Abstract
Thirty-one surface soil samples were collected from Liaohe estuarine wetland in October 2008 and May and August 2009. The concentrations of 16 polycyclic aromatic hydrocarbons (PAHs), identified as priority pollutants by the US EPA, were measured by gas chromatography. PAHs were predominated by three- and four-ring compounds. The total PAH concentrations ranged from 704.7 to 1,804.5 μg/kg with a mean value of 1,001.9 μg/kg in October 2008, from 509.7 to 1,936.9 μg/kg with an average of 887.1 μg/kg in May 2009, and from 293.4 to 1,735.9 μg/kg with a mean value of 675.4 μg/kg in August 2009. The PAH concentration detected at most sites shared the same pattern, with maximum concentrations during the autumn (October) and minimum concentrations during the summer (August). The ecological risk assessment of PAHs showed that adverse effects would occasionally occur in the soils from Liaohe estuarine wetland based on the effects range low (ERL)/effects range median and the toxic equivalency factors. The results revealed that some of the individual PAHs were in excess of ERL which implied possible acute adverse biological effects. The BaPeq values in some sites surpassed the Dutch target value. Therewith, quite a part of soils in the wetland were subjected to potential ecological risks.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Polycyclic aromatic hydrocarbons (PAHs) are a large group of organic pollutants that consist of two or more fused aromatic rings and may stimulate adverse effects in human beings and other organisms. PAHs generally possess high chemical stability and hydrophobic properties, which result in enhanced accumulation and a high capacity for distribution in the environment (Ahmed et al. 2007). Many individual PAHs are genotoxic (White et al. 1998) and may cause mutations and certain types of cancer (Dragan et al. 2007). PAHs are usually introduced into the environment as a result of anthropogenic activities which increased dramatically in the last two decades, mainly including pyrolytic processes, such as the incomplete combustion processes involving carbon fuels and materials. PAHs are generally highly lipophilic, and soil ecosystem is a major reservoir. The contamination of soil with organic compounds such as PAHs is an issue of great environmental concern.
Wetland is environment that mankind depends on for existence, but wetland is suffering from pollution and plants and animals species, and self-purification capacity of wetland is continuously in the reduction status. Therefore, studies on PAH concentration characteristics and their ecological risk assessment in the wetland would be very informative. On the basis of large amount of previous studies, Long et al. (1995) proposed effects range low (ERL) and effects range median (ERM) to evaluate the potential eco-risk of organic pollutants. Halek et al. (2008) established a toxicity equivalency factor (TEF) to assess the risk of mixtures containing PAHs. In this paper, 16 PAHs, including naphthalene (Nap), acenaphthene (Ace), acenaphthylene (Acy), fluorene (Flu), phenanthrene (Phe), anthracene (Ant), fluoranthene (Flt), pyrene (Pyr), benzo(a)anthracene (BaA), chrysene (Chr), benzo(b)fluoranthene (BbF), benzo(k)fluoranthene (BkF), benzo(a)pyrene (BaP), indeno(1,2,3,cd)pyrene (Ind), dibenzo(a,h)anthracene (DBA), and benzo(g,h,i)perylene (Bpe), were measured to assess environmental risk by ERL/ERM and TEF method.
Study areas
Liaohe estuarine wetland, in northeastern China, has a geographic area of approximately 3,150 km2. The wetland is situated at latitude of 40°41′ to 41°27′ N and the longitude of 121°34′ to 122°29′ E. Reed was the dominant plant in Liaohe estuarine wetland. The climate of Liaohe estuarine wetland is temperate monsoon, with four seasons (cold winter, windy spring, and hot, humid summers with well-distributed rainfall). The average annual temperature is 8.3°C. The mean annual rainfall is 611.6 mm, of which 62.9% is received during summer. The evaporation can be more than ten times higher than precipitation in spring.
Soil moisture is at a peak value during the summer rain season, while soil salinity and pH are reduced during the same period. These factors return to average annual levels in late August (Ren and Han 2003). Bare beach, seepweed (Suaeda heteroptera), common reed, meadow, rice paddy, maize, and woods form a distinctive sequential arrangement of vegetation types. In addition, environmental gradients of soil salinity and pH are remarkable (Liu et al. 2004).
Materials and methods
Sample collection and preparation
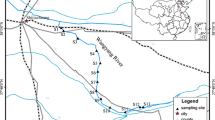
Thirty-one samples were collected from Liaohe estuarine wetland in October 2008 and May and August 2009. Sites of LH2–LH3 and LH7–LH9 were taken in Salina wetland area. Sites LH1, LH4–LH6, and LH29–LH31 were located in S. heteroptera degraded area. Samples of LH10–LH13 and LH17–LH25 were collected from reed field area. Sites LH14, LH15, and LH16 were selected in reed degraded area. Sites LH26, LH27, and LH28 were located in reed field oil well area. The locations of sampling station were shown in Fig. 1.
Surface soils (0–20 cm depth) were taken with a stainless steel soil auger after removal of the uppermost plant cover and transported to the laboratory. The samples were freeze-dried and then sieved through a 100-mesh stainless sieve. The representative samples were obtained after coning quartering. The samples were stored in the precombusted amber glass jar until extraction and analysis for PAHs.
Chemicals
Chromatographic grade dichloromethane, n-hexane, etc. were used as solvents for sample processing and analysis. A PAH standard mixture containing 16 PAHs which specified in US EPA method 610 and deuterated PAH internal standard mixture (naphthalene-d8, acenaphthene-d10, phenanthrene-d10, and chrysene-d12) were purchased from Supelco, USA.
Sample extraction and cleanup
Soil samples were extracted by ultrasonication, a method developed and recommended by various authors (Trapido 1999; Agarwal et al. 2006; Ray et al. 2008). About 2.0 g of soil sample was mixed with 2.0 g anhydrous sodium sulfate and then extracted in an ultrasonic bath for 20 min with 20 mL n-hexane/dichloromethane (1:1 v/v). The extracts were concentrated to a volume of 1 mL by rotary vacuum evaporation and solvent-exchanged to hexane. The concentrated extracts were purified with a glass chromatographic column packed anhydrous sodium sulfate, silica gel/alumina (1:1 M/M). The samples were charged onto the column in about 2 mL hexane and eluted sequentially with 30 mL hexane/dichloromethane (3:7 v/v). The PAHs containing fraction were preconcentrated to about 1 mL by a rotary evaporator and concentrated to exactly 1 mL by gentle nitrogen stream.
GC analysis
The concentrated extract was separated into fractions using gas chromatography (GC), which have been developed and optimized. A Shimadzu 2010 GC-FID equipped with a SPB-5 column (30 m × 0.32 mm × 0.25 μm) was used for all analyses. Nitrogen was used as the carrier gas with a flow rate of 1.1 mL/min. The GC conditions for analysis were as follows: A 1.0-μl aliquot of the extract was injected while the injection port temperature was maintained at 250°C and operated in splitless mode. The oven temperature program started at 80°C with 1 min hold and covered a range from 80°C to 255°C at 15°C/min with 1 min hold. Then the temperature was increased to 265°C at 1°C/min with 1 min hold and finally increased to 295°C at 2.5°C/min and held for 5 min.
Quality control
The procedural blanks, spiked blanks, and sample duplicates were routinely analyzed with field samples, and no interferences were detected. Instrumental detection limits were determined from linear extrapolation from the lowest standard in the calibration curve using the area of a peak having a signal-to-noise ratio of 3, which ranged from 5 to 12 μg/kg, depending on the individual PAH. Analytical methods were checked for the precision and accuracy. All the samples were analyzed in quadruplicate. Results expressed as percentage of relative standard deviation, which varied from 1.547% to 18.096%. The recovery efficiency was checked by analyzing soil samples spiked with known amount of PAH standard. Recoveries of 16 PAHs ranged from 74.25% to 92.15% in soil samples. Procedural blanks were performed periodically to prevent contamination.
Results and discussion
The distribution characteristics of PAHs
All 16 PAHs in each soil sample were quantified on the base of dry weight unless special mention. The total concentrations of 16 PAHs ranged from 704.7 to 1,804.5 μg/kg with a mean value of 1001.9 μg/kg in October 2008, from 509.7 to 1,936.9 μg/kg with a mean value of 887.1 μg/kg in May 2009, and from 293.4 to 1,735.9 μg/kg with a mean value of 675.4 μg/kg in August 2009 (Fig. 2). The PAH concentration detected at most stations shared the same pattern, with maximum concentrations during the autumn (October) and minimum concentrations during the summer (August). The reduction in soil contaminant levels during the warm season is facilitated by volatilization, degradation, and uptake into new biological growth. Moreover, vertical movements within the soil occur during the summer and can lead to the redistribution and dilution of the surface loads into the soil profile (McLachlan et al. 2002). All of these factors can lead to a seasonal cycle in the concentration of contaminants in soils, as observed in this study for the sum of PAHs. Single compound trends (Fig. 3) are more complex: Most of the compounds follow the general trend shown by the sum of all PAHs, but other compounds have a maximum level in spring (Nap, Acy, Fle, Phe, BaA, BkF). For these compounds, a different seasonal trend of emission sources and depositional phenomena can be hypothesized.
The low molecular weight (two- and three-ring) PAHs have a significant acute toxicity, whereas some of the higher molecular weight PAHs are carcinogenic (Neff 1979; Witt 1995). Generally, three- and four-ring PAHs appear dominant in the studied soils with median concentration values generally higher than the 55–60% of the PAH total concentrations. Higher proportions of PAH species with three rings (32.4–38.3%) were detected. Compositions and relative abundance of individual PAH in soils of Liaohe estuarine wetland were quite similar (Fig. 4) in three seasons. Among all 16 PAHs, Nap was predominant specie in samples from most of the sites. Moreover, BaP, one of the most potent carcinogenic PAHs, varied from 28.3 to 52.4 μg/kg with an average of 37.7 μg/kg in October 2008, from non-detectable levels to 44.2 μg/kg with an average of 15.048 μg/kg in May 2009, and from non-detectable levels to 77.2 μg/kg with an average of 14.471 μg/kg in August 2009. Concentration of Bap observed in all the sites in October 2008 was found to be higher as compared to the Dutch target value (25 μg/kg).
Sixteen PAHs were selected to compare total PAH concentrations with available data from other areas in the world (Table 1). The concentrations of PAHs in the study area are in the moderate range of the global levels. Observed concentrations were comparable to the 16 PAH average concentrations of 3,731 and 1,172.7 μg/kg in the soils and sediments of New Orleans (Mielke et al. 2001) and surface soil of north suburban Shanghai (Sun et al. 2008), respectively. However, lower concentrations (71.1, 395, and 417.4 μg/kg, sum of 16 species) have been reported in the northern wetland of the Yellow River Delta (Yuan et al. 2008), arable soil in Poland (Barbara et al. 2008), and soil of Baiyangdian area (Zhao et al. 2009), respectively. In this study, some recommendations or guidelines were used to determine the environmental status of Liaohe estuarine wetland. The PAH concentration of about 100 μg/kg is typical in the areas where no anthropogenic pollution sources occur (Trapido 1999). Also it has been suggested the typical endogenous PAHs in soil, resulting from plant synthesis and natural fires between the ranges of 1–10 μg/kg (Edwards 1983). Overall, soils from Liaohe estuarine wetland were contaminated above the natural level. The values in most stations were much higher than the guideline values in soils of Dutch (20–50 μg/kg), mathematically indicating that majority of the sites contained relatively high PAH concentrations. The pollution degree of 16 PAHs from sites LH14, LH18, LH19, and LH22–LH31 in October 2008, sites LH25–LH28 in May 2009, and sites LH23 and LH26–LH28 in August 2009 was pollution class III (1,000–5,000 μg/kg) according to Polish soil standards (Skrbic et al. 2005).
Ecological risk assessment
The ERL and the ERM values were used for assessment of soil with a ranking of low to high impact values (Long et al. 1995; Jon et al. 2005). The ERL and ERM criteria were established using the 10th and 50th percentile in a database of increasing concentrations associated with adverse biological effects, in which ERL (4,022 μg/kg) stands for the potential eco-risk probability is 10% and 50% for ERM (44,792 μg/kg) is the another transition point. Now, both of them are considered as the Eco-risk Guidelines. The ERL and ERM delineate three relative safety ranges. The concentrations of total PAH concentrations and each of individual PAHs were far below the ERM, indicating no frequent acute adverse biological effect would happen in Liaohe estuarine wetland. The concentrations of individual PAH recorded in the study ranged from ND levels to 458.7 μg/kg and were much lower than the ERM values. The concentrations of total PAH concentrations were lower than the ERL values. However, some of the individual PAHs were in excess of ERL which implied possible acute adverse biological effects. As shown in Table 2, the maximum concentrations of the individual PAH were lower than the ERL except for Nap, Acy, Ace, and Fle, and the latter showed values above ERL but below ERM. These findings indicated that there was at least one PAH in a site that exceeded ERL, and occasional adverse effects may occur in the study area (Long et al. 1995).
The TEFs have been developed and introduced to facilitate risk assessment and regulatory control of exposure to PAH mixtures (Zhang et al. 2009). The development of TEFs can increase the reliability of the evaluation of the toxicity of a sample containing a mixture of PAHs (Sandro et al. 2010). Since BaP is well characterized toxicologically, whereas comparatively less information is available for most of the other PAHs (Lodovici et al. 2003), the risks of PAHs have often been calculated on the basis of BaP concentration. In the present study, the carcinogenic potency of a PAH can be expressed as its BaP-equivalent (BaPeq) concentration, which is calculated from the TEF of the PAHs relative to the carcinogenic potency of BaP. The list of TEFs of individual PAHs compiled by Tsai et al. (2004) was adopted in this study. The calculated TEFs for Nap, Acy, Ace, Flu, Phe, Ant, Flt, Pyr, BaA, Chr, BbF, BkF, Bap, Ind, DBA, and Bpe are 0.001, 0.001, 0.001, 0.001, 0.001, 0.01, 0.001, 0.001, 0.1, 0.01, 0.1, 0.1, 1, 0.1, 1, and 0.01, respectively. The toxicity of PAH is defined by the concentration of individual PAH compounds (Ci) in a mixture times their corresponding TEF and determining the concentration of total PAHs expressed as BaPeq. These concentrations are then arithmetically summed as a surrogate indicator to provide an indication of the toxic potential of the samples expressed as toxic equivalents.
In this study, the total PAH BaPeq in different sampling station are showed in Fig. 5. BaPeq values calculated for soil samples in this study varied from 52.1 to 84.3 μg/kg, a mean value of 64.0 μg/kg in October 2008, from 8.6 to 59.2 μg/kg with a mean value of 29.7 μg/kg in May 2009, and from 7.8 to 80.9 μg/kg with a mean value of 28.4 μg/kg in August 2009. In comparison with other studies, the mean value (28.4 μg/kg) in soils of Liaohe estuarine wetland was lower than those in surface soils of Agra, India (650 μg/kg-BaPeq) (Amit and Ajay 2006), soil around airport in India (1,021 μg/kg-BaPeq) (Ray et al. 2008), soil from Tarragona County of Spain (124 μg/kg-BaPeq) (Nadal et al. 2004), and roadside soil of Shanghai, China (892 μg/kg-BaPeq) (Jiang et al. 2009). BaP is one of the most important PAHs in the carcinogenic group. In the soil samples in August 2009, BaP accounted for 50.88% of the total BaPeq with only 2.1% of the total concentration. Among different PAHs, contribution to the total BaPeq decreased in the order: BaP (50.88%) > BaA (13.13%) > BbF (12.85%) > BkF (11.51%). The reference total carcinogenic potency was calculated as a sum of multiplied Dutch target concentrations for unpolluted soil with appropriate Bapeq. The toxicity and carcinogenetic of the investigated sites in 3 months was estimated by comparing the total carcinogenic potency with reference once. In October 2008, the value were 1.6∼2.6 times higher than the Dutch target value (32.96 μg/kg), indicating the increased carcinogenic burden of soils from these sites. The result suggests that the carcinogenic potency of PAHs should be given more attention due to potential environmental risk in the study area, and appropriate remedial measures should be carried out to minimize the adverse effects of long-term exposure on local inhabitants. In May and August 2009, the average total BaPeq value is generally comparable to the Dutch target value. The total BaPeq detected in 38.7% and 22.6% of the sites exceed the Dutch target value.
Conclusions
Due to continuously increasing anthropogenic activities, management of the pollution from soils in wetland areas is attracting more attention. The distributions of PAHs in the environment and potential human health risks have become the focus of much attention. In this study, PAH concentrations, possible adverse ecological effects, and their seasonal variation were identified in the soils from Liaohe estuarine wetland. Analyses of 31 surface soil samples showed that the levels for 16 PAHs ranged from 704.7 to 1,804.5 μg/kg dry weight, with the mean value of 1,001.9 μg/kg dry weight in October 2008, from 509.7 to 1,936.9 μg/kg with a mean value of 887.1 μg/kg in May 2009, and from 293.4 to 1,735.9 μg/kg with a mean value of 675.4 μg/kg in August 2009. The PAH levels for all sites were higher than the target value set by Dutch government for unpolluted soil. Three- and four-ring PAHs were predominant in Liaohe estuarine wetland soils. The PAH concentration detected at most stations shared the same pattern, with maximum concentrations during the autumn and minimum concentrations during the summer. The results indicate that no frequent acute adverse biological effect would happen in Liaohe estuarine wetland. High carcinogenic potency of PAHs in soils is an indication of high degree of risk. Appropriate remedial measures were required to minimize the adverse effects.
References
Agarwal, T., Khillare, P. S., & Shridhar, V. (2006). PAHs contamination in the bank sediment of the Yamuna River, Delhi, India. Environmental Monitoring and Assessment, 123, 151–166.
Ahmed, E. N., Tarek, O. S., Azza, K., Amany, E. S., & Aly, M. A. (2007). The distribution and sources of polycyclic aromatic hydrocarbons in surface sediments along the Egyptian Mediterranean coast. Environmental Monitoring and Assessment, 124, 343–359.
Amit, M., & Ajay, T. (2006). Polycyclic aromatic hydrocarbons (PAHs) concentrations and related carcinogenic potencies in soil at a semi-arid region of India. Chemosphere, 65, 449–456.
Barbara, M. K., Bozena, S., Agnieszka, K. P., & Henryk, T. (2008). Monitoring of the total content of polycyclic aromatic hydrocarbons (PAHs) in arable soils in Poland. Chemosphere, 73, 1284–1291.
Dragan, C., Mirjana, R., Anka, J., & Dušan, A. (2007). Levels of PAHs in the soils of Belgrade and its environs. Environmental Monitoring and Assessment, 125, 75–83.
Edwards, N. T. (1983). Polycyclic aromatic hydrocarbons (PAHs) in the terrestrial environment. Journal of Environmental Quality, 12, 427–441.
Halek, F., Nabi, G., & Kavousi, A. (2008). Polycyclic aromatic hydrocarbons study and toxic equivalency factor (TEFs) in Tehran, IRAN. Environmental Monitoring and Assessment, 143, 303–311.
Jiang, Y. F., Wang, X. T., & Wang, F. (2009). Levels, composition profiles and sources of polycyclic aromatic hydrocarbons in urban soil of Shanghai, China. Chemosphere, 75, 1112–1118.
Jon, M. H., Joy, M. B., Michael, B., & Robert, J. (2005). Sediment quality survey of the Choctawhatchee Bay system in the Florida panhandle. Marine Pollution Bulletin, 50, 889–903.
Kimbrough, K. L., & Dickhut, R. M. (2006). Assessment of polycyclic aromatic hydrocarbon input to urban wetlands in relation to adjacent land use. Marine Pollution Bulletin, 52, 1355–1363.
Liu, Q. S., Liu, G. H., & Li, H. G. (2004). Analysis on characteristics of soil salinization in Liaohe River Delta. Acta Pedologica Sinica, 41, 190–195.
Lodovici, M., Venturini, M., Marini, E., Grechi, D., & Dolara, P. (2003). Polycyclic aromatic hydrocarbons air levels in Florence, Italy, and their correlation with other air pollutants. Chemosphere, 50, 377–382.
Long, E. R., MacDonald, D. D., Smith, S. L., & Calder, F. D. (1995). Incidence of adverse biological effects within ranges of chemical concentrations in marine and estuary sediments. Environmental Management, 19, 81–97.
McLachlan, M. S., Czub, G., & Wania, F. (2002). The influence of vertical sorbed phase transport on the fate of organic chemicals in surface soils. Environmental Science & Technology, 36, 4860–4867.
Mielke, H. W., Wang, G., Gonzales, C. R., Le, B., Quach, V. N., & Mielke, P. W. (2001). PAH and metal mixtures in New Orleans soils and sediments. Science of the Total Environment, 281, 217–227.
Nadal, M., Schuhmacher, M., & Domingo, J. L. (2004). Levels of PAHs in soil and vegetation samples from Tarragona County, Spain. Environmental Pollution, 132, 1–11.
Neff, J. M. (1979). Polycyclic aromatic hydrocarbons in the aquatic environment sources, fates and biological effects (p. 262). London: Applied Science.
Ray, S., Khillare, P. S., Agarwal, T., & Shridhar, V. (2008). Assessment of PAHs in soil around the international airport in Delhi, India. Journal of Hazardous Materials, 156, 9–16.
Ren, Y. M., & Han, H. R. (2003). Salinity trends of salt marsh in coastal regions at the lower reaches of Liaohe River. In Northeast University Press (Ed.), The improvement and utilization of littoral saline soil in Liaohe Delta (pp. 48–64). Technical Consultative Committee of People’s Government of Panjin: Shenyang.
Sandro, F., Marcell, M., Erissen, C. D., Daniele, B. S., & Karina, S. M. (2010). Distribution of polycyclic aromatic hydrocarbons in marine sediments and their potential toxic effects. Environmental Monitoring and Assessment, 168, 205–213.
Skrbic, B., Cvejanov, J., & Durisic, M. N. (2005). Polycyclic aromatic hydrocarbons in surface soils of Novi Sad and bank sediment of the Danube River. Journal of Environment Science and Health, Part A Environmental Science, 40, 29–42.
Sun, X. J., Shi, C., Xu, S. Y., Pan, F. F., Wang, P. H., Ma, J. L., et al. (2008). Concentration and sources of polycyclic aromatic hydrocarbons in surface soil of north suburban Shanghai, China. Research of Environmental Sciences, 21(4), 140–144.
Trapido, M. (1999). PAHs in Estonian soil: contamination and profiles. Environmental Pollution, 105, 67–74.
Tsai, P. J., Shih, T. S., Chen, H. L., Lee, W. J., Lai, C. H., & Liou, S. H. (2004). Assessing and predicting the exposures of polycyclic aromatic hydrocarbons (PAHs) and their carcinogenic potencies from vehicle engine exhausts to highway toll station workers. Atmospheric Environment, 38, 333–343.
White, P. A., Rasmussen, J. B., & Blaise, C. (1998). Genotoxic substances in the St. Lawrence system I: industrial genotoxins sorbed to particulate matter in the St. Lawrence, St. Maurice and Saguenay Rivers, Canada. Environmental Toxicology and Chemistry, 17, 286–303.
Witt, G. (1995). Polycyclic aromatic hydrocarbons in water and sediment of Baltic sea. Marine Pollution Bulletin, 31, 237–248.
Yuan, H. M., Zhao, G. M., Pang, S. J., Gao, G. Y., & Ye, S. Y. (2008). Polycyclic aromatic hydrocarbons (PAHS) exposure and their source analysis in the northern wetland of the Yellow River Delta. Marine Geology & Quaternary Geology, 28(6), 57–62.
Zhang, P., Song, J. M., & Yuan, H. M. (2009). Persistent organic pollutant residues in the sediments and mollusks from the Bohai Sea coastal areas, North China: An overview. Environment International, 35, 632–646.
Zhao, J., Zhou, H. D., Lu, J., Wang, Y. C., Hu, C. H., & Yuan, H. (2009). Distribution and source of polycyclic aromatic hydrocarbons (PAHs) in soils of Baiyangdian area. Chinese Journal of Ecology, 28(5), 901–906.
Acknowledgments
This work was supported by the Foundation of Water Pollution Control and Management of Major Special Science and Technology (2008ZX07208-009) and Program for New Century Excellent Talents in University.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lang, Y., Wang, N., Gao, H. et al. Distribution and risk assessment of polycyclic aromatic hydrocarbons (PAHs) from Liaohe estuarine wetland soils. Environ Monit Assess 184, 5545–5552 (2012). https://doi.org/10.1007/s10661-011-2360-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10661-011-2360-8