Abstract
The fluoride level in drinking water is an important parameter and has to be controlled in order to prevent dental and skeletal fluorosis. The objective of this study is to assess fluoride content and other water quality parameters in the samples taken from open wells, tube wells, and karezes of Mastung, Mangochar, and Pringabad areas of Balochistan province. A total number of 96 drinking water samples out of 150 were found unfit for human consumption. Area-wise analysis show that the samples from 39 sites from Mastung, 12 from Mangochar, and 13 from Pringabad were found in the risk of dental fluorosis of mild to severe nature. However, 12 sampling sites from Mastung, 8 from Mangochar, and 2 from Pringabad were identified as the risks of mottling and skeletal fluorosis or other bone abnormalities. The highest concentration of F− has been observed as 14 mg L−1 in Mastung. Correlation analysis show that fluoride solubility in drinking water is pH dependent; and the salts of Ca2+, Na+, K+, Cl−, and SO4 2− contribute to attain the favorable pH for dissolution of fluoride compounds in drinking water. Principal component analysis shows that the geochemical composition of the rocks is only responsible for groundwater contamination. On the basis of the results, defloridation of the identified sampling sites and continuous monitoring of drinking water at regular basis is recommended at government level to avoid further fluorosis risks.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Fluoride (F−) is a natural element that may cause both beneficial and adverse effects on skeletal tissues (bones and teeth). Drinking water is the biggest contributor to F− exposure. It comes in water naturally as a result of the geological composition of bedrock and soil. The naturally occurring fluoride can dissolve easily into groundwater as it moves through gaps and pore spaces between rocks. The other significant sources of exposure are dental products, food, air pollution (as a result of combustion of F− rich coal), pharmaceuticals, and pesticides. The World Health Organization (WHO) recommended 0.8–1.2 mg L−1 as the desired optimal level (target value) for F− to promote public health benefits for preventing tooth deterioration and 1.5 mg L−1 as the guideline value for a minimal chance for dental fluorosis (WHO 2006). The risk of dental fluorosis increases with fluoride levels in drinking water above 1.5 mg L−1. Crippling skeletal fluorosis may arise when fluoride levels exceed 10 mg L−1. Fluorosis may also damage the structure and functions of non-skeletal systems, such as brain, liver, kidney, and spinal cord (Wang et al. 2004; Guan et al. 1998; Dote et al. 2000). The EPA’s enforceable standard allowed in public water supplies to protect against risks from exposure to too much fluoride is 4.0 mg L−1 (EPA 2012). Because of the marginal difference between beneficial and harmful concerns of F−, it is of great importance to determine F− content in drinking water accurately.
The Mastung (coordinates: 29°48′ 0″ N, 66° 50′ 60″ E), Pringabad (coordinates: 29° 52′ 33″ N, 66°51′41″E), and Manguchar (coordinates: 29° 20′ 48″ N, 66° 38′ 21″ E) are three adjoining areas located at N-25 road of Balochistan province. The former two are located in Mastung district, while the latter is in Kalat district. All the three areas are surrounded by high mountain ranges of shirinab and chiltan formations of jurassic age. These formations mainly contain limestone. A large number of abnormalities like dental and skeletal fluorosis have been noticed in the inhabitants of these areas. According to the National Institute of Population Studies, the projected population of Mastung district in 2012 was 269,000 residents (Aijaz et al. 2011). The inhabitants of below 14 years age are considered as children, and they are about 46.8 % of the total population. Almost all of the population of the selected areas is using water from the unprotected and remote sources of open wells, tube wells, and karezes. The water in these areas is being used for drinking without any treatment. The aim of the present study is to identify the mapping risk of fluorosis considering population exposed and the potential indicators of high fluoride in water using water quality parameters. Statistical analysis was also performed to examine the correlations between the fluoride level and the other water quality parameters.
Materials and methods
Study area
Total 150 representative samples were collected from three groundwater sources, i.e. open wells, tube wells, and karezes of the three adjacent localities of Balochistan province during August–November 2014. Sample No. 1–90 were taken from Mastung, 91–115 from Manguchar, and 116–150 from Pringabad localities. The source of sample No. 1–10 and 101–133 was open wells. Sample No. 11–80 and 134–150 were taken from karezes, and 81–100 were collected from tube wells. The representative samples were stored in clean plastic bottles at 4 ± 1 °C.
Analysis of physicochemical parameters
The chemical composition of drinking water was estimated using several analytical techniques. A pH meter (Orion 720 A) was used to measure the pH of drinking water samples. Total dissolved solids (TDS) were measured with a Jenway 4320 conductivity meter. The Orion 960 Autochemistry system was used to measure chloride (Cl−), and fluoride (F−) in the samples. Ion selective electrodes against Ag/AgCl reference electrode are used in the autochemistry system, which make it possible to measure the total amount of free and complex-bound F− and Cl− dissolved in water. Sodium (Na+) and potassium (K+) ions were measured by flame photometer (Model 360, Sherwood Scientific Ltd.). Calcium (Ca2+) and magnesium (Mg2+) ions were estimated using the atomic absorption spectrophotometer (PE-2380). Sulphate (SO4 2−), carbonate (CO3 2−), and bicarbonate (HCO3 −) ions were measured in accordance with the standard methods (APHA, AWWA & WEF 1992). The detection limit for cations and anions was 0.1 mg L−1. All the measurements were made in triplicate to ensure accuracy and precision in the data, and most of the RSD were less than 5 %. The results were compared with the limits recommended by WHO.
Statistical analysis
The correlation analysis was performed to identify the possible relations between the water quality parameters using Minitab version 14. Principal component analysis (PCA) was applied to the data to predict the source of contamination using XLSTAT statistical software for Excel.
Results and discussion
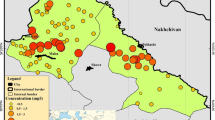
The contents of F−1 in drinking water samples of the studied area are shown in Fig. 1a, b. Among 150 drinking water samples, 54 samples (36 % of the total) were identified containing the concentration of F− within the guideline value of WHO (1.50 mg L−1), while 96 samples (64 % of the total) exceeded the guideline value. High groundwater F− content is associated with igneous and metamorphic rocks such as granites and gneisses (WHO 2006). These alarming results suggest that area-wise discussion of F− content is prerequisite to identify the contaminant sources and the level of contamination.
a F− content (mg L−1) in drinking water of three different areas of Balochistan province (dotted line show maximum permissible limits recommended by WHO 2004a, b). Samples: ○ Mastung open well,  Mastung kareze, ● Mastung tube well, Δ Mangochar open well, ▲ Mangochar tube well, □ Pringabad open well,
Mastung kareze, ● Mastung tube well, Δ Mangochar open well, ▲ Mangochar tube well, □ Pringabad open well,  Pringabad kareze. b Frequency distribution of F− content (mg L−1) in the studied areas. Samples:
Pringabad kareze. b Frequency distribution of F− content (mg L−1) in the studied areas. Samples:  Mastung;
Mastung;  Magochar; ■ Pringabad
Magochar; ■ Pringabad
Area-wise analysis shows that 29 samples from Mastung (32.22 %), 5 samples from Mangochar (20.00 %), and 20 samples from Pringabad (57.14 %) have the F− content within the optimal level. Low concentration of F− in drinking water provides protection against dental caries through a topical mode of action. The pre- and post-eruptive protective effects of F− starts at 0.5 mg L−1 and has been observed up to a concentration of about 2 mg L−1 (WHO 2004a, b).
The samples from 39 sampling sites from Mastung (43.33 %), 12 from Mangochar (48.00 %), and 13 from Pringabad (37.14 %) were found unfit for drinking purpose as the F− content of these samples were found in the range of 2.00–5.00 mg L−1. At these concentrations, adverse effects of dental fluorosis are expected. The severity of dental fluorosis may increase with increases in fluoride concentration. It starts with white line striations on the teeth followed by brown patches and, in severe cases, the brittling of enamel occur instantly. However, systematic control of F− in these water reserves is necessary. The remaining sampling sites, i.e. 12 from Mastung (13.33 %), 8 from Mangochar (32 %), and 2 from Pringabad (5.71 %) were identified, severely contaminated with F− (6.00 − 14.00 mg L−1); and are at high risk of skeletal fluorosis or other bone abnormalities. The symptoms of skeletal fluorosis started from pain in different joints, then limits joint movement, leading to stiffness and skeletal crippling. The highest concentration of F− was observed as 14, 10, and 7 mg L−1 in Mastung, Mangochar, and Pringabad localities, respectively. High concentrations of F− in these areas are associated with dissolution of substantial deposits of fluorite (total estimated reserves about 100,000 t) in Balochistan province (Mineral profile of Balochistan 2009). Besides dental and skeletal fluorosis, other signs such as nervousness, depression, and muscle weakness may also appear in connection with high F− intake. Five stations of Mastung have been identified at the risk of crippling skeletal fluorosis (F− level = 11–14 mg L−1). Area-wise risk assessment of F− contamination is shown in Table 1. As boiling water and the classical charcoal-based water filtration systems used in most homes do not remove fluoride from water; therefore, distillation and reverse osmosis techniques are suggested for removing fluoride from the identified potential locations.
Figure 2a shows that pH values of the majority of the samples are within WHO (1963) acceptable limits (6.5–8.5). The pH values have been ranged from 6.5 to 8.5 (average 7.4), 6.5 to 7.5 (average 7.0), and 5.8 to 8.0 (average 7.0) in the samples collected from Mastung, Mangochar, and Pringabad areas, respectively. Seven samples of Mastung are found at the border line of the upper permissible limit for pH (i.e. 8.5). The apparent effect in these water samples is slippery feeling and soda-like taste. Five samples of Mastung and two samples of Manguchar are found at the lower permissible limit for pH (i.e. 6.5). pH values of five samples of Pringabad have been observed in the range of 5.8–6.2. These samples possess a bitter and metallic taste and show the risk of corrosion in a water supply system. No health-based guideline value is proposed for pH of drinking water as it has no direct impact on the consumer’s health. However, the quality of the samples showing pH near to upper or lower permissible limits is not satisfactory. The samples should be monitored periodically to control the corrosion in water supply systems.
a pH of drinking water of studied areas of Balochistan province (dotted line show maximum permissible limits recommended by WHO 2004a, b). Samples: ○ Mastung open well,  Mastung kareze, ● Mastung tube well, Δ Mangochar open well, ▲ Mangochar tube well, □ Pringabad open well,
Mastung kareze, ● Mastung tube well, Δ Mangochar open well, ▲ Mangochar tube well, □ Pringabad open well,  Pringabad kareze. b TDS level in drinking water of the studied areas (legends show the same areas as are mentioned in (a))
Pringabad kareze. b TDS level in drinking water of the studied areas (legends show the same areas as are mentioned in (a))
TDS of drinking water represents the sum of Ca2+, Mg2+, Na+, and K+ cations and CO3 2−, HCO3 −, Cl−, SO4 2−, and NO3 − anions as the principal inorganic constituents. WHO (2003) recommends 500 and 2000 mg L−1 as the desirable and maximum permissible levels, respectively. TDS level affects the palatability of drinking water as follows: excellent, < 300 mg L−1; good, 300–600 mg L−1; fair, 600–900 mg L−1; poor, 900–1200 mg L−1; and unacceptable, > 1200 mg L−1 (WHO 2003). Water with extremely low concentrations of TDS may also be unacceptable because of its flat, insipid taste and lack of useful minerals. There is no recommendation for generally accepted lower limit, but 80 mg/L may be used. It is evident from Fig. 2b that 27 samples from Mastung have the acceptable TDS level (80–1200 mg L−1), 40 samples have the objectionable TDS (1200–2000 mg L−1), and 23 samples exceeded the maximum contamination limits (MCL) of 2000 mg L−1. Eleven samples of Manguchar show excellent to poorly acceptable levels, 7 samples have objectionable, and 7 samples exceeded the MCL for TDS. Eighteen samples from Pringabad have acceptable TDS values, 7 have objectionable level, and 10 samples crosses MCL. As no anthropogenic activity has been reported in studied locations, therefore, minerals of the soil are considered as the possible source for TDS. The TDS levels of natural sources from different geological regions have been reported from 30 to 6000 mg L−1, depending on the solubility of minerals. (WHO 2003).
An elevated TDS concentration does not mean that the water is a health hazard; it only shows that the samples may cause aesthetic or nuisance problems associated with staining, taste, or precipitation. The presence of high levels of TDS (>500 mg L−1) in underground water of studied sites may be unpleasant for consumers owing to the taste and excessive scaling in water pipes, heaters, boilers, and household appliances. The scaling is associated with certain components of TDS, such as Cl−, SO4 2−, Mg2+, Ca2+, CO3 2− etc. and responsible to shorten the service life of the appliances. Therefore, in the areas where TDS level is very high, the responsible constituents should be monitored by local public health authorities as a regular activity to maintain the level in the acceptable limit. The treatment of drinking water containing a high amount of TDS depends on the nature of the cations and anions. The treatment process may not reduce the concentration of TDS, but it only reduces the aesthetic problems with water.
The levels of principal cations and anions in groundwater of the studied areas are shown in Fig.3. Calcium and magnesium contribute in the hardness of water. Only 4 samples from Mastung and 2 from Mangochar were identified within the maximum accepted limit (75 mg L−1), set by WHO (1963) (Olayanju et al. 2011). The concentration of Ca2+ was found higher than the excessive limit of WHO (200 mg L−1) in 53, 15, and 16 samples from Mastung, Mangochar, and Pringabad, respectively. The excessive amount of Ca2+ should be removed by treatments like chemical softening, reverse osmosis, electrodialysis, and ion exchange (De Zuane 1997).
Principal cations and anions in drinking water of studied areas (double lines show maximum permissible limits recommended by WHO 2004a, b). Samples: ○ Mastung open well,  Mastung kareze, ● Mastung tube well, Δ Mangochar open well, ▲ Mangochar tube well, □ Pringabad open well,
Mastung kareze, ● Mastung tube well, Δ Mangochar open well, ▲ Mangochar tube well, □ Pringabad open well,  Pringabad kareze (Ms, Mn, and Pr represent to Mastung, Mangochar, and Pringabad, respectively)
Pringabad kareze (Ms, Mn, and Pr represent to Mastung, Mangochar, and Pringabad, respectively)
Magnesium has considered as non-toxic to humans at the concentration expected in water. Mg2+ salts show laxative and diuretic effects at a high dose. Sixteen samples of Mastung were identified to cross the maximum acceptable level of 50 mg L−1, out of which 2 have above the maximum allowable level of 150 mg L−1 (WHO 1963; Olayanju et al. 2011). The presence of higher amounts of Ca2+ and Mg2+ represents the hardness of water and shows that these samples are not suitable for drinking purpose.
High intake of sodium (Na) is considered harmful for patients suffering from renal, cardiac, and circulatory diseases. No maximum limit has suggested by WHO; however, 200 mg L−1 Na+ is listed as a potential complaint for taste. 51 samples (more than half) from Mastung, 2 samples from Mangochar, and 4 samples from Pringabad were identified as containing a high concentration of Na+. Ion exchange, demineralization process, and limitation of pollution sources are recommended for affected drinking water sources. Only 5 samples of Mastung were identified to have an exceeded amount of K+ (>250 mg L−1). It shows that Ca2+ and Na+ cations are responsible for high TDS levels in selected sites. It is important that drinking water does not serve as the main source of Ca2+, Mg2+, Na+, and K+; it only provides supplemental nutrients to the diet. Therefore, it is better to fulfill the nutritional requirement of these nutrients from food.
The main source of human exposure to chloride (Cl−) is the addition of sodium chloride to food; drinking water provides relatively lower contribution. High Cl− in drinking water promotes to the rate of corrosion of metals in drinking water; as the result, it increases the concentrations of metals in the supply system. The health authorities have not proposed health-based guideline value for Cl− in drinking water as it has a slight importance from a public health perspective. Conversely, Cl− concentration is related to Na+ ions, and higher than 250 mg L−1 develops a detectable taste in water and linked to heart and kidney diseases (Kumar and Puri 2012). A total number of 39 samples (34 from Mastung and 5 from Pringabad) were identified with a concentration of Cl− above 250 mg L−1.
No adverse human health effects have been reported for the limits of SO4 2− in drinking water. However, a laxative effect at concentrations of 1000–1200 mg L−1 has been observed, which does not cause diarrhoea, dehydration, or weight loss. The lowest taste threshold concentration for SO4 2− is reported as 250 mg L−1 as a sodium salt (WHO 2004a, b). Figure 3 shows that 38 samples from Mastung, 3 from Mangochar, and 8 from Pringabad have a concentration greater than 250 mg L−1. The presence of high SO4 2− may contribute to the taste and corrosion of a water supply system.
Bicarbonate (HCO3 −) and carbonate (CO3 2−) contribute to the alkalinity of water. No aesthetic level or maximum acceptable concentration in drinking water has been set for these anions. HCO3 − of Ca2+ and Mg2+ decompose in steam boilers to form scale and release corrosive carbon dioxide gas. The concentrations of CO3 2− in samples were identified as 57–299, 85–194, and 65–299 mg L−1 in Mastung, Mangochar, and Pringabad localities, respectively. HCO3 − was estimated in the range of 131–641, 167–365, and 102–355 mg L−1 in Mastung, Mangochar, and Pringabad, respectively.
Statistical analysis
Correlation analysis
Correlation analysis has been performed on the individual data of each area. Significant correlations of F− have been observed with pH in samples from Mastung (r 2 = 0.291, p = 0.005), Mangochar (r 2 = 0.406, p = 0.044), and Pringabad (r 2 = −0.552, p = 0.001). The results indicate that F− solubility in drinking water is pH dependent. The other significant correlations of F− in Mastung were observed with TDS (r 2 = 0.449, p < 0.001), Na+ (r 2 = 0.454, p < 0.001), K+ (r 2 = 0.647, p < 0.001), Cl− (r 2 = 0.216, p = 0.041), and SO4 2− (r 2 = 0.267, p = 0.011). This indicates that high amount of F− is present in those water samples which contain high amount of dissolved solids. In other words, F− contributes in TDS. Similarly, Na+, K+, Cl−, and SO4 2− are identified to form positive correlation with the F−. On the other hand, the data of Mangochar show significant correlations of F− with TDS (r 2 = −0.662, p < 0.001) and Ca2+ (r 2 = −0.444, p = 0.026). It shows that those water samples of Mangochar which contain high amount of F− also contain high amount of dissolved salts of Ca2+ ions. No other significant correlation has been observed for Pringabad samples.
PCA
The Principal Component Analysis (PCA) was applied to the data to visualize the possible correlations among the variables and principal components (PCs). The eigenvalues are shown as clustered columns at primary y-axis, and the cumulative variability (%) is shown as the line with markers at secondary y-axis in the scree plot (Fig. 4a). It is evident from the figure that the first four principal components (PC1-PC4) are significant on the basis of the eigenvalue (>1) and represent 66.85 % of the initial variability of the data. The significant PCs are shown as filled columns, while non-significant components (P5-PC11) are shown as unfilled columns in the scree plot.
Principal component analysis. a Scree plot b Biplot showing score and loading plots simultaneously (λ1, and λ2 are eigenvalues and EV1, and EV2 explained variance %). Samples: ○ Mastung open well,  Mastung kareze, ● Mastung tube well, Δ Mangochar open well, ▲ Mangochar tube well, □ Pringabad open well,
Mastung kareze, ● Mastung tube well, Δ Mangochar open well, ▲ Mangochar tube well, □ Pringabad open well,  Pringabad kareze
Pringabad kareze
The correlations between the PCs and the original variables were estimated. A correlation value above 0.5 is considered important in this study. The first principal component (PC1) explained 32.12 % of the total variability and is strongly correlated with K+ ions (0.812), followed by Na+ (0.77), SO4 2− (0.76), pH (0.65), F− (0.54), and Mg2+ (0.52). This suggests that drinking water samples of high Na+, K+, and Mg2+ contains high SO4 2− and F− ions. The second component (PC2) (14.50 % of the variance) was found a function of CO3 2− (0.75), Ca2+ (−0.55), and HCO3 − (0.53). This observation indicates that the presence of CO3 2− and HCO3 − in these samples decreases the availability of Ca2+ ions. The third component (PC3) has observed as a measure of Cl− (0.65) and TDS (−0.51) with 10.74 % of the total variability. It shows that the TDS values of those samples are higher which contains the lower amount of Cl− ions. The fourth component (PC4) was correlated to Mg2+ (0.63) with 9.49 % variance.
Figure 4b represents scatter biplot of PC1 and PC2 components. The trends of the data show that the three study sites are clustered and intermixed with each other. As no anthropogenic activities have been reported in the studied areas, therefore, it is evidenced that the rocks present in these areas possibly contribute to the F− contamination in groundwater. Hence, it is concluded that the three sources of groundwater are interlinked to each other and apparently comes from the layers of the same environment. However, the chemical composition of drinking water of Mastung exhibited a wide range of variations which is attributed to the difference in geochemical composition of the rocks of this area.
Conclusions
The inhabitants of Mastung, Mangochar, and Pringabad rely on drinking water from unprotected and remote sources of groundwater. With the exception of 36 % sampling sites, the rest of the sites were identified as contaminated and unfit for human consumption. About 64 % locations of the sites were found at the risk of dental caries, dental fluorosis, or skeletal fluorosis. Area-wise analyses show that groundwater of 43.33 % sampling sites from Mastung, 48.00 % from Mangochar, and 37.14 % from Pringabad sampling sites are contaminated with high level of F−. The salts of Ca2+, Na+, K+, Cl−, and SO4 2− were identified to contribute in TDS and pH of groundwater. Correlation analysis shows that F− solubility in drinking water is pH dependent, and high amount of F− is present in those samples of Masung which contains high amount of TDS, Na+, K+, Cl−, and SO4 2−. Conversely, high amount of F− has been observed in those samples of Mangochar which contains high amount of dissolved salts of Ca2+ ions. On the basis of the results, it is concluded that the rocks present in the three areas possibly contribute to F− contamination in groundwater. Additionally, the three sources of groundwater (open well, karez, and tube well) are interlinked to each other and comes from the layers of the same surroundings. However, even no comprehensive health surveys reported for abnormalities in the skeletal tissues in studied areas from which the overall extent of the problem could be assessed. Due to the thin dividing line between the beneficial and hazardous effects of fluoride, drinking water schemes for public health should be introduced by the local authority in the country and monitored continuously to retain the appropriate amounts of F− and other cations and anions contribute to the quality of drinking water.
References
Aijaz, M., Scheik, Z., & Azfar, S. F. (2011). Mastung District Government. Mastung - Integrated District Development Vision. IUCN Pakistan, Quetta, Pakistan. https://cmsdata.iucn.org/downloads/pk_mastung_iddv.pdf. Accessed 30 June 2015.
APHA, AWWA & WEF. (1992). Standard methods for examination of water and wastewater (18th ed.). Washington: American Public Health Association, Washington, DC.
De Zuane, J. (1997). Chemical parameters—inorganics. In Handbook of drinking water quality (2nd ed., p. 119). New York: John Wiley & Sons. doi:10.1002/9780470172971.ch3.
Dote, T., Kono, K., Usuda, K., Nishiura, H., Tagawa, T., Miyata, K., et al. (2000). Toxicokinetics of intravenous fluoride in rats with kidney damage caused by high-dose fluoride exposure. International Archives of Occupational and Environmental Health, 73(Suppl), 90–92.
EPA (United States Environmental Protection Agency) (2012). 2012 Edition of the drinking water standards and health advisories (p. 8). Office of water U.S. Environmental Protection Agency Washington, DC. http://water.epa.gov/action/advisories/drinking/upload/dwstandards2012.pdf. Accessed 25 Feb 2015.
Guan, Z. Z., Wang, Y. N., Xiao, K. Q., Dai, D. Y., Chen, Y. H., Liu, J. L., et al. (1998). Influence of chronic fluorosis on membrane lipids in rat brain. Neurotoxicology and Teratology, 20(5), 537–542.
Kumar, M., & Puri, A. (2012). A review of permissible limits of drinking water. Indian Journal of Occupational and Environmental Medicine, 16(1), 40–44.
Mineral profile of Balochistan (2009). http://www.gsp.gov.pk/images/mineralprofileofbalochistan.pdf. Accessed 22 Feb 2015.
Olayanju, G. M., Ayuk, M. A., & Adelusi, A. O. (2011). Geoelectrical mapping of the groundwater regime around the Federal Polytechnic Ado-Ekiti, South Western Nigeria. Journal of Geology and Mining Research, 3(8), 201–210.
Wang, A. G., Xia, T., Ru, R. A., Chen, X. M., & Yang, K. D. (2004). Antagonistic effect of selenium on oxidative stress, DNA damage, and apoptosis induced by fluoride in human hepatocytes. Fluoride, 37(37), 107–116.
WHO (World Health Organization) (1963). Guidelines of drinking water quality recommendations: 1, Geneva.
WHO (World Health Organization) (2003). Total dissolved solids in drinking water: Background document for development of WHO guidelines for drinking-water quality (p. 1). http://www.who.int/water_sanitation_health/dwq/chemicals/tds.pdf. Accessed 25 Jan 2015.
WHO (World Health Organization) (2004a). Fluoride in drinking-water: Background document for development of WHO guidelines for drinking-water quality. (p. 5). http://www.who.int/water_sanitation_health/dwq/chemicals/fluoride.pdf. Accessed 25 Jan 2015.
WHO (World Health Organization) (2004b). Sulfate in Drinking-water: Background document for development of WHO guidelines for drinking-water quality. (p. 1). http://www.who.int/water_sanitation_health/dwq/chemicals/sulfate.pdf. Accessed 25 Jan 2015.
WHO. (World Health Organization). (2006). Fluoride in drinking-water (p. 7, 32). London: IWA Publishing.
Conflict of interest
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chandio, T.A., Khan, M.N. & Sarwar, A. Fluoride estimation and its correlation with other physicochemical parameters in drinking water of some areas of Balochistan, Pakistan. Environ Monit Assess 187, 531 (2015). https://doi.org/10.1007/s10661-015-4753-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-015-4753-6