Abstract
The process of bioethanol production has several economic drawbacks such as the cost of enzymes, i.e., cellulase, which hydrolyze cellulose to produce fermentable sugars. In this study, to reduce the production cost of cellulase, agricultural wastes such as wheat straw (WS) and rice straw (RS) were used as carbon sources for cellulase production through solid-state fermentation. First, the standard cellulase production conditions were optimized, the initial moisture content of the solid medium was 60%, the biomass particle size was 100 µm to 4 mm size mixed, and the optimal carbon/nitrogen (C/N) ratio in the medium of WS and RS was 59.0 and 41.2, respectively. Subsequently, to simplify process handling, eliminating trace elements such as MgSO4, CaCl2, FeSO4, MnSO4, ZnSO4, CoCl2, and phosphorous compounds such as KH2PO4 in the general Mandel’s medium was used for cellulase production medium and compared with the standard medium. Maximum 5.4 filter paper unit (FPU)/g-substrate (WS) and 5.3 FPU/g-substrate (RS) were attained under trace element eliminating medium; these were similar to that from the standard medium of WS (4.8 FPU/g-substrate) and RS (5.7 FPU/g-substrate). Moreover, the produced crude cellulases from RS were evaluated for their potential in hydrolysis of alkaline-treated RS. The performances of the crude cellulases were similar to those of commercial cellulases.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Lignocellulosic biomass as the carbon–neutral resources is a promising resource for the production of bioethanol and other beneficial chemicals [1, 2]. The conversion of lignocellulosic biomass to bioethanol entails the following three main steps: pretreatment to remove lignin and reduce the recalcitrance of cellulose, enzymatic hydrolysis to release fermentable sugars such as glucose, and fermentation to produce bioethanol [3]. Enzymatic hydrolysis is conducted using cellulase, which breaks down cellulose into glucose [4]. Cellulase is an industrially important enzyme for bioethanol production, food processing (more optimized extraction and clarification of fruit juice), the detergent industry (accelerating detergency), the pulp and paper industry (biomechanical pulping), and the textile industry (biopolishing of textile fibers) [5,6,7]. However, the cellulase production process is quite costly; it accounts for 50% of the overall cost of hydrolysis [6, 7]. Therefore, the production cost of bioethanol is greatly affected by the cost of cellulase [8].
Solid-state fermentation (SSF) has numerous advantages, such as high volumetric productivity and low moisture content in the medium, and occurs in a natural state for enzyme production [9, 10]. SSF has numerous economic and practical advantages over submerged fermentation. Particularly, SSF can utilize heterogeneous byproducts such as agricultural wastes and industrial wastes based on cellulosic materials [11]. As the expression of the produced cellulase is greatly influenced by physical state and chemical properties of the carbon source [12], identical microorganisms growing on different types of cellulosic materials can yield varying amounts of cellulase [13].
Wheat straw (WS) and rice straw (RS) are lignocellulosic agricultural wastes that are abundantly available worldwide and are considered to be potential feedstocks for the production of ethanol. Approximately 850 million tons of WS and 669 million tons of RS are produced in Asia per annum. [14]. Therefore, these straw wastes are promising resources for the production of ethanol and cultivation of cellulase-producing fungi. Recently, herbaceous biomass solid among various special biomass solids was used as cellulase production medium nutrients by SSF [15,16,17,18,19,20,21]. In these studies, a general standard nutrient additive called Mandel’s medium containing nitrogen and various mineral salts was used with solid biomass. Scaling up and manufacturing on a large scale with low cost and easy handling necessitate the use of the popular and abundant cellulosic biomass worldwide as well as limiting the additives into the biomass medium.
In this study, we used the poplar herbaceous cellulose biomass, wheat straw (WS), and rice straw (RS) as natural cellulosic substrates for cellulase production through SSF using Trichoderma reesei as cellulase producer. First, the fermentation parameters such as the initial medium moisture content, particle sizes, and carbon/nitrogen ratio in the medium were optimized, and the total cellulase titer and detailed activities, i.e., endoglucanase, carboxymethyl cellulase (CMCase), β-glucosidase, and xylanase activities, of the cellulolytic enzymes produced from WS and RS were evaluated. Next, under optimized condition, to reduce handling nutrient, elements in the WS and RS production medium were minimized. Finally, the detailed activities of cellulase produced from the minimized biomass medium were evaluated, and the hydrolysis ability was tested.
2 Materials and methods
2.1 Cellulosic substrate materials for the production of cellulase
Cellulosic materials for the production of cellulase were WS and RS. The WS and RS were provided by a local farm in Tokushima City; they were cut to pieces measuring 20 mm × 10 mm × 1 mm and then ground using a model D3V-10 cutter mill (Osaka Chemical Co. Ltd., Osaka, Japan) for 5 s to obtain particles ranging from 100 µm to 4 mm in size. Furthermore, 100 g of ground RS was washed with 2 L of ion-exchange water after being dried in an oven at 100 °C; the residue was ground for 15 s.
2.2 Microorganism
SSF was carried out using Trichoderma reesei (T. reesei), which is commonly used for commercial cellulase production and has been researched extensively because of its high cellulase production capability [22,23,24]. T. reesei ATCC 56765 was purchased from the American Type Culture Collection. The strain was cultivated on potato dextrose agar (PDA) medium in Petri dishes at 28 °C for 1 week.
2.3 Chemical analysis of wheat straw and rice straw
The cellulosic materials were analyzed for their chemical compositions as follows. The cellulose content in the sample was determined based on the monomer content (glucose) measured after hydrolysis (72 wt% H2SO4 followed by dilution). The sample was evaluated using the Klason lignin measurement method [25], where 1 g of the material was added to 15 mL of 72 wt% H2SO4 and stored at room temperature for 4 h. The residue was then placed in a 1 L conical flask containing 560 mL of distilled water and autoclaved for 1 h at 121 °C. After cooling, the solid and liquid portions were separated by centrifugation, and the glucose in the liquid was analyzed by high-performance liquid chromatography with a refractive index detector and a model HPX-87H column (Bio-Rad, Hercules, CA, USA) at 65 °C. The mobile phase was 5.0 mM H2SO4 at a flow rate of 0.6 mL/min. The solid portion was washed with distilled water, dried at approximately 105 °C with a heater to a constant weight, and weighed as an acid-insoluble material (lignin). The amount of hemicellulose was calculated by subtracting the cellulose content from the total sugar content. The total sugar content was determined using the phenol–sulfuric acid method [26]. To determine the ash content, 5 g of the sample was placed in a sintered crucible, which was placed inside a muffle furnace at 600 °C for 2 h followed by cooling in a desiccator. Ash content was calculated using the following formula:
Ash (%) = [(Weight of ash with crucible (g) – Weight of dry crucible (g)] / Weight of the sample] × 100
All analytical determinations were performed in triplicate, and the calculations are shown.
The mineral K was measured by atomic absorption spectrometry. The minerals Ca, Mg, Fe, Zn, Mn, and P were measured by inductively coupled plasma optical emission spectroscopy. The minerals analyses were performed by Japan Functional Food Analysis and Research Center (Fukuoka, Japan).
2.4 Carbon-to-nitrogen ratio measurement
The total carbon and nitrogen of WS and RS samples were measured using the combustion method by MICRO CORDER (JM10, J-SCIENCE LAB, Osaka, Japan). The total carbon and nitrogen content on the weight basis of samples was used to calculate the carbon-to-nitrogen (C/N) ratio. The C/N ratio of the medium was calculated using the following formula:
C/N ratio = [Weight of carbon in WS or RS (g) + weight of carbon in pepton, urea, and ammonium sulfate (g)] / [Weight of nitrogen in WS or RS (g) + weight of nitrogen in pepton, urea, and ammonium sulfate (g)].
2.5 Inoculum preparation
Inoculum preparation was performed in 300 Erlenmeyer flasks containing 100 mL of Mandels and Weber growth medium [27] with slight modifications consisting of the following compositions (g/L): KH2PO4, 2.0; (NH4)2SO4, 1.4; MgSO4.7H2O, 0.005; FeSO4.7H2O, 0.005; MnSO4.H2O, 0.0016; ZnSO4.7H2O, 0.0015; CoCl2.6H2O, 0.004; CaCl2.2H2O, 0.3; urea, 0.3; peptone, 0.1; glucose, 5.0; and Avicel, 10. Five loopfuls of mycelial conidia cultures from the PDA medium of the Petri dish were added and shaken at 140 rpm at 30 °C in an incubator shaker for 48 h. After incubation, the inoculum was placed in a test tube and separated by centrifugation (3500 rpm for 15 min). The collected solid cells were used for SSF.
2.6 Solid-state fermentation
Dry substrate (5.0 g) was added to a 100 mL Erlenmeyer flask. The flask was autoclaved at 121 °C for 20 min for sterilization. After cooling, 0.06 g of the cells (dry cell weight) was suspended in the concentrated production medium (5 mL) and aseptically added to the substrates. The concentrated production medium had the following composition (in g/L): KH2PO4, 20; (NH4)2SO4, 14; MgSO4.7H2O, 0.05; FeSO4.7H2O, 0.05; MnSO4.H2O, 0.016; ZnSO4.7H2O, 0.015; CoCl2.6H2O, 0.04; CaCl2.2H2O, 0.3; urea, 3.0; and peptone, 1.0. The moisture content was adjusted with 2.5 mL and 14 mL of sterilized distilled water to levels of 60% or 80%. The cultures were incubated at 30, 32, or 36 °C for 9 days. The incubated samples were collected at 24-h intervals and checked for cellulase and xylanase activities.
2.7 Enzyme extraction
At the end of incubation, the fungal enzyme was extracted. For each 5 g of fermented substrate, 50 mL of sodium citrate buffer (50 mM and pH 4.8) was added and agitated in a shaker for 1 h. The mixture was centrifuged at room temperature (25 °C) at 3500 rpm for 15 min to collect the clear supernatant as a crude enzyme solution.
2.8 Enzyme assays
The total cellulase activity was determined by filter paper unit (FPU) activity according to the standardized National Renewable Energy Laboratory analytical procedure [28]. The assay was carried out by adding 0.5 mL of enzyme solution to a test tube containing 1 mL of sodium citrate buffer (50 mM and pH 4.8) and a Whatman No. 1 filter paper strip (1 cm × 6 cm and 50 mg). The mixture was incubated at 50 °C for 60 min, and the released reducing sugar was determined using the 3,5-dinitrosalicylic acid (DNS) method [29]. One unit of the activity was defined as the amount of enzyme that releases 1 μmol of glucose per min under assay conditions. Endoglucanase activity, that is, carboxymethyl cellulase activity (CMCase), was measured with 1% (w/v) carboxymethyl cellulose in 50 mM sodium citrate buffer (50 mM and pH 4.8) [30]. The definition of activity in this case is similar to that of FPU activity. For the xylanase assay, suspension of beech wood xylan of 1% (w/v) in the buffer mentioned above was used as a substrate. One unit of xylanase activity was defined as the amount of enzyme that releases 1 μmol of xylose per min under assay conditions [31]. The β-glucosidase assay was carried out with 1 mL of p-nitrophenyl -β-D-glucopyranoside (pNPG, 2 mM, Sigma-Aldrich) as substrate, which was digested using 0.1 mL of enzyme solution at 50 °C for 5 min. The reaction was stopped by adding 2 mL of sodium carbonate solution (1 M), and the amount of p-nitrophenol was determined by reading the absorbance at 405 nm [32]. One unit of β-glucosidase activity was defined as the amount of enzyme that liberated 1 μmol of pNPG per min under assay conditions.
2.9 Enzymatic hydrolysis of alkaline-treated rice straw
Alkaline-treated RS was used as the substrate for the enzymatic hydrolysis test by producing cellulase from RS.
After grinding and washing with water treatment of RS, alkaline treatment was conducted at a ratio of 1:10 using 3% (w/v) of NaOH, that is, 10 g of RS in 100 mL of 3% (w/v) of NaOH. The RS in the NaOH solution was then autoclaved at 121 °C for 30 min. Afterwards, the treated residue was neutralized to an approximate pH of 7.0 using a diluted HCl solution, washed with distilled water, and oven dried at 100 °C for 24 h and stored at room temperature (25 °C) prior to use.
The crude enzyme mixture produced by T. reesei ATCC 56765 from RS medium was produced under optimal conditions of an incubation temperature of 30 °C and substrate moisture content of 60% and extracted after 6 days of growth. Commercial cellulases were used as controls. Acremonium cellulase (derived from Acremonium cellulolyticus, 269 FPU activity/g-enzyme) was purchased from Meiji Seika Pharma Co., Ltd. (Osaka, Japan).
Enzymatic hydrolysis was performed using 10 mL of 0.1 M sodium acetate buffer (pH 5.0) at 50 °C in a rotary shaker operating at 160 rpm. The substrate concentrations and enzyme loading were 50 g/L and 26 FPU/g of dry substrate, respectively. The hydrolyzed samples were obtained at 24-h intervals and centrifuged to remove the solid residue. The supernatant was analyzed for glucose using the mutarotase glucose oxidase method (Glucose C-II test, Wako Pure Chemicals Co., Ltd., Japan). All enzymatic hydrolysis experiments were performed in triplicate, and the means were calculated.
The hydrolysis yield (%) by enzymatic hydrolysis was calculated using the following equation:
(Amount of glucose produced (g)/Amount of cellulose in the substrate (g) × 1.1) × 100.
3 Results and discussion
3.1 Chemical composition of WS and RS
To confirm the chemical component in the experimental material, chemical analyses of WS and RS (washed with water) were performed. The amounts of cellulose, hemicellulose, lignin, crude protein, and ash were determined. Moreover, elemental composition, i.e., carbon content and nitrogen content in the WS and RS washed with water were also determined. The results are summarized in Table 1. The cellulose contents of WS and RS were 35.9% and 35.1%, respectively. The carbon content (%) and nitrogen content (%) in the WS and RS washed with water were as follows: 40.0 and 0.23, 41.1, and 0.55.
3.2 Optimization of solid-state fermentation conditions for cellulase production by T. reesei using wheat straw and rice straw
3.2.1 Initial moisture content
For the assessment of the effective utilization of agricultural byproducts, WS and RS were used as solid substrate carbon sources for the production of cellulase.
Initial moisture content is an important parameter for cellulase production by SSF. In this study, initial moisture content of 60% and 80% using WS as incubation medium was investigated. Figure 1 shows the time course of cellulase activity as FPU activity during incubation. Many researchers observed that the optimum temperature for cellulase production using T. reesei species was approximately 30 °C [33, 34]. Verma et al. reported that temperatures above or below 30 °C were somewhat unfavorable for T. reesei, cellulase producer, which might be due to their physiological nature and protein synthesis capability. At a higher temperature (35 °C), enzyme biosynthesis decreases owing to thermal deactivation. At a lower temperature (25 °C), the transport of nutrients in the cells is hindered, and the microbial enzyme production capacity decreased [6]. Therefore, subsequent experiments were performed at 30 °C. The maximum activity of 4.8 FPU/g-dry WS was obtained on day 9 under 60% of moisture content. Conversely, for the moisture content of 80%, although they have similar cellulase activity to that of moisture content of 60%, the activities were variable (large error bars). Many studies have reported on the initial moisture content of cellulase production by SSF using Trichoderma spp. Salomão et al. reported that relatively high enzymatic activities were obtained at an incubation temperature of 28 °C to 50% of initial moisture content of sugarcane bagasse solid medium using Trichoderma koningii [35]. Verma et al. also reported that optimal initial moisture content was approximately 60% using wheat bran as solid substrate for cellulase production by T. reesei and Neurospora crassa co-culture [36].
3.2.2 Particle sizes
The effect of the particle sizes of WS and RS on cellulase production by T. reesei were evaluated using powder particles (< 500 μm, sieved after grinding), powder and fiber particles mixed (100 μm to 4 mm, only grinding), and pieces (measuring 20 mm × 10 mm × 1 mm) as substrates. With RS as a solid substrate, the residue after washing with water was used as substrate, because no production of cellulase was observed in cases where RS was not washed with water (data not shown). Figure 2 shows the time course of cellulase activity as FPU activity during incubation using WS and RS as substrates. For both WS and RS, FPU activity profiles with powder, and fiber particles and only powder particles were similar; however, with pieces, there was no bigger increase than those obtained with powder and fiber or powder particles.
A relatively small substrate size provides a relatively large surface area for growth and action of microorganisms and is beneficial for heat transfer and uptake of oxygen [37]. Verma et al. reported that a particle size of 850 μm (in the case of wheat bran) was effective for cellulase activity [36]. Vanajakshi et al. also suggested that both interparticle porosity and surface area exhibit a high medium particle size range, which encourages the growth of microorganisms and cellulase production owing to more optimized mass and heat transfer [38]. A mixture of powder and fiber particles (100 μm to 4 mm) was used throughout this study to simplify the preparation of the substrate because it is necessary to sieve the sample to produce powder particles (< 500 μm).
3.2.3 Carbon-to-nitrogen ratio
According to the modified Mandel’s medium used in this study (standard medium), the C/N ratios of WS and RS were 59.0 and 41.2, respectively. The compositions of different cultivation media and their C/N ratios of WS and RS mediums are summarized in Table 2. In the nitrogen-reduced medium (C/N ratio; 14.2 and 13.1 for WS and RS), urea and ammonium sulfate were half of the standard medium. In the nitrogen-increased medium (C/N ratio; 86.7 and 52.7), urea and ammonium sulfate were six times higher than those of the standard medium. Figure 3 shows the time course of cellulase activity as FPU activity during incubation using WS and RS as substrates under various C/N ratios. Unstable cellulase production was observed at C/N rations of 14.2 (WS) and 13.1 (RS). Conversely, no drastic increments were observed at C/N ratios of 86.7 (WS) and 52.7 (RS). These results showed that C/N ratios of 59.0 (WS) and 41.2 (RS), i.e., standard medium composition, were favorable for the production of cellulase. It is important to calculate the C/N ratio using biomass solid substrate and other medium additives (Mandel’s medium), because carbon content and nitrogen content differ depending on the biomass solid substrate. For example, Moran-Aguilar et al. used sugarcane bagasse and brewery spent grain as cellulase fermentation substrate by Aspergillus niger (A. niger), carbon content, and nitrogen content in the biomass substrates which are reported as 42.55% and 0.25%, 44.38%, and 3.61%, respectively. Using these carbon and nitrogen contents, C/N = 149 (sugarcane bagasse) and C/N = 12.1 (brewery spent grain) could be calculated [39]. Although the nitrogen content varies greatly depending on the substrate, only a few studies have analyzed carbon and nitrogen contents of the substrates and calculated and discussed the C/N ratio values. Amaro-Reyes et al. carried out co-culture cellulase fermentation of A. niger and T. reesei using Bermuda grass and corn cob mixture as solid-state substrate. Maximum FPU activity (23.2 FPU/g-substrate) was observed at C/N = 30, and no significant activity difference was observed at C/N ratios of 60 and 120. However, the lowest FPU activity was observed at a C/N ratio of 3 (half of the highest) [40]. Determining a suitable C/N ratio is essential to producing cellulase for each substrate. The growth of T. reesei on WS and RS medium during 9 days of incubation at optimal culture condition (initial moisture 60%, mixture of powder and fiber particles (100 μm to 4 mm), C/N = 59.0 for WS and 41.2 for RS) is shown in Fig. 4. Both WS and RS medium growths of hyphae were observed within 4 days, forming white fluffy aerial mycelia. Finally, the mycelia turned greenish.
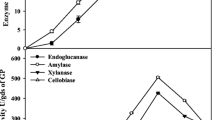
Furthermore, a cellulolytic enzyme system is complex and comprises endoglucanase (CMCase), exoglucanase, β-glucosidase, and xylanase that acts synergistically to degrade cellulosic substrate [41, 42]. An efficient enzyme system should contain balanced activities of these cellulases; therefore, FPU (total cellulase titer), CMCase, β-glucosidase, and xylanase activities were evaluated in this study. Figure 5 shows the time course of the produced FPU, CMCase, β-glucosidase, and xylanase activities from WS and RS as the carbon source under optimized fermentation conditions, initial moisture content of 60%, particle size ranging from 100 μm to 4 mm, and C/N ratio of 59.0 (WS) and 41.2 (RS). With CMCase, the maximum activities of 246 U/g-WS and 205 U/g-RS were obtained on day 10. The β-glucosidase activity increased to 3.0 U/g-WS and 3.5 U/g-RS on day 9; the xylanase activity increased to 2563 U/g-WS and 2111 U/g-RS on days 9 and 10. These production amounts were similar to that reported by Dhillon et al. using RS as the solid fermentation substrate by T. reesei Rut C-30 [43]; 16.1 FPU/g-RS of FPU activity, 58.2 U/g-RS of CMCase, 12.6 U/g-RS of β-glucosidase activity, and 1299 U/g-RS of xylanase activity were attained at 96 h of incubation time at 30 °C. Taherzadeh-Ghahfarokhi et al. also reported that 2.5 FPU/g-WS and 1.9 FPU/g-RS, 7.5 U/g-WS and 1.8 U/g-RS of CMCase activity, and 400 U-g-WS and 200 U/g-RS of xylanase activity were obtained from WS and RS solid medium by T. reesei ATCC 13631 at 28 °C (data of β-glucosidase activity not shown) [15]. However, these activity values varied depending on the microorganism and fermentation parameters used. In this study, high CMCase activity and xylanase activity were observed compared to other studies.
3.3 Easy-to-handle medium for cellulase production by T. reesei using wheat straw and rice straw
To reduce handling steps, the simplification of medium components was carried out using WS and RS as solid fermentation substrates under optimized fermentation conditions: initial moisture content of 60%, 100 μm to 4 mm of particle size mixed, and C/N ratio of 59.0 (WS) and 41.2 (RS). Figure 6 shows the time courses of cellulase activity as FPU activities during incubation using WS (a) and RS (b) standard medium (with Mandel’s medium), WS and RS standard medium without trace elements compounds (MgSO4, CaCl2, FeSO4, MnSO4, ZnSO4, CoCl2) in the Mandel’s medium, and WS and RS standard medium without trace elements and KH2PO4. Both WS and RS mediums without trace element compounds, with similar activity production behavior, and with maximum FPU activities were observed to those of standard medium of WS and RS. Conversely, both WS and RS medium without trace element compounds and KH2PO4 and with relatively low maximum FPU activities than that of the standard medium were observed. These results showed that trace element compounds in the Mandel’s medium could be removed from the WS and RS medium; however, KH2PO4 must be added into the WS and RS medium. Wen et al. also reported that when dairy manure (with sufficient content trace elements) was used as cellulase production nutrients, the elimination of CaCl2, MgSO4, and other trace elements such as Fe, Zn, Co, and Mn from the original salt solution (Mandel’s medium) had no negative impact on the cellulase production, and KH2PO4 elimination did reduce cellulase production [44]. The contents of potassium and phosphorus from the manure were low. Therefore, metal elemental features (except for Co) in WS and RS washed with water were determined and are presented in Table 3. Regarding trace elements such as Ca, Mg, Fe, Zn, and Mn, all the metals except for Ca present enough in the WS and RS as solid fermentation substrate compared with standard medium (modified Mandel’s medium). Belén et al. reported that Fe, Mn, and Zn are regarded as cofactors in the growth and development metabolism of the microorganism [45]. Shu et al. found Mn is crucial in cellular functions such as cell wall synthesis, sporulation, and the production of secondary metabolites [46]. As T. reesei could utilize these trace elements from WS or RS, the elimination of trace elements via the standard Mandel medium had no negative influence on cellulase production. Based on the result, medium composition using WS and RS for the cellulase production can be simplified and cost-effective. Conversely, K and P were significantly less than those of the standard medium; their contents were 13.3 mg/g-WS and 3.83 mg/g-RS and only 0.71 mg/g-WS and 0.47 mg/g-RS, respectively. This necessitates the compensation of K and P using KH2PO4 into the natural WS and RS as cellulase fermentation medium. Generally, because KH2PO4 (with K2HPO4) is added to buffer the pH of the medium (in liquid medium case) [47], elimination of KH2PO4 may not directly affect the quality of secondary metabolites, i.e., cellulase ability. Therefore, in the subsequent hydrolysis ability test, cellulase hydrolysis ability and the detailed activities such as CMCase, β-glucosidase, and xylanase activities derived from standard Mandel’s medium, the medium without trace element, and the medium without trace elements and KH2PO4 were compared. The cellulase activity (FPU) of RS was slightly higher than that of WS; therefore, hydrolysis tests were carried out using cellulase obtained from RS.
3.4 Hydrolysis ability test with cellulase produced from RS medium by T. reesei
Hydrolysis tests for cellulosic materials were performed using a cellulase solution prepared from RS via the standard Mandel’s medium (with trace elements), RS via a standard medium without trace elements, and RS via a standard medium without trace elements and KH2PO4, respectively, to confirm the cellulose in the RS biomass hydrolysis ability of the cellulase produced by T. reesei ATCC 56765 under SSF. Cellulase was extracted from each medium on day 6. As a reference for hydrolysis ability, commercial cellulases such as Acremonium cellulase (AC) was used. Before the hydrolysis test of the cellulose in RS, to reduce the high lignin content, alkaline treatment with 3 wt% of NaOH solution was performed on RS at 121 °C for 30 min. The chemical compositions of the raw and alkali-treated RS are summarized in Table 4. After the treatments, the content of lignin in the RS decreased to 1.5%; however, the cellulose content increased to 64.3%. Low lignin content is advantageous for enzymatic hydrolysis by cellulase [48, 49]. Figure 7 shows the hydrolysis yield time profiles from the alkali-treated RS at incubation times during 120 h by produced cellulase and commercial cellulase (AC). The substrate concentration and cellulase loading were 50 g/L and 26 FPU/g-substrate, respectively. A similar high hydrolysis yield was observed, and almost 100% of hydrolysis yield were attained at 96 h for cellulose produced from RS via a standard medium without trace elements and RS via standard medium without trace elements and KH2PO4. With the cellulase dosage of 26 FPU/g-substrate, corresponding individual cellulase activities from cellulase produced from 3 kinds of medium and AC were summarized in Table 5. The produced cellulase contained similar CMCase and higher xylanase activities than those of commercial cellulase AC. In contrast, AC contained higher β-glucosidase of 1169 U/g-enzyme than that of produced cellulase from RS, 20–55.6 U/g-enzyme. Based on this result, it was confirmed that the cellulase quality (hydrolysis ability) derived from Mandel’s medium without trace element and KH2PO4 was similar to that derived from Mandel’s medium without trace element, and KH2PO4 had no effect on the quality of cellulase. However, although the cellulase produced exhibited low β-glucosidase activities, the actual hydrolysis yields of alkali-treated RS among the cellulase produced from RS medium and AC were similar. Hu et al. reported that cellulase activity produced via SSF from textile waste as a fermentable substrate comprised a CMCase of 254 U/g-substrate and β-glucosidase of 31,500 U/g-substrate under 25 FPU/g-substrate, which were higher than those of a commercial cellulase, Celluclast 1.5 L from Novozymes (USA), with a CMCase of 114 U/g-substrate and β-glucosidase of 1633 U/g-substrate under 25 FPU/g-substrate. The actual hydrolysis yield of textile waste, cotton/polyester = 80/20, was lower (65%) than that of Celluclast 1.5 L (75%) at an incubation time of 72 h [21]. Kabel et al. reported that enzymatic hydrolysis is more dependent on the characteristics of the substrate than on measured standard enzyme activities [50].
4 Conclusion
This study demonstrated that agricultural wastes such as WS and RS are potential substrates for cellulase production through SSF using T. reesei. We developed a cellulase production medium that was easy to operate based on the amount of trace metal contents, i.e., ash, in WS and RS. Regarding the role of lignin in lignocellulosic materials, components besides cellulose, namely, lignin and hemicellulose, are also presumed to have certain functions in cellulase production in the cellulase producer. Wang et al. reported that cellulase production was performed by A. niger and T. reesei using cotton or a cotton and polyester mixture as carbon substrates. Where only cotton or the cotton and polyester mixture was used as substrates (no lignin and hemicellulose), cellulase activity was low at 1.5 FPU/g-cotton by A. niger and 1.0 FPU/g-mixture by T. reesei. Alternatively, cellulase activity from cotton medium with sawdust (0.1% w/v) or wheat bran (1% w/v) increased and was 8.5 FPU/g-cotton and 3.4 FPU/g-cotton, respectively. Moreover, the cellulase activity from cotton and polyester mixture medium with either sawdust (0.1% w/v) or wheat bran (1% w/v) increased and was found to be 7.1 FPU/g-mixture and 8.1 FPU/g-mixture, respectively [51].
Furthermore, the cellulase produced from easy-to-handle RS medium was found to be efficient in hydrolyzing agricultural wastes indicating their potential for sustainable utilization for the remediation of cellulose biomass into valuable products. Scale-up experiments and cost and energy estimations are necessary components for the commercialization of these processes. This will be the focus of the next study.
Data availability
Not applicable.
Change history
20 November 2022
The comma in the organism strain nos. such as T. reesei 56,765 and 13,631 have been removed.
References
Bryngemark E (2019) Second generation biofuels and the competition for forest raw materials: a partial equilibrium analysis of Sweden. For Policy Econ 109:102022. https://doi.org/10.1016/j.forpol.2019.102022
Robak K, Balcerek M (2020) Current state-of-the-art in ethanol production from lignocellulosic feedstocks. Microbiol Res 240:126534. https://doi.org/10.1016/j.micres.2020.126534
Parisutham V, Kim TH, Lee SK (2014) Feasibilities of consolidated bioprocessing microbes: from pretreatment to biofuel production. Bioresour Technol 161:431–440. https://doi.org/10.1016/j.biortech.2014.03.114
Gupta A, Verma JP (2015) Sustainable bio-ethanol production from agro-residues: a review. Renew Sustain Energy Rev 41:550–567. https://doi.org/10.1016/j.rser.2014.08.032
Kuhad RC, Gupta R, Singh A (2011) Microbial cellulases and their industrial applications. Enzyme Res 2011:280696. https://doi.org/10.4061/2011/280696
Verma N, Kumar V, Bansal MC (2018) Utility of Luffa cylindrica and Litchi chinensis peel, an agricultural waste biomass in cellulase production by Trichoderma reesei under solid state cultivation. Biocatal Agric Biotechnol 16:483–492. https://doi.org/10.1016/j.bcab.2018.09.021
Niyonzima FN (2019) Detergent-compatible bacterial cellulases. J Basic Microbiol 59:134–147. https://doi.org/10.1002/jobm.201800436
Reith JH, Den Uil H, Van Veen H, De Laat W, Niessen JJ, De Jong E, Elbersen HW, Weusthuis RA, Van Dijken JP, Van Raamsdonk LWD (2002) Co-production of bio-ethanol, electricity and heat from biomass residues. In: Proceedings of the 12th European Conference on Biomass for Energy, Industry and Climate Protection, 17–21 June 2002, Amsterdam, The Netherlands, 1118–1123.
Krishna C (2005) Solid-state fermentation systems—an overview. Crit Rev Biotechnol 25:1–30. https://doi.org/10.1080/07388550590925383
Cen P, Xia L (1999) Production of cellulase by solid-state fermentation. In: Recent Progress in Bioconversion of Lignocellulosics, 69–92. Springer. https://doi.org/10.1007/3-540-49194-5_4
Raimbault M (1998) General and microbiological aspects of solid substrate fermentation. Electron J Biotechnol 1:26–27
Oke MA, Annuar MSM, Simarani K (2016) Enhanced endoglucanase production by Bacillus aerius on mixed lignocellulosic substrates. BioResources 11(3):5854–5869. https://doi.org/10.15376/Biores.11.3.5854-5869
Wagner AO, Schwarzenauer T, Illmer P (2013) Improvement of methane generation capacity by aerobic pre-treatment of organic waste with a cellulolytic Trichoderma viride culture. J Environ Manage 129:357–360. https://doi.org/10.1016/j.jenvman.2013.07.030
Swain MR, Singh A, Sharma AK, Tuli DK (2019) Bioethanol production from rice- and wheat straw: an overview. In: Bioethanol Production from Food Crops, 213–231. Academic Press https://doi.org/10.1016/B978-0-12-813766-6.00011-4
Taherzadeh-Ghahfarokhi M, Panahi R, Mokhtarani B (2019) Optimizing the combination of conventional carbonaceous additives of culture media to produce lignocellulose-degrading enzymes by Trichoderma reesei in solid state fermentation of agricultural residues. Renew Energy 131:946–955. https://doi.org/10.1016/j.renene.2018.07.130
Idris ASO, Pandey A, Rao SS, Sukumaram RK (2017) Cellulase production through solid-state tray fermentation, and its use for bioethanol from sorghum stover. Bioresour Technol 242:265–271. https://doi.org/10.1016/j.biortech.2017.03.092
Biswal D, Mandavgane SA (2021) Biomass wastes: a potential feedstock for cellulase production. Curr Status Future Scope Microbial Cellulases https://doi.org/10.1016/B978-0-12-821882-2. 00017-X
Santos GB, Filho ASF, Rodrigues JRS, Souza RR (2022) Cellulase production by Aspergillus niger using urban lignocellulosic waste as substrate: Evaluation of different cultivation strategies. J Environ Manag 305:114431. https://doi.org/10.1016/j.jenvman.2022.114431
Darabzadeh N, Hamidi-Esfahani Z, Hejazi P (2019) Optimization of cellulase production under solid-state fermentation by a new mutant strain of Trichoderma reesei. Food Sci Nutr 7:572–578. https://doi.org/10.1002/fsn3.852
Ezeilo UR, Wahab RA, Tin LC, Zakaria II, Huyop F, Mahat NA (2020) Fungal-assisted valorization of raw oil palm leaves for production of cellulase and xylanase in solid state fermentation media. Waste Biomass Valorization 11:3133–3149. https://doi.org/10.1007/s12649-019-00653-6
Hu Y, Du C, Leu SY, Jing H, Li X, Lin CSK (2018) Valorisation of textile waste by fungal solid state fermentation: an example of circular waste-based biorefinery. Resour Conserv Recycl 129:27–35. https://doi.org/10.1016/j.resconrec.2017.09.024
Reczey K, Szengyel ZS, Eklund R, Zacchi G (1996) Cellulase production by T. reesei. Bioresour Technol 57:25–30. https://doi.org/10.1016/0960-8524(96)00038-7
Olsson L, Christensen TMIE, Hansen KP, Palmqvist EA (2003) Influence of the carbon source on production of cellulases, hemicellulases and pectinases by Trichoderma reesei Rut C-30. Enzyme Microb Technol 33:612–619. https://doi.org/10.1016/S0141-0229(03)00181-9
Ahamed A, Vermette P (2009) Effect of culture medium composition on Trichoderma reesei’s morphology and cellulase production. Bioresour Technol 100:5979–5987. https://doi.org/10.1016/j.biortech.2009.02.070
TAPPI T222 om-88 (1992) Acid-insoluble lignin in wood and pulp. Technical Association of the Pulp and Paper Industries Test Methods. TAPPI Press, Atlanta, Georgia, TAPPI 1992–1993.
Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28:350–356. https://doi.org/10.1021/ac60111a017
Mandels M, Weber J (1969) The production of cellulases. In: Cellulases and Their Applications, 391–414. American Chemical Society https://doi.org/10.1021/ba-1969-0095.ch023
Adney B, Baker J (2008) Measurement of cellulase activities. Laboratory Analytical Procedure, Technical Report NREL/TP-510–42628.
Miller GL (1959) Use of dinitrosalicylic acid recagent for determination of reducing sugar. Anal Chem 31:426–428. https://doi.org/10.1021/ac60147a030
Ghose TK (1987) Measurement of cellulase activities. Pure Appl Chem 59:257–268. https://doi.org/10.1351/pac198759020257
Ghose TK, Bisaria VS (1987) Measurement of hemicellulase activities. Part 1: xylanases. Pure Appl Chem 59:1739–1751. https://doi.org/10.1351/pac198759121739
Herr D (1979) Secretion of cellulase and β-glucosidase by Trichoderma viride ITCC-1433 in submerged culture on different substrates. Biotechnol Bioeng 21:1361–1371. https://doi.org/10.1002/bit.260210805
Klein D, Eveleigh DE (1998) Trichoderma and Gliocladium. Vol. 1: Basic Biology, Taxonomy and Genetics, vol.1, Taylor and Francis Ltd., London.
Ezeilo UR, Lee CT, Huyop F, Zakaria II, Wahab RA (2019) Raw oil palm frond leaves as cost-effective substrate for cellulase and xylanase productions by Trichoderma asperellum UC1 under solid-state fermentation. J Environ Manage 243:206–217. https://doi.org/10.1016/j.jenvman.2019.04.113
Salomão GSB, Agnezi JC, Paulino LB, Hencker LB, De Lira TS, Tardioli PW, Pinotti LM (2019) Production of cellulases by solid state fermentation using natural and pretreated sugarcane bagasse with different fungi. Biocatal Agric Biotechnol 17:1–6. https://doi.org/10.1016/j.bcab.2018.10.019
Verma N, Kumar V (2020) Impact of process parameters and plant polysaccharide hydrolysates in cellulase production by Trichoderma reesei and Neurospora crassa under wheat bran based solid state fermentation. Biotechnol Reports 25:e00416. https://doi.org/10.1016/j.btre.2019.e00416
Xin F, Geng A (2010) Horticultural waste as the substrate for cellulase and hemicellulase production by Trichoderma reesei under solid-state fermentation. Appl Biochem Biotechnol 162:295–306. https://doi.org/10.1007/s12010-009-8745-2
Vanajakshi J, Subhakar C, Jetty A (2009) Media engineering for the production of cellulases by a novel strain Aspergillus sp IICT-F 141 using wheat bran as raw material. J Pure Appl Microbiol 3(2):477–483
Moran-Aguilar MG, Costa-trigo I, Galderón-Santoyo M, Domínguez JM, Aguilar-Uscanga MG (2021) Production of cellulases and xylanases in solid-state fermentation by different strains of Aspergillus niger using sugarcane bagasse and brewery spent grain. Biochem Eng J 172:108060. https://doi.org/10.1016/j.bej.2021.108060
Amaro-Reyes A, Gracida J, Huizache-Peña N, Elizondo-García N, Salazar-Martínez J, Almendárez BEG, Regalado C (2016) On-site hydrolytic enzymes production from fungal co-cultivation of Bermuda grass and corn cob. Bioresour Technol 212:334–337. https://doi.org/10.1016/j.biortech.2016.04.070
Hölker U, Höfer M, Lenz J (2004) Biotechnological advantages of laboratory-scale solid-state fermentation with fungi. Appl Microbiol Biotechnol 64:175–186. https://doi.org/10.1007/s00253-003-1504-3
Esterbauer H, Steiner W, Labudova I, Hermann A, Hayn M (1991) Production of Trichoderma cellulase in laboratory and pilot scale. Bioresour Technol 36:51–65. https://doi.org/10.1016/0960-8524(91)90099-6
Dhillon GS, Oberoi HS, Kaur S, Bansal S, Brar SK (2011) Value-addition of agricultural wastes for augmented cellulase and xylanase production through solid-state tray fermentation employing mixed-culture of fungi. Ind Crop Prod 34:1160–1167. https://doi.org/10.1016/j.indcrop.2011.04.001
Wen Z, Liao W, Chen S (2005) Production of cellulase by Trichoderma reesei from dairy manure. Bioresour Technol 96:491–499. https://doi.org/10.1016/j.biortech.2004.05.021
Belén M, Salgado JM, Fodríguez N, Cortés S, Converti A, Domínguez JM (2016) Biotechnological productions of citric acid. Braz J Microbiol 41:53–70. https://doi.org/10.1590/S1517-83822010000400005
Shu P, Johnson MJ (1948) The interdependence of medium constituents in citric acid production by submerged fermentation. J Bacteriol 56:577–585. https://doi.org/10.1128/jb.56.5.577-585.1948
Yu XB, Nam JH, Yum HS, Koo YM (1998) Optimization of cellulase production in batch fermentation by Trichoderma reesei. Biotechnol Bioprocess Eng 3:44–47. https://doi.org/10.1007/BF02932483
Sasaki C, Okumura R, Asada C, Nakamura Y (2014) Steam explosion treatment for ethanol production from branches pruned from pear trees by simultaneous saccharification and fermentation. Biosci Biotechnol Biochem 78(1):160–166. https://doi.org/10.1080/09168451.2014.877818
Ladeira Azar RIS, Bordignon-Junior SE, Laufer C, Specht J, Ferrier D, Kim D (2020) Effect of lignin content on cellulolytic saccharification of liquid hot water pretreated sugarcane bagasse. Molecules 25:623–634. https://doi.org/10.3390/molecules25030623
Kabel MA, Van der Maarel MJEC, Klip G, Voragen AGJ, Schols HA (2006) Standard assays do not predict the efficiency of commercial cellulase preparations towards plant materials. Biotechnol Bioeng 93:56–63. https://doi.org/10.1002/bit.20685
Wang H, Kaur G, Pensupa N, Uisan K, Du C, Yang X, Lin CSK (2018) Textile waste valorization using submerged filamentous fungal fermentation. Process Saf Environ Prot 118:143–151. https://doi.org/10.1016/j.psep.2018.06.038
Acknowledgements
This study is based on the results obtained from a project, JPNP20011, commissioned by the New Energy and Industrial Technology Development Organization (NEDO).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. The roles are shown as follows: conceptualization, all of authors; methodology, C. Sasaki and K. Matsuura; formal analysis and investigation, C. Sasaki; writing — original draft preparation, C. Sasaki; writing — review and editing, all of authors; funding acquisition, K. Matsuura and T. Omasa; and supervision, T. Omasa. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sasaki, C., Matsuura, K. & Omasa, T. Cellulase production on easy-to-handle solid media containing agricultural waste and its application for enzymatic hydrolysis of cellulosic biomass. Biomass Conv. Bioref. (2022). https://doi.org/10.1007/s13399-022-03518-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13399-022-03518-6