more on the western part (Mukherjee 1969; Snead 1985).
Study area
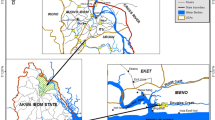
The present study areas and the sampling locations are bounded by longitudes 88°10′E and 89°30′E at the surface. In the Indian Territory, the sampling stations have been chosen along the Hugli estuary including and adjoining areas belonging to Indian Sundarban. The nine sampling locations, e.g., Lot 8 (S1) Gangadharpur (S2), Gangasagar (S3), Fresargunge (S4), Jharkhali (S5), Gosaba (S6), Canning (S7), Sandeshkhali (S8) and Gushighata (S9) belong to the different wave tidal environments and anthropogenic stresses (as in Fig. 1a).
The sampling stations in Bangladesh territory follow a north to south direction covering nine separate locations within the Bangladesh Sundarban. Some of these stations have included the points emergence of some distributory channels with the main north to south flowing Martaja River estuary opening in the Bay of Bengal. The sampling stations are Khalispur (S1), Labanchara (S2), Mongla port (S3), Dhangmari (S4), Karamjal (S5), Chandpy (S6), Estuary (S7), Hiron Point (S8) and Sea (S9). All the sampling locations of Bangladesh territory also have different and distinctive wave-tide climate together with the impacts of anthropogenic stresses exerted by the Mongla Port and other activities like agriculture, industries and reclamation. The present paper is the first attempt to get a comprehensive comparative account of the concentrations of PAH congeners in sediments of the estuarine and deltaic systems of the two countries. The distance between the Hugli estuary and the Martaja estuary at the sea face is ~140 km. The study stations belong to the same chunk of 6,000 km2 area of mangrove forest- the Sundarban, a UNESCO World Heritage Site and a well-known mangrove wetland in the world.
Sampling and analytical procedure
Samples were collected with the help of a stainless steel spade, stored in polyethylene bags, and transported to the laboratory. Once in the laboratory, samples were oven-dried at 50 °C (Sarkar et al. 2012; Zuloaga et al. 2009) and were divided into two parts: one for physicochemical analysis and the other for PAH analysis. The sediment samples were stored in labeled polyethylene bags kept in iceboxes and transported to the laboratory where they were frozen to −20 °C. Within 2 days, a portion of each sample was placed in a ventilated oven at a very mild temperature (max. 40 °C). Dried samples were then disaggregated using an agate mortar and pestle and divided into two aliquots. One aliquot was sieved through 63 μm metallic sieve and stored in hermetic plastic bags until elemental analysis. All visible marine organisms and coarse shell fragments, sea grass leaves and roots when present were removed manually. The other aliquot was unsieved and used for sediment quality parameters (organic carbon, pH, % of silt, clay and sand). Organic carbon (Corg) content of the soil was determined following a rapid titration method (Walkey and Black 1934) and pH with the help of a deluxe pH meter (model no. 101E) using combination glass electrode manufactured by M.S. Electronics (India) Pvt. Ltd. Mechanical analyses of sediment were done by sieving in a Ro-Tap Shaker (Krumbein and Pettijohn 1938) manufactured by W.S. Tyler Company, Cleveland, Ohio, and statistical computation of textural parameters was done by using the formulae of Folk and Ward (1957). Approximately 1.0 g of dry sediment sample was weighed and submitted to microwave-assisted extraction in 15 ml of acetone at 21 psi during 15 min and at 80 % of microwave power in a MDS-2000 microwave (CEM, Matthews, NC, USA), according to Bartolomé et al. (2005). 25 μl of a mixture of acenaphthene-d10, chrysene-d12 and phenanthrene-d10 at 20 μg ml−1 in acetone was added as surrogates. The extract was then concentrated under a gentle stream of nitrogen and loaded onto a 1-g Florisil® cartridges (Supelco, Walton-on-Thames, UK) for further clean-up of the extract. PAHs were eluted in a (4:1) n-hexane:toluene mixture. The elutes were concentrated to dryness, re-dissolved in 500 μl of isooctane and kept in the dark at −18 °C until analysis.
The extracted compounds were analyzed on a 6890 N chromatograph coupled to a 5973 mass spectrometer with a 7683 autosampler (Agilent Technologies, Avondale, PA, USA). Two microliters of the sample were injected in the splitless mode (2 min) at 270 °C into a 30 m × 0.25 mm × 0.25 μm HP-5 capillary column. For the separation of PAHs, the temperature program was: 60 °C for 2 min, increase at 10 °C min−1 to 290 °C with a final hold for 10 min. The carrier gas was helium (C-50) and was kept at a constant flux of 1.5 ml min−1. The mass spectrometer was operated in the electron impact ionization mode at 70 eV. The interface was kept at 300 °C and the ionization source and the quadrupole at 230 and 150 °C, respectively. Measurements were performed in the SIM mode; the m/z values for each congener are included in Table 1. Seven-point internal calibration curves were built in the range of 0.05–16 μg ml−1 for the target compounds. The proposed analysis method was validated after application to NIST 1944 certified reference sediment. The recoveries obtained were in good agreement with the certified values (Bartolomé et al. 2005) and relative standard deviations were lower than 17 % for all the analytes, except for naphthalene (35 %). Reagent blanks were performed for every ten samples and limits of detection (LOD) were estimated as three times the signal of the blanks plus three times the standard deviation and were in the 0.5–11 ng (Bartolomé et al. 2005). For every ten samples, replicates of the NIST 1944 were also performed in order to guarantee that the analyses were under control during the processing of the samples.
Results and discussion
Sediment geochemistry and PAH accumulation
The sediment quality parameters (pH, organic carbon and textural properties) of the different samples vary among them, as depicted in Tables 2 (for sediment samples collected from Martaja estuary, Bangladesh) and 3 (for sediment samples collected from Hugli estuary, India).Values of pH range from slightly acidic to basic (pH 6.5–8.5) for the samples from Hugli River estuary and adjoining areas of India but mainly basic for Martaja estuary samples, Bangladesh (pH 7.5–7.9); the acidic nature is partly due to the oxidation of FeS2 and FeS to SO4 −2 and partly results from the decomposition of mangrove litter and hydrolysis of tannin in mangrove plants releasing various kinds of organic acids (Liao 1990). Organic carbon (OC) values were below 1 % in all stations for Hugli estuary samples but were above 1 % for Martaja estuary samples except at S9. Very low organic carbon values in the intertidal zone sediments of Sundarban wetland, India, were also recorded in previous studies (Sarkar et al. 2004; Chatterjee et al. 2007). These low values of organic carbon are probably related to the poor absorbability of organics on negatively charged quartz grains, which predominate in the intertidal sediments of this estuarine environment (Bhattacharya and Sarkar 2003; Sarkar et al. 2004). Regarding textural composition, all the studied stations at the two countries also exhibit wide variations with differences in the proportions of silt and clay content. Textural differences might be attributed to vigorous estuarine mixing, suspension–resuspension and flocculation–deflocculation processes. These variations might influence the PAH accumulation in the sediments (Tables 4, 5), where the processes involved are quite complex in nature. Other important factors are the chemical properties of the compounds, especially their water solubility, sediment composition and organic carbon content.
Distribution of PAHs in sediments
Concentrations of 16 PAHs measured in surface sediments of 9 sampling sites in Martaja river estuary, Bangladesh, and 9 sampling sites in Hugli river estuary, India, have been summarized in Tables 4 and 5, respectively. PAH concentrations ranged from 0 to 641 ng g−1 in Martaja River, Bangladesh, sediments and 0 to 1,839.5 ng g−1 for Hugli River sediment samples, India. The prevalent level of PAH concentration is lower than that of Mediterranean coastal sediments of Egypt (Barakat et al. 2011), Huaihe River sediments, China (Fu et al. 2011) and Liaoning, Jilin Heilongjiang provinces, northeast China (Lin et al. 2012). Figure 2a and b show the distribution pattern of two-, three-, four-, five- and six-ring PAH compounds in the individual stations of Bangladesh and India, respectively. For Martaja sediments, as it can be seen in Fig. 2a, there is a dominance (60–70 % of the total PAHs) of high molecular weight PAH compounds (HPAHs) (i.e., 3–4, 5–6 rings) at stations S1, S2, S3, S4 and S9. It can be referred here that the low molecular weight PAH compounds (LPAHs) were degraded more rapidly in the sediments (Jones et al. 1986) in these five sampling sites. In comparison, the three-, four-, five- and six-ringed PAHs and their alkyl groups have been found to be more refractory in a natural environment which is essential in preserving the source features (Sauer and Boehm 1991). The predominance of HMW PAHs at majority of the study sites in Martaja estuary, Bangladesh, was the evidence of the strong influence of pyrogenic sources for these contaminants (Kimbrough and Dickhut 2006).
This conclusion was supported by a previous research, which found automobile emissions and coal-dominated PAH sources in the southern Chesapeake Bay region, USA (Dickhut et al. 2000). On the other hand, the distribution of two-, three-, four-, five- and six-ringed PAHs in the individual stations of Hugli River estuary, India, is completely different from that of Bangladesh. Different trends of PAH distribution were observed as evidenced in Fig. 2b. For example, stations S1, S2 and S5, S6 show low contribution of two-ring PAH compounds compared to three-, four-, five- and six-ring PAH compounds, i.e., dominance of high molecular PAH molecules. Dominance of two-ring PAH compounds could be found in station S8, while station S4 shows very low contribution of five- and six-ring PAH compounds. The dominance of high molecular PAHs, i.e., three-, four-, five- and six-ring PAH compounds was pronounced at stations S1, S2, S3, S5, S6, S7 and S9. Thus, it could be stated that the source of PAHs generating different ring structures are various in case of Hugli River sediments. These mixed sources were also found in the previous observations of the same area (Sarkar et al. 2007; Binelli et al. 2008, etc.). Coal is the dominant source of PAHs as in other river sediments (Walker and Dickhut 2001). However, automotive sources of PAHs also dominate (Dickhut et al. 2000). Coal has distinctly high levels of fluoranthene and pyrene with lower level of other HMW PAHs. In contrast, auto-emissions have similar levels of fluoranthene and pyrene, relative to benz(ghi)perylene and only moderately lower levels of both benzo(a)anthracene and benzo(a) pyrene (Kimbrough and Dickhut 2006).
Assessment of diagnostic ratios
PAHs can be generated by three main processes: combustion at a very high temperature of organic matter, release of petroleum or diagenetic processes (degradation of the organic matter) (Neff 1979). However, for PAHs contamination, it is difficult to discriminate between pyrolytic products and petroleum-derived ones, due to the parameters complexity governing PAHs distribution in the marine sediments. In general, petrogenic PAHs are more available than the pyrolytic ones, because the first ones are introduced directly into the marine environment in dissolved, colloidal forms or fairly associated with suspended matter, whereas the last ones are probably strongly associated with the particles issued from pyrolytic sources. Besides, pyrogenic PAHs are characterized by the dominance of the high molecular weight (HMW) (PAHs 5–6 rings) (Muel and Saguem 1985), while petroleum hydrocarbons are dominated by the low molecular weight (LMW) (PAHs 2–4 rings) (Neff 1979; Wise et al. 1988; Berner et al. 1990).
In the coastal area of West Bengal, the industrial and anthropogenic activities constitute the main source of PAHs. Several methods have been used to identify the origin and the sources of PAHs in marine sediments, such as the characteristic of PAHs profiles or the characteristic ratios between PAH compounds. The use of PAH ratios is not feasible, due to the variety of processes contributing to the distribution of PAHs in marine sediments. In spite of it, information on major pollution sources for a particular area can help to infer the primary factors, which cause the appearance of these compounds in the environment. Diagnostic ratios provide only qualitative information about the contribution of various sources with regard to PAH contaminations (Wang et al. 2010). A first step to discriminate between petrogenic and pyrolytic contamination consisted in comparing LPAHs and HPAHs compound abundances (Perra et al. 2011). In order to estimate the origin of the contamination, the LMW/HMW ratio was the first index calculated (Tables 4, 5), where LMW = Naph + Acy + Ace + Flu + Phen + Anth + Flu + Pyr + B[a] A + Chry and HMW = B[b + k]Fl + B[a]P + DB[ah]A + B[ghi]P + IndP. It was revealed that most values of the LMW/HMW ratio are lower than 1 indicating a pyrolytic origin of PAHs excepting S5, S6 and S8 for Martaja river samples (LMW/HMW = 1.25, 1.05 and 1.89, respectively) and S4 for Hugli River samples (LMW/HMW = 2.5), where PAHs contamination could arise from a petrogenic source. Another method to estimate the origin of the PAHs in sediments uses the ratios Phen/Anth and Flu/Pyr. Phenanthrene and anthracene are the two structural isomers. Because of their different physicochemical properties, they could behave differently in the environment and could lead to different values for their Phen/Anth ratio (Gschwend and Hites 1981). Pyrolysis of organic matter at very high temperature generates PAHs, characterized by a low Phen/Anth ratio (<10), while the slow maturation of petroleum at lower temperatures leads to much larger values of the Phen/Anth ratio (>25) (Soclo 1986; Raoux 1991). In conclusion, phenanthrene is thermodynamically more stable than anthracene, so a Phen/Anth ratio <10 is characteristic of a pyrolytic pollution (Baumard et al. 1999; Budzinski et al. 1997; Sicre et al. 1987). In the same way, the Flu/Pyr ratio is also discriminating. Fluoranthene is less thermodynamically stable than pyrene and a predominance of fluoranthene over pyrene is characteristic of pyrolytic products, while in petroleum-derived PAHs, pyrene is more abundant than fluoranthene (Budzinski et al. 1997). During the combustion process, pyrolytic products are usually characterized by a fluoranthene/pyrene ratios >1 (Baumard et al. 1999). The ratios of phenanthrene to anthracene (Phen/Anth) and fluoranthene to pyrene (Flu/Pyr) have been applied to assess the contribution of petroleum or combustion sources of PAHs. A Phen/Anth ratio of <10 and Flu/Pyr ratio of >1 have also been evidenced in the majority of the cases, which strongly support pyrogenic origins of PAHs (Baumard et al. 1998). Sediment samples with Phen/Anth <10 and Flu/Pyr <1 were characteristic of a mixed pattern of pyrogenic and petrogenic contamination. It is probably prudent to observe that all the samples display a dominance of the pyrolytic PAHs. The likely sources of these are a combination of local point source anthropogenic inputs and the ubiquitous pyrolytic PAHs deposition that results from global Aeolian transport (Jaward et al. 2004). The latter will be present in greater proportion in the samples with low, compared to those higher, total PAH concentrations. The distribution of individual PAHs may be used to differentiate between pyrolytic and petrogenic sources. These are characterized by certain molecular indices based on the concentration ratios of selected PAHs (Colombo et al. 1989). However, one difficulty in identifying the PAH sources is the possible coexistence of several of them and the transformation processes that PAHs can undergo before deposition in the sediments (Butler and Crossley 1981). The wide range of PAH ratios found in sediments of both Martaja (Bangladesh) and Hugli estuary (India) may suggest a mixed source of hydrocarbons of both petrogenic and pyrolytic origin, although some post-depositional alterations within the sediment cannot be ruled out (Bicego et al. 2006). In order to provide a better estimate of PAH sources, a series of ratios are worked out. PAH sources to wetland sediments were similar. Moreover, it can be seen that they reflect predominant combustion sources and particularly, originated from grass, wood and coal.
The large use of coal and wood for domestic cooking, preparing molasses and husking rice by local inhabitants was reported by Chatterjee et al. (2009) from Indian Sundarban. The combustion source of PAH was also endorsed by Liu et al. (2007) from China. In addition, this also acts as a potential local atmospheric source of PAHs. A comparative account of the PAH contamination status of the two countries shows that, in general, there is a great difference in the distribution pattern and concentration range of PAH congeners in two countries. The sediment samples from Sundarban wetland, India, showed higher range of PAH concentration (208.3–12,993.1 ng g−1) than that of Bangladesh samples (208.4–4,687.9 ng g−1). According to the number of aromatic rings, the 16 PAH compounds were divided into four groups representing two-, three-, four-, five- and six-ring PAHs. For sediment samples of Martaja River, Bangladesh, a higher proportion of PAH species with three- and four-ring structures for majority of the stations and five to six rings for the station S2 was detected. Compositions and relative abundance of individual PAH in sediments of the Hugli River, India, were quite similar, except for site S1, S3 and S4, where the two-ring PAH were predominant. The result suggests that the PAH contamination in the two countries come essentially from same identical source and is indicative of a pyrolytic origin. Among all 9 PAHs, acenaphthene, naphthalene and fluoranthene were predominant species in the nine sampling sites of Bangladesh, which accounted approximately for 0.42–29.36, 0–22.16 and 13.69–19.34 %, respectively, of total PAHs. In addition, pyrene and benzo[a]pyrene were prevalent in samples from most of the sites. Conversely, the trend is different for sediments in Indian sites. Naphthalene predominated at the level of 0–95.73 % followed by phenanthrene (1.07–26.91 %) and fluoranthene (1.2–19.87 %).
Generally, biomass combustion process and the release of uncombusted petroleum products are the two main sources of anthropogenic PAHs found in the environment. In the present study, three typical pyrogenic PAHs (i.e., naphthalene, acenaphthalene and fluoranthene) were present at high concentrations. Furthermore, in both the cases, level of fluoranthene was higher than pyrene and that of indeno [1,2,3-cd]pyrene was higher for Bangladesh samples than benzo[ghi]perylene and lower for Indian samples.
Potential toxicological assessment
The effects range low (ERL) and the effects range median (ERM) values were used for impact assessment on aquatic sediment with a ranking of low to high impact values (Long et al. 1995). The measured concentrations of PAHs were compared with the existing ERL and ERM values. Results showed that naphthalene, anthracene, pyrene and benzo(a)pyrene concentrations were below the ERL of values for Martaja sediment samples (Table 6). But in the case of Hugli river sediment samples none of the PAH values showed below ERL values at 100 % cases (Table 7). These findings indicated that the studied sites have some potential biological impact. Long et al. (1995) refined the method but did not change the basic conceptual approach. TEL/PEL values (threshold effects level and probable effects level) were determined by the Florida Department of Environmental Protection (FDEP 1994). The method was similar to the method for deriving ERL/ERM values, but both ‘‘effect’’ and ‘‘no effect’’ data were used in calculating TEL and PEL values. Essentially, the TEL corresponds to the ERL and the PEL to the ERM. The TEL was calculated as the geometric mean of the 15th percentile concentration of the effects set and the 50th percentile concentration of the no-effects dataset. Similarly, the PEL was the geometric mean of the 50th percentile of the effects data and the 85th percentile of the no-effects data. The TEL (or ERL) and PEL (or ERM) values define three concentration ranges for a chemical, including those that were rarely (below the TEL or ERL), occasionally (above the TEL or ERL, but below a PEL or ERM) and frequently (above the PEL or ERM) associated with adverse effects. When contaminant concentrations exceed PEL or ERM, sediment samples were predicted to be toxic. The comparison of chemical concentrations of PAHs with sediment quality guidelines (SQGs) was summarized in Tables 6 and 7. As it can be seen, the evaluation of ecotoxicological risk can change according to considered SQGs. For Martaja river sediments, Bangladesh, PAH concentration showed 11.11–100 % of cases exceeding the ERL values. ERM value was exceeded in the case of dibenzo(a,h)anthracene for 11.11 % cases, whereas PEL values exceeded for 22.22 % cases for acenaphthene and dibenzo (a,h) anthracene. For Hugli River samples, India, PAH contamination levels exceeded the ERM values of naphthalene (6.25 %), fluorene (6.25 %), pyrene (6.25 %), benzo(a)anthracene (6.25 %), chrysene (6.25 %), benzo(a)pyrene (6.25 %) and dibenzo (a,h) anthracene (18.75 %) while PEL level exceeded for naphthalene (68.75 %), acenaphthylene (12.5 %), fluoranthene (12.5 %), pyrene (6.25 %), benzo(a)anthracene (6.25 %), chrysene (6.25 %) and dibenzo(a,h) anthracene (12.5 %). In case of Hugli river samples, India, PAH concentration showed 44.44–88.87 % of cases exceeding the ERL values. For fluoranthene, benzo(a)anthracene and pyrene, 11.11 % of the cases for each congener exceeded the ERM values but PEL values for those congeners were in the range of 11.11–22.22 % . Some PAHs and especially their metabolic products are of great concern due to their documented carcinogenicity. These potentially carcinogenic PAHs (CPAHs) include BaA, BaP, BbF, BkF, DahA and InP (Savinov et al. 2003). The total concentrations of CPAHs in sediments from the Sundarban of Bangladesh and Indian subcontinent varied from 57 to 2,050.5 and 7.7 to 7,901 ng g−1 d.w. with an average of 658 and 1,152 ng g−1 and accounted for 49.9 and 50.6 % of the total PAHs concentrations, respectively. Among the CPAHs, BaP is the only PAH for which toxicological data are sufficient for the derivation of a carcinogenic potency factor (Peters et al. 1999). The toxic equivalency factors (TEFs) were used to quantify the carcinogenicity of other PAHs relative to BaP and to estimate BaP-equivalent doses (BaPeq dose) (Nadal et al. 2004). Calculated TEFs for BaA, BaP, BbF, BkF, InP, DahA and Chr are 0.1, 1, 0.1, 0.01, 0.1, 1 and 0.001, respectively, according to the USEPA. In this study, we converted the above mentioned seven PAH concentrations into one toxic concentration for each site using the corresponding TEFs.
The total BaP toxicity equivalent (TEQ) for all PAHs was calculated as: TEQcarc = Σ (Ci × TEFcarc), where Ci was the concentration of individual PAHs (ng g−1 d.w.) and TEFi was its corresponding TEF. Total TEQ values calculated for samples from the Bangladesh and Indian Sundarban varied from 13.68 to 1,014.75 and 1.31 to 2,451 ng g−1 d.w. with an average of 221.02 and 358.63 ng g−1, respectively. The maximum total TEQ value was found at sample location S2 for Bangladesh and S1 for India. Among different PAHs, contribution to the total TEQ decreased in the order: B[b]F (53.7 %) > D[ah]A (45.53 %) > BaP (44.47 %) > Chr (43.4 %) > B[k]F (38.3 %) > BaA (31.5 %) > Ind (10.5 %) in Bangladesh and Ind(59.7 %) > B[k]F (52.4 %) > BaP (46.5 %) > B[b[F (45.85 %) > Chr (44.5 %) > D[ah]A (38.87 %) > B[a]A (34.51 %) in India. In comparison with other studies (Table 8), the total TEQ values in sediments of the Bangladesh were lower than that detected in the Gulf of Gemlik, Marmara Sea, Turkey (Ünlü and Alpar 2009). However, the TEQ levels were higher than those of other literature reported sites, such as the Meiliang Bay, Taihu Lake, China (Qiao et al. 2006), the Guba Pechenga, Barents, Sea, Russia (Savinov et al. 2003), the coastal lagoons in central Vietnam (Giuliani et al. 2008) and Jialu River, China (Fu et al. 2011). In case of Indian Sundarban, the values of TEQs were found higher than all literature reported sites.
Conclusion
The analyses of the surface sediments from Indian as well as Bangladesh Sundarban environments provided very useful and important information for evaluating PAH contamination levels and probable potential sources. Data obtained reveal that the wetland appeared to be moderately polluted by PAHs in the Indian side and less polluted in the Bangladesh side. Based on the use of diagnostic source ratios, it can be referred that the PAH source in sediments was mixed comprising contamination by local vehicle combustion, biomass burning and domestic and industrial coal combustion. However, in some specific sites, a pyrogenic as well as petrogenic pattern was also observed, probably due to the mixture of industrial and urban discharges. The data provide background information that should be useful in designing future strategies for inter-governmental environmental protection of this vast wetland. Compared with SQGs values, it is revealed that the concentration of PAHs exceeded exclusively at the site Lot 8 of Indian Sundarban. This would exert adverse biological effects and demands immediate preventive measures to avoid the ecological risks on marine organisms especially the benthic species. Further research should be performed to investigate the effects of these compounds on the biogeochemical cycles of this wetland. Though high molecular PAHs are highly mutagenic and carcinogenic and low molecular PAHs are less mutagenic, low and moderate molecular PAHs presented much more ecosystem risk than high molecular PAHs in the present study.
References
Barakat AO, Mostafa A, Wade TL, Sweet ST, El Sayed NB (2011) Distribution and characteristics of PAHs in sediments from the Mediterranean coastal environment of Egypt. Mar Pollut Bull 62(9):1969–1978
Bartolomé L, Cortazar E, Raposo JC, Usobiaga A, Zuloaga O, Etxebarria N, Fernández LA (2005) Simultaneous microwave-assisted extraction of polycyclic aromatic hydrocarbons, polychlorinated biphenyls, phthalate esters and nonylphenols in sediments. J Chromatogr A 1068:229–236
Baumard P, Budzinski H, Garrigues P, Sorbe JC, Burgeot T, Bellocq J (1998) Concentrations of PAHs (polycyclic aromatic hydrocarbons) in various marine organisms in relation to those in sediments and to trophic level. Mar Pollut Bull 36:951–960
Baumard P, Budzinski H, Garrigues P, Dizer H, Hansen PD (1999) Polycyclic aromatic hydrocarbons in recent sediments and mussels (Mytilus edulis) from the Western Baltic Sea: occurrence, bioavailability and seasonal variations. Mar Environ Res 47:17–47
Berner BA Jr, Bryner NP, Wise SA, Mulholland GH, Lao RC, Fingas MF (1990) Polycyclic aromatic hydrocarbon emissions from combustion of crude oil on water. Environ Sci Technol 24:1418–1427
Bhattacharya A, Sarkar SK (2003) Impact of overexploitation of shellfish: northeastern coast of India. Ambio 32:70–75
Bicego M, Murino V, Pelillo M, Torsello A (2006) Special issue on similarity-based classification. Pattern Recognit 39:1813–1948
Binelli A, Sarkar SK, Chatterjee M, Riva C, Parolini M, Bhattacharya BD, Bhattacharya A, Satpathy KK (2008) A comparison of sediment quality guidelines for toxicity assessment in the Sundarban wetlands (Bay of Bengal, India). Chemosphere 73:1129–1137
Budzinski H, Jones I, Bellock J, Piérard C, Garrigues P (1997) Evaluation of sediment contamination by polycyclic aromatic hydrocarbons in the Gironde estuary. Mar Chem 58:85–97
Butler JD, Crossley F (1981) Reactivity of polycyclic aromatic hydrocarbons adsorbed on soot particles. Atmos Environ 15:91–94
Chatterjee M, Silva Filho EV, Sarkar SK, Sella SM, Bhattacharya A, Satpathy KK, Prasad MVR, Chakraborty S, Bhattacharya BD (2007) Distribution and possible source of trace elements in the sediment cores of a tropical macrotidal estuary and their ecotoxicological significance. Environ Int 33:346–356
Chatterjee M, Canario J, Sarkar SK, Brancho V, Bhattacharya AK, Saha S (2009) Mercury enrichments in core sediments in Sunderban mangroves, northeastern part of Bay of Bengal and their ecotoxicological significance. Environ Geol 57:1125–1134
Colborn T, vom Saal FS, Soto AM (1993) Developmental effects of endocrine disrupting chemicals in wildlife and humans. Environ Health Perspect 101:378–384
Colombo JC, Pelletier E, Brochu C, Khalil M, Cataggio JA (1989) Determination of hydrocarbon sources using n-alkanes and polyaromatic hydrocarbon distribution indices. Case study: Rio de la Plata estuary, Argentina. Environ Sci Technol 23:888–894
Dickhut RM, Canuel EA, Gustafoson KE, Liu K, Arzayus KM, Walker SE, Edgecombe G, Gaylor MO, Macdonald EH (2000) Automative sources of carcinogenic polycyclic aromatic hydrocarbons associated with particulate matter in the Chesapeake Bay region. Environ Sci Technol 34:4635–4640
Eisma D (1998) Intertidal deposits. River mouths, tidal flats, and coastal lagoons. CRC Press, New York
FDEP (Florida Department of Environmental Protection) (1994) Florida coastal sediment contaminants atlas. Office of Intergovernmental Programs, Tallahassee
Folk RL, Ward WC (1957) Brazos River bar: a study in the significance of grain size parameters. J Sediment Petrol 27:3–26
Fu J, Ding Y-H, Li L, Sheng S, Wen T, Yu L-J, Chen W, An S-Q, Zhu H-L (2011) Polycyclic aromatic hydrocarbons and ecotoxicological characterization of sediments from the Huaihe River. China J Environ Monit 13:597–604
Giuliani S, Sprovieri M, Frignani M, Cu NH, Mugnai C, Bellucci LG, Albertazzi S, Romano S, Feo ML, Marsella E (2008) Presence and origin of polycyclic aromatic hydrocarbon in sediments of nine coastal lagoons in central Vietnam. Mar Pollut Bull 56:1504–1512
Goodbred SL Jr (2003) Response of Ganges dispersal system to climate change: a source-to sink view since the last interstade. Sediment Geol 162:83–104
Gschwend PM, Hites RA (1981) Fluxes of the polycyclic aromatic hydrocarbons to marine and lacustrine sediments in the northeastern United States. Geochim Cosmochim Acta 45:2359–2367
Hites RA, Laflamme RE, Farrington JW (1977) Sedimentary polycyclic aromatic hydrocarbon: the historical record. Science 198:829–831
Jaward FM, Farrar NJ, Harner T, Sweetman AJ, Jones KC (2004) Passive air sampling of polycyclic aromatic hydrocarbons and polychlorinated naphthalenes across Europe. Environ Toxicol Chem 23:1355–1364
Jones DM, Rowland SJ, Douglas AG, Howells S (1986) An examination of the fate of Nigerian crude oil in surface sediments of the Humber estuary by gas chromatography and gas chromatography–mass spectrometry. Environ Anal Chem 24:227–247
Kannan K, Johnson-Restrepo B, Yohn SS, Giesy JP, Long DT (2005) Spatial and temporal distribution of polycyclic aromatic hydrocarbons in sediments from Michigan inland lakes. Environ Sci Technol 39:4700–4706
Kimbrough KL, Dickhut RM (2006) Assessment of polycyclic aromatic hydrocarbon input to urban wetlands in relation to adjacent land use. Mar Pollut Bull 52(11):1355–1363
Krumbein WC, Pettijohn FJ (1938) Manual of sedimentary petrology. Appleton-Century Crafts, New York
Liao JF (1990) The chemical properties of the mangrove Solonchak in the northeast part of Hainan Island. Acta Sci Nat Univ Sunyats (Supp) 9:67–72
Lin T, Qin Y, Zheng B, Li Y, Zhang L, Guo Z (2012) Sedimentary record of polycyclic aromatic hydrocarbons in a reservoir in Northeast China. Environ Pollut 163:256–260
Liu S, Tao S, Liu W, Liu Y, Dou H, Zhao J, Wang L, Wang J, Tian Z, Gao Y (2007) Atmospheric polycyclic aromatic hydrocarbons in north China: a wintertime study. Environ Sci Technol 41:8256–8261
Long ER, MacDonald DD, Smith SL, Calder FD (1995) Incidence of adverse biological effects within ranges of chemical concentrations in marine and estuarine sediments. Environ Manag 19(1):81–97
Luo XJ, Mai BX, Yang QS, Fu JM, Sheng GY, Wang ZS (2004) Polycyclic aromatic hydrocarbons (PAHs) and organochlorine pesticides in water columns from the Pearl River and the Macao harbor in the Pearl River Delta in South China. Mar Pollut Bull 48:1102–1115
Muel B, Saguem S (1985) Determination of 23 polycyclic aromatic hydrocarbons in atmospheric particulate matter of the Paris area and photolysis by sun light. Int J Environ Anal Chem 19:111–131
Mukherjee KN (1969) Nature and problems of neo-reclamation in the Sundarbans. Geogr Rev India 31(4):1–9
Nadal M, Schuhmacher M, Domingo JL (2004) Levels of PAHs in soils and vegetation samples from Tarragona County, Spain. Environ Pollut 132:1–11
Neff JM (1979) Polycyclic aromatic hydrocarbons in the aquatic environment, sources, fates, and biological effects. Applied Science, London
Perra G, Pozo K, Guerranti C, Lazzeri D, Volpo V, Corsolini S, Focardi S (2011) Levels and spatial distribution of polycyclic aromatic hydrocarbons (PAHs) in superficial sediment from 15 Italian marine protected areas (MPA). Mar Pollut Bull 62(4):874–877
Peters CA, Knightes CD, Brown DG (1999) Long-term composition dynamics of PAH-containing NAPLs and implications for risk assessment. Environ Sci Technol 33:4499–4507
Qiao M, Wang CX, Huang SB, Wang DH, Wang ZJ (2006) Composition, sources, and potential toxicological significance of PAHs in the surface sediments of Meiliang Bay, Taihu Lake, China. Environ Int 32:28–33
Raoux C (1991) Modélisation du mécanisme de contamination par des hydrocarbures aromatiques polycycliques (HAP) des sédiments marins côtiers de Méditerranée: conséquences sur la biodisponibilité des HAP dans le milieu marin. PhD Thesis, Nr 565, University bordeaux I, Bordeaux, France
Santschi PH, Presley BJ, Wade TL, Garcia-Romero B, Baskaran M (2001) Historical contamination of PAHs, PCBs, DDTs, and heavy metals in Mississippi River Delta, Galveston Bay and Tampa Bay sediment cores. Mar Environ Res 52:51–79
Sarkar SK, Franciscovic-Bilinski S, Bhattacharya A, Saha M, Bilinski H (2004) Levels of elements in the surficial estuarine sediments of the Hugli river, northeast India and their environmental implications. Environ Int 30:1089–1098
Sarkar SK, Saha M, Takada H, Bhattacharya A, Mishra P, Bhattacharya B (2007) Water quality management in the lower stretch of the river Ganges, east coast of India: an approach through environmental education. J Clean Prod 15:1559–1567
Sarkar K, Binelli A, Chatterjee M, Bhattacharya BD, Parolini M, Riva C, Jonathan MP (2012) Distribution and ecosystem risk assessment of polycyclic aromatic hydrocarbons (PAHs) in core sediments of Sundarban mangrove wetland, India. Polycycl Aromat Compd 32:1–26
Sauer TC, Boehm PD (1991) The use of defensible analytical chemical measurements for oil spill natural resource damage assessment. In: Proceedings of the 1991 International Oil Spill Conference, San Diego, CA, March 4–7, USCG, API, and EPA, pp 363–369
Savinov VM, Savinova TN, Matishov GG, Dahle S, Naes K (2003) Polycyclic aromatic hydrocarbons (PAHs) and organochlorines (OCs) in bottom sediments of the Guba Pechenga, Barents Sea, Russia. Sci Total Environ 306:39–56
Seruto C, Sapozhnikova Y, Schlenk D (2005) Evaluation of the relationships between biochemical endpoints of PAH exposure and physiological endpoints of reproduction in male California Halibut (Paralichthys californicus) exposed to sediments from a natural oil seep. Mar Environ Res 60:454–465
Sharif AKM, Mustafa AI, Amin NM, Shafiullah S (1991) Trace metals in tropical marine fish from the Bay of Bengal. Sci Tot Environ 107:135–142
Sharif AKM, Mustafa AI, Amin NM, Shafiullah S (1993a) Lead and cadmium contents in ten species of tropical marine fish from the Bay of Bengal. Sci Tot Environ 133:193–199
Sharif AKM, Alamgir M, Mustafa AI, Hossain MA, Amin MN (1993b) Trace element concentration in ten species of freshwater fish of Bangladesh. Sci Tot Environ 138(1–3):177–186
Sicre MA, Marty JC, Saliot A (1987) Aliphatic and aromatic hydrocarbons in different sized aerosols over the Mediterranean Sea: occurrence and origin. Atmos Environ 21:2247–2259
Simpson CD, Mosi AA, Cullen WR, Reimer KJ (1996) Composition and distribution of polycyclic aromatic hydrocarbons in surficial marine sediments from Kitimat Harbour, Canada. Sci Tot Environ 181(3):265–278
Snead RE (1985) Pakistan. In: Bird ECF, Schwartz ML (eds) The world’s coastline. Van Nostrand, New York, pp 735–740
Soclo HH (1986) Etude de la distribution des hydrocarbures aromatiques polycycliques dans les sédiments récents. Identification des sources. PhD Thesis, Nr 50, University Bordeaux I, Bordeaux, France
Ünlü S, Alpar B (2009) Evolution of potential ecological impacts of the bottom sediment from the Gulf of Gemlik; Marmara Sea, Turkey. Bull Environ Contam Toxicol 83:903–906
Van Metre PC, Mahler BJ (2005) Trends in hydrophobic organic contaminants in urban and Reference Lake sediments across the United States, 1970–2001. Environ Sci Technol 39:5567–5574
Walker SE, Dickhut RM (2001) Sources of PAHs to sediments of the Elizabeth River, VA. Soil Sediment Contam 10(6):611–632
Walkey A, Black TA (1934) An estimation Degitijaraff method for determining soil organic matter and proposed modification of the chromic acid titration method. Soil Sci 37:23–38
Wang MS, Chen SJ, Lai YC, Huang KL, Chang-Chien GP (2010) Characterization of persistent of organic pollutants in ash collected from different facilities of a municipal solid waste incinerator. Aerosol Air Qual Res 10:391–402
Wise SA, Hilpert LR, Rebbert RE, Sander LC, Schantz MM, Chesler SN, May WE (1988) Standard reference materials for the determination of polycyclic aromatic hydrocarbons. Fresenius Z Anal Chem 332:573–582
Yamashita N, Kannan K, Imagawa T, Villeneuve DL, Hashimoto S, Miyazaki A, Giesy JP (2000) Vertical profile of polychlorinated dibenzo-p-dioxins, dibenzofurans, naphthalenes, biphenyls, polycyclic aromatic hydrocarbons, and alkyphenols in a sediment core from Tokyo Bay, Japan. Environ Sci Technol 35:3560–3567
Yunker MB, Macdonald RW, Vingarzan R, Mitchell RH, Goyette D, Sylvestre S (2002) PAHs in the Fraser River basin: a critical appraisal of PAH ratios as indicators of PAH source and composition. Org Geochem 33:489–515
Zuloaga O, Prieto A, Usobiaga A, Sarkar SK, Chatterjee M, Bhattacharya BD, Bhattacharya A, Alam A (2009) Polycyclic aromatic hydrocarbons in intertidal marine bivalves of Sunderban mangrove wetland, India: an approach to bioindicator species. Water Air Soil Pollut 201:305–318
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zuloaga, O., Prieto, A., Ahmed, K. et al. Distribution of polycyclic aromatic hydrocarbons in recent sediments of Sundarban mangrove wetland of India and Bangladesh: a comparative approach. Environ Earth Sci 68, 355–367 (2013). https://doi.org/10.1007/s12665-012-1743-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12665-012-1743-7