Abstract
Elevated levels of arsenic in groundwater have caused serious health problems in many countries of the world. In the present study, the level of arsenic and associated physicochemical parameters of groundwater of Sahibganj district along the river Ganga in Jharkhand, India was assessed in three seasons. The groundwater was found to be Ca–HCO3 − type with low redox potential and high total dissolved solids load. Arsenic concentration was found to be highest in postmonsoon ranging between 1 and 133 µg L−1 compared to (2 to 98 µg L−1) monsoon and (7 to 115 µg L−1) premonsoon. Depth-wise heterogeneity suggested an incremental trend of arsenic with depth. Arsenic was found to be positively correlated to depth, PO4 3− and Fe suggesting both natural and anthropogenic role in release mechanism. Principal Component Analysis helped further to support this observation. Spatial distribution of As indicated discrete patches with high concentrations. Health risk analysis revealed high to very high carcinogenic and non-carcinogenic risk for adults and children in the communities dependent on groundwater of the study area. The present observation will help in defining strategies for managing the arsenic problems of Sahibganj in future.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Arsenic contamination of groundwater is an alarming problem worldwide. Globally As (arsenic)-contaminated water has caused serious chronic human diseases like dermatological problems, hyperpigmentation, keratosis, gangrene and cancer (Rahman et al. 2006). Natural calamity due to arsenic toxicity has prompted World Health organisation to set the guideline value for As as 10 µg L−1 for drinking water (WHO 2007), though a few countries have developed their own guideline values to meet compliance. With time, the concern for arsenic contamination of soil and ground water and its transfer to human and other forms of lives through agriculture and other means has gained much attention. Food represents a further potential exposure pathway to arsenic in instances where crops are irrigated with high arsenic groundwater, grown in contaminated fields or where food is cooked using arsenic-contaminated water (Halder et al. 2012; Chakraborty et al. 2014). However, the pathway of exposure may vary from region to region depending on human activities, geological composition of the aquifer and bioavailability of arsenic and presence of other heavy metals (Álvarez et al. 2003). In India, cases of arsenic toxicity due to drinking of arsenic-contaminated water were first reported from Chandigarh in early 1976. In 1984, a large number of arsenic-induced skin lesion cases were reported from Kolkata, West Bengal. Since then many studies reported that a considerable part of all the states along the Ganga plain (Uttar Pradesh, Bihar, Jharkhand, West Bengal), some parts of the Brahmaputra plain (Assam) and most parts of Bangladesh in the Padma-Meghna-Brahmaputra (PMB) plain are arsenic affected (Chakraborti et al. 2004). Regarding the source and mobilisation of arsenic in groundwater, a number of propositions are there. Though it is believed the natural release is a major phenomenon, which depends on natural process of geological formation, it has been found that rock water interaction, weathering and biological transformations play a vital role in arsenic release (Oinam et al. 2011). The mineralogy and reactivity of geological formation in aquifers along with anthropogenic inputs control the geochemical mobility of arsenic in groundwater environment (Bhattacharya et al. 2009). In India, arsenic in Gangetic plain is believed to be naturally originating from eroded Himalayan sediments, and entering into the solution following reductive release from solid phases under anaerobic conditions (Polizzotto et al. 2008). Many authors have reported arsenic-rich pyrite in the sediment samples of Ganges delta of West Bengal to be the causative factor for arsenic release (Chakraborti et al. 2001), while others have put forward that arsenic is mobilised either by slow reduction of iron oxyhydroxides or sorbed arsenate by detrital organic carbon (Nickson et al. 1998) or through sulphide oxidation reactions that is induced by the massive increase of irrigation pumping in dry-season (Chowdhury et al. 1999). Based on the data compilation from Bangladesh and West Bengal, DPHE (1999) reported that the average total As concentrations in fluvio deltaic sediments are 15.9 and 10.3 mg Kg−1 for onshore and offshore samples, respectively (Ravencroft et al. 2005).
Studies on the ground water quality in a major part of the middle Gangetic plains of India have indicated the presence of elevated concentrations of arsenic in dug wells (Nayak et al. 2008; Chauhan et al. 2009). Fifteen districts in Bihar exhibited high arsenic contamination on either side of the banks of river Ganga (Kinniburgh and Smedley 2001). In Jharkhand, only Sahibganj, situated on the western bank of river Ganges, facing Maldah district of West Bengal in the eastern bank has been reported to be arsenic affected (Nayak et al. 2008). First occurrence of arsenic contamination in Sahibganj was reported by Chakraborti et al. (2004). National Metallurgical laboratory with UNICEF has also reported about the presence of arsenic in Sahibganj in 2005 (Nickson et al. 2007). Deterioration of health condition in Jharkhand state of the Middle Gangetic Plain (Huq et al. 2001) and Central Ground Water Board (2008) of arsenic contamination in Sahibganj block of Sahibganj district also indicated significant arsenic contamination. Nayak et al. (2008) has reported 88 % out of 367 studied biological samples (nail, hair and urine) to contain arsenic above normal level in Sahibganj district in the gangetic plains with occurrences of arsenical skin lesion and neurological disorders due to arsenic intoxication. However, detailed study of arsenic contamination in groundwater with other associated hydrochemical parameters of the district has been very limited and sporadic till date to our knowledge. Hence, the current study was attempted with the following objectives: 1) determine the spatial (horizontal and vertical) and seasonal variation of total arsenic concentration in groundwater samples of three blocks of Sahibgunj along the river bank with the physical and chemical factors which can potentially influence the arsenic release into groundwater and 2) assess the potential health risk including non-carcinogenic and carcinogenic impacts due to the level of arsenic contamination in groundwater of the study area.
Materials and Methods
Sampling Site Description
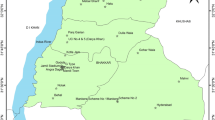
The present area, i.e. three blocks namely Sahibganj, Rajmahal and Udhwa of district situated in the western bank of river Ganga, is located in the north east of Jharkhand state in India (Fig. 1). It is bounded by Bhagalpur and Godda district in the west, Murshidabad and Maldah district of West Bengal in the east and river Ganges and Katihar district of Bihar in the north. It covers an area of approximately 1599 sq km (37.185 m above sea level) and extends between 87°25′ and 87°54′ East Longitude and 24°42′ to 25°21′ North Latitude. The terrains are dominantly undulating and mainly consist of hills, valley and undulating land. Geologically the area has basaltic trap, laterite and alluvium (Central Ground Water Board 2008). Alluvium occurs in the northern and eastern boundary of the district which is composed of sand and subordinate clay. Laterites are mainly of insitu origin and have been formed by sub-aerial erosion of underlying basalts under favourable climatic condition in which the massive basaltic component is hard and compact in nature with insignificant primary porosity and permeability (Central Ground Water Board 2008). The area is characteristic of semi-confined aquifers in joints and fractures of shear zones in 15- to 40-m depth and confined aquifers below 40 m depth due to the presence of highly impermeable basaltic beds (Central Ground Water Board 2008). Major drainage lines are Gumani and Udhawa nala. Important crops grown in flood plains are rice, wheat, maize and jute. During recent year, people have started growing vegetable and seasonal fruits.
Collection and Preservation of Samples
Groundwater samples were collected from the tubewells of three blocks of Sahibganj district during three seasons (premonsoon, monsoon and postmonsoon) from December 2013 to September 2014. Sampling locations were recorded using Garmin etrex30 global positioning system (GPS), and the depth of the tube wells was noted from the consultation with the local villagers and Ground Water Board, Jharkhand. Groundwater was collected after pumping out the water 40–80 times depending on tubewell depth to remove the partially oxidised water out of the tubewell ascent pipe, and immediately, onsite measurement of pH, Temperature, electrical conductivity (EC), total dissolved solids (TDS), dissolved oxygen (DO), oxidation reduction potential (ORP), ammonium, nitrate and chloride was done using Flow cell and portable instrument (YSI multi-parameter analyser 6,050,000) at 20 sampling locations, in two sets. One set of water samples was filtered using Whatman filter paper (0.45 µ), collected in polypropylene bottles, acidified with HNO3 to pH 2 and brought to the laboratory for elemental analysis (Loring and Rantala 1992), and another unacidified set was utilised for bicarbonate, phosphate and sulphate analysis by standard APHA, AWWA protocols. All the groundwater samples were stored at 4 °C prior to analysis.
Elemental Analysis of the Water Samples
Acidified filtered samples were used directly for arsenic, Fe and Mn analysis by using ICP-OES (Inductively coupled plasma–optical emission spectrometry), the technique by which elements (water soluble) are analysed in its elemental forms using spectrophotometer and plasma. In our study, we have used Perkin Elmer Optima 2100 DV ICP-OES Spectrophotometer for analysis of the sample using argon gas and plasma zone temperature of 8000–10,000 °C. The standard curve (multi-element) is prepared using Merck supra pure and sample acidified with 5 % HNO3.
Contour Mapping for Spatial Distribution of Arsenic in the Study Area
The coordinate values of the sample locations were taken using GPS. The coordinates were then placemarked on Google Earth and a polygon was digitised on Google earth surrounding the sample location to be identified as study area. Both the KML files were then exported to SHP format so as to be processed in ARC GIS 10. Interpolation of data obtained for Arsenic, Phosphate and iron was created using IDW method in ARC GIS 10. Study area was used as a polyline for limiting the boundaries of interpolation.
Health Risk Assessment
The degree of health risk of metal or any metalloid is usually expressed as carcinogenic or non-carcinogenic health risk (USEPA 2009). Bortey-Sam et al. (2015) stated that hazard and exposure combines together to cause a risk and according to WHO definition, a risk factor is any attribute, characteristic or exposure of an individual that increases the likelihood of developing a disease or injury. The two principal toxicity risk factors are the slope factor (SF) for carcinogen risk characterisation and the reference dose (RfD) for noncarcinogen risk characterisation (USEPA 1997, 1999).
Considering oral RfD (mg Kg−1 day−1) for arsenic 3.00E-04 and oral SF (mg Kg−1 day−1)−1 1.50 as per toxicity index (USEPA IRIS http://www.epa.gov/iris/), the HQ (Hazard quotient) is calculated taking into consideration the average concentration of arsenic in three seasons. If the value of HQ exceeds 1, it indicates potential non-carcinogenic effects on health and if it is less than 1, the exposed population is supposed to be free from any health risk as a result of exposure (USEPA 2001; ECETOC 2001).
The formula for calculating HQ is as follows:
where ADD (Average Daily Dose) (in mg Kg−1 day−1) is the estimation of magnitude, frequency and duration of human exposure to a heavy metal or metalloid in the environment (USEPA 1992).
where C is the geometric mean concentration (mg L−1) of heavy metal or metalloid, IR is the water intake rate (3.49 and 2.14 L day−1 for adults and children respectively in India) (Hossain et al. 2013), EF is the exposure frequency (365 days year−1), ED is the exposure duration (70 years for adults and 10 years for children respectively), BW is the average body weight (48.56 kg for adults and 31 kg for children, respectively, in Jharkhand) (Shome et al. 2014) and AT is the average time (25,550 days, i.e. EF × ED for adults and 3650 days for children) (Bortey-Sam et al. 2015.
The carcinogenic probability was calculated as the product of ADD (mg/Kg/day) and SF (mg/Kg/day/)−1. The scale of characterisation was done according to Table 1 (USEPA 1999).
Statistical Analysis
Statistical analysis, including Pearson’s correlation, Single factor ANOVA and regression, was performed using microsoft excel. PCA (Principal component analysis) was performed in SPSS 17 software. Piper diagram or Trilinear diagram was constructed by Groundwater chart software of USGS.
Results and Discussion
Physicochemical Characteristics of Groundwater
The physicochemical characteristics of the groundwater of Sahibganj district situated in the river basin of Ganges for premonsoon, monsoon and postmonsoon seasons are presented in Table 2(a–c). Physical and chemical parameters which generally help in understanding the rock water interaction, anthropogenic influences and mechanism of arsenic release in groundwater (Kumar et al. 2010) showed significant variation in the present study. pH was found to be ranging between 6.2 and 7.7 in premonsoon, 6.9 to 8.2 in monsoon and 7.6 to 8.6 in postmonsoon season. The observation indicated slightly acidic to mildly alkaline nature of groundwater in premonsoon season, whereas nearly all the samples in monsoon and postmonsoon season were found to be slightly alkaline. Low pH is generally due to application of acid producing synthetic fertilisers like (NH4)2SO4 and Ca(H2PO4)2 (Laluraj and Gopinath 2006), which might be possible here, since these three blocks are situated along the bank of river Ganges are predominantly agricultural lands. Only Udhwa village showed pH values of 8.6 in postmonsoon, higher than WHO permissible limit of 8.5 (WHO 2004).
Oxidation reduction potential, an approximate indication of the redox potential of any solution and a major modifier of water chemistry including speciation of arsenic (APHA 1998) in the present study, was found to be considerably low in all the three seasons in all the sampling locations of Sahibganj, ranging between −20 and −197 mV in premonsoon, −1 to −193 mV in monsoon and −38 to −199 mV in postmonsoon. Dehari, which exhibited the highest arsenic concentration among the villages, showed the most negative ORP values in premonsoon and monsoon season and significantly low value in postmonsoon season. Similar observation of prevalence of arsenic in strong or moderate reducing aquifers has been found in previous reports of Gangetic basin (McArthur et al. 2004; Nath et al. 2008). However, seasonal variation of ORP was not prominent.
Total dissolved solids and EC generally represent the total ionic species of water. In the present study area, EC varied between 539 and 2388 µS cm−1 with an average value of 1162 µS cm−1 in premonsoon, 406 to 2220 µS cm−1 and mean 1011 µS cm−1 in monsoon and 372 to 1313 µS cm−1 and 924 µS cm−1 in postmonsoon season. TDS ranged within 338 and 1529 mg L−1in premonsoon, 165 to 1106 mg L−1 in monsoon and 189 to 818 mg L−1 in postmonsoon. Higher EC values in monsoon might be attributed to enhanced chemical weathering and lengthier residence time of groundwater in the aquifers (Oinam et al. 2011). Ravindra and Garg’s (2007) theory of ion exchange and solubilisation in the aquifer with agricultural runoff to be responsible for higher EC could be applicable here, since the study area is composed of older alluvium.
The concentration of major anions in the present study area was found in the order of HCO3 − > Cl− > SO4 2− > NO3− > PO4 3− in majority of the samples. Bicarbonate (HCO3 −) was the main anionic constituent of the groundwater samples ranging from 285 to 740 mg L−1 in premonsoon, 246 to 565 mg L−1 in monsoon and 124–520 mg L−1 in postmonsoon season. HCO3 − representing the major source of alkalinity generally prevails due to the dissolution of CO2 and carbonates, reaction of silicates with carbonic acid (Ranjan et al. 2013) and oxidation of organic matter (Jeong 2001). With regard to arsenic release mechanism, it normally causes the leaching of arsenic into groundwater by carbonation of arsenic sulphide minerals (Hossain et al. 2004).
Cl− which generally arises from precipitation (Ranjan et al. 2013) was found to be highly variable ranging between 8 and 415 mg L−1, 8 and 283 mg L−1 and 5 and 370 mg L−1 in premonsoon, monsoon and postmonsoon, respectively. All values were below the permissible limit of 250 mg L−1 except Kathalbari (Premonsoon and postmonsoon) and Begumganj (in monsoon). Sulphate (SO4 2−) which originates from oxidation of sulphite (Ranjan et al. 2013) ranged between 3 and 129 mg L−1 in premonsoon, 3 and 254 mg L−1 in monsoon and 4 and 105 mg L−1 in postmonsoon season, showing higher concentration in monsoon season, which might be due to increased agricultural runoff containing elevated concentration of SO4 2− (Kumar 2008). Otherwise, sulphate concentration was lower in other seasons and this corroborates with lower ORP environment in the groundwater inhibiting sulphide oxidation. NO3−, arising from biological oxidation of nitrogenous substances produced from wastes (Ranjan et al. 2013), varies from 1.6 to 21.5 mg L−1 in premonsoon, 0.8 to 26.9 mg L−1 in monsoon and 2.4 to 20.3 mg L−1 in postmonsoon season.
PO4 3− varied between 0.04 and 0.19 mg L−1 in premonsoon, 0.009 to 0.20 mg L−1 in monsoon and 0.01 to 0.24 mg L−1 in postmonsoon season.
Major cations here followed the order of Ca > Na > Mg > K. The groundwater seemed to be dominated with Ca ions followed by Na, Mg and K being the lowest abundant. The mean concentrations of the major cations calculated from the data of three seasons (mentioned in Table 2a–c.) were Ca2+ (44 to 196 mg L−1), Mg2+ (16 to 144 mg L−1), Na+ (8.7 to 67.8 mg L−1) and K+ (2.16 to 38.9 mg L−1). The trilinear Piper diagram constructed by summarising the concentration of the cations and anions of the present study area of three seasons suggested the groundwater to be Ca–HCO3 type (Fig. 2). Piper diagrams generally give an idea about the hydrogeochemical composition of various groundwater sources (Kar et al. 2010). Bicarbonate was reported to be the most dominant anion in the Ganges delta plain with comparatively higher concenytrations in deep wells in earlier studies (Kar et al. 2010).
Elemental Composition of groundwater
Fe concentrations of the groundwater samples were below WHO permissible limit (1000 mg L−1 WHO 2004). Mean concentration of Fe ranged from 54 to 344 µg L−1 in premonsoon, 55 to 154 µg L−1 in monsoon and 55 to 280 µg L−1 in postmonsoon season. ANOVA suggested significant seasonal variation for Fe (F = 5.609, F crit = 3.158) (Table 3). Generally reduction of arsenopyrites by oxygen deficient groundwater or percolation of precipitation leads to higher concentration of Fe in groundwater (Oinam et al. 2011). The mean manganese concentration in all three seasons is higher than the acceptable limit of WHO (0.05 mg L−1) (WHO 2004) and BIS (0.1 mg L−1) (BIS 2009). It ranges from 0.13 to 0.8 mg L−1 in premonsoon, 0.05 to 1.17 mg L−1 in monsoon and 0.02 to 0.99 in postmonsoon season. The high concentration of manganese is due to the ubiquity of manganese in the soil and rocks.
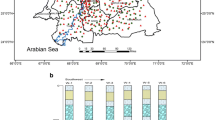
Mean arsenic concentration of groundwater was found to be the highest in the postmonsoon season and similar observation in postmonsoon was reported by Oinam et al. (2011) in Bishnupur district, Manipur. As concentrations ranged from 7 to 115 µg L−1 in premonsoon with all the sampling locations except English village exhibiting concentrations above WHO standard (10 µg L−1) and eight out of twenty sampling points above BIS permissible limit (50 µg L−1). It was as high as 115 µg L−1 in Dehari followed by Badi Koderjanna and Ghat Jamni (97 and 96 µg L−1, respectively) in premonsoon season. However, arsenic contamination in monsoon was found to be slightly lower though statistically not significant (Table 3) than the other two seasons ranging between 2 and 98 µg L−1 with the highest concentration being 98 µg L−1 in Dehari. Lowering of arsenic concentration in monsoon might be due to dilution of groundwater by more infiltration of precipitation. 17 out of 20 sampling points showed arsenic concentration above 10 µg L−1 and 7 locations above 50 µg L−1. In postmonsoon season, again Badi Koderjanna exhibited the maximum concentration of arsenic (133 µg L−1) followed by Dehari (117 µg L−1), Ghat Jamni (94 µg L−1) and Keswa (88 µg L−1). Range of arsenic concentration varied between 1 and 133 L−1. 9 sampling points showed arsenic above BIS limit of 50 µg L−1. Spatial distribution of arsenic in the study area suggested elevated concentrations along the bank of river Ganges.
Depth-Wise Heterogeneity of Arsenic
Arsenic concentration suggested a positive increment with depth of the tubewells (Fig. 3), though in the present study depth of the tube wells were limited to 40 m, which according to previous studies in West Bengal and Bangladesh has been known to be formed of grey sediments of the Holocene age up to depth of 100 m (Bhattacharya et al. 1997; DPHE 1999; van Geen et al. 2003). The higher concentrations of arsenic in the sampling locations like Dehari (mean = 110 µg L−1, depth 36 m), Badi Koderjanna (mean = 97 µg L−1, depth 31 m), Ghat Jamni (mean = 84.33 µg L−1, depth 29 m) and Keswa (mean = 73.67 µg L−1, depth 20 m) were all found at greater depths. Up to 15 m depth, the average concentration of As was 27.33 µg L−1; however, As increased steadily with depth after that and reached an average of 110 µg L−1 at 36-m depth. Pearson correlation coefficient between arsenic and depth was 0.82 (p ≤ 0.05) in premonsoon, 0.69 (p ≤ 0.05) in monsoon and 0.80 (p ≤ 0.05) in postmonsoon showing positive significant correlation (Table 4). Regression curves also indicated strong dependence of arsenic on depth (R 2 = 0.64, p < 0.05) (Fig. 4). Similar trend has been observed in West Bengal Bangladesh, up to 30–40 m of depth (Kar et al. 2010). The presence of arsenic up to 40-m depth indicated probable source in the aquifer, which is released due to dissolution (Kar et al. 2010).
Probable Release Mechanism
Pearson correlation analysis of the three seasons, premonsoon, monsoon and postmonsoon was performed to analyse the relationship between pairs of hydrochemical parameters of the study area. Correlation co-efficients were considered to be showing strong positive or negative correlation when the values were near 1 (+ or –) at a significance level of p ≤ 0.05. During all the three seasons, positive correlation was observed between arsenic—depth (0.822 in premonsoon, 0.695 in monsoon and 0.804 in postmonsoon) (p < 0.05) and depth—phosphate (0.721 in premonsoon, 0.774 in monsoon and 0.655 in postmonsoon) (p < 0.05) and depth—Fe (0.44 in premonsoon, 0.465 in monsoon and 0.784 in postmonsoon) (p < 0.05). Arsenic showed strong positive correlation with phosphate (0.717 in premonsoon, 0.657 in monsoon and 0.809 in postmonsoon) (p < 0.05) (Table 3). Phosphate, which generally leach into the groundwater from agricultural runoff containing fertilisers, helps in release of arsenic in groundwater (Oinam et al. 2011). In the present deposited alluvium tracts, there are several agricultural fields, so phosphate contamination can be easily happening here due to percolation of runoff, which ultimately goes and competes with arsenic for the adsorption sites in Fe and Mn oxides and thus mobilise the arsenic into groundwater (Katsoyiannis and Katsoyiannis 2006). Stollenwerk et al. (2007) has also proposed similar theory of reductive dissolution mechanism of Fe (hydr)oxides to be responsible for arsenic release in groundwater. As was also found to be moderately positively correlated to Fe in premonsoon (0.534) (p < 0.05), and strongly positively correlated in postmonsoon (0.900) (p < 0.05). The relation between As and Fe can be attributed to the fact that Fe(III) oxides are the most likely electron acceptors for As(III) in reducing environment according to Stollenwerk et al. (2007). However, statistical analysis did not exhibit significant correlation between arsenic and HCO3−, suggesting no probable role of HCO3− in the release of arsenic in the present study area. Strong positive correlation between EC and TDS was also observed in all the three seasons (0.972 in premonsoon, 0.937 in monsoon and 0.865 in postmonsoon). High values of EC indicate prevalence of inorganic ions like H+, Na+, K+, Mg2+, Ca2+, Cl−, SO4 2− and HCO3 −, etc., that have major influence on the conductivity of water.
In monsoon season in addition to the above-mentioned parameters, SO4 2− was found to be strongly positively correlated to Cl−, which might be due to higher rate of leaching of ions due to precipitation and runoff.
Positive correlation was not found between As–SO4 2− and As–Cl−, hence suggesting no probable role in arsenic release mechanism in the present study area.
Principal Component Analysis
In premonsoon season, predominant components or factors were 1 and 2. Factor 1 and 2 explained 26.77 and 20.9 % of the variability in data, respectively (Fig. 5). Strong loadings were found for EC (0.78) and TDS (0.75) in the factor 1, and medium loadings were shown by depth (0.46), As (0.72), HCO3 (0.65), PO4 3− (0.56), Mg (0.68), Ca (0.56), Fe (0.64) and K (0.67). This indicates that the sources can be geological and anthropogenic (Sikdar and Chakraborty 2008). Geological sources can be weathering of soil and rock minerals, whereas anthropogenic source can be pesticides or fertilisers. Depending on the factor loadings, Factor 2 can be termed as ‘solid factor’ as medium loadings were shown by ORP, TDS, SO42−, NO3−, Cl−, Mg and Ca (Mukherjee et al. 2011; Jiang et al. 2015). These anions and cations can be present in the water in dissolved forms. In monsoon season, however, factor 1, 2 and 3 explained 18.75, 14.7 and 9.16 % of the variability of the total data, respectively. None of the parameters showed significant loading in any of the factors (Fig. 5). This signifies that in this season, sources may vary widely. This is may be due to the fact that in this season, water from various sources percolates and influence the groundwater quality. In postmonsoon season, factor 1 explains 32.21 % and factor 2 explains 26.1 % of the data structure. Factor 1 is showing strong loadings of depth (0.853), arsenic (0.961), PO −34 (0.827), NO3 − (0.816) and Fe (0.898). Medium loadings are shown by Mn, K, HCO3 and ORP. It seems that major ions are associated with hydrochemical variables from the mineral weathering and water–rock interactions in the aquifer. Strong loadings of phosphate, iron and arsenic also suggest the possible replacement mechanism of arsenate from the iron oxide bearing minerals by phosphate (Sikdar and Chakraborty 2008; Sappa et al. 2014). Factor 2 is showing strong loadings of EC (0.878), TDS (0.943), Ca (0.895) and Mg (0.905). This again signifies the dissolution of minerals.
Contour Map of Spatial Distribution of Arsenic in the Study Area
The spatial analysis of As, Fe and PO43− showed discrete patches of high concentration of arsenic and phosphate (Fig. 6a–c). High arsenic concentrations were found in villages of Dehari, Badi Koderjanna, Ghat jamni, Keswa and Hazipur diara in all the three seasons. Majority of the areas showed concentration above WHO and BIS permissible limit. Fe distribution, however, showed a more uniform pattern in the study area with high concentrations at Dehari, BadiKoderjanna, Kabutorkhopi, Gadai, GhatJamni, keswa and Kathalbari.
Health Risk Assessment
Health risk assessment calculation showed very high arsenic-induced non-carcinogenic detrimental health risk for both adults and children (Table 5) in all the twenty sampling locations of the three blocks of Sahibganj district. HQ values ranged from 1.284 (adults) and 1.230 (children) at English to as high as 26.270 (adults) and 25.200 (children) at Dehari village, which contained the highest geometric mean value of As concentration (0.110 mg L−1). Children are yet more vulnerable to non-carcinogenic arsenic toxicity, since they consume more per unit of body weight than adults (ENHIS 2007).
The carcinogenic risk for drinking arsenic laden water for both adults and children was found to be equally very high in the study area. Risk level in adults ranged from 5.78E-04 (high risk) in English village to 1.18E−02 (very high risk) (Tables 1, 5) in Dehari. In fact cancer risk was found to be very high in all the village communities except English and Udhwa. Similar observation was observed for children also. Cancer risk in case of children ranged from 5.60E-04 (high risk) in English to 1.14E-02 (very high risk) in Dehari (Tables 1, 5) (USEPA 1999). Mean Cancer risk for the exposed communities suggested very high risk of 4.63E-03 in adults and 4.45E-03 in children. Carcinogenic risk of arsenic-contaminated groundwater in India has been extensively reviewed by Mazumdar (2008), where it was reported that occurrences of skin cancer and cancer of lung and urinary bladder are quite common due to long-term exposure to arsenic. Moreover, the present study area is located in tropical region, where the local communities are exposed to relatively high temperature, humidity and sunrays. As a result, their water intake is high (average 3.49 litres for adults and 2.14 litres for children) (Hossain et al. 2013) leading to higher ADD and consequently higher carcinogenic and non-carcinogenic risk (Bortey-Sam et al. 2015).
Conclusion
The physicochemical characteristics and total arsenic concentration of groundwater of three blocks of Sahibganj situated along the river bank of Ganga in north east of Jharkhand in India have been evaluated. Overall observation suggests that the groundwater is slightly alkaline in nature with low ORP, high electrical conductivity and TDS with spatial as well as seasonal variation. The groundwater is Calcium bicarbonate type with dominance of bicarbonate. Phosphate concentration was high and showed a significant positive correlation with arsenic. Arsenic concentration was found to be much higher than the WHO or BIS permissible limit in majority of the villages in the study area. Arsenic was also seen to be positively correlated with iron and phosphate. PCA results demonstrated the role of both geological condition and anthropogenic inputs to influence the water quality. These observations hinted at reductive dissolution of Fe oxyhydroxides due to high phosphate concentration from agricultural input to be the probable mechanism of arsenic release from the sediments along river Ganges. Arsenic was also found to increase with depth up to 100 m from the surface. Calculation of carcinogenic and non-carcinogenic effects of the arsenic concentration in the communities exposed to the groundwater for drinking and other purpose indicated high risk with an average of more than 1 in a 1000 population. The results of this study including the spatial, seasonal and depth heterogeneity of arsenic will help formulating strategies to avoid the highly arsenic polluted groundwater sources and exploit new sources as mitigation strategies to arsenic toxicity.
References
Álvarez E, Fernández Marcos ML, Vaamonde C, Fernández-Sanjurjo MJ (2003) Heavy metals in the dump of an abandoned mine in Galicia (NW Spain) and in the spontaneously occurring vegetation. Sci Total Environ 313:185–197. doi:10.1016/S0048-9697(03)00261-4
APHA (1998) Standard methods for the examination of water and waste water, vol 20. American Public Health Association, Washington, DC, pp 413–426
Bhattacharya P, Chatterjee D, Jacks G (1997) Occurrence of arsenic contaminated groundwater in alluvial aquifers from the delta plains, eastern India: option for safe drinking water supply. Int J Water Resour Dev 13:79–92
Bhattacharya P, Samal AC, Majumdar J, Santra SC (2009) Accumulation of arsenic and its distribution in rice plant (Oryza sativa L.) in GangeticWest Bengal, India. PaddyWater Environ. doi:10.1007/s10333-009-0180-z
Bortey-Sam N, Nakayama SMM, Ikenaka Y, Akoto O, Yohannes YB, Baidoo E, Mizukawa H, Ishizuka M (2015) Human health risks from metals and metalloid viaconsumption of food animals near gold mines in Tarkwa, Ghana: estimation of the daily intakes and target hazardquotients (THQs). Ecotoxicol Environ Saf 111:160–167
Bureau of Indian Standards (2009) Drinking water–specification (Second Revision of IS 10500)
Central Ground Water Board (2008) “Ground water information booklet” Sahebganj district, Jharkhand state. Ministry of Water Resources (Govt. of India). Mid–Eastern Region, Patna
Chakraborti D, Basu GK, Biswas BK, Chowdhury UK, Rahman MM, Chowdhury TR, Chanda CR, lodh D, Ray SL (2001) Characterisation of arsenicbearing sediments in Gangetic Delta of West Bengal, India. In: Chappell WR, Abernathy CO, Calderon RL (eds) Arsenic exposure and health effects. Elsevier, New York, pp 27–52
Chakraborti D, Sengupta MK, Rahman MM, Ahamed S, Chowdhury UK, Hossain A, Mukherjee SC, Pati S, Saha KC, Dutta RN (2004) Groundwater arsenic contamination and its health effects in the Ganga–Meghna–Brahmaputra Plain. J Environ Monit 6(6):74N–83N
Chakraborty S, Alam MO, Bhattacharya T, Singh YN (2014) Arsenic accumulation in food crops: a potential threat in Bengal Delta Plain. Water Qual, Expos Health 6:233–246
Chauhan D, Nickson R, Iyengar L, Sankararamakrishnan N (2009) Groundwater geochemistry and mechanism of mobilization of arsenic into the groundwater of Ballia district, UP. India Chemos 75(1):83–89
Chowdhury TR, Basu GK, Mandal BK, Biswas BK, Samanta G (1999) Arsenic poisoning in the Ganges delta. Nature 401:545–546
DPHE (Department of Public Health Engineering) (1999) Groundwater studies for arsenic contamination in Bangladesh. Main report and volumes S1–S5, report on Phase I. Dhaka, Bangladesh
ECETOC (European Centre for Ecotoxicology of Chemicals) (2001) Aquatic toxicity of mixtures. Technical report, 80. Brussels
ENHIS (European Environment and Health Information System) (2007) Exposure of children to chemical hazards in food. Fact Sheet No. 4.4, CODE: RPG4_Food_EX1. World Health Organization
Halder D, Bhowmick S, Biswas A, Chatterjee D, Nriagu J, Guha Mazumder D et al (2012) Risk of arsenic exposure from drinking water and dietary components: implications for risk management in rural Bengal. Environ Sci Technol. doi:10.1021/es303522s
Hossain AM, Akai J, Sakugawa H (2004) Mobilization of arsenic from subsurface sediments by effect of bicarbonate ions in groundwater. Chemosphere 54(6):753–762
Hossain MA, Rahman MM, Murrill M, Das B, Roy B, Dey S, Maity D, Chakraborti D (2013) Water consumption patterns and factors contributing to water consumption in arsenic affected population of rural West Bengal, India. Sci Total Environ 463–464:1217–1224
Huq SMI, Ara QAJ, Islam K, Zaher A, Naidu R (2001) The possible contamination from arsenic through food chain. In: Groundwater Arsenic Contamination in the Bengal Delta Plain of Bangladesh. In: Proceedings of the KTH-Dhaka University Seminar, University of Dhaka, Bangladesh. Jacks G, Bhattacharya P, Khan A.A (Eds.). KTH Special Publication, TRITA-AMI Report 3084, ISSN 1400-1306, ISRN KTH/AMI/REPORT 3084-SE, ISBN: 91-7283-076-X, © 2001, KTH, pp 91–96
Jeong CH (2001) Effect of land use and urbanization on hydrochemistry and contamination of groundwater from Taejon area, Korea. J Hydrol 253:194–210
Jiang Y, Huaming G, Yongfeng J, Yongsheng C, Chao H (2015) Principal component analysis and hierarchical cluster analyses ofarsenic groundwater geochemistry in the Hetao basin, Inner Mongolia. Chemie der Erde, Geochem 75:197–205
Kar S, Maity JP, Jean J-S, Liu C-C, Nath B, Yang H-J, Bundshuh J (2010) Arsenic-enriched aquifers: occurrences and mobilization of arsenic in groundwater of Ganges Delta Plain, Barasat, West Bengal, India. Appl Geochem 25:1805–1814
Katsoyiannis IA, Katsoyiannis AA (2006) Arsenic and other metal contamination of groundwaters in the industrial area of Thessaloniki, Northern Greece. Environ Monit Assess 123:393–406
Kinniburgh DG, Smedley PL (eds) (2001) Arsenic contamination of groundwater in Bangladesh, vol 4. British Geological Survey, Keyworth
Kumar P (2008) Targeting safe aquifers for Drinking water with special reference to arsenic contamination in Bhagalpur (Bihar) and Ghazipur (Uttar Pradesh) India. M. Phil thesis, Jawaharlal Nehru University, New Delhi
Kumar M, Kumar P, Ramanathan AL, Bhattacharya P, Thunvik R, Singh UK, Tsujimura M, Sracek O (2010) Arsenic enrichment in groundwater in the middle Gangetic plain of Ghazipur district in Uttar Pradesh, India. J Geochem Explor 105:83–94
Laluraj CM, Gopinath G (2006) Assessment on seasonal variation of groundwater quality of phreatic aquifers–a river basin system. Environ Monit Assess 117:45–57
Loring DH, Rantala RTT (1992) Manual for the geochemical analyses of marine sediments and suspended particulate matter. Earth-Science Reviews, 32: 2350283, and 1995, Regional Seas, Reference methods for marine pollution studies no. 63, United Nations Environment Programme
Mazumder DNG (2008) Chronic arsenic toxicity and human health. Indian J Med Res 128(4):436–447
McArthur JM, Banerjee DM, Hudson-Edwards KA, Mishra R, PurohitR Ravenscroft P et al (2004) Natural organic matter in sedimentary basins and its relation to arsenic in anoxic groundwater: the example of West Bengal and its worldwide implications. Appl Geochem 19:1255–1293
Mukherjee Rana JI, Kumar Nirmal, Manishita Das, Kumar RN (2011) Assessment of hydrochemical characteristics of a tropical wetland system using principal component analysis: a case study. The IUP J Environ Sci 5(1):37–50
Nath B, Stüben D, Basu Mallik S, Chatterjee D, Charlet L (2008) Mobility of arsenic in West Bengal aquifers conducting low and high groundwater arsenic. Part I: comparative hydro chemical and hydro geological characteristics. Appl Geochem 23:977–995
Nayak B, Das B, Mukherjee SC, Pal A, Ahamed S, Hossain MA, Maity P, Dutta RN, Dutta S, Chakraborti D (2008) Groundwater arsenic contamination in Sahibgunj district of Jharkhand state, India in middle Ganga plain and adverse health effects. Toxicol Environ Chem 90(4):673–694
Nickson R, McArthur J, Burgess W, Ahmed KM, Ravenscroft P, Rahman M (1998) Arsenic poisoning of Bangladesh groundwater. Nature 395:338
Nickson R, Sengupta C, Mitra P, Dave SN, Banerjee AK, Bhattacharya A, Basu S, Kakoti N, Moorthy NS, Wasuja M, Kumar M, Mishra DS, Ghosh A, Vaish DP, Srivastava AK, Tripathi RM, Singh SN, Prasad R, Bhattacharya S, Deverill P (2007) Current knowledge on the distribution of arsenic in groundwater in five states of India. J Environ Sci Health A Toxic Hazard Subst Environ Eng 42:1707–1718
Oinam JD, Ramanathan AL, Linda A, Singh G (2011) A study of arsenic, iron and other dissolved ion variations in the groundwater of Bishnupur District, Manipur. India Environ Earth Sci 62:1183–1195
Polizzotto ML, Kocar BD, Benner SG, Sampson M, Fendorf S (2008) Near-surface wetland sediments as a source of arsenic release to ground water in Asia. Nature 454:505–508
Rahman M, Vahter M, Wahed MA, Sohel N, Yunus M, Streatfield PK et al (2006) Prevalence of arsenic exposure and skin lesions. A population based survey in Matlab, Bangladesh. J Epidemiol Community Health 60(3):242–248
Ranjan RK, Ramanathan AL, Parthasarthy P, Kumar A (2013) Hydrochemical characteristics of groundwater in the plains of Phalgu river in Gaya, Bihar, India. Arab J Geosci 6:3257–3267
Ravenscroft P, Burgess WG, Ahmed KM, Burren M, Perrin J (2005) Arsenic in groundwater of the Bengal Basin, Bangladesh: distribution, field relations, and hydrogeological setting. Hydrogeol J 13(5–6):727–751
Ravindra K, Garg VK (2007) Hydro-chemical survey of groundwater of Hisar City and assessment of defluoridation methods used in India. Environ Monit Assess 132:33–43
Sappa G, Ergul S, Ferranti F (2014) Geochemical modeling and multivariate statistical evaluation of trace elements in arsenic contaminated groundwater systems of Viterbo Area, (Central Italy). SpringerPlus 3:237
Shome S, Roy P, Pal M, Bhati M (2014) Variation of adult and weight in India: state and zone wise analysis. Human Biol Review 3(3):242–257
Sikdar PK, Chakraborty S (2008) Genesis of arsenic in groundwater of North Bengal Plain using PCA: a case study of English Bazar Block, Malda District, West Bengal. India Hydrol Process 22:1796–1809
Stollenwerk KG, Briet GN, Welch AH, Yount JC, Whitney JW, Foster AL, Uddin M, Nehal M, Ratan K, Ahmed N (2007) Arsenic attenuation by oxidized aquifer sediments in Bangladesh. Sci Total Environ 379(2–3):133–150
USEPA (US Environmental Protection Agency) (1992) Definitions and general principles for exposure assessment. Guidelines for exposure assessment. Office of Pesticide Programs, Washington, D.C.
USEPA (US Environmental Protection Agency) (1997) Exposure factors handbook (EPA/600/P-95/002Fa) (update to Exposure factors handbook (EPA/600/8–89/043). Environmental Protection Agency Region I, Washington, D.C.
USEPA (US Environmental Protection Agency) (1999) A risk assessment–multiway exposure spreadsheet calculation tool. United States Environmental Protection Agency, Washington, D.C.
USEPA (US Environmental Protection Agency) (2001) Baseline human health risk assessment, Vasquez Boulevard and I–70 superfund site, Denver CO. http://www.epa.gov/region8/superfund/sites/VB-170-Risk.pdf
USEPA (US Environmental Protection Agency) (2009) National primary/secondary and drinking water regulations. Washington, D.C
van Geen A, Ahmed KM, Seddique AA, Shamsudduha M (2003) Community wells to mitigate the current arsenic crisis in Bangladesh. Bull World Health Org 82:632–638
WHO (2004) Guideline for drinking-water quality, 3rd edn (Recommendations). World Health Organization, Geneva
WHO (2007) Joint FAO/WHO Expert Standards Program Codex Alimentation Commission, Geneva. http://www.who.int
Acknowledgments
The authors would like to acknowledge the Department of Science and Technology, New Delhi for funding the SERB Young Scientist project SR/FTP/ES-2/2013 entitled “Arsenic enrichment in agricultural soils with potential impacts on crops and food security of Sahibganj, Jharkhand, India” for undertaking this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Alam, M.O., Shaikh, W.A., Chakraborty, S. et al. Groundwater Arsenic Contamination and Potential Health Risk Assessment of Gangetic Plains of Jharkhand, India. Expo Health 8, 125–142 (2016). https://doi.org/10.1007/s12403-015-0188-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12403-015-0188-0