Abstract
The main aim of this study is to utilize municipal solid waste (MSW) and date molasses as a culture medium to reduce the production cost of enzymes. A novel cellulase-producing fungus, Trichoderma reesei Al-K1 149, was isolated from the date molasses. MSW and date molasses were processed and used as the substrate (1:1 ratio) in solid-state fermentation. The proximate composition of the substrate revealed that the MSW was enriched with cellulostic material and contributed about 33% of the available biomass. Elements such as Ca, K, S, P, Mg, Fe, Cu, and Na were found in the MSW. Fungal cellulase production was at its maximum after 96 h of incubation with the yields of β-glucanase (98 ± 3.9 U/gds), carboxymethyl cellulase (CMCase) (241 ± 12.8 U/gds), and filter paperase (FPase) (31.2 ± 3.1 U/gds). The combination of municipal solid waste and date molasses was found to be the best source of nitrogen and carbon for the biosynthesis of cellulase by T. reesei Al-K1 149. The optimal temperature and moisture content of the medium for cellulase production by T. reesei Al-K1 149 were 40 °C and 60%, respectively. The optimal pH and inoculum were 6.0 and 8% (v/w), respectively. The optimized culture condition was used to produce cellulases in a laboratory-scale tray reactor, and enzyme production was enhanced twofold compared to the unoptimized medium. The cellulolytic ability was tested in biomass saccharification with various plant materials (palm sawdust, palm leaves, palm fruit waste, and filter paper) and saccharified plant materials effectively. These findings revealed that the enzymes secreted by strain Al-K1 149 may have significant value for the industrial saccharification process.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Municipal solid waste (MSW) bio-prospecting is one of the important subjects in environmental protection [1]. In England, more than 23 million tons of kitchen waste was generated in 2009–2010, which amounts to over 1000 Kg of kitchen waste per house [2]. The present methods for MSW management are landfill, incineration, anaerobic digestion, and composting. In recent years, the conversion of MSW into biogas by anaerobic conversion has attracted much more attention as a successful method to minimize environmental pollution and generate more renewable fuel. Interestingly, some MSW contain about 50% lignocellulosic material and anaerobic fermentation may not be effective in this process [3]. One of the important alternatives to methods is to hydrolyze the available lignocellulosic material into simple sugar. Then bioconversion of simple sugars into ethanol is a possible alternate of waste management. A ton of MSW generates about 150 L of ethanol after the fermentation of lignocellulosic from the waste [3]. Production of ethanol from MSW biomass is one of the viable technologies for renewable energy and the major constraints in cellulase production are low yield and the cost of cellulases [4]. In recent years, there has been an increasing interest in using various thermophilic fungi and bacteria for the production of cellulases, saccharification processes and fermentation of various lignocellulostic materials due to their broad substrate range and high operating temperature [5, 6]. Generally, the complete hydrolysis of cellulose substrate can be achieved by a combination of glucosidase, endoglucanases and exoglucanases [6]. Hence, it is desirable that the selected organism synthesize these types of enzymes. The Kingdom of Saudi Arabia shared more than 4 million tons of date’s production and the contribution was about 72% of the Global share [7]. In KSA, palm trees cover about 162,000 hectares with about 23 million palm trees [8]. Other than fruits, leaves, trunks can be effectively used as raw materials for industrial processes. Date palm, Phoenix dactylifera is rich in lignin (25.82%), hemicelluloses (29.13%) and cellulose (45.3%) [9]. The lignocellulosic materials are processed through chemical, physical and mechanical pre-treatments for saccharification by microbial cellulases [10].
Solid-state fermentation (SSF) is defined as the transformation of biomass in the near absence of free water or in the absence of water [6]. Recently, SSF has received more attention to explore the production of value-added products such as, biological lead molecules, biological control substances, enzymes and biopolymers [11]. Wilson [12] and Srivastava et al. [13] reported the optimization of hemicellulases, cellulase production and economic biofuel production. Both SSF and submerged fermentation have been widely used in the production of cellulases [14]. SSF required minimum equipment support was easy to operate, and was useful to handle large numbers of solid wastes, such as lignocellulose materials for enzyme production. Hence, SSF has been widely applied in the fermentation of food processing wastes and agricultural residues. The substrates, such as, oil palm biomass, sugar cane bagasse and wheat straw have been evaluated for cellulolytic enzyme production using Trichoderma reesei and A. niger. The fungus, T. reesei reportedly utilized water hyacinth, sugar cane bagasse, oil palm empty fruit branches, and rice bran substrates for cellulase production [15,16,17]. In a study, wheat straw was used as the substrate for cellulase production by A. niger [18]. Substrates such as, coconut coir pith, banana fruit stalk, rice husk, corn cob residue, rice straw, cow dung and banana peel have been utilized for cellulase production [14, 19, 20]. The Kingdom of Saudi Arabia is the second-largest producer of palm dates in the world. The annual production of dates was approximately 1.07 million tonnes [21]. In order to store the produced dates, date producers opted to store them as molasses. Date syrup or date molasses has a shelf life of about 24 months if stored under prescribed environmental conditions. After 24 months, it starts to crystallise and becomes inedible. It is rich in reducing sugars. In date molasses, fructose and glucose are the two major components [22]. The amount of these sugars varied based on the type of tree. For instance, glucose and fructose were determined to be the major sugar components and contributed 51.80% and 47.50%, respectively. The other components, such as fructose, sucrose, and galacturonic acid, were found in trace amounts [23]. Although date molasses and MSW contain large quantities of lignocellulosic material, there has been very limited work on the mixture of date molasses and MSW to produce cellulolytic enzyme using SSF. In this study, an attempt has been made to use the mixture of date molasses - MSW as the substrate for the production of cellulases.
Materials and Methods
Materials
The major chemicals, including, sodium hydroxide pellet, and glucose, were purchased from SigmaeAldrich, Co. USA. Carboxymethyl cellulose, acetic acid, and sodium acetate, methanol, and dichloromethane were purchased from Merck, Germany. The bacteriological culture medium was purchased from Himedia, India. The fungal DNA extraction kit (AllPrep DNA extraction kit) was purchased from Qiagen, Germany. Date molasses was purchased from the local market and municipal solid waste was collected in Chennai, India. Acetic acid, sodium acetate, citric acid, trisodium citrate, succinic acid, sodium succinate, disodium hydrogen phosphate, sodium dihydrogen phosphate, tris base, and hydrochloric acid were Analytical grade and these were used for the preparation of buffers between pH ranges 4.0 and 8.0.
Date Molasses and Municipal Solid Waste
Date molasses and MSW were used as substrates for cellulase production. The selection of these substrates was based on nutrient contents and availability. The MSW was autoclaved and an organic rich fraction was obtained after 30 min of incubation. Date molasses was directly used without filtration and MSW was sieved individually using a standard sieve (2 mm diameter). The sieved substrate was dried at 70 °C for 12 h. Date molasses and MSW were mixed at a 1:1 ratio and used as the substrate until otherwise stated.
Determination of Components MSW and Date Molasses
Hemicellulose and cellulose were tested using acid hydrolysis based on the methods suggested by the National Renewable Energy Laboratory (NREL/TP-500-42618). About 100 mg of sample was mixed with 1 mL of sulphuric acid (12 M) and incubated for 1 h at 37 °C. Then 10 mL of double distilled water was added, and the sample was incubated in a water bath for 2 h [24]. Finally, the hydrolyzed sugars were tested using a High Performance Liquid Chromatography (Shimadzu, Japan). (1100 series) equipped with a refractive index detector (RID) (Agilent, CA). A Shodex sugar SP0810 column was used for analysis. HPLC grade water was sterilized using 0.2 μm filter and degassed using a sonicator (Analab Scientific Instruments Private Limited, India) for 20 min. About 10 µL of sample was injected, and the flow rate was set to 0.5 mL/min. The lignin content of the sample was tested by the acetyl bromide method, as suggested by Sluiter et al. [24]. The absorbance of the sample was tested at 280 nm, and lignin was used as the standard [25]. The nitrogen content of the sample was tested using a nitrogen analyzer (G6 Leonardo, Bruker, Germany) [26]. The total lipid content of the sample was tested by the method of Folch with little modification [27]. MSW was extracted with methanol and dichloromethane at 1:2 (v/v) ratios and incubated for 3 h at 30 ± 2 °C. The trace element of MSW was tested after the extraction of the MSW sample with 20 mL of concentrated nitric acid. The available trace element was determined as described by Hokura et al. [28]. Proximate analysis of date molasses was carried out. The components, such as tannin, pectin, sugar, ash and protein content were analyzed from date molasses [29].
Pre-Treatment of Substrate by Alkali and Acid
About 1 kg of substrate was mixed with 2% sodium hydroxide (100 mL) or 1% sulphuric acid (100 mL) and kept in an autoclave for 15 min, 30 min, respectively. After 30 min, samples were neutralized using 1 M H2SO4 or 1 M NaOH. The solid fraction of the sample was retained, rinsed twice by adding double distilled water and centrifuged. Finally, the sample was dried at ambient temperature.
Isolation and Characterization of Fungus for Cellulolytic Enzyme Production
A total of 34 fungi were isolated from date molasses and cellulase screening was carried out. Date molasses was serially diluted and plated on potato dextrose agar plates. Then it was incubated at 28 ºC for five days. The isolated colonies were selected and further grown on potato dextrose agar. Based on productivity, one strain (Al-K1 149) was selected for characterization. A cellulase producing potent fungal strain was purified as described earlier [30]. 18 S rDNA was amplified and sequenced using a forward and a reverse primer [31]. The amplified PCR amplicon was purified and sequenced using Applied Biosystems (USA). The 18 S rDNA genes were sequenced at SciGenom Labs, Cochin, India.
Inoculum
Ten grams of substrate were transferred into 90 mL of double distilled water in a 250 mL Erlenmeyer flask. The medium was sterilized for 20 min at 121℃ and incubated at 28 ± 2°C. In this culture medium, a plug (about 1 × 1 cm2) of 8-day old mycelia was gently inoculated and incubated in a shaker incubator at 175 rpm for three days.
Production of Cellulases in SSF
Solid state fermentation was performed in a 250-mL Erlenmeyer containing 5.0 g of substrate. The dry substrate was moistened with sodium phosphate buffer (pH 6.5, 0.1 M) and the moisture content of the substrate was maintained at a ratio of 1:1.5. The culture medium was mixed, autoclaved at 121 °C for 30 min and inoculated with inoculum. Erlenmeyer flasks were incubated for 96 h under static culture conditions. After 96 h, cellulases were extracted by adding 50 mL of double distilled water and shaking the fermented medium on an orbital shaker for 30 min. The contents were filtered using a metallic sieve and further centrifuged (10,000 g, 4 °C). The clear supernatant was used as the crude enzyme [32].
Enzyme Assays
The crude enzyme was subjected to carboxymethyl cellulase (CMCase), β-glucosidase and filter paper activity (FPase) assay. A unit of b-glucosidase, FPase and CMCase was defined as being equivalent to the enzyme that liberates 1 µmole of glucose from salicin, Whatman filter paper, and carboxymethyl cellulose, respectively, in 0.05 M acetate buffer (pH 4.0) under standard assay conditions using dinitrosalicylic acid reagent method [33]. Enzyme activities were expressed as U/g dry substrate (U/gds) tested in the Erlenmeyer flask [34].
Optimization of Physical Factors for Cellulases Production in SSF
The mixture of MSW and date molasses is used as the substrate for enzyme production, unless otherwise stated. The time course of cellulase synthesis was evaluated by preparing various sets of Erlenmeyer flasks (250 – mL) containing 5 g of substrate adjusted to 70% moisture content. The fermentation experiment was performed as described previously [35]. The Erlenmeyer flask was incubated for five days, and the production profile of cellulases was monitored every 24 h. The selected substrate (date molasses and MSW) is rich in carbon, nitrogen sources, and essential minerals. Hence, nutrient requirements were not considered for optimization studies, and only physical factors were optimized. The effect of different fermentation temperatures (25–50 °C), moisture content (40–80%), pH (4.0–8.0) and inoculum level (1–10%) on cellulase yield was analyzed. To adjust the pH, various buffers were used at a 0.1 M concentration (citrate buffer 4.0, acetate buffer 5.0, succinate buffer 6.0, phosphate buffer 7.0, and tris-buffer 8.0).
Fermentation of date molasses and MSW in a tray bioreactor
The optimized culture medium was selected, and the process parameters were validated using an open trays bioreactor. The open tray bioreactor consists of rectangular shaped stainless steel trays with a 4 cm depth and a 10 × 5 cm size. The tray reactor consists of three layers of trays, and the top of the tray was gently covered with a lid, and the bottom of the tray was arranged in such a way as to allow air inflow (Fig. 1). The solid medium was carefully mixed and transferred into a tray reactor. In all trays, solid substrate was filled, and the optimum level of inoculum was introduced. These stainless steel trays are autoclavable and compatible. After complete sterilization, 10% inoculums were introduced and incubated at 28 ± 2 ºC for four days. Enzyme was extracted as described previously, and CMCase, FPase and β-glucosidase activity were assayed.
Saccharification of Biomass
In this study, lignocellulosic materials such as palm sawdust, palm leaves, palm fruit waste and filter paper were used for saccharification process. The cellulolytic enzymes were obtained from lignocellulosic fermentation in tray bioreactor with T. reesei Al-K1 149. Commercial cellulase was used as the standard to validate the saccharification process. The saccharification process was performed at 45 °C at pH 5.0. The experiments were performed in a 250 mL Erlenmeyer flask containing various substrates (plam saw dust, palm leaves, palm fruit waste and filter paper), and 50 mL acetate buffer (pH 5.0, 50 mM). Then the Erlenmeyer flask was kept on a water bath for 5 min for constant heat transfer throughout the flask. To this end, cellulases obtained from the tray reactor were applied, and commercial enzymes were also added and validated. The saccharification process was continued for 24 h and the reducing sugar level was evaluated by the DNS method as suggested by Miller [33] using a UV-Visible spectrophotometer.
Results and Discussion
Composition of Substrates
In this study, the chemical composition of MSW was evaluated as suggested previously in materials and methods. In MSW, hydrocarbons contributed about 60%, showing that they were rich sources of carbon, which could be effectively applied as a novel medium for the production of biofuels. The co-fermentation of municipal sludge and date molasses was evaluated as a promising way to combine renewable energy production and waste management. The co-fermentation of food waste and municipal sludge [36], waste-activated sludge-distillation residue [37], green waste, and municipal solid waste [38] were used as culture media to improve productivity and yield of biomolecules. Kamyab et al. [39] used activated sludge and date syrup as inexpensive substrates for biohydrogen production. The stiff and loose properties of co-substrates led to improved fungal growth and product formation. The cellulose content of the MSW was about 33% which was similar to the results by Hussin et al. [40] and Barlaz et al. [41]. In some cases, the cellulose content in MSW was found to be high, where MSW obtained mainly from the milling, wood, and paper industries [42, 43]. In this study, the lignin content of the MSW sample was about 13% (Fig. 2), which was similar to the study of Hussin et al. [40]. The hemicellulose content of the MSW was determined to be 16%. However, in some studies, a low level of hemicellulose availability was reported. For example, Ham et al. [44], and Price et al. [45] reported low availability of hemicellulose in the MSW. Moreover, lignocellulose accounts for more than 30% of municipal solid waste [46]. Lipid and total protein content contributed about 9.1%, 6.1%, respectively. di Bitonto et al. [47] reported low lipid content (4–10%) in MSW and it was higher (13%) in other MSW [48]. The chemical composition of the compost varied based on the source and country of origin [14]. The lipid content of the MSW was extremely low compared to previous reports. Generally, MSW derived from hotels, households, vegetable markets, fruit markets and juice processing units has a lower lipid content [36]. The contribution from total protein content was about 6.5%. This result was in accordance with the observations made previously by Ponsá et al. [49] and Hartmann and Ahring [50]. In this study, the composition of the trace element was described in (Table 1).
The elements such as Ca, K, S, P, Mg, Fe, Cu, and Na were determined from the MSW. The sulphur content was 4217 ± 13.2 mg/kg and the determined Ca level was 13,005 ± 198 mg/kg in MSW. The toxic metals such as Al, Hg, Au, Cd, and Ag were determined at a very low level which was similar to that of the previous study [51]. The date molasses was composed of tannin, pectin, sugar, ash and protein. The pH of the date molasses ranged between 4.7 and 5.4. Pectin content was about 0.32%, and tannin content was 0.45%. Reducing sugar contributed 69%, and the share of ash content was approximately 1.9%. The chemical and physical properties of the date molasses were described in (Table 2).
Physico-Chemical Factors Affecting Cellulase Production
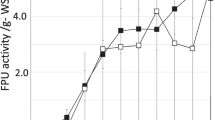
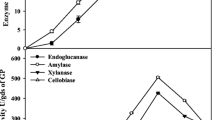
In the study, the influence of the fermentation period on cellulase production was optimized. In the case of fungi, the fermentation period is one of the important factors for the biosynthesis of enzymes. Cellulase production was at its maximum after 96 h of incubation with the yields of β-glucanase (98 ± 3.9 U/gds), CMCase (241 ± 12.8 U/gds) and FPase (31.2 ± 3.1 U/gds) as described in (Fig. 3). After four days of incubation, cellulase yield decreased gradually due to the drop in the medium pH and the release of proteolytic enzymes. In a study, Singhania et al. [15] also reported the maximum cellulase yield in T. reesei after 96 h incubation (102.65 U/gds). In another study, Trichoderma sp. was used for cellulase production on apple pomace as the substrate and showed a dramatic increase in the enzyme yield after 120 h of incubation (2.3 U/gds) [52]. The amount of cellulase production varied based on the substrate selected for fermentation. Campos et al. [53] used green coconut fibers for the production of carboxy methyl celluase (5.19 U/g) and filter paperase (1.19 U/g). Environmental factors affected enzyme production and the growth of microorganisms. In this study, enzyme production by T. reesei Al-K1 149 on biowaste was optimized with respect to moisture content, temperature, inoculum and pH of the substrate. In SSF, the optimum temperature range for enzyme production has been reported to be 25 to 30 °C [15]. Maximum yields of β-glucosidase (104 ± 3.2 U/gds), FPase (39.1 U/gds) and CMCase (264 U/gds) were found after 96 h at 40 °C, as described in (Fig. 4a). In A. niger YL128, the optimum temperature was 30 °C for cellulase production [54]. In a study, saw dust was used as the substrate for cellulase production by A. niger and obtained the maximum yield at 28 °C. In Trichoderma harzianum, enzyme biosynthesis is enhanced with an increase in temperature up to optimum (35 °C) [55]. This incubation temperature was lower (32 °C) in Aspergillus fumigates for cellulase production [56]. Water is an important factor for microbial activity, and the depletion of available water affects functional changes in microorganisms. In SSF, both low and high moisture levels affected enzyme productivity. A lower moisture level causes a complete reduction in the solubility of available nutrients in the substrate, high water tension, and a higher moisture content of the solid substrate which decreased porosity, lowers oxygen transfer etc. [57]. The optimum moisture content was 60% and the enhanced yield was 108.4 ± 13.3 U/gds (β-glucosidase), 46.4 ± 11.4 U/gds (FPase) and 278 ± 10.5 U/gds (CMCase) as described in (Fig. 4b). In a study, 50% moisture content was reported as optimum for maximum FPase production in SSF [58] in the case of A. niger culture. In another study, moisture content ranging between 40 and 60% was optimum for CMCase and FPase yield for Trichoderma koningii in SSF [59]. In Aspergillus sp. S4B2F, cellulase yield was maximum when it was cultured at a 60% moisture level [60]. In this study, the pH of the substrate was adjusted between 4.0 and 8.0 using suitable buffers, and the results are depicted in (Fig. 5a). The highest enzyme yields were 117.4 ± 5.1 U/gds (β-glucosidase), 54.8 ± 8.2 U/gds (FPase) and 281 ± 10.8 U/gds (CMCase) at pH 6.0. At the acidic pH level (4.0), enzyme biosynthesis was decreased, and it gradually increased up to pH 6.0, then declined. The enzyme yields obtained in this study were highly consistent with previous studies on cellulase production by Trichoderma sp. and Aspergillus niger [61]. Likewise, the solid medium inoculated with 8% inoculum showed the highest enzyme yields of 120 ± 8.3 U/gds (β-glucosidase), 60.1 ± 13.2 U/gds (FPase) and 250 ± 10.3 U/gds (CMCase) which were decreased at the higher inoculum level (10%) as described in (Fig. 5b). Lower enzyme production at higher inoculum levels was mainly due to nutritional imbalance or an initial high concentration of conidial cells and anaerobic conditions in the medium, while the decreased yield at lower inoculum sizes was mainly due to the availability of fewer conidial cells, which are not enough to utilize the available fermentation medium [62]. In a study, Iqbal et al. [55] reported 10% inoculum as optimum carboxymethyl cellulase production from T. harzianum, and enzyme production was reduced above the optimum level.
Production of Cellulases in a Tray Bioreactor
Tray bioreactors have been used for the production of various enzymes. In a study, Nahid et al. [63] extensively studied the application of tray bioreactors on enzyme production. Also, Mitra et al. [64] compared the yield of enzymes in different types of tray bioreactors. Gupta and Kar [65] analyzed the use of tray bioreactor on cellulolytic enzyme production. Also, Dhillon et al. [66] have used tray reactor for the production of xylanase and cellulase using co-cultured fungi. In this study, the tray reactor enhanced the production of cellulases over twofold than unoptimized conditions. Earlier, tray bioreactors were used for the production of fermented products such as, soy sauce, koji and tempeh, and various enzymes in SSF. Brijwani et al. [67] used Aspergillus oryzae and Trichoderma reesei for co-fermentation using agro industrial wastes. The combination of wheat bran and soybean has been utilized for cellulase production. And, the optimum composition of C: N ratio was obtained for the production of enzymes and the controlled release of nutrients. Dhillon et al. [66] recently used apple pomace as the substrate for the production of β-glucosidase and the process parameters were optimized by response surface methodology. Then, the optimized medium was applied for the production of enzymes in the tray bioreactor [68].
Saccharification of Agro-Wastes
Lignocellulosic materials such as palm sawdust, palm leaves, palm fruit waste, and filter paper were used for saccharification process. In this study, saccharification process was maximum up to 12 h of incubation and declined slowly (Fig. 6). In this case, until 12 h incubation reducing sugar level was found to be high, and about 89% saccharification was obtained within the 12 h incubation time. Among the substrates used, cellulases released the maximum amount of reducing sugar in the reaction mixture containing palm fruit as the substrate. Also, enzyme activity in the reaction mixture was gradually declining up to 12 h of incubation. So, up to 12 h was optimum for saccharification process and later efficacy declined. In a study, Annamalai et al. [69] used pretreated rice straw for the saccharification process using cellulase obtained from Bacillus carboniphilus CAS 3. In another study, Gokhale et al. [70] reported that the enzymatic activity of cellulolytic enzymes increased to maximum up to 50 °C and decreased at higher temperatures, because of enzyme denaturation at higher temperatures. Also, Lan et al. [71] analyzed the saccharification process of cellulose wastes using cellulases from Trichoderma viride and the yield was compared with the control. Fungal cellulases and saccharification efficacy would help to reduce the use of various chemicals used in the enzyme industry in the saccharification process.
Conclusions
A hyperactive cellulolytic bacterial strain was isolated from date molasses and identified as Trichoderma reesei Al-K1 149. Municipal solid waste and date molasses (1:1 ratiowere used as substrates to improve enzyme production. The cellulase production was maximum after 96 h of incubation with the yields of β-glucanase (98 ± 3.9 U/gds), carboxymethyl cellulase (CMCase) (241 ± 12.8 U/gds) and filter paperase (FPase) (31.2 ± 3.1 U/gds). Optimum temperature (40 °C) and moisture content (60%) improved cellulase production. In the successive optimization trials, physical factors were optimized and over two fold enzyme yield was obtained in tray reactor. Subsequently, the enzymes used for saccharification of palm saw dust, palm leaves, palm fruit waste, and filter paper. The results indicated that the fungal strain can be considered for biomass saccharification and industrial applications.
Data Availability
Not applicable.
References
Vergara, S.E., Tchobanoglous, G.: Municipal solid waste and the environment: A global perspective. Ann. Rev. Environ. Resour. 37, 277–309 (2012)
Keeling, C.: Canterbury Region Waste Data Report 2009/2010. Environment Canterbury. (2011)
Li, S., Zhang, X., Andresen, J.M.: Production of fermentable sugars from enzymatic hydrolysis of pretreated municipal solid waste after autoclave process. Fuel. 92(1), 84–88 (2012)
Shankar, A., Saini, S., Sharma, K.K.: Fungal-integrated second-generation lignocellulosic biorefinery: Utilization of agricultural biomass for co-production of lignocellulolytic enzymes, mushroom, fungal polysaccharides, and bioethanol. Biomass Conv Biorefin. 14(1), 1117–1131 (2024)
Mansy, A.E., El-Desouky, E., El-Gendi, H., Abu-Saied, M.A., Taha, T.H., Amer, R.A.: Cellulosic fiber waste feedstock for bioethanol production via bioreactor-dependent fermentation. Fermentation. 9(2), 176 (2023)
Chandel, H., Kumar, P., Chandel, A.K., Verma, M.L.: Biotechnological advances in biomass pretreatment for bio-renewable production through nanotechnological intervention. Biomass Con Biorefin. 14(3), 2959–2981 (2024)
FAO.: Food and Agriculture Organization of the United Nations. Food and Agriculture Organization Statistical Databases (FAOSTAT); (2013)
El-Habba, M.S., Al-Mulhim, F.: The competitiveness of the Saudi Arabian date palm: An analytical study. Afr. J. Agric. 8, 5260–5267 (2013)
Al-Mefarrej, H.A., Abdel-Aal, M.A., Nasser, R.A.: Chemical evaluation of some lignocellulosic residues for pulp and paper production. American-Eurasian J. Agric. Environ. Sci. 13, 498–504 (2013)
Jahromi, M.F., Liang, J.B., Rosfarizan, M., Goh, Y.M., Shokryazdan, P., Ho, Y.W.: Effects of Aspergillus Niger (K8) on nutritive value of rice straw. Afr. J. Biotechnol. 9(42), 7043–7047 (2010)
Lizardi-Jiménez, M.A., Hernández-Martínez, R.: Solid state fermentation (SSF): Diversity of applications to valorize waste and biomass. 3 Biotech. 7(1), 44 (2017)
Wilson, D.B.: Cellulases and biofuels. Curr. Opin. Biotechnol. 20, 295–299 (2009)
Srivastava, N., Srivastava, M., Mishra, P.K., Gupta, V.K., Molina, G., Rodriguez-Couto, S., Manikanta, A., Ramteke, P.W.: Applications of fungal cellulases in biofuel production: Advances and limitations. Ren. Sustain. Ener Rev. 82, 2379–2386 (2018)
Vijayaraghavan, P., Arun, A., Al-Dhabi, N.A., Vincent, S.G.P., Arasu, M.V., Choi, K.C.: Novel Bacillus subtilis IND19 cell factory for the simultaneous production of carboxy methyl cellulase and protease using cow dung substrate in solid-substrate fermentation. Biotechnol. Biofuel. 9, 1–13 (2016)
Singhania, R.R., Sukumaran, R.K., Pillai, A., Prema, P., Szakacs, G., Pandey, A.: Solid-state fermentation of lignocellulosic substrates for cellulase production by Trichoderma Reesei NRRL 11460. Ind. J. Biotechnol. 5(3), 332–336 (2006)
Alam, M.Z., Mamun, A.A., Qudsieh, I.Y., Muyibi, S.A., Salleh, H.M., Omar, N.M.: Solid state bioconversion of oil palm empty fruit bunches for cellulase enzyme production using a rotary drum bioreactor. Biochem. Eng. J. 46(1), 61–64 (2009)
Latifian, M., Hamidi-Esfahani, Z., Barzegar, M.: Evaluation of culture conditions for cellulase production by two Trichoderma reesei mutants under solid-state fermentation conditions. Bioresour Technol. 98(18), 3634–3637 (2007)
Pensupa, N., Jin, M., Kokolski, M., Archer, D.B., Du, C.: A solid state fungal fermentation-based strategy for the hydrolysis of wheat straw. Bioresour Technol. 149, 261–267 (2013)
Couto, S.R., Sanromán, M.A.: Application of solid-state fermentation to ligninolytic enzyme production. Biochem. Eng. J. 22, 211–219 (2005)
Klein-Marcuschamer, D.: The challenge of enzyme cost in the production of lignocellulosic biofuels. Biotechnol. Bioeng. 109, 1083–1087 (2012)
Taghizadeh-Alisaraei, A., Motevali, A., Ghobadian, B.: Ethanol production from date wastes: Adapted technologies, challenges, and global potential. Renew. Ener. 143, 1094–1110 (2019)
El-Sharnouby, G.A., Aleid, S.M., Al-Otaibi, M.M.: Liquid sugar extraction from date palm (Phoenix dactylifera L.) fruits. Int. J. Food Process. Technol. 5, 1–5 (2014)
Siddeeg, A., Zeng, X.A., Ammar, A.F., Han, Z.: Sugar profile, volatile compounds, composition and antioxidant activity of Sukkari date palm fruit. J. Food Sci. Technol. 56, 754–762 (2019)
Sluiter, A., Hames, B., Ruiz, R., Scarlata, C., Sluiter, J., Templeton, D.: Determination of sugars, byproducts, and degradation products in liquid fraction process samples. Golden: Nat. Renew. Ener Lab. 11, 65–71 (2006)
Sluiter, A., Hames, B., Ruiz, R., Scarlata, C., Sluiter, J., Templeton, D., Crocker, D.L.A.P.: Determination of structural carbohydrates and lignin in biomass. Lab. Anal. Proce. 1617(1), 1–16 (2008)
Campbell, C.R., Plank, C.O.: Determination of total nitrogen in plant tissue by combustion. Plant Anal Ref Proc for S US Southern Coop. Ser. Bull. 368, 20–22 (1992)
Cequier-Sánchez, E., RODRiguez, C.O., Zarate, R.: Dichloromethane as a solvent for lipid extraction and assessment of lipid classes and fatty acids from samples of different natures. J. Agric. Food Chem. 56(12), 4297–4303 (2008)
Hokura, A., Matsuura, H., Katsuki, F., Haraguchi, H.: Multielement determination of major-to-ultratrace elements in plant reference materials by ICP-AES/ICP-MS and evaluation of their enrichment factors. Anal. Sci. 16(11), 1161–1168 (2000)
Van Soest, P.V., Robertson, J.B., Lewis, B.A.: Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy. Sci. 74(10), 3583–3597 (1991)
Melo, S.C.O., Pungartnik, C., Cascardo, J.C.M., Brendel, M.: Rapid and efficient protocol for DNA extraction and molecular identification of the basidiomycete Crinipellis Perniciosa. Genet. Mol. Res. 5(4), 851–855 (2006)
Kurtzman, C.P., Robnett, C.J.: Identification of clinically important ascomycetous yeasts based on nucleotide divergence in the 5’end of the large-subunit (26S) ribosomal DNA gene. J. Clin. Microbiol. 35, 1216–1223 (1997)
Dhillon, G.S., Kaur, S., Brar, S.K., Verma, M.: Potential of apple pomace as a solid substrate for fungal cellulase and hemicellulase bioproduction through solid-state fermentation. Ind. Crop Prod. 38, 6–13 (2012)
Miller, G.L.: Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 31(3), 426–428 (1959)
Mandels, M., Andreotti, R., Roche, C.: January. Measurement of saccharifying cellulase. In Biotechnol. Bioeng. Symp.;(United States) (Vol. 6). Army Natick Development Center, MA.6, 21–23 (1976)
Kalaiyarasi, M., Vijayaraghavan, P., Raj, S.R.F., Vincent, S.G.P.: Statistical approach for the production of protease and cellulase from Bacillus cereus KA3. Bioprocess Eng. 1(4), 93–103 (2017)
Al-Dhabi, N.A., Esmail, G.A., Valan Arasu, M.: Co-fermentation of food waste and municipal sludge from the Saudi Arabian environment to improve lactic acid production by Lactobacillus rhamnosus AW3 isolated from date processing waste. Sustainability. 12(17), 6899 (2020)
Wilinska-Lisowska, A., Ossowska, M., Czerwionka, K.: The influence of co-fermentation of agri-food waste with primary sludge on biogas production and composition of the liquid fraction of digestate. Energies. 14(7), 1907 (2021)
Semeraro, B., Summa, D., Costa, S., Zappaterra, F., Tamburini, E.: Bio-delignification of green waste (GW) in co-digestion with the organic fraction of municipal solid waste (OFMSW) to enhance biogas production. Appl. Sci. 11(13), 6061 (2021)
Kamyab, S., Ataei, S.A., Tabatabaee, M., Mirhosseinei, S.A.: Optimization of bio-hydrogen production in dark fermentation using activated sludge and date syrup as inexpensive substrate. Int. J. Green. Ener. 16(10), 763–769 (2019)
Hussin, A.M., Collins, S.R.A., Merali, Z., Parker, M.L., Elliston, A., Wellner, N., Waldron, K.W.: Characterisation of lignocellulosic sugars from municipal solid waste residue. Biomass Bioen. 51, 17–25 (2013)
Barlaz, M.A., Eleazer, W.E., Odle, W.S., Qian, X., Wang, Y.S.: Biodegradative Analysis of Municipal Solid Waste in laboratory-scale Landfills. United States Environmental Protection Agency (1997)
Wang, Y.S., Byrd, C.S., Barlaz, M.A.: Anaerobic biodegradability of cellulose and hemicellulose in excavated refuse samples using a biochemical methane potential assay. J. Ind. Microbiol. Biotechnol. 13(3), 147–153 (1994)
Barlaz, M.A., Schaefer, D.M., Ham, R.: Bacterial population development and chemical characteristics of refuse decomposition in a simulated sanitary landfill. Appl. Environ. Microbiol. 55(1), 55–65 (1989)
Ham, R.K., Norman, M.R., Fritschel, P.R.: Chemical characterization of fresh kills landfill refuse and extracts. J. Environ. Eng. 119(6), 1176–1195 (1993)
Price, G.A., Barlaz, M.A., Hater, G.R.: Nitrogen management in bioreactor landfills. Waste Manage. 23(7), 675–688 (2003). (1993)
Farmanbordar, S., Amiri, H., Karimi, K.: Synergy of municipal solid waste co-processing with lignocellulosic waste for improved biobutanol production. Waste Manage. 118, 45–54 (2020)
di Bitonto, L., Antonopoulou, G., Braguglia, C., Campanale, C., Gallipoli, A., Lyberatos, G., Ntaikou, I., Pastore, C.: Lewis-Brønsted acid catalysed ethanolysis of the organic fraction of municipal solid waste for efficient production of biofuels. Bioresour Technol. 266, 297–305 (2018)
Barampouti, E.M., Mai, S., Malamis, D., Moustakas, K., Loizidou, M.: Liquid biofuels from the organic fraction of municipal solid waste: A review. Renew. Sustain. Ener Rev. 110, 298–314 (2019)
Ponsá, S., Gea, T., Sánchez, A.: Anaerobic co-digestion of the organic fraction of municipal solid waste with several pure organic co-substrates. Biosys Eng. 108(4), 352–360 (2011)
Hartmann, H., Ahring, B.K.: Strategies for the anaerobic digestion of the organic fraction of municipal solid waste: An overview. Water Sci. Technol. 53, 7–22 (2006)
Slack, R.J., Bonin, M., Gronow, J.R., Van Santen, A., Voulvoulis, N.: Household hazardous waste data for the UK by direct sampling. Environ. Sci. Technol. 41(7), 2566–2571 (2007)
Sun, H., Ge, X., Hao, Z., Peng, M.: Cellulase production by Trichoderma sp. on apple pomace under solid state fermentation. Afr. J. Biotechnol. 9, 163–166 (2010)
Campos, A.O., Asevedo, E.A., Souza Filho, P.F., Santos, E.S.D.: Extraction of Cellulases produced through solid-state fermentation by Trichoderma reesei CCT-2768 using green coconut fibers pretreated by Steam Explosion combined with Alkali. Biomass. 4(1), 92–106 (2024)
Ja’afaru, M.I., Fagade, O.E.: Optimization studies on cellulase enzyme production by an isolated strain of Aspergillus Niger YL128. Afr. J. Microbiol. Res. 4(24), 2635–2639 (2010)
Iqbal, H.M., Asgher, M., Ahmed, I., Hussain, S.: Media optimization for hyper-production of carboxymethyl cellulase using proximally analyzed agroindustrial residue with Trichoderma Harzianum under SSF. IJAVMS. 4, 47–55 (2010)
Gilna, V.V., Khaleel, K.M.: Biochemistry of cellulase enzyme activity of aspergillus fumigatus from mangrove soil on lignocellulosics substrate. Rec Res. Sci. Technol. 3, 132–134 (2011)
Anto, H., Trivedi, U.B., Patel, K.C.: Glucoamylase production by solid-state fermentation using rice flake manufacturing waste products as substrate. Bioresour Technol. 97(10), 1161–1166 (2006)
Chandra, M.S., Viswanath, B., Reddy, B.R.: Cellulolytic enzymes on lignocellulosic substrates in solid state fermentation by Aspergillus Niger. Indian J. Microbiol. 47, 323–328 (2007)
Liu, J., Yang, J.: Cellulase production by T. Koningii. Food Technol. Biotechnol. 45, 420–425 (2007)
Soni, S.K., Batra, N., Bansal, N., Soni, R.: Bioconversion of sugarcane bagasse into second generation bioethanol after enzymatic hydrolysis with in-house produced cellulases from aspergillus sp. S4B2F. BioRes. 5(2), 741–757 (2010)
Gautam, S.P., Bundela, P.S., Pandey, A.K., Khan, J., Awasthi, M.K., Sarsaiya, S.: Optimization for the production of cellulase enzyme from municipal solid waste residue by two novel cellulolytic fungi. Biotechnol. Res. Int. 1, 1–8 (2011)
Azzouz, Z., Bettache, A., Djinni, I., Boucherba, N., Benallaoua, S.: Biotechnological production and statistical optimization of fungal xylanase by bioconversion of the lignocellulosic biomass residues in solid-state fermentation. Biomass Con Biorefin 1–13 (2020)
Nahid, P., Vossoughi, M., Roostaazad, R., Ahmadi, M., Zarrabi, A., Hosseini, S.M.: Production of glucoamylase by Aspergillus Niger under solid state fermentation (research note). Int. J. Eng. 25(1), 11–17 (2012)
Mitra, R.D., Silva, C.M., Youvan, D.C.: Fluorescence resonance energy transfer between blue-emitting and red-shifted excitation derivatives of the green fluorescent protein. Gene. 173(1), 13–17 (1996)
Gupta, U., Kar, R.: Optimization and scale up of cellulase free endo xylanase production by solid state fermentation on corn cob and by immobilized cells of a thermotolerant bacterial isolate. Jordon J. Biol. Sci. 1(3), 129–134 (2008)
Dhillon, G.S., Brar, S.K., Valero, J.R., Verma, M.: Bioproduction of hydrolytic enzymes using apple pomace waste by A. Niger: Applications in biocontrol formulations and hydrolysis of chitin/chitosan. Bioprocess. Biosyst Eng. 34, 1017–1026 (2011a)
Brijwani, K., Oberoi, H.S., Vadlani, P.V.: Production of a cellulolytic enzyme system in mixed-culture solid-state fermentation of soybean hulls supplemented with wheat bran. Process. Biochem. 45, 120–128 (2010)
Khanahmadi, M., Arezi, I., Amiri, M.S., Miranzadeh, M.: Bioprocessing of agro-industrial residues for optimization of xylanase production by solid-state fermentation in flask and tray bioreactor. Biocat Agric. Biotechnol. 13, 272–282 (2018)
Annamalai, N., Rajeswari, M.V., Balasubramanian, T.: Enzymatic saccharification of pretreated rice straw by cellulase produced from Bacillus carboniphilus CAS 3 utilizing lignocellulosic wastes through statistical optimization. Biomass Bioen. 68, 151–160 (2011b) (2014)
Gokhale, A.A., Lu, J., Lee, I.: Immobilization of cellulase on magnetoresponsive graphene nano-supports. J. Mol. Cat B: Enzy. 90, 76–86 (2013)
Lan, T.Q., Wei, D., Yang, S.T., Liu, X.: Enhanced cellulase production by Trichoderma viride in a rotating fibrous bed bioreactor. Bioresour Technol. 133, 175–182 (2013)
Funding
The authors extend their appreciation to the Researchers supporting project number (RSP2024R185), King Saud University, Riyadh, Saudi Arabia.
Author information
Authors and Affiliations
Contributions
Conceptualization: MSE, P.V; Writing review and editing: KMA; Methodology: S.A, VP; Resources: KMA, MAA; Data curation: S.A. VP; Writing original draft: VP; Investigation: VP, Supervision: SA.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Alarjani, K.M., Elshikh, M.S., Alghmdi, M.A. et al. Valorization of Date Molasses and Municipal Solid Waste for the Production of Cellulases by Trichoderma reesei Al-K1 149 in a Tray Reactor. Waste Biomass Valor (2024). https://doi.org/10.1007/s12649-024-02665-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12649-024-02665-3