Abstract
Incidence of high fluoride (F−) in groundwater (>1.5 mg/L) in two tribal belts of eastern India, one around Chukru in the Palamau district of Jharkhand and the other around Karlakot in the Nuapada district of Odisha, has been studied. The maximum concentration of F− in groundwater from dug wells and tube wells is 10.30 mg/L in Chukru and 4.62 mg/L in Karlakot. The groundwaters are mildly alkaline with pH ranges of 7.52–8.22 and 7.33–8.20 in Chukru and Karlakot, respectively. The F− concentration is positively correlated with pH, electrical conductivity and SO4 2− in both areas. The high F− in groundwater resulted mainly from dissolution of biotite and fluorapatite in quartzofeldspathic gneiss. The ionic dominance pattern (in meq/L) is mostly in the order Ca2+ > Na+ > Mg2+ > K+ among cations and HCO3 − > SO4 2− > < Cl− > F− among anions in the Karlakot groundwater. Preliminary adsorption experiments were conducted on natural haematite iron ore and synthetic magnetite to evaluate their potential for F− removal from water. Effects of different parameters such as contact time, pH, adsorbent dose and initial F− concentration on the adsorption capacity of these materials were investigated. Strong dependence of F− removal on pH was observed for both the adsorbents. With natural haematite iron ore, the maximum F− removal of 66 % occurred at an initial pH of 3.2 for a solution with F− concentration of 3 mg/L, adsorbent dose of 7 g/L and overnight contact time. The haematite iron ore was observed to increase the pH of the F− solution. Adsorption equilibrium was not achieved with this adsorbent even after a contact time of 45.2 h. In the case of synthetic magnetite, 84 % F− removal was achieved after 2 min of contact time for a solution with F− concentration of 6 mg/L, adsorbent dose of 10 g/L and initial pH of 7. The results indicate high potential of both natural haematite iron ore and synthetic magnetite as adsorbents of F− in water.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Excessive intake of fluoride (F−) over a long period of time has negative effects on human health and results in a disease called fluorosis, which is known for the worst form of bone disorder and body crippling (WHO 1997; Cerklewski 1997; Barbier et al. 2010). Ingestion of F− predominantly comes from drinking water sources, especially groundwater. Enrichment of F− in groundwater is generally attributed to prolonged rock–water interaction (Nordstrom et al. 1989; Chae et al. 2006). Hydrous silicate minerals such as biotite, muscovite, hornblende and tourmaline can contain significant amount of F− in their crystal structure. This is because the ionic radius of F− (0.136 nm) is nearly the same as that of hydroxide ion (OH−) and as a result, the two ions commonly substitute for one another. These silicate minerals as well as some non-silicate F-bearing minerals such as fluorapatite and fluorite, usually occur as accessory phases in various igneous and metamorphic rocks. Chemical weathering of these phases during rock–water interaction results in the release of F− to groundwater. The release is a function of several factors including the mineralogy of rock, hydrogeological condition, groundwater chemistry, interaction period of groundwater with aquifer material, and the dissolution kinetics of F-bearing minerals. The solubility of fluorite in fresh water is low and its dissolution rate is remarkably slow under normal temperature and pressure conditions (Nordstrom and Jenne 1977). Therefore, high F− concentration in groundwater is more likely to result from the dissolution of silicate minerals and fluorapatite.
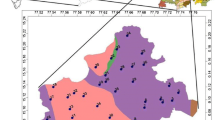
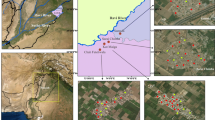
Vast stretches of peninsular India are occupied by granitic and gneissic rocks of Precambrian age (older than 540 million years) in which F-bearing minerals commonly occur as accessory phases. Prolonged chemical weathering of these rocks over a period of millions of years have resulted in elevated contents of F− in groundwater in several parts of India. Consequently, fluorosis is endemic in 17 states of the country, the problem being most pronounced in the states of Andhra Pradesh, Jharkhand, Odisha, Gujarat, Madhya Pradesh, Punjab, Rajasthan, Tamil Nadu, and Uttar Pradesh (UNICEF 1999; Saxena and Ahmed 2003; Meenakshi and Maheshwari 2006; Susheela 2007). Fluoride problem in groundwater is acute in the Palamau and Garwa districts of Jharkhand (Avishek et al. 2010), and in the Nuapada (also known as Nawapara), Kalahandi, Koraput, Bolangir and Nayagarh districts in Odisha (Das et al. 2000; Kundu et al. 2001; Pattnaik and Mishra 2002; Srivastava and Tiwari 2006). The main source of drinking water in these areas is groundwater in the form of dug wells and tube wells. The majority of the population in these areas are poor rural communities that do not have alternative sources of water for their domestic and other uses. The present work focuses on the assessment of F− contamination of groundwater in two tribal belts of eastern India where fluorosis is rampant, one around Chukru in the Palamau district of Jharkhand and the other around Karlakot in the Nuapada district of Odisha (Fig. 1).
Several studies have emphasised the role of rock–water interaction in the F− contamination of groundwater in different parts of India (Mamatha and Rao 2010; Kantharaja et al. 2012; Padhi and Muralidharan 2012; Shyam and Kalwannia 2012; Avtar et al. 2013; Chidambaram et al. 2013; Rao et al. 2013; Vikas et al. 2013). These authors have attributed the F− enrichment in groundwater mainly to the chemical breakdown of F-bearing minerals. While they have indicated different minerals such as fluorite, fluorapatite, biotite, muscovite and hornblende as possible contributors of F− to the groundwater, the present study identifies the actual F−-bearing phases in the rocks based on electron probe microanalysis. Further, the study combines geology, mineralogy of the rocks and geochemical analysis of the groundwater to explain the evolution of physicochemical parameters of the groundwater.
Several methods have been studied for the removal of excess F− from drinking water above the permissible limit (1.5 mg/L as per the Indian Bureau of Standards), and these include adsorption, coagulation/precipitation, ion exchange and reverse osmosis (Mohapatra et al. 2009; Jagtap et al. 2012). Certain disadvantages associated with the precipitation and reverse osmosis processes are: generation of unwanted chemicals, waste disposal issues, low economic viability, high maintenance cost, fouling, scaling, and membrane degradation. Ion exchange and adsorption are widely accepted technologies as these are quite effective in reducing F− concentrations to values within the permissible limit. Adsorption has additional advantage due to simple procedure, low operational and maintenance costs. Several natural/synthetic/waste materials have been tested for their adsorption/absorption characteristics (Rahmani et al. 2010; Bhatnagar et al. 2011; Gitari et al. 2013; Loganathan et al. 2013). Iron oxides/hydroxides are important adsorbents due to the available active sites for adsorption of F− (Mohapatra et al. 2012). It is crucial that technology be developed for defluoridation of groundwater that is cheap and easily available to rural communities. One of the ways to develop a cheaper technology is to come up with cheap and high capacity adsorbents based on locally available materials. In the present study, preliminary adsorption experiments for F− removal have been conducted using synthetic magnetite and natural haematite iron ore, which are locally available. The effects of some of the major parameters of adsorption such as contact time, adsorbent dose, pH and initial fluoride concentration on F− removal have been investigated.
Geomorphology and geological setting
Chukru area
The topography of the Chukru area is almost flat with the lowest contour having an elevation of 220 m above mean sea level (MSL). A few isolated hillocks occur in the area and the highest elevation is 323 m above MSL. A perennial river, namely the North Koel, flows in a northerly direction (Fig. 1b). The drainage pattern is dendritic.
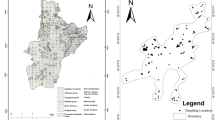
Geologically, the area falls in the northwestern part of the Chhotanagpur Granite Gneiss Complex of Precambrian age (Chatterjee and Ghose 2011), and is mainly occupied by biotite quartzofeldspathic gneiss (Fig. 2a). The essential minerals in the gneiss are quartz, K-feldspar and plagioclase, while biotite and apatite are common accessory phases. Muscovite is occasionally found as an accessory phase. Hornblende granite is exposed in the southern part of the area and is mineralogically composed of quartz, plagioclase and hornblende. Bands of amphibolite (hornblende + plagioclase + quartz) and calcitic marble occur locally.
Karlakot area
The topography of the Karlakot area is marked by a major N–S trending elongated hill in the west, while the central and eastern parts are mostly plain with a few isolated hillocks. The highest peak in the area is 668 m above MSL and the lowest contour has an elevation of 220 m above MSL. Two perennial rivers, namely Indra and Sundar, flow in a southeasterly direction and join in the east-central part of the area (Fig. 1c). The drainage pattern is dendritic.
Geologically, the area is located at the eastern periphery of the Archaean Bastar Craton close to its thrust contact with the Proterozoic Eastern Ghats Mobile Belt (EGMB) (Biswal et al. 2007) (Fig. 2b). The dominant rock types in the cratonic part are quartzofeldspathic gneiss and foliated granite, whereas those in the EGMB include tonalitic granulite and clinopyroxene-bearing felsic granulite. The quartzofeldspathic gneiss contains quartz, K-feldspar and plagioclase as essential minerals and biotite and apatite as accessory minerals. The mineral assemblage in tonalitic granulite is quartz + plagioclase + garnet, whereas that in clinopyroxene-bearing felsic granulite is quartz + plagioclase + clinopyroxene + garnet. A N–S trending elongated body of nepheline syenite has intruded the central part of the area, whereas amphibolite (hornblende + plagioclase) occurs as small enclaves in different parts of the area. The nepheline syenite has the mineral assemblage K-feldspar + plagioclase + nepheline + hornblende.
Most of the villages affected by F− pollution in both Chukru and Karlakot areas occur on a lithology of quartzofeldspathic gneiss. Weathering of this bed rock has given rise to a zone of soil cover (sandy loam to clayey loam) and saprolite of up to 15 m thickness. This zone acts as shallow unconfined aquifers, whereas groundwater occurs in semi-confined to confined condition in the underlying zone of fractured bed rock (Sikdar et al. 2007).
Both Chukru and Karlakot areas have subtropical, monsoon climate and are marked by three distinct seasons—summer, rainy (monsoon) and winter. The monsoon rain starts in mid-June and lasts till September with an average annual rainfall of 1,200–1,300 mm.
Materials and methods
Groundwater sampling
Groundwater in the Chukru area was collected from sixteen wells, including nine dug wells and seven tube wells, spread over seven villages, namely Bhakhari, Chukru, Jorkat, Kauria, Nauatikar, Pindarahi and Sua (Fig. 1b). In the Karlakot area, sampling was done from sixteen sites, out of which eleven are tube wells and three are dug wells scattered over eleven villages, namely Amera, Bolda, Kalimati, Karlakot, Khaira, Kirejhola, Kirekela, Kotamal, Purakhut, Rajna and Tangripara (Fig. 1c). The other two sampling sites in the Karlakot area (PG1a, PG1b) are located near the Patalganga spring (N20o15′08′′: E82o31′49′′), which occurs outside the area in Fig. 1c.
Sampling was done in two phases in both Chukru and Karlakot areas. In the former, samples were collected in December, 2004 (winter) and May, 2005 (summer), whereas in the latter, sampling was done in December, 2007 (winter) and early June, 2008 (summer).
Chemical analysis of groundwater
Groundwater samples of the Chukru area collected during winter were analysed for pH, electrical conductivity (EC), total hardness (Ca2+ + Mg2+), alkalinity (HCO3 −), Cl−, SO4 2−, NO3 − and F−, whereas those collected during summer were analysed only for F−. Samples of the Karlakot area collected during both the seasons were analysed for pH, EC, Ca2+, Mg2+, Na+, K+, alkalinity, Cl−, SO4 2−, and F−. Analyses were carried out as per the standard procedures (APHA 1998). Measurement of pH and conductivity were done in the field with pH meter (Control Dynamics) and Conductivity meter (Systronics 306), respectively, after calibration with standard solutions. Other parameters were determined in the lab. Alkalinity was measured by acid titration, whereas total hardness, Ca2+ and Mg2+ were determined by complexometric titration with EDTA. Na+ and K+ were analysed by Inductively Coupled Plasma—Optical Emission Spectrometer (Jobin–Yvon Element 2). Cl− and SO4 2− were measured by argentometric titration and turbidimetric method, respectively. NO3 − was determined by UV–VIS spectrophotometer (Shimadzu UV-160A).
Groundwater samples from the Chukru area collected during winter were analysed for F− by both SPADNS method (JASCO V530 spectrophotometer) and ion-selective electrode (ISE) (Thermo Orion 720A), whereas all other samples were analysed by ISE only. In the ISE technique, a Total Ionic Strength Adjusting Buffer Grade III (TISAB III) was mixed with samples in order to maintain the ionic strength and pH constant and also to decomplex metal–F complexes present in the sample. The later role of TISAB III is most crucial since the ISE, comprising lanthanum fluoride membrane, is sensitive only to free F− ions. The composition of TISAB III solution was 383 g ammonium acetate, 19.8 g of CDTA and 211 mL of 12 N HCl per litre. The balance of cations and anions (in meq/l) in the Karlakot groundwater, expressed as (∑cations − ∑anions)/(∑cations + ∑anions), falls in the range of +10 % to −3 %.
Mineral chemical analysis
Major and minor element composition of minerals were determined in thin sections of rocks using a Cameca SX-Five electron probe micro-analyser (EPMA) at the Indian Institute of Technology, Bombay. The operating parameters were: acceleration voltage of 15 kV, beam current of 20 nA and beam diameter of 1 μm. Standards included both natural and synthetic minerals and data correction was made by the X-PHI method. Fluorine and chlorine were measured using PC0 and LPET crystals, respectively.
Apatite, biotite and plagioclase in quartzofeldspathic gneiss were analysed in two samples each from Chukru (CK3, CK18) and Karlakot (KR1 and KR2). Muscovite occurs in sample CK3 which was also analysed. In addition, analysis of hornblende, plagioclase and nepheline was carried out in two samples of nepheline syenite from Karlakot (KR3 and KR17). The locations of the analysed samples are shown in Fig. 2.
Fluoride removal experiments
Preliminary F− adsorption tests were carried out using natural iron ore and synthetic magnetite. The iron ore used is from the Noamundi open cast iron ore mines of M/s Tata Steel in Jharkhand. Geologically, the iron ore is from a rock formation of Banded Haematite Jasper belonging to the Iron Ore Group of Archaean age. A few kilograms of reddish brown, powdery iron ore (dominantly haematite) was collected for the present study. XRD pattern of the ore shows the presence of additional phases such as goethite, gibbsite and silica. The ore, when washed with water, released some preexisting F− that was adsorbed by natural processes. Therefore, before conducting the experiments, the iron ore was washed with 1 % NaOH. The ore was then ground to fine size and the material of 125–212 micron size range was used for experiments. Groundwater samples from the Chukru area were used for the F− removal experiments with iron ore by varying contact time and initial pH.
Synthetic magnetite was prepared using FeCl2, FeCl3, 2 M HCl and 1 M NH4OH. Artificially fluoridated water was used for the F− removal experiments with synthetic magnetite by varying adsorbent dose, contact time, initial pH, initial F− concentration and temperature. All the experiments were done in plastic bottles, which were shaken by end over end shaker.
Results and discussion
Mineral chemistry
Mineral compositions of rocks from both Chukru and Karlakot areas are presented in Table 1. Apatite in the quartzofeldspathic gneisses contains F anion in the range of 1.774–1.927 out of 2(OH, F, Cl) per formula unit, and hence can be termed fluorapatite. The atomic F/Ca ratio is 0.19 for Chukru fluorapatite and 0.18 for Karlakot fluorapatite. Biotite in the quartzofeldspathic gneisses of both the areas contains significant F except when it has highly iron-rich composition. Chukru biotite has higher F content [0.378 out of 4(OH, F, Cl) per formula unit; sample CK18] than Karlakot biotite [0.182–0.225 out of 4(OH, F, Cl) per formula unit; samples KR1, KR2]. Biotite in the Chukru sample CK3 is highly iron-rich [X Fe = Fe/(Fe + Mg) = 0.80] compared to that in all other samples, which have X Fe = 0.53–0.61. Both biotite and muscovite in this sample are devoid of F although the rock contains significant amount of fluorapatite. Hornblende in nepheline syenite of Karlakot is also highly iron-rich (X Fe = 0.94–0.98) and contains negligible or no F.
Plagioclase in quartzofeldspathic gneisses of both the areas is oligoclase in composition with X Ca [=Ca/(Na + Ca)] in the range of 0.14–0.26. Plagioclase in Karlakot nepheline syenite is almost pure albite with X Ca = 0.01. Nepheline in this syenite is significantly potassic with K/(K + Na) = 0.19.
Groundwater chemistry
Groundwater analyses are presented in Table 2. The groundwater is weakly alkaline with pH in the ranges of 7.52–8.22 (winter) in Chukru and 7.33–8.20 (winter) and 7.40–8.12 (summer) in Karlakot. The EC falls in the ranges of 527–1,780 μS/cm (winter) in Chukru and 548–1,743 μS/cm (winter) and 297–1,575 μS/cm (summer) in Karlakot. Except for a few locations in both Chukru and Karlakot areas, the F− concentration is above the permissible limit of 1.50 mg/L. The F− values obtained from the SPADNS method for the winter samples of the Chukru are, in most cases, somewhat higher than those from the ISE method. For the purpose of comparison, F− values from the ISE method have been described below. In the Chukru groundwater, except for the tube well location RT13, F− concentration falls in the ranges of 2.43–10.30 mg/L (winter) and 2.43–10.00 mg/L (summer). At RT13, the groundwater has relatively low F− with values of 0.77 mg/L and 0.90 mg/L in winter and summer, respectively. The F− concentration in the Karlakot groundwater falls in the ranges of 0.80–4.46 mg/L (winter) and 0.70–4.62 mg/L (summer). The seasonal variation of F− is negligible in Chukru (Fig. 3a), while it is significant in several wells of Karlakot with higher F− values in winter than in summer (Fig. 3b).
The dominant carbonate species present in water is universally a function of pH. Carbonic acid is dominant at pH <6.3, while CO3 2− becomes dominant at pH >10.3, and in between these pH values HCO3 − is the major carbonate species in water. The pH range of the studied groundwater (7.33–8.22) indicates that the dominant carbonate species is HCO3 −. Strong bases such as SO4 2− and Cl− occur in low concentrations relative to HCO3 −. The ionic dominance pattern (in meq/l) is in the order Ca2+ > Na+ > Mg2+ > K+ among cations and HCO3 − > SO4 2− > < Cl− > F− among anions in most samples. The concentration (in meq/l) of alkali earth ions (Ca2+ + Mg2+) exceeds that of alkali ions (Na+ + K+). Piper plot of the Karlakot samples shows that the groundwater is mostly Ca–HCO3 Type (Fig. 4a). From the K+ versus Na+ diagram (in meq/l), it can be seen that Karlakot groundwater is relatively sodic with samples mostly having K+/Na+ < 0.2 (Fig. 4b). The ratio of Ca2+ to Na+ (in meq/l) is >1 (Fig. 4c), which indicates that calcium could not have resulted from stoichiometric breakdown of oligoclase in quartzofeldspathic gneiss alone because it has low Ca/Na ratio (0.16–0.35). The other minerals which could have contributed Ca2+ to groundwater are apatite in quartzofeldspathic gneiss and hornblende in nepheline syenite.
Bivariate plots of F− versus other parameters of groundwater show that F− concentration is positively correlated with pH, EC and SO4 2− in both Chukru and Karlakot areas (Fig. 5). Increase of F− with increasing Na+ is observed in the Karlakot groundwater (Fig. 6a). These relationships indicate that F− enrichment is related to geochemical processes releasing Na+ and SO4 2− and removing H+ ions. Fluoride is adsorbed by clay in acidic medium; however, in alkaline medium, it is desorbed and thus, alkaline water promotes leaching of F− into groundwater (Simard and Lafrance 1996; Agarwal et al. 2002).
In order to evaluate the chemistry of Karlakot groundwater with respect to fluorite saturation, the activities of F− and Ca2+ were calculated using the WATEQ4F program of Ball and Nordstrom 1991 (updated in 2012) The bivariate plot of these activities is shown in Fig. 6b along with the fluorite dissolution line and fluorite saturation line constrained by the reaction: CaF2 = Ca2+ + 2F−. The equilibrium constant of this reaction, \( \text{K} = (a_{\text{Ca} ^{2+}})\cdot(a_{\text{F}^{-}})^{2}\) implies that the activity of F− in the presence of fluorite is inversely proportional to that of Ca2+. Most of the Karlakot groundwater samples plot close to the fluorite saturation line suggesting that the upper limit of F− concentration is constrained by fluorite equilibrium. The data points fall far off the fluorite dissolution line (line 1) but close to the fluorapatite dissolution line (line 2) which indicates that fluorapatite was a significant contributor of F− in Karlakot groundwater. However, the trend of data points differs from that of the fluorapatite dissolution line which can be explained if there was a contribution of Ca2+ from another mineral such as oligoclase (line 3). The data points can also be explained by contribution of F− solely from biotite (line 4) and Ca2+ from oligoclase (line 3).
The chemical composition of the groundwater in both Chukru and Karlakot areas is controlled by rock weathering with both fluorapatite and biotite as possible contributors of F−. According to Guidry and Mackenzie (2003), dissolution of fluorapatite is controlled by aqueous solution state, temperature and pH range of 2–8.5. In this regard, the pH conditions of Chukru (7.52–8.22) and Karlakot (7.33–8.20) are favourable for dissolution of fluorapatite and promote preferential removal of F and Ca from its crystal structure (Chairat et al. 2007). Regarding biotite as a contributor of F−, laboratory studies have demonstrated that biotite dissolution of granitic rocks can result in F− concentrations of up to 6 mg/L in water (Saxena and Ahmed 2001; Chae et al. 2006; Kularatne and Pitawala 2012). Although biotite in the studied gneisses contains lesser amount of F than fluorapatite, the higher modal abundance of biotite compared to fluorapatite makes it the major source of F− in groundwater. Muscovite and hornblende in the rocks are devoid of F and hence, could not have contributed F− to the groundwater.
The composition of K- and Na-bearing minerals in rocks needs to be discussed to explain the low K+/Na+ ratio of Karlakot groundwater. The main K-bearing minerals are biotite and K-feldspar in quartzofeldspathic gneiss with a higher modal abundance of the latter phase. Thus, K-feldspar is the major contributor of K+ in the Karlakot groundwater. Significant Na can come from plagioclase (oligoclase) in quartzofeldspathic gneiss, and hornblende, plagioclase (albite) and nepheline in nepheline syenite. However, the latter rock type is volumetrically minor in the Karlakot area. Therefore, oligoclase in quartzofeldspathic gneiss is considered to be the main contributor of Na+ in groundwater. In such a scenario, the low K+/Na+ ratio of Karlakot groundwater can be explained by two alternative models, one in which K-feldspar is subordinate to oligoclase in quartzofeldspathic gneiss, the other in which K-feldspar did not dissolve to a great extent during chemical weathering. The former alternative is less likely because K-feldspar has higher modal abundance than oligoclase in the rock. This leads us to infer that the dissolution of K-feldspar was less intense compared to that of oligoclase during chemical weathering.
Fluoride removal
To understand the kinetic behaviour in adsorption studies, the adsorbent dose should be such that around 50–70 % of adsorbate (F−) is taken up by the adsorbent. Accordingly, adsorbent dose was chosen in the present study. Experimental variables and results of three sets of experiments (1a–c) involving haematite iron ore as adsorbent in Chukru groundwater samples are given in Table 3. For experiments 1a and 1b, 11.9 g of iron ore was kept in 1.75 L of groundwater. In experiment 1a, the removal of F− from an initial concentration of 3.26 mg/L was measured at time intervals of 1–3 h with pH adjusted close to 5 at the beginning of each time interval. The final F− concentration after a cumulative contact time of 12.5 h was 1.02 mg/L. The decrease in F− concentration with time is nearly linear (Fig. 7a). The results of Experiment 1a show that adsorption equilibrium was not achieved even after 12.5 h of contact time and therefore a further experiment (Experiment 1b) was conducted specifically to observe uptake behaviour after longer contact time. Experiment 1b was similar to experiment 1a except that it had two large time gaps, one of 15 h and another of 16 h duration. The initial F− concentration of 2.97 mg/L decreased to 0.97 mg/L after a cumulative contact time of 45.2 h. A decrease in the rate of F− removal is observed during the large time gaps compared to the small time gaps (Fig. 7b). It can be observed that F− uptake was occurring even up to 45.2 h contact time, which indicates that F− was also getting transported inside the pores of adsorbent and possibly getting complexed (inner sphere complexation). Experiment 1c included a set of 40 experiments, each with 0.35 g of iron ore and 50 mL of groundwater, but at different initially adjusted pH values in the range of 2.69–10.46. Three batches of groundwater samples were used with F− concentrations of 2.70, 3.00 and 3.15 mg/L, respectively. Contact time was overnight. The results demonstrate strong dependence of F− removal on pH with the maximum removal of 66 % occurring at a pH of 3.23 (Fig. 7c). Table 3 shows an increase in pH as the F− solution interacts with haematite iron ore. This is probably because F− replaces OH− in some hydrous phase such as goethite or gibbsite present in the ore.
Adsorption of F− in Chukru groundwater samples by natural haematitic iron ore. F− concentration in water remaining after adsorption has been plotted. Plots show the effects of contact time (a), large gap in contact time (b), and initial pH (c). See Table 3 for experimental data
Results of five sets of experiments (2a–e) involving synthetic magnetite as adsorbent in artificially fluoridated water are given in Table 4. The volume of water used was 200 ml for each experiment. Parameters such as contact time (1 h), initial pH (7.0) and initial F− concentration (6.0 mg/L) were kept constant except when their individual effects were studied. Experiment set 2a involved five experiments with different amounts of adsorbent (0.2–1.0 g). The F− concentration decreases with increasing adsorbent dose (Fig. 8a). Experiment set 2b comprised six experiments with 2 g of adsorbent dose and different contact times (2–60 min). The F− removal is 84 % after 2 min of contact time. The rate of F− removal is low after a contact time of 20 min (Fig. 8b). The data fit well with second-order reaction kinetics. In the experiment set 2c, five experiments were conducted with 0.3 g of adsorbent and different initial pH (6.5–8.5). Nearly linear decrease in F− concentration is observed with decreasing pH (Fig. 8c). Experiment sets 2d and 2e both involved varying initial F− concentrations (2–10 mg/L), but while the former was conducted at room temperature (~25 °C), the latter was conducted at 50 °C. Adsorption isotherms for these two sets of experiments have been drawn as per the Freundlich equation: x/m = KC n where, x/m is the weight of adsorbate (F−) divided by the weight of adsorbent (magnetite) (in mg/g); C is the F− concentration (in mg/L) remaining after adsorption, and K and n are constants. The isotherm plot shows that the highest fraction of F− is adsorbed at the highest F− concentration, corresponding to the steepest part of the isotherm (Fig. 8d). The adsorption is enhanced at higher temperature.
Adsorption of F− in artificially fluoridated water by synthetic magnetite. F− concentration in water remaining after adsorption has been plotted. Plots show the effects of adsorbent dose (a), contact time (b), initial pH (c), and initial F− concentration (d). See text for explanation of x/m and C, and Table 4 for experimental data
Conclusions
The groundwaters of the Chukru and Karlakot areas have a problem of F− contamination that is controlled by natural conditions of rock–water interaction. Most of the F− affected localities in these areas occur on a lithology of quartzofeldspathic gneiss. Fluoride is derived mainly from the dissolution of biotite and fluorapatite in the rock. In a study of seasonal variations of F− in Karlakot groundwater, Pattnaik and Mishra (2002) observed a fall of up to 23 % in F− concentration from monsoon season (August, 1999 and July, 2000) to winter season (November, 1999 and February, 2001). On the other hand, the present study shows a fall of up to 24 % in F− concentration in groundwater from winter season (December, 2007) to summer season (June, 2008) in several wells of Karlakot. The reason for such variations is not known and detailed studies on the rainfall pattern and seasonal fluctuations in groundwater level are required to address this issue.
In the majority of groundwater samples from Karlakot, the ionic dominance pattern (in meq/l) is in the order Ca2+ > Na+ > Mg2+ > K+ among cations and HCO3 − > SO4 2− > < Cl− > F− among anions. The minerals which have mainly contributed different cations to the groundwater during chemical weathering are: oligoclase and apatite for Ca2+, oligoclase and nepheline for Na+, K-feldspar and biotite for K+, and biotite for Mg2+. The F− concentration in groundwater shows positive correlation with pH, electrical conductivity and SO4 2−, while the upper limit of F− concentration is constrained by fluorite equilibrium.
The F− removal experiments show that both natural iron ore and synthetic magnetite have potential for use as adsorbents of F− in water. A suitable adsorbent should not only have high F− adsorption capacity and cost-effectiveness but also be amenable to easy desorption of the adsorbed F− and capable of efficient regeneration for reuse. Since F− adsorption decreases at high pHs, bases having high pHs can be used for desorption of F− from synthetic iron ore. For natural iron there is no need of regeneration because the material is inexpensive and readily available in several parts of India.
References
Agarwal M, Rai K, Shrivastav R, Dass S (2002) A study of fluoride sorption by montmorillonite and kaolinite. Water Air Soil Pollut 141:247–261
APHA (1998) Standard methods for the examination of water and wastewater, 20th edn. American Public Health Association, Washington
Avishek K, Pathak G, Nathawat MS, Jha U, Kumari N (2010) Water quality assessment of Majhiaon block of Garwa district in Jharkhand with special focus on fluoride analysis. Environ Monit Assess 167:617–623
Avtar R, Kumar P, Surjan A, Gupta LN, Roychowdhury K (2013) Geochemical processes regulating groundwater chemistry with special reference to nitrate and fluoride enrichment in Chhatarpur area, Madhya Pradesh, India. Environ Earth Sci 70:1699–1708
Ball JW, Nordstrom DK (1991) User’s manual for WATEQ4F, with revised thermodynamic data base and test cases for calculation speciation of major, trace, and redox elements in natural waters. US Geological Survey Open-File Report 91–193, p 189
Barbier O, Arreola-Mendoza L, Del Razo LM (2010) Molecular mechanisms of fluoride toxicity. Chem Biol Interact 188:319–333
Bhatnagar A, Kumar E, Sillanpaa M (2011) Fluoride removal from water by adsorption–a review. Chem Eng J 171:811–840
Biswal TK, De Waele B, Ahuja H (2007) Timing and dynamics of the juxtaposition of the Eastern Ghats Mobile Belt against the Bhandara Craton, India: A structural and zircon U–Pb SHRIMP study of the fold-thrust belt and associated nepheline syenite plutons. Tectonics 26, TC4006. doi:10.1029/2006TC002005
Cerklewski FL (1997) Fluoride bioavailability: nutritional and clinical aspects. Nutr Res 17:907–929
Chae GT, Yun ST, Kwon MJ, Kim YS, Mayer B (2006) Batch dissolution of granite and biotite in water: implication for fluorine geochemistry in groundwater. Geochem J 40:95–102
Chairat C, Schott J, Oelkers EH, Lartigue JE, Harouiya N (2007) Kinetics and mechanism of natural fluorapatite dissolution at 25 C and pH from 3 to 12. Geochim Cosmochim Acta 71:5901–5912
Chatterjee N, Ghose NC (2011) Extensive early Neoproterozoic high-grade metamorphism in North Chotanagpur Gneissic Complex of the Central Indian Tectonic Zone. Gondwana Res 29:279–363
Chidambaram S, Prasad MBK, Manivannan R, Karmegam U, Singaraja C, Anandhan P, Prasanna MV, Manikandan S (2013) Environmental hydrogeochemistry and genesis of fluoride in groundwaters of Dindigul district, Tamilnadu (India). Environ Earth Sci 68:333–342
Das S, Mehta BC, Samanta SK, Das PK, Srivastava SK (2000) Fluoride hazards in ground water of Orissa, India. J Environ Health 42:40–46
Gitari WM, Ngulube T, Masindi V, Gumbo JR (2013) Defluoridation of groundwater using Fe3+-modified bantonite clay: optimization of adsorption conditions. Desal Water Treat. doi:10.1080/19443994.2013.855669
Guidry MW, Mackenzie FT (2003) Experimental study of igneous and sedimentary apatite dissolution: control of pH, distances from equilibrium, and temperature on dissolution rates. Geochim Cosmochim Acta 67:2949–2963
Jagtap S, Yenkie MK, Labhsetwar N, Rayalu S (2012) Fluoride in drinking water and defluoridation of water. Chem Rev 112:2454–2466
Kantharaja DC, Lakkundi TK, Basavanna M, Manjappa S (2012) Spatial analysis of fluoride concentration in groundwaters of Shivani watershed area, Karnataka state, South India, through geospatial information system. Environ Earth Sci 65:67–76
Kularatne KUKS, Pitawala HMTGA (2012) Leaching of fluoride from biotite mica in soil: implications for fluoride in shallow groundwater. Int Sch Res Netw Soil Sci 2:7. doi:10.5402/2012/739051
Kundu N, Panigrahi MK, Tripathy S, Munshi S, Powell M, Hart BR (2001) Geochemical appraisal of fluoride contamination of groundwater in the Nayagarh district, Orissa, India. Environ Geol 41:451–460
Loganathan P, Vigneswaran S, Kandasamy J, Naidu R (2013) Defluoridation of drinking water using adsorption processes. J Hazard Mater 248–249:1–19
Mamatha P, Rao SM (2010) Geochemistry of fluoride rich groundwater in Kolar and Tumkur districts of Karnataka. Environ Earth Sci 61:131–142
Meenakshi, Maheshwari RC (2006) Fluoride in drinking water and its removal. J Hazard Mater B137:456–463
Mohapatra M, Anand S, Mishra BK, Giles DE, Singh P (2009) Review of fluoride removal from drinking water. J Environ Manag 91:67–77
Mohapatra M, Hariprasad D, Mohapatra L, Anand S, Mishra BK (2012) Mg-doped nanoferrihydrite–a new adsorbent for fluoride removal from aqueous solutions. Appl Surf Sci 258:4228–4236
Nordstrom DK, Jenne EA (1977) Fluoride solubility in selected geothermal waters. Geochim Cosmochim Acta 41:175–188
Nordstrom DK, Ball JW, Donahoe RJ, Whittemore D (1989) Groundwater chemistry and water-rock interactions at Stripa. Geochim Cosmochim Acta 53:1727–1740
Padhi S, Muralidharan D (2012) Fluoride occurrence and mobilization in geo-environment of semi-arid Granite watershed in southern peninsular India. Environ Earth Sci 66:417–479
Pattnaik M, Mishra S (2002) Effect of fluoride in groundwater around Karlakot village of Nuapada district, Orissa: a geoenvironmental assessment. Soc Geosci Allied Technol Bull 2:32–35
Rahmani AN, Nouri J, Ghadiri SK, Mahvi AH, Zare MR (2010) Adsorption of fluoride from water by Al3+ and Fe3+ pretreated natural Iranian zeolites. Int J Environ Res 4:607–614
Rao NS, Subrahmanyam A, Rao GB (2013) Fluoride-bearing groundwater in Gummanampadu Sub-basin, Guntur district, Andhra Pradesh, India. Environ Earth Sci 70:575–586
Saxena VK, Ahmed S (2001) Dissolution of fluoride in groundwater: a water–rock interaction study. Environ Geol 40:1084–1087
Saxena VK, Ahmed S (2003) Inferring the chemical parameters for the dissolution of fluoride in groundwater. Environ Geol 43:731–736
Shyam R, Kalwannia GS (2012) Health risk assessment of fluoride with other parameters in groundwater of Sikar city (India). Environ Earth Sci 65:1275–1282
Sikdar PK, Adhikari SK, Bhattacharya BB (2007) Lineament density modelling to identify high potential aquifers in hard rock areas. J Geol Soc India 69:1118–1131
Simard RR, Lafrance P (1996) Fluoride sorption and desorption indices in Quebec soils. Commun Soil Sci Plant Anal 2:853–866
Srivastava SK, Tiwari SK (2006) Distribution of high fluoride in ground water of Orissa. Soc Geosci Allied Technol Bull 7:18–26
Susheela AK (2007) A Treatise on Fluorosis. Fluorosis Research and Rural Development Foundation, New Delhi, 133 p
UNICEF (1999) States of the art report on the extent of fluoride in drinking water and the resulting endemicity in India. Report by Fluorosis and Rural Development Foundation for UNICEF, New Delhi
Vikas C, Kushwaha R, Ahmad W, Prasannakumar V, Reghunath R (2013) Genesis and geochemistry of high fluoride bearing groundwater from a semi-arid terrain of NW India. Environ Earth Sci 68:289–305
WHO (1997) Guideline for drinking water quality health criteria and other supporting information, 2nd edn. World Health Organisation, Geneva
Acknowledgments
Mr. Prabir K. Naik of the Central Ground Water Board, Bhubaneshwar is thanked for help during field work in the Karlakot area. Grants under FIST and IRPHA programmes of the Department of Science and Technology, Govt. of India for establishing the ICP–AES and EPMA facilities, respectively, at IIT Bombay are gratefully acknowledged. Dr. Mugera Gitari is thanked for valuable suggestions for improving the quality of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Patel, S.C., Khalkho, R., Patel, S.K. et al. Fluoride contamination of groundwater in parts of eastern India and a preliminary experimental study of fluoride adsorption by natural haematite iron ore and synthetic magnetite. Environ Earth Sci 72, 2033–2049 (2014). https://doi.org/10.1007/s12665-014-3112-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12665-014-3112-1