Abstract
Sepsis-associated encephalopathy causes brain dysfunction that can result in cognitive impairments in sepsis survivor patients. In previous work, we showed that simvastatin attenuated oxidative stress in brain structures related to memory in septic rats. However, there is still a need to evaluate the long-term impact of simvastatin administration on brain neurodegenerative processes and cognitive damage in sepsis survivors. Here, we investigated the possible neuroprotective role of simvastatin in neuroinflammation, and neurodegeneration conditions of brain structures related to memory in rats at 10 days after sepsis survival. Male Wistar rats (250–300 g) were submitted to cecal ligation and puncture (CLP, n = 42) or remained as non-manipulated (naïve, n = 30). Both groups were treated (before and after the surgery) by gavage with simvastatin (20 mg/kg) or an equivalent volume of saline and observed for 10 days. Simvastatin-treated rats that survived to sepsis showed a reduction in the levels of nitrate, IL1-β, and IL-6 and an increase in Bcl-2 protein expression in the prefrontal cortex and hippocampus, and synaptophysin only in the hippocampus. Immunofluorescence revealed a reduction of glial activation, neurodegeneration, apoptosis, and amyloid aggregates confirmed by quantification of GFAP, Iba-1, phospho Ser396-tau, total tau, cleaved caspase-3, and thioflavin-S in the prefrontal cortex and hippocampus. In addition, treated animals presented better performance in tasks involving habituation memory, discriminative, and aversive memory. These results suggest that statins exert a neuroprotective role by upregulation of the Bcl-2 and gliosis reduction, which may prevent the cognitive deficit observed in sepsis survivor animals.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In sepsis, peripheral overproduction of pro-inflammatory cytokines and nitric oxide through the activation of endothelial cells contributes to an increase in blood-brain barrier (BBB) permeability (Handa et al. 2008). Inflammatory mediators may reach the central nervous system (CNS) through the activation of primary afferent, vagus, and trigeminal nerves (neural pathway), or by penetrating via the choroid plexus and circumventricular organs (CVOs), structures devoid of a BBB (humoral pathway) (Sonneville et al. 2013). The presence of these mediators in the CNS alters neurotransmitter synthesis, promotes microglial activation, neuronal apoptosis, and activation of immunological cascades (Hshieh et al. 2008; Semmler et al. 2008; Taccone et al. 2010; Comim et al. 2013; Oliveira-Pelegrin et al. 2013; da Costa et al. 2017). All these mechanisms contribute to the establishment of an inflammatory process in the CNS that, even in the absence of an in situ infection, will culminate in a diffuse cerebral dysfunction denominated sepsis-associated encephalopathy (SAE) (van Gool et al. 2010; Kettenmann et al. 2013).
SAE, which can reach in some cases 70% of sepsis patients, may be an independent predictor of mortality (Sprung et al. 1990; Maramattom 2007; Young et al. 1990) and can be associated with cognitive impairment in a large proportion of survivors (Calsavara et al. 2013; Chaudhry and Duggal 2014). Approximately 45% of surviving patients with severe sepsis present cognitive impairment after 1 year of hospital discharge (Hopkins et al. 1999; Hopkins et al. 2005), and even after eight years, cognitive deficits such as memory impairment are still observed in some of those patients (Iwashyna et al. 2010; Adam et al. 2013). This persistent cognitive dysfunction in sepsis survivor patients diagnosed with SAE is accompanied by hippocampal atrophy and electroencephalogram disturbances (Semmler et al. 2013). In experimental models of SAE, damages to hippocampus and cortex were associated to impaired long-term potentiation (LTP) and reduced learning and memory (Comim et al. 2011a, b; Imamura et al. 2011; Field et al. 2012). Some authors (Olivieri et al. 2018; Schwalm et al. 2014; Gasparotto et al. 2018) have proposed that brain deposition of the amyloid-β (Aβ) peptide, which is known to occur under inflammatory conditions through cytokine-mediated upregulation of β-amyloid cleaving enzyme-1 (BACE-1) (Guo et al. 2002; Chen et al. 2012; Sastre et al. 2003), may contribute to cognitive dysfunctions in sepsis survivors.
Based on the cognitive impact of SAE, and the consequent risk for the development of dementias, it becomes essential to develop strategies to attenuate the neurodegenerative processes triggered by SAE (Benveniste et al. 2001; Ransohoff 2016). In this scenario, statins, in addition to be clinically employed in vascular diseases via the well-known inhibition of HMG-CoA reductase, also exhibit anti-inflammatory and antioxidant effects (Catalão et al. 2017; Kim et al. 2002; Qin et al. 2019; Reis et al. 2017),and therefore have been investigated as an option to treat the late consequences of SAE.
The pleiotropic effects of statins are known to be in part related to the inhibition of the synthesis of isoprenoid compounds, such as farnesyl pyrophosphate (FPP) and geranylgeranyl pyrophosphate (GGPP), which limits G protein prenylation and their consequent binding to the plasma membrane to trigger cellular signaling cascades leading to production of inflammatory, endothelial mediators, and reactive oxygen species (ROS) (Greenwood et al. 2006). Nonetheless, clinical studies relating statins and sepsis are still rather controversial about the benefits of these compounds (Martin et al. 2007; Kopterides and Falagas 2009; Piechota et al. 2013; Wan et al. 2014; Deshpande et al. 2015; Mehl et al. 2015). Recent studies have reported that treatment with statins led to decreased glial activation, reduction of microvascular damage and apoptosis, restoration of balance in the redox system, and regulation of mitochondrial bioenergetics in brain structures related to the memory of rats and mice 48 h after sepsis (Catalão et al. 2017; Reis et al. 2017). However, these studies did not analyze whether these brain alterations persist in the form of long-term impaired memory observed in animals that survive days after sepsis. Despite of those promising results, important gaps still need to be filled in order to obtain a consensus regarding the use of statins as a therapeutic strategy to face neuroinflammation and cognitive damage in sepsis survivors (Piechota et al. 2013). For instance, quantification of biomarkers of neurodegeneration in parallel to behavioral tests in SAE in rats was not performed yet, and in our view, this approach would allow a better comprehension of the molecular effects of statins.
Here, we investigated the effects of simvastatin in cecal ligation and puncture (CLP)–induced polymicrobial sepsis in 10-day survivor rats by monitoring neuroinflammatory and neurodegenerative events through quantification of dementia-related biomarkers in structures responsible for learning and memory.
Materials and Methods
Animals
Male Wistar rats (280 ± 30 g) provided by the Animal Facility of the Campus of Ribeirão Preto, University of São Paulo, were housed in controlled temperature (25 ± 1 °C) and photoperiodic (12:12 h night:day cycle) conditions, with food (Nuvilab CR-1, NUVITAL) and tap water available ad libitum. All experiments were carried out according to the National Council of Animal Experiment Control (CONCEA) and with approval by the Institutional Animal Care and Use Committee at the School of Dentistry of Ribeirão Preto, University of São Paulo (protocol number: 2019.1.51.58.6). We used humane endpoints in shock research (Nemzek et al. 2004) as criteria to euthanize CLP animals in high suffering, immediately before or soon after the studied time points defined in this study.
Cecal Ligation and Puncture Surgery and Drug Administration
Animals were randomly assigned to one of two groups, CLP, or naïve (non-manipulated animals). All experiments were performed at the same time of day (08:00–10:00). Sepsis was induced in experimental rats by the surgical procedure of CLP according to our previous studies (Catalão et al. 2017) and showed that untreated septic animals had 70% mortality rate, while simvastatin-treated animals 40%. This difference in the mortality rate between septic groups was not significant (data not shown).
Experimental Protocol
Simvastatin (Merck Sharp & Dohme, UK) dissolved in sterile saline or saline only was administered by gavage, daily at 14:00, 4 days before and 10 days after CLP surgery. The same protocol, without surgery, was applied to the group of naïve rats. We chose 10 days after CLP-induced sepsis (cecum perforated once with a 14-gauge needle) because this is considered by several authors to be the initial time point that marks the full recovery of the animals considering them as survivors of sepsis (Barichello et al. 2005; Cassol et al. 2010; Steckert et al. 2015). Additionally, the dose of 20 mg/kg simvastatin was determined from a pharmacokinetic study using different doses (20 mg /kg, 40 mg/kg, and 80 mg/kg) according to our previous study (Catalão et al. 2017). Since the oral route is the most common way to administer statins to patients who use this medication continuously, we used oral gavage as a route of administration to simulate what occurs in clinical practice. Moreover, simvastatin is a lipophilic statin with a high penetration capacity across the blood-brain barrier (Saheki et al. 1994; Vuletic et al. 2006), and there are several findings putting in evidence its action on the CNS, including of our group (Stein et al. 2015; Catalão et al. 2017; Reis et al. 2017; Zheng et al. 2018). After 10 days, the survivor animals were split into two groups. In one group, the animals were anesthetized and fixed in the stereotaxic apparatus to collect the cerebrospinal fluid (CSF) as described by Consiglio and Lucion (2000). Subsequently, the animals were decapitated or anesthetized and perfused with PBS (0.01 M) for blood collection and brain removal. CSF and blood were used for the determination of nitrate and cytokine concentrations. The brains collected from the decapitated animals were removed and immediately fixed in paraformaldehyde (4%), following specific protocols for immunohistochemistry or immunofluorescence. The prefrontal cortex and hippocampus of the perfused animals were dissected for determination of glial activation, neurodegenerative, and apoptotic biomarkers. Finally, the other animal group was randomized to perform the behavioral test after 10 days of simvastatin treatment.
Nitrate and Cytokine Determination
Total nitrate was determined by means of the purge system of a Sievers Instruments Nitric Oxide Analyzer (NOA model 280i, Boulder, CO, USA), as described in previous work of this laboratory (Wahab et al. 2016).
IL-1β and IL-6 concentrations were determined using specific enzyme-linked immunosorbent assay (ELISA) kits for each cytokine (R&D Systems, Minneapolis, MN, USA) according to the manufacturer’s instructions. The detection limits for IL-1β and IL-6-specific ELISA kits were 5, 10, and 5 pg/mL, respectively. The samples were analyzed in a microplate reader (Synergy™ H1, BioTek® Instruments, Inc.).
Immunofluorescence and Immunohistochemical Assays
The animals were perfused with 250 mL of PBS followed by 250 mL of fixative solution (4% paraformaldehyde in 0.1 mol/L phosphate buffer). For immunofluorescence, the brains were removed, post-fixed in a fixative solution for 4 h, placed in PBS containing 30% sucrose, and stored at 4 °C. The brain coronal sections were cut with a cryostat (Microm HM 505 E) and the free-floating sections (40 μm) were submitted to an antigen retrieval protocol, for 30 min at 70 °C. After washing three times with PBS, nonspecific binding sites were blocked for 60 min with 5% normal goat serum and 0.3% Triton X-100 in PBS. Posteriorly, the sections were incubated overnight at 4 °C with either Iba-1 (WAKO, 1:1000) or GFAP (Millipore, 1:7000) antibody. After rinsing again, the sections were incubated for 2 h at 4 °C with goat anti-rabbit Alexa Fluor 488 conjugate (Vector, 1:1000). Finally, the sections were mounted on gelatin-coated slides and covered with antifade mounting medium (ProLong® Gold Antifade Mountant, Thermo Fisher Scientific) containing DAPI for nuclear staining.
For immunohistochemical analyses, the brains were post-fixed in paraformaldehyde (4%) for 2 days at 4 °C and then kept in alcohol 70%. After dehydration, the s sections were incubated overnight at 4 °C with a cleaved caspase-3 antibody (Cell Signaling, 1:300), followed by incubation with a secondary HRP-conjugated antibody (Abcam, 1:1000). Subsequently, they were reacted with diaminobenzidine (DAB (Sigma-Aldrich, D5905)) for 2 min. Staining specificity was checked by the omission of the primary antibody in some sections, resulting in the complete elimination of the immunoreaction signal. Images were captured using an AxioCam MRc system (Zeiss) coupled to the Zeiss KS300 microscope. The anatomical description of brain regions was done according to the rat brain atlas of Paxinos and Watson (2005).
Thioflavin-S Histochemistry
Brain sections were mounted on glass slides and allowed to completely air dry prior to staining. Subsequently, the slides were washed with 70% and 80% ethanol for 1 min each and incubated in filtered (0.2 μm filter) thioflavin-S (Sigma-Aldrich, T1892) solution (1% in 80% of ethanol) for 15 min. The slides were again washed with 80% and 70% ethanol for 1 min each, followed by two washes with distilled water. Finally, after 2 h of drying in the dark, the slides were cover slipped and sealed with clear nail polish. Green fluorescence-stained plaques were visualized by fluorescence microscopy, and images were captured using an AxioCam MRc system (Zeiss) coupled to the Zeiss KS300 microscope. Extracts of the prefrontal cortex and hippocampus were prepared in RIPA buffer containing protease inhibitors (Sigma-Aldrich) and centrifuged at 2000×g for 2 min at 4 °C. The supernatant (5 μL) was incubated for 5 min in 195 μL PBS containing 200 μM thioflavin-S. A standard curve of Aβ42 (Sigma-Aldrich, A9810) (0–11 μM) was prepared in PBS and amyloid fibril formation was monitored by thioflavin-S fluorescence for 24 h (Xue et al. 2017). Thioflavin-S binding to amyloid fibrils was determined at 450/482 nm excitation/emission (Naiki et al. 1989) in a fluorescence spectrophotometer (Synergy 2, BioTek Instruments, Inc., Winooski, USA).
Western Blot Assays
The prefrontal cortex and hippocampus were dissected from brain samples and immersed in RIPA buffer, containing a 10% protease inhibitor cocktail and 0.5% of phenylmethylsulfonyl fluoride (Sigma-Aldrich). Following homogenization and centrifugation, the supernatant was collected. Proteins (30 μg/sample) were separated electrophoretically (125 V, 90 min) in 12% SDS-polyacrylamide gels. After electrophoresis, proteins were blotted to a nitrocellulose membrane (0.45 μm pore size; Millipore) in a tank blotting system (125 V, 90 min). The membranes were kept in blocking solution (BSA 5% in PBS, with 0.2% Tween 20) for 1 h and then incubated overnight at 4 °C with specific primary antibodies for Iba-1 (WAKO, 1:2000), GFAP (Millipore, 1:1000), phospho Ser396-tau (Abcam, 1:1000), total tau (Abcam, 1:1000), synaptophysin (Cell Signaling, 1:3000), Bcl-2 (Santa Cruz, 1:1000), and cleaved caspase-3 (Cell Signaling, 1:1000), and then incubated for 2 h at 4 °C with a secondary HRP-conjugated antibody (Abcam, 1:10000). A chemiluminescence reaction kit (GE Healthcare) was used for detection, and immunolabeled protein bands were visualized in a ChemiDoc MP System (Bio-Rad) and analyzed by the ImageLab 5.2.1 software. A β-actin-specific antibody was used for normalization of the samples. The western blot assays and analysis were done according to the previous works of this laboratory (Santos-Junior et al. 2018; Catalão et al. 2019).
Open Field Task
The animal habituation to an open field was tested in an acrylic arena (46 × 46 × 46 cm) surrounded by infrared sensors for detecting the position of the animal during the monitoring period. Thus, it was possible to calculate the average speed and the total distance walked using dedicated software (Insight, Ribeirão Preto, Brazil). In the training session, the animals were placed in the center of the arena to explore it for 5 min. In the test session, 24 h later, they were returned to the arena to explore it for another 5 min. The total distance walked and rearings performed in both sessions were counted. The decrease in distance walked and rearings between the two sessions was taken as a measure of the retention of habituation (Vianna et al. 2000).
Object Recognition Task
An object recognition task assay was carried out as described in previous studies (Barker and Warburton 2011). Habituation was observed by placing the animals for 20 min in a wooden box (50 × 50 × 90 cm) without any of the objects. On the following day, the animals were again placed in the empty box to explore it for 3 min. A training session was conducted by placing an individual rat for 5 min in the box with two identical objects (objects A1 and A2; Double Lego Toys) positioned in the bottom of the box in the left and right corner, respectively. After 3 h in the test session, the rat was allowed to explore the box for 5 min in the presence of one familiar (A) and one novel (B) object. The objects were distinct in shape and color. The exploratory preference was defined as the percentage of the total exploration time of the animal spent investigating object A or the novel object, and from this, we calculated for each animal the ratio TB/(TA + TB) (TA = time spent exploring the familiar object A; TB = time spent exploring the novel object B).
Contextual Fear Conditioning Task
The apparatus used was an acrylic box (50 × 25 × 25 cm) whose floor consisted of 18 parallel-caliber stainless-steel bars (1 mm diameter), spaced 1 cm apart, and connected to an automatic shock generator (scrambler, Insight, Ribeirão Preto, Brazil). The conditioning session consisted of a habituation time (5 min) and 10 shocks to the paws (1.0 mA, 1 s) with a 60-s interval between them. During habituation, the basal freezing time of the animal (defined as the complete immobilization of the animal, except for respiratory movements) was quantified. In the intervals between the shocks, the freezing time was quantified in fractions of 15 s. After 24 h and 5 days, the animals were re-exposed to the same context of the conditioning session, but without the application of shocks, and its freezing reaction was counted every 15 s during 8 min to evaluate the short-term memory (STM) and long-term memory (LTM), respectively.
Statistical Analysis
All results are expressed as mean ± SEM. Nitrate, cytokines, synaptophysin, Bcl-2, phospho Ser396-tau, total tau, GFAP, Iba-1, amyloid fibrils, cleaved caspase-3, and contextual fear conditioning task results were analyzed by one-way analysis of variance (ANOVA) followed by Tukey’s post hoc test for multiple comparisons. Behavioral tests, such as open field task and object recognition task, were analyzed using an unpaired Student’s t test for data with normal distribution, or a Mann–Whitney test in case of a nonparametric data distribution. The software used was GraphPad© Prism 7.00 (San Diego, CA, USA). Results were considered statistically significant when P < 0.05 (*P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001).
Results
Plasma and CSF Levels of Nitrate and Cytokines in 10-Day Sepsis Survivor Animals Following Simvastatin Treatment
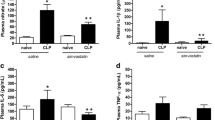
Sepsis caused an increase in CSF nitrate levels (F(3, 12) = 7.859; P > 0.01), IL-1β (F(3, 16) = 6921; P < 0.01), and IL-6 (F(3, 13) = 14.05; P < 0.01). When simvastatin was administered, we observed a decrease in CSF nitrate levels (F(3, 12) = 7.859; P < 0.05), IL-1β (F(3, 16) = 6921; P < 0.05), and IL-6 (F(3, 13) = 14.05; P < 0.001) when compared with vehicle-treated CLP animals. There was no significant difference in plasma nitrate and cytokine levels between groups (Fig. 1).
Effect of treatment with simvastatin (20 mg/kg, p.o.) or saline 4 days before and 10 days after CLP surgery on nitrate and pro-inflammatory cytokine levels in the plasma and CSF of sepsis survivor rats. Sepsis caused an increase in nitrate (d), IL-1β (e), and IL-6 (f) levels in the CSF. Simvastatin treatment prevented the increase in nitrate and in these cytokines in the CSF. Nitrate (a), IL-1β (b), and IL-6 (c) levels in the plasma were not affected in any of the groups. Bars indicate mean ± SEM (n = 5–7 animals per group). One-way ANOVA with Tukey’s multiple comparison test correction.*P < 0.05 (d, e) vs. CLP+Sal. **P < 0.01 vs. Ctr+Sal (d, e, f) and Ctr+Sv (d, e, f). ***P < 0.001 vs. CLP+Sal (f). Ctr+Sal (naïve animals treated with saline); CLP+Sal (septic animals treated with saline); Ctr+Sv (naïve animals treated with simvastatin); CLP+Sv (septic animals treated with simvastatin)
Glial Activation in Prefrontal Cortex and Hippocampus of 10-Day Sepsis Survivor Animals Following Simvastatin Treatment
The number of reactive astrocytes stained with GFAP was significantly increased in the prefrontal cortex (F(3, 17) = 12; P < 0.001) and hippocampus (F(3, 20) = 4.181; P < 0.05) of the sepsis survivor animals (Fig. 2a, b, and c) compared with naïve animals. This was also the case for microglia immunolabeled with Iba-1 in the prefrontal cortex (F(3, 22) = 8.496; P < 0.01), dentate gyrus, and CA1 region (hippocampus: F(3, 17) = 15.95; P < 0.001) (Fig. 2d, e, and f). Simvastatin administration prevented astrogliosis in both structures (prefrontal cortex: F(3, 17) = 12; P < 0.05; hippocampus: F(3, 20) = 4.181; P < 0.05) and mitigated microglia activation (prefrontal cortex: F(3, 22) = 8.496; P < 0.01; hippocampus: F(3, 17) = 15.95; P < 0.05).
Effect of treatment with simvastatin (20 mg/kg, p.o.) or saline 4 days before and 10 days after CLP surgery on glial activation in sepsis survivor rats. Photomicrographs of different regions of rat brains immunostained for GFAP (a) and Iba-1(d): PF, prefrontal cortex; DG, dentate gyrus; CA1, cornu ammonis area 1. In all these regions, the CLP+Sal group showed reactive astrocytes with marked hypertrophic processes. In contrast, the CLP+Sv group showed scattered astrocytes with thin astrocyte processes, similar to the picture seen in Ctr+Sal and Ctr+Sv groups (a). Simvastatin administration prevented astrogliosis in the prefrontal cortex (b) and hippocampus (c) of the sepsis survivor animals by western blot analysis. Bars indicate mean ± SEM (n = 5–7 animals per group). One-way ANOVA with Tukey’s multiple comparison test correction. *P < 0.05 vs. Ctr+Sal (c), Ctr+Sv (c) and CLP+Sal (b, c). ***P < 0.001 vs. Ctr+Sal (b) e Ctr+Sv (b). In the CLP+Sal group, microglia presented typical ameboid shape of activation with round bodies and scarce dendrites in all these regions. In contrast, the CLP+Sv group showed suppression of these activation microglial. The Ctr+Sal and Ctr+Sv groups showed resident microglia with fine and short processes (d). Scale bar, 20 μm. Simvastatin administration decreased significantly the amount of Iba1+ microglia in the prefrontal cortex (e) and hippocampus (f) of the sepsis survivor animals by western blot analysis. Bars indicate mean ± SEM (n = 5–7 animals per group). One-way ANOVA with Tukey’s multiple comparison test correction. *P < 0.05 vs. CLP+Sal (f). **P < 0.01 vs. Ctr+Sal (e), Ctr+Sv (e) and CLP+Sal (e). ***P < 0.001 vs. Ctr+Sal (f) e Ctr+Sv (f). Ctr+Sal (naïve animals treated with saline); CLP+Sal (septic animals treated with saline); Ctr+Sv (naïve animals treated with simvastatin); CLP+Sv (septic animals treated with simvastatin)
Levels of Neurodegeneration Biomarkers in the Prefrontal Cortex and Hippocampus of 10-Day Sepsis Survivor Animals Following Simvastatin Treatment
The sepsis survivor animals showed increased brain levels of phospho Ser396-tau protein (prefrontal cortex: F(3, 20) = 27.42; P < 0.001; hippocampus: F(3, 20) = 4.884; P < 0.05) (Fig. 3d and f), species associated to abnormal Tau hyperphosphorylation and neurodegeneration (Bramblett et al. 1993). Total tau levels were also elevated in sepsis survivor animals (prefrontal cortex: F(3, 20) = 7.302; P < 0.01; hippocampus: F(3, 20) = 11.58; P < 0.001) (Fig. 3e and g). Amyloid aggregates were readily found in the brains of sepsis survivors (prefrontal cortex: F(3, 20) = 6.905; P < 0.05; hippocampus: F(3, 24) = 7.9; P < 0.01) (Fig. 3a, b, and c). Simvastatin treatment alleviated the increase in both phospho Ser396-tau (prefrontal cortex: F(3, 20) = 27.42; P < 0.001; hippocampus: F(3, 20) = 4.884; P < 0.05) and total tau levels (prefrontal cortex: F(3, 20) = 7.302; P < 0.05; hippocampus: F(3, 20) = 11.58; P < 0.01). A similar positive effect of simvastatin was observed in thioflavin-S-positive amyloid aggregates burden in the prefrontal cortex (F(3, 20) = 6.905; P < 0.01) and the CA1 region of hippocampus (F(3, 24) = 7.9; P < 0.05).
Effect of treatment with simvastatin (20 mg/kg, p.o.) or saline 4 days before and 10 days after CLP surgery on amyloid aggregates burden and neurodegenerative biomarkers in sepsis survivor rats. Photomicrographs of different regions of rat brains stained for thioflavin-S: PF, prefrontal cortex; DG, dentate gyrus; CA1, cornu ammonis area 1. In the CLP+Sal group, cells displayed a large amount of amyloid aggregates in all these regions. However, the CLP+Sv group displayed a reduction in the deposition of these amyloid aggregates. The Ctr+Sal and Ctr+Sv groups displayed no significant amount of amyloid aggregates (a). Scale bar, 20 μm. Simvastatin administration decreased significantly the amount of amyloid fibrils in the prefrontal cortex (b) and hippocampus (c) of the sepsis survivor animals by fluorometric determination. Bars indicate mean ± SEM (n = 5–7 animals per group). One-way ANOVA with Tukey’s multiple comparison test correction. *P < 0.05 vs. Ctr+Sal (b); Ctr+Sv (b, c) and CLP+Sal (c). **P < 0.01 vs. Ctr+Sal (c) and CLP+Sal (b). The brain of septic animals showed an increase in the expression of phospho Ser396-tau and total tau protein. Simvastatin-treated septic animals showed a decrease in these markers in both the prefrontal cortex (d and e) and hippocampus (f and g). Bars indicate mean ± SEM (n = 5–7 animals per group). One-way ANOVA with Tukey’s multiple comparison test correction. *P < 0.05 vs. Ctr+Sal (f); Ctr+Sv (f) and CLP+Sal (e, f). **P < 0.01 vs. Ctr+Sal (e); Ctr+Sv (e) and CLP+Sal (g). ***P < 0.001 vs. Ctr+Sal (d, g); Ctr+Sv (d, g) and CLP+Sal (d). Ctr+Sal (naïve animals treated with saline); CLP+Sal (septic animals treated with saline); Ctr+Sv (naïve animals treated with simvastatin); CLP+Sv (septic animals treated with simvastatin)
Immunostaining of Cleaved Caspase-3 in the Prefrontal Cortex and Hippocampus of 10-Day Sepsis Survivor Animals Following Simvastatin Treatment
Sepsis led to an increase in the expression of cleaved caspase-3 in the rat brains after sepsis (prefrontal cortex: F(3, 23) = 8.82, P < 0.01; hippocampus: F(3, 25) = 19.76, P < 0.001) (Fig. 4b and c). Simvastatin treatment mitigated apoptosis in the prefrontal cortex (F(3, 23) = 8.82, P < 0.01) (Fig. 4b) and the hippocampus (F(3, 25) = 19.76, P < 0.001) (Fig. 4c). This condition was also observed in the immunohistochemistry assay (Fig. 4a).
Effect of treatment with simvastatin (20 mg/kg, p.o.) or saline 4 days before and 10 days after CLP surgery on apoptosis in brains of sepsis survivor rats. Photomicrographs of different regions of rat brains immunostained for cleaved caspase-3: PF, prefrontal cortex; DG, dentate gyrus; CA1, cornu ammonis area 1. In the CLP group, there was intense labeling of apoptotic cells in all these regions. In the CLP+Sv group, there was a significant reduction in the increase of these apoptotic cells. The Ctr+Sal and Ctr+Sv groups displayed no significant amount of apoptotic cells immunolabeled for the cleaved caspase-3 (a). Scale bar, 10 μm. Simvastatin administration decreased significantly the amount of cleaved caspse-3 protein in the prefrontal cortex (b) and hippocampus (c) of the sepsis survivor animals by western blot analysis. Bars indicate mean ± SEM (n = 5–7 animals per group). One-way ANOVA with Tukey’s multiple comparison test correction. **P < 0.01 vs. Ctr+Sal (b); Ctr+Sv (b) and CLP+Sal (b). ***P < 0.001 vs. Ctr+Sal (c); Ctr+Sv (c) and CLP+Sal (c). Ctr+Sal (naïve animals treated with saline); CLP+Sal (septic animals treated with saline); Ctr+Sv (naïve animals treated with simvastatin); CLP+Sv (septic animals treated with simvastatin)
Synaptophysin and Bcl-2 Levels in the Prefrontal Cortex and Hippocampus of 10-Day Sepsis Survivor Animals Following Simvastatin Treatment
The prefrontal cortex of the sepsis survivor animals showed a decrease in synaptophysin (F(3, 19) = 4.387; P < 0.05) and Bcl-2 (F(3, 19) = 4.624; P < 0.05) levels (Fig. 5a, b). In the hippocampus, only synaptophysin levels showed a decrease (F(3, 19) = 6.481; P < 0.05) (Fig. 5c). Simvastatin treatment led to increased Bcl-2 levels in the prefrontal cortex (F(3, 19) = 4.624; P < 0.05) (Fig. 5b) and the hippocampus (F(3, 15) = 13.8; P < 0.001) (Fig. 5d), but prevented the decrease in synaptophysin levels only in the hippocampus (F(3, 19) = 6.481; P < 0.01) of sepsis survivor animals (Fig. 5c).
Effect of treatment with simvastatin (20 mg/kg, p.o.) or saline 4 days before and 10 days after CLP surgery on neurotransmission and anti-apoptotic markers in brains of sepsis survivor rats. The brain of septic animals showed a decrease in synaptophysin and Bcl-2 expression. Simvastatin-treated septic animals showed an increase in Bcl-2 in both the prefrontal cortex (b) and hippocampus (d). The synaptophysin expression increased only in the hippocampus (c) and was not altered in the prefrontal cortex (a). Bars indicate mean ± SEM (n = 5–7 animals per group). One-way ANOVA with Tukey’s multiple comparison test correction. *P < 0.05 vs. Ctr+Sal (a, b, c); Ctr+Sv (a, b, c) and CLP+Sal (b). **P < 0.01 vs. CLP+Sal (c). ***P < 0.001 vs. CLP+Sal (d). Ctr+Sal (naïve animals treated with saline); CLP+Sal (septic animals treated with saline); Ctr+Sv (naïve animals treated with simvastatin); CLP+Sv (septic animals treated with simvastatin)
Behavioral Assessment Through Habituation to Open Field Task in 10-Day Sepsis Survivor Animals
Simvastatin-treated septic animals showed a significant decrease in the distance walked (t = 1.95; df = 6; P < 0.05) (Fig. 6a) and the number of rearings (t = 4.761; df = 6; P < 0.01) (Fig. 6b) between the training and test sessions. These differences were also observed in the groups of non-septic animals, which indicate that in those groups, there was habituation. In contrast, the septic group did not show differences between the training and test sessions in both assessments, indicating damages with respect to the retention of a spatial habituation. In the open field task, there were no differences in distance walked and rearings between the groups in the training session, demonstrating absence of motor damage.
Effect of treatment with simvastatin (20 mg/kg, p.o.) or saline 4 days before and 10 days after CLP surgery on habituation memory by an open field task test (a and b), on discriminative memory assessed by an object recognition task test (c and d) and on aversive memory assessed by a contextual fear conditioning task test (e) in sepsis survivor rats. Sepsis caused a weakening in the retention of habituation, whereas simvastatin treatment prevented this impairment, as seen through the differences in the distance walked (a) and the number of rearings (b) between training and test sessions. Bars indicate mean ± SEM (n = 5–7 animals per group). Unpaired Student’s t test or Mann–Whitney test. *P < 0.05 and **P < 0.01 compared with the training session in the same group. Exploration index of the identical objects (A and A′) in the training session (c) did not present significant differences in any of the experimental groups. Simvastatin-treated septic animals and non-septic animals, but not septic rats, showed a preference for the new object (C) in relation to the familiar object (A), in the test session (d). Bars indicate mean ± SEM (n = 5–7 animals per group). Unpaired Student’s t test or Mann–Whitney test. ***P < 0.001 and ****P < 0.0001 compared with the familiar object (A) in the same group. Shown are the results for the freezing rate of the animals during (COND), 24 h (MCP = short-term memory), and 5 day after the conditioning (MLP = long-term memory) (e). Untreated septic animals showed a significantly lower freezing rate at 24 h and 5 days post-conditioning. Simvastatin administration was able to prevent the MCP and MLP impairments. Bars indicate mean ± SEM (n = 5–7 animals per group). One-way ANOVA with tukey’s multiple comparison test correction. *P < 0.05 vs. Ctr+Sv and CLP+Sal. **P < 0.01 vs. Ctr+Sv. ****P < 0.0001 vs. Ctr+Sal and CLP+Sal. Ctr+Sal (naïve animals treated with saline); CLP+Sal (septic animals treated with saline); Ctr+Sv (naïve animals treated with simvastatin); CLP+Sv (septic animals treated with simvastatin)
Behavioral Assessment Through Object Recognition Task in 10-Day Sepsis Survivor Animals
The septic animals did not present differences (t = 1.044; df = 10; P > 0.05) in the object recognition index; i.e., they did not spend a significantly higher percentage of time exploring the novel object (C) in comparison with the familiar object (A), indicating that sepsis causes impairment to the discriminative memory. In contrast, septic animals treated with simvastatin presented significant differences (t = 10.27; df = 10; P < 0.001) for the object recognition index, showing preference for the new object (Fig. 6 c and d).
Behavioral Assessment Through Contextual Fear Conditioning in Sepsis Survivor Animals
During the conditioning phase to the aversive stimulus, no significant differences were found in the freezing rate between the experimental groups, demonstrating integrity in the phase of memory acquisition. However, septic animals showed a significant decrease in this rate 24 h (F(3, 24) = 6.037; P < 0.05) and 5 days (F(3, 24) = 17.29; P < 0.0001) after the conditioning, suggesting impaired short-term (STM) and long-term (LTM) aversive memory (Fig. 6e). Treatment with simvastatin prevented the reduction in the animals’ freezing reaction evaluated both for the STM (F(3, 24) = 6.037; P < 0.05) and LTM (F(3, 24) = 17.29; P < 0.0001) tests (Fig. 6e).
Discussion
Inflammatory mediators produced during sepsis can reach the CNS and, through the activation of glial cells, contribute to installing a neuroinflammatory process characterized as SAE (van Gool et al. 2010; Kettenmann et al. 2013). In this study, IL-1β, IL-6, and nitrate levels were measured in the CSF and plasma 10 days after sepsis induction in rats in order to evaluate the possible action of simvastatin in survivor animals. Although we found no significant difference in the plasma levels of these inflammatory mediators, their increase in CSF leads us to infer that, even with systemic recovery, there is an important sustained neuroinflammatory process in these surviving animals, and the prior and continuous use of simvastatin was effective in reducing this condition. It is known that the synergistic interaction between IL-1β and other cytokines leads to a higher level of neurotoxicity, which is associated with changes in the performance of behavioral tasks, as observed in septic animals affected by SAE (Allan et al. 2005; Calsavara et al. 2013). Glial cell activation seems to play a key role in SAE pathophysiology (Akiyama et al. 2000; Sonneville et al. 2013). As already reported in previous studies, after 48 h of sepsis induction, the brains of rats and mice showed intense oxidative stress and microglial and astrocytic activation that was mitigated by simvastatin administration (Catalão et al. 2017; Reis et al. 2017). In this present study, we observed that this effect persisted in the brains of rats even at 10 days after they had survived sepsis.
In fact, chronic inflammation plays an essential role in neuronal apoptosis and nuclear factor-κappaB (NF-κB) is a determining factor in its regulation. Recent studies report that statins may act on the regulation of NF-κB signaling pathway by SIRT1 (silent information regulator 1) activation and inhibiting the M1microglia phenotype (Tian et al. 2019; Pan et al. 2018; Zhuo et al. 2018; Lu et al. 2019). This modulation is likely to occur by suppressing microglia activity and blocking the pIκBα/pNF-κB signaling pathway, decreasing downstream inflammatory cytokines (Lu et al. 2019). Besides, NF-κB inactivation associated with SIRT1 elevation inhibits p53-dependent apoptosis in endothelial progenitor cells (Du et al. 2014) and increases Bcl-2 level followed by decreased Bax content in mice fed a high-fat diet (Liu et al. 2019). In our study, although we did not investigate the NF-κB/SIRT1 signaling pathway, we observed a decrease on the Iba-1 expression accompanied by reduction of cytokines, cleaved caspase-3, and increase of Bcl-2 in the brain of simvastatin-treated septic animals. These results reinforce the pleiotropic effects of statins mediated by reduced glial activation.
It is possible that this mechanism explains the decrease of IL-1β levels and the reduction in GFAP protein expression involved in the astrocytic activation (Pekny et al. 2016). Reactive astrocytes can increase expression of genes of the complement cascade and release an unidentified neurotoxin that induces neuronal and oligodendrocyte death leading to cognitive impairment (Liddelow and Barres 2017). The pleiotropic effect of simvastatin in reducing microglial activation may have contributed to the downregulation of astrogliosis, since the cytokine release by activated microglia induces the neurotoxic phenotype of astrocytes (Arranz and De Strooper 2019; Liddelow et al. 2017).
Chronic inflammation is often linked to degenerative conditions, and a common outcome of such condition is cognitive dysfunction, as in Alzheimer’s disease (AD) (McManus and Heneka 2017; Heneka et al. 2018; Heneka 2019). This link led us to investigate the levels of two major AD biomarkers in the pathophysiology of SAE (Calsolaro and Edison 2016). In the 10-day sepsis survivor animals, we observed a considerable increase in amyloid fibrils and phospho Ser396-tau, making it possible to infer that sustained neuroinflammation caused by glial activation culminates in neurodegeneration. It is possible that the observed appearance of amyloid plaques is related to an impairment in the clearance mechanisms of the β-amyloid peptide induced by inflammation, a fact that would increase the risk for the development of a CNS amyloidosis such as sporadic AD (Mawuenyega et al. 2010). In the case of Tau hyperphosphorylation, although we have not observed a significant increase in the phospho-tau/total tau ratio, the detection of elevated levels of phospho Ser396-Tau strongly suggests a propensity to formation of Tau aggregates and consequent neurodegeneration, since phosphorylation at this residue has been shown to modulate Tau conformation towards a more aggregation-prone structure (Chukwu et al. 2018). The accumulation of β-amyloid aggregates could also constitute the molecular culprit responsible for the cognitive impairment in sepsis survivors, since it is known that these aggregates cause synaptic dysfunction, through reduction in synapse density (Shankar and Walsh 2009). Supporting this notion, we have observed a significant reduction in synaptophysin (an important synaptic vesicular glycoprotein) in both the prefrontal cortex and hippocampus of the sepsis survivor animals tested in this study. Therefore, through its pleiotropic effects, simvastatin administered to survivor animals was potentially able to normalize the brain levels of Aβ42, thus reducing the formation of amyloid aggregates and the consequent abnormal tau phosphorylation, besides restoring synaptophysin levels in the hippocampus. This idea is reinforced by the fact that Aβ aggregates induce excessive ROS formation in hippocampal neurons, and simvastatin has been able to attenuate brain oxidative stress in septic animals (Catalão et al. 2017; Figueiredo et al. 2013).
In cerebral dysfunctions, including SAE, glial cells are involved in NF-κB and inducible nitric oxide synthase (iNOS) activation mechanisms, triggering cellular toxicity and neuronal death (Gorina et al. 2011). Additionally, an increase in the hypoxia-inducible factor-1α (HIF-1α) modulates the expression of pro-apoptotic genes, including caspase-3, a common effector playing a central role in all apoptosis pathways (Oliveira-Pelegrin et al. 2014; Jänicke et al. 1998; Elmore 2007). In our study, we observed a reduction in the cleaved caspase-3 protein in the prefrontal cortex and hippocampus of septic animals treated with simvastatin. Similar results obtained in a recent study, also performed in our laboratory, postulated that the decrease in apoptotic markers seen in the brain of septic animals treated with simvastatin was a consequence of the restoration of redox system balance and regulation of mitochondrial bioenergetics (Catalão et al. 2017). Since apoptotic events are preceded by mitochondrial disturbances and are associated with reactive oxygen/nitrogen species (ROS/RNS) production, compounds with antioxidant properties, such as HMG-CoA reductase inhibitors, play a key role in the long-term brain dysfunction prevention imposed by the SAE (Catalão et al. 2017; Reis et al. 2017; Greenwood et al. 2006).
In addition, we observed in our study an increase in the expression of Bcl-2 (a protein involved in anti-apoptotic mechanisms) in the brain of septic survival animals treated with simvastatin. It is possible that the increase in the levels of this protein is related to the reduction of nitrate levels in the CSF, since an elevation in NO metabolites accompanied by increased myeloperoxidase (MPO) activity has been shown to contribute to the apoptosis of neurons and astrocytes (Ambrosini et al. 2005). Furthermore, an exaggerated elevation in nitrate levels with consequent formation of oxidant species has been seen capable of altering the balance between pro- and anti-apoptotic proteins of the prefrontal cortex and hippocampus of septic rats (Semmler et al. 2005; Semmler et al. 2007; Weberpals et al. 2009; Brown and Neher 2010). For example, upregulation of Bax (intracellular pro-apoptotic protein) expression and downregulation of Bcl-2 expression are associated with NO-mediated neurotoxic mechanisms (Matsuzaki et al. 1999; Tamatani et al. 1998). Additionally, neurons with neurofibrillary tangle formation showed reduced levels of Bcl-2, demonstrating the important role this protein plays in neurodegenerative mechanisms (Satou et al. 1995). Several studies have shown that the protective effect of simvastatin is due to increase in Bcl-2 gene expression, and its ability to suppress apoptosis is related to the decrease of the Bax/Bcl-2 ratio (Johnson-Anuna et al. 2005, 2007; Franke et al. 2007). Although we have not analyzed Bax protein in this study, the neuroprotective effect of simvastatin was demonstrated through increasing Bcl-2 protein levels followed by decreased cleaved caspase-3 protein, a common effector in all apoptotic pathways (Elmore 2007; Jänicke et al. 1998). Interestingly, this anti-apoptotic effect of simvastatin seems to occur independent from inhibition of the mevalonate pathway, but is likely due to the stimulation of endothelin-1 and nuclear factor of activated T cells 3 (NFATc3) (Butterick et al. 2010). However, at high concentrations, simvastatin inhibits TNF-α-induced NF-κB activation in a dose-dependent manner by reducing Bcl-2 levels in human myeloid KBM-5 cells (Ahn et al. 2007). Thus, changes in Bcl-2 levels, independent of the mevalonate pathway, can also be explained by activation of its gene expression by the transcription factor NF-κB (Viatour et al. 2003), and high simvastatin concentrations appear to be related to pro-apoptotic effects (Wood et al. 2013). Moderate and low doses of statins, such as those adopted in this study (20 mg/kg), may attenuate cell apoptosis via the elevation of SIRT1 and subsequent inactivation of NF-κB activity (Liu et al. 2019).
One of the advantages of oral administration of simvastatin is the reduction in off-target effects, as it has a high absorption by the intestinal mucosa and a high degree of first-pass hepatic extraction, protecting peripheral tissues from unexpected side effects (Vickers et al. 1990; Blum 1994). However, the various changes that occur in the liver during sepsis may increase the likelihood of the most common side effect of statin use, that is, elevation of liver aminotransferases, contributing to further deterioration of liver function, as demonstrated by several clinical studies (Vasudevan et al. 2005; Elhayany et al. 2012; Chaipichit et al. 2015). A limiting factor in our study was not having measured liver function biomarkers to assess the degree of simvastatin toxicity in our treatment protocol. Nevertheless, we believe that the brain alterations of simvastatin we report on this study can occur with or without these side effects.
From clinical studies, it is known that sepsis survivors may present cognitive disabilities, such as memory and learning deficits that are often confused with neurodegenerative diseases or other dementias (Jackson et al. 2004; Granja et al. 2005; Hopkins et al. 2005; Hough and Curtis 2005). In this study, behavioral tests were performed in order to evaluate the impact of simvastatin on cognitive damages imposed by SAE in sepsis survivor animals. It is worthy of note that during these tests, we did not observe any limiting symptoms, such as locomotor disability in animals treated with simvastatin, since the continuous use of statins may trigger metabolic myopathies, such as rhabdomyolysis generating muscle weakness and compromising behavioral tasks (Ayanian et al. 1988).
The untreated sepsis survivors in this present study showed deficits in memory retention when performing the behavioral tasks at 10 days after sepsis survival. Several behavioral studies using different time points have already indicated such a long-term functional and cognitive decline mechanism in SAE (Barichello et al. 2007; Hernandes et al. 2014; Mina et al. 2014; Schwalm et al. 2014; Michels et al. 2015). However, there are controversies of whether the cognitive and memory deficits found in brain dysfunction are direct consequences of systemic inflammation, or are generated by resident brain cells, such as microglia or astrocytes (Michels et al. 2015). Previous studies from our and another laboratory have demonstrated that systemic inflammation plays a critical role in glial activation through the production of pro-inflammatory mediators, and that NO can reach the CNS by crossing the BBB, which is more permeable in the septic condition (Catalão et al. 2017; Yang et al. 2015).
Subsequently, as demonstrated so far, in sepsis survivors, a sustained glial activation will produce more cytokines, chemokines, and ROS/RNS, supporting a neuroinflammatory environment characterized by high toxicity and leading to the development of a neurodegenerative condition associated with synaptic dysfunction, tissue death, and cognitive impairment. In addition to this mechanism, studies have shown that changes in various receptors, such as those G protein–coupled receptors (GPCRs), may be responsible for the progression of cognitive decline (Xu et al. 2012). The modification of these receptors could trigger a signaling cascade producing messenger substances capable of modulating certain receptors associated with amyloid plaque formation and tau neurotoxicity (Xiong et al. 2004). This mechanism has been used to support investigations of the possible neuroprotective effect of statins in neurodegenerative diseases, since the action of this drug is related to the inhibition of G protein prenylation (Li et al. 2012; Posada-Duque et al. 2013; Ostrowski et al. 2016; Jeong et al. 2018). In AD, sustained glial activation plays a key role in neuroinflammation causing alterations in phagocytosis with consequent insufficiency in the removal of Aβ peptides and synaptic dysfunction (McQuade and Blurton-Jones 2019). We believe that a similar mechanism occurs in SAE because we saw elevations in Iba-1 and GFAP proteins and an increase in amyloid fibrils and a reduction in synaptophysin in the prefrontal cortex and hippocampus of our animals. Therefore, therapeutic strategies capable of modulating activated microglia and reactive astrocytes may be beneficial to prevent cognitive dysfunctions (Shetty et al. 2019). Reinforcing this hypothesis and corroborating our results, microglial inhibition by intracerebroventricular injection of minocycline was seen to decrease acute cerebral oxidative damage and inflammation, preventing long-term cognitive dysfunction in sepsis survivor rats (Michels et al. 2015). In addition, the use of an IL-1β inhibitor (IL-1βra) in septic rats was able to reverse the increase in BBB permeability and pro-inflammatory cytokine levels in the prefrontal cortex, hippocampus, and striatum, preventing cognitive impairment (Mina et al. 2014).
Finally, we believe that reducing gliosis has contributed to improve animals’ performance on behavioral tasks since sustained glial activation can trigger neuroinflammation with subsequent neurodegeneration resulting in cognitive dysfunction and long-term memory deficits (Widmann and Heneka 2014). Decreased glial activation is likely to be the result of simvastatin’s anti-inflammatory and antioxidant action through downregulation of protein prenylation, which in turn leads to suppression of NADPH oxidase (NOX) activity and reduction of pro-inflammatory cytokines (Fracassi et al. 2019). Additionally, the anti-apoptotic effect of simvastatin observed by increasing Bcl-2 protein followed by decreased cleaved caspase-3 protein appears to be independent of the mevalonate/isoprenoid/cholesterol pathway and occurs through a transcriptional mechanism stimulating Bcl-2 gene expression (Johnson-Anuna et al. 2005, 2007; Franke et al. 2007; Butterick et al. 2010). Therefore, we believe that the combination of these mechanisms may partly explain the simvastatin-induced neuroprotection observed in sepsis survivor rats.
In conclusion, simvastatin administered 4 days before and 10 days after septic induction proved to be effective in reducing inflammation, preventing the installation of biomarkers typical of neurodegenerative diseases, and reducing apoptotic mediators produced by sustained glial activation in sepsis survivor animals. In addition, simvastatin was able to restore the levels of synaptophysin in the hippocampus and provided evidence for anti-apoptotic effects by increasing Bcl-2 and reducing cleaved caspase-3 levels. Finally, its combined effects alleviated cognitive dysfunctions related to habituation, discriminative memory, and aversive reactions, as demonstrated by specific behavioral tests. Thinking translationally, since a significant proportion of sepsis survivors are expected to develop cognitive dysfunctions, and statins are currently widely consumed, it is possible to speculate that those individuals taking statin regularly would be less susceptible to develop cognitive decline after a septic event.
Therefore, it would be relevant to consider, from the therapeutic point of view, the maintenance of this drug during sepsis treatment. Nevertheless, studies related to the pharmacokinetics of HMG-CoA inhibitors, as well as their bioavailability and interaction with brain isoprenoid molecules, should be performed to clarify question about the effectiveness of their pleiotropic effects in neuropathologies.
References
Adam N, Kandelman S, Mantz J, Chrétien F, Sharshar T (2013) Sepsis-induced brain dysfunction. Expert Rev Anti-Infect Ther 11(2):211–221. https://doi.org/10.1586/eri.12.159
Ahn KS, Sethi G, Aggarwal BB (2007) Simvastatin potentiates TNF-alpha-induced apoptosis through the down-regulation of NF-kappaB-dependent antiapoptotic gene products: role of IkappaBalpha kinase and TGF-beta-activated kinase-1. J Immunol 178(4):2507–2516. https://doi.org/10.4049/jimmunol.178.4.2507
Akiyama H, Barger S, Barnum S, Bradt B, Bauer J, Cole GM, Cooper NR, Eikelenboom P, Emmerling M, Fiebich BL, Finch CE, Frautschy S, Griffin WS, Hampel H, Hull M, Landreth G, Lue L, Mrak R, Mackenzie IR, McGeer P, O’Banion MK, Pachter J, Pasinetti G, Plata-Salaman C, Rogers J, Rydel R, Shen Y, Streit W, Strohmeyer R, Tooyoma I, van Muiswinkel F, Veerhuis R, Walker D, Webster S, Wegrzyniak B, Wenk G, Wyss-Coray T (2000) Inflammation and Alzheimer’s disease. Neurobiol Aging 21(3):383–421
Allan SM, Tyrrell PJ, Rothwell NJ (2005) Interleukin-1 and neuronal injury. Nat Rev Immunol 5(8):629–640. https://doi.org/10.1038/nri1664
Ambrosini A, Louin G, Croci N, Plotkine M, Jafarian-Tehrani M (2005) Characterization of a rat model to study acute neuroinflammation on histopathological, biochemical and functional outcomes. J Neurosci Methods 144(2):183–191. https://doi.org/10.1016/j.jneumeth.2004.11.002
Arranz AM, De Strooper B (2019) The role of astroglia in Alzheimer’s disease: pathophysiology and clinical implications. Lancet Neurol 18(4):406–414. https://doi.org/10.1016/s1474-4422(18)30490-3
Ayanian JZ, Fuchs CS, Stone RM (1988) Lovastatin and rhabdomyolysis. Ann Intern Med 109(8):682–683
Barichello T, Martins MR, Reinke A, Feier G, Ritter C, Quevedo J, Dal-Pizzol F (2005) Cognitive impairment in sepsis survivors from cecal ligation and perforation. Crit Care Med 33(1):221–223; discussion 262-223. https://doi.org/10.1097/01.ccm.0000150741.12906.bd
Barichello T, Martins MR, Reinke A, Constantino LS, Machado RA, Valvassori SS, Moreira JCF, Quevedo J, Dal-Pizzol F (2007) Behavioral deficits in sepsis-surviving rats induced by cecal ligation and perforation. Braz J Med Biol Res 40(6):831–837
Barker GR, Warburton EC (2011) Evaluating the neural basis of temporal order memory for visual stimuli in the rat. Eur J Neurosci 33(4):705–716. https://doi.org/10.1111/j.1460-9568.2010.07555.x
Benveniste EN, Nguyen VT, O’Keefe GM (2001) Immunological aspects of microglia: relevance to Alzheimer’s disease. Neurochem Int 39(5–6):381–391
Blum CB (1994) Comparison of properties of four inhibitors of 3-hydroxy-3-methylglutaryl-coenzyme A reductase. Am J Cardiol 73(14):3D–11D. https://doi.org/10.1016/0002-9149(94)90626-2
Bramblett GT, Goedert M, Jakes R, Merrick SE, Trojanowski JQ, Lee VMY (1993) Abnormal tau phosphorylation at Ser396 in Alzheimer’s disease recapitulates development and contributes to reduced microtubule binding. Neuron 10(6):1089–1099
Brown GC, Neher JJ (2010) Inflammatory neurodegeneration and mechanisms of microglial killing of neurons. Mol Neurobiol 41(2–3):242–247. https://doi.org/10.1007/s12035-010-8105-9
Butterick TA, Igbavboa U, Eckert GP, Sun GY, Weisman GA, Müller WE, Wood WG (2010) Simvastatin stimulates production of the antiapoptotic protein Bcl-2 via endothelin-1 and NFATc3 in SH-SY5Y cells. Mol Neurobiol 41(2–3):384–391. https://doi.org/10.1007/s12035-010-8122-8
Calsavara AC, Rodrigues DH, Miranda AS, Costa PA, Lima CX, Vilela MC, Rachid MA, Teixeira AL (2013) Late anxiety-like behavior and neuroinflammation in mice subjected to sublethal polymicrobial sepsis. Neurotox Res 24(2):103–108. https://doi.org/10.1007/s12640-012-9364-1
Calsolaro V, Edison P (2016) Neuroinflammation in Alzheimer’s disease: current evidence and future directions. Alzheimers Dement 12(6):719–732. https://doi.org/10.1016/j.jalz.2016.02.010
Cassol OJ, Comim CM, Petronilho F, Constantino LS, Streck EL et al (2010) Low dose dexamethasone reverses depressive-like parameters and memory impairment in rats submitted to sepsis. Neurosci Lett 473(2):126–130. https://doi.org/10.1016/j.neulet.2010.02.036
Catalão CHR, Santos-Júnior NN, da Costa LHA, Souza AO, Alberici LC, Rocha MJA (2017) Brain oxidative stress during experimental sepsis is attenuated by simvastatin administration. Mol Neurobiol 54(9):7008–7018. https://doi.org/10.1007/s12035-016-0218-3
Catalão CHR, Souza AO, Santos-Júnior NN, da Silva SC, da Costa LHA, Alberici LC, Rocha MJA, da Silva Lopes L (2019) Kaolin-induced hydrocephalus causes acetylcholinesterase activity dysfunction following hypothalamic damage in infant rats. Brain Res 1724:146408. https://doi.org/10.1016/j.brainres.2019.146408
Chaipichit N, Krska J, Pratipanawatr T, Jarernsiripornkul N (2015) Statin adverse effects: patients’ experiences and laboratory monitoring of muscle and liver injuries. Int J Clin Pharm 37(2):355–364. https://doi.org/10.1007/s11096-015-0068-5
Chaudhry N, Duggal AK (2014) Sepsis associated encephalopathy. Adv Med 2014:762320–762316. https://doi.org/10.1155/2014/762320
Chen CH, Zhou W, Liu S, Deng Y, Cai F, Tone M, Tone Y, Tong Y, Song W (2012) Increased NF-κB signalling up-regulates BACE1 expression and its therapeutic potential in Alzheimer’s disease. Int J Neuropsychopharmacol 15(1):77–90. https://doi.org/10.1017/S1461145711000149
Chukwu JE, Pedersen JT, Pedersen L, Volbracht C, Sigurdsson EM et al (2018) Tau antibody structure reveals a molecular switch defining a pathological conformation of the tau protein. Sci Rep 8(1):6209. https://doi.org/10.1038/s41598-018-24276-4
Comim CM, Barichello T, Grandgirard D, Dal-Pizzol F, Quevedo J, Leib SL (2013) Caspase-3 mediates in part hippocampal apoptosis in sepsis. Mol Neurobiol 47(1):394–398. https://doi.org/10.1007/s12035-012-8354-x
Comim CM, Cassol-Jr OJ, Constantino LS, Felisberto F, Petronilho F, Rezin GT, Scaini G, Daufenbach JF, Streck EL, Quevedo J, Dal-Pizzol F (2011a) Alterations in inflammatory mediators, oxidative stress parameters and energetic metabolism in the brain of sepsis survivor rats. Neurochem Res 36(2):304–311. https://doi.org/10.1007/s11064-010-0320-2
Comim CM, Constantino LS, Petronilho F, Quevedo J, Dal-Pizzol F (2011b) Aversive memory in sepsis survivor rats. J Neural Transm (Vienna) 118(2):213–217. https://doi.org/10.1007/s00702-010-0502-8
Consiglio AR, Lucion AB (2000) Technique for collecting cerebrospinal fluid in the cisterna magna of non-anesthetized rats. Brain Res Brain Res Protoc 5(1):109–114
da Costa LHA, Júnior NNDS, Catalão CHR, Sharshar T, Chrétien F et al (2017) Vasopressin impairment during sepsis is associated with hypothalamic intrinsic apoptotic pathway and microglial activation. Mol Neurobiol 54(7):5526–5533. https://doi.org/10.1007/s12035-016-0094-x
Deshpande A, Pasupuleti V, Rothberg MB (2015) Statin therapy and mortality from sepsis: a meta-analysis of randomized trials. Am J Med 128(4):410–417.e411. https://doi.org/10.1016/j.amjmed.2014.10.057
Du G, Song Y, Zhang T, Ma L, Bian N et al (2014) Simvastatin attenuates TNF-α-induced apoptosis in endothelial progenitor cells via the upregulation of SIRT1. Int J Mol Med 34(1):177–182. https://doi.org/10.3892/ijmm.2014.1740
Elhayany A, Mishaal RA, Vinker S (2012) Is there clinical benefit to routine enzyme testing of patients on statins? Expert Opin Drug Saf 11(2):185–190. https://doi.org/10.1517/14740338.2012.630659
Elmore S (2007) Apoptosis: a review of programmed cell death. Toxicol Pathol 35(4):495–516. https://doi.org/10.1080/01926230701320337
Field RH, Gossen A, Cunningham C (2012) Prior pathology in the basal forebrain cholinergic system predisposes to inflammation-induced working memory deficits: reconciling inflammatory and cholinergic hypotheses of delirium. J Neurosci 32(18):6288–6294. https://doi.org/10.1523/JNEUROSCI.4673-11.2012
Figueiredo CP, Clarke JR, Ledo JH, Ribeiro FC, Costa CV, Melo HM, Mota-Sales AP, Saraiva LM, Klein WL, Sebollela A, de Felice FG, Ferreira ST (2013) Memantine rescues transient cognitive impairment caused by high-molecular-weight aβ oligomers but not the persistent impairment induced by low-molecular-weight oligomers. J Neurosci 33(23):9626–9634. https://doi.org/10.1523/JNEUROSCI.0482-13.2013
Fracassi A, Marangoni M, Rosso P, Pallottini V, Fioramonti M, Siteni S, Segatto M (2019) Statins and the brain: more than lipid lowering agents? Curr Neuropharmacol 17(1):59–83. https://doi.org/10.2174/1570159X15666170703101816
Franke C, Nöldner M, Abdel-Kader R, Johnson-Anuna LN, Gibson Wood W, Müller WE, Eckert GP (2007) Bcl-2 upregulation and neuroprotection in guinea pig brain following chronic simvastatin treatment. Neurobiol Dis 25(2):438–445. https://doi.org/10.1016/j.nbd.2006.10.004
Gasparotto J, Girardi CS, Somensi N, Ribeiro CT, Moreira JCF, Michels M, Sonai B, Rocha M, Steckert AV, Barichello T, Quevedo J, Dal-Pizzol F, Gelain DP (2018) Receptor for advanced glycation end products mediates sepsis-triggered amyloid-β accumulation, tau phosphorylation, and cognitive impairment. J Biol Chem 293(1):226–244. https://doi.org/10.1074/jbc.M117.786756
Gorina R, Font-Nieves M, Márquez-Kisinousky L, Santalucia T, Planas AM (2011) Astrocyte TLR4 activation induces a proinflammatory environment through the interplay between MyD88-dependent NFκB signaling, MAPK, and Jak1/Stat1 pathways. Glia 59(2):242–255. https://doi.org/10.1002/glia.21094
Granja C, Lopes A, Moreira S, Dias C, Costa-Pereira A, Carneiro A, JMIP Study Group (2005) Patients’ recollections of experiences in the intensive care unit may affect their quality of life. Crit Care 9(2):R96–R109. https://doi.org/10.1186/cc3026
Greenwood J, Steinman L, Zamvil SS (2006) Statin therapy and autoimmune disease: from protein prenylation to immunomodulation. Nat Rev Immunol 6(5):358–370. https://doi.org/10.1038/nri1839
Guo JT, Yu J, Grass D, de Beer FC, Kindy MS (2002) Inflammation-dependent cerebral deposition of serum amyloid a protein in a mouse model of amyloidosis. J Neurosci 22(14):5900–5909
Handa O, Stephen J, Cepinskas G (2008) Role of endothelial nitric oxide synthase-derived nitric oxide in activation and dysfunction of cerebrovascular endothelial cells during early onsets of sepsis. Am J Physiol Heart Circ Physiol 295(4):H1712–H1719. https://doi.org/10.1152/ajpheart.00476.2008
Heneka MT (2019) Microglia take centre stage in neurodegenerative disease. Nat Rev Immunol 19(2):79–80. https://doi.org/10.1038/s41577-018-0112-5
Heneka MT, McManus RM, Latz E (2018) Inflammasome signalling in brain function and neurodegenerative disease. Nat Rev Neurosci 19(10):610–621. https://doi.org/10.1038/s41583-018-0055-7
Hernandes MS, D’Avila JC, Trevelin SC, Reis PA, Kinjo ER et al (2014) The role of Nox2-derived ROS in the development of cognitive impairment after sepsis. J Neuroinflammation 11:36. https://doi.org/10.1186/1742-2094-11-36
Hopkins RO, Weaver LK, Collingridge D, Parkinson RB, Chan KJ, Orme JF Jr (2005) Two-year cognitive, emotional, and quality-of-life outcomes in acute respiratory distress syndrome. Am J Respir Crit Care Med 171(4):340–347. https://doi.org/10.1164/rccm.200406-763OC
Hopkins RO, Weaver LK, Pope D, Orme JF, Bigler ED et al (1999) Neuropsychological sequelae and impaired health status in survivors of severe acute respiratory distress syndrome. Am J Respir Crit Care Med 160(1):50–56. https://doi.org/10.1164/ajrccm.160.1.9708059
Hough CL, Curtis JR (2005) Long-term sequelae of critical illness: memories and health-related quality of life. Crit Care 9(2):145–146. https://doi.org/10.1186/cc3483
Hshieh TT, Fong TG, Marcantonio ER, Inouye SK (2008) Cholinergic deficiency hypothesis in delirium: a synthesis of current evidence. J Gerontol A Biol Sci Med Sci 63(7):764–772
Imamura Y, Wang H, Matsumoto N, Muroya T, Shimazaki J, Ogura H, Shimazu T (2011) Interleukin-1β causes long-term potentiation deficiency in a mouse model of septic encephalopathy. Neuroscience 187:63–69. https://doi.org/10.1016/j.neuroscience.2011.04.063
Iwashyna TJ, Ely EW, Smith DM, Langa KM (2010) Long-term cognitive impairment and functional disability among survivors of severe sepsis. JAMA 304(16):1787–1794. https://doi.org/10.1001/jama.2010.1553
Jackson JC, Gordon SM, Ely EW, Burger C, Hopkins RO (2004) Research issues in the evaluation of cognitive impairment in intensive care unit survivors. Intensive Care Med 30(11):2009–2016. https://doi.org/10.1007/s00134-004-2422-2
Jeong A, Suazo KF, Wood WG, Distefano MD, Li L (2018) Isoprenoids and protein prenylation: implications in the pathogenesis and therapeutic intervention of Alzheimer’s disease. Crit Rev Biochem Mol Biol 53(3):279–310. https://doi.org/10.1080/10409238.2018.1458070
Johnson-Anuna LN, Eckert GP, Keller JH, Igbavboa U, Franke C, Fechner T, Schubert-Zsilavecz M, Karas M, Müller WE, Wood WG (2005) Chronic administration of statins alters multiple gene expression patterns in mouse cerebral cortex. J Pharmacol Exp Ther 312(2):786–793. https://doi.org/10.1124/jpet.104.075028
Johnson-Anuna LN, Eckert GP, Franke C, Igbavboa U, Müller WE, Wood WG (2007) Simvastatin protects neurons from cytotoxicity by up-regulating Bcl-2 mRNA and protein. J Neurochem 101(1):77–86. https://doi.org/10.1111/j.1471-4159.2006.04375.x
Jänicke RU, Sprengart ML, Wati MR, Porter AG (1998) Caspase-3 is required for DNA fragmentation and morphological changes associated with apoptosis. J Biol Chem 273(16):9357–9360
Kettenmann H, Kirchhoff F, Verkhratsky A (2013) Microglia: new roles for the synaptic stripper. Neuron 77(1):10–18. https://doi.org/10.1016/j.neuron.2012.12.023
Kim EH, Jang MH, Shin MC, Lim BV, Kim HB, Kim YJ, Chung JH, Kim CJ (2002) Acupuncture increases cell proliferation and neuropeptide Y expression in dentate gyrus of streptozotocin-induced diabetic rats. Neurosci Lett 327(1):33–36
Kopterides P, Falagas ME (2009) Statins for sepsis: a critical and updated review. Clin Microbiol Infect 15(4):325–334. https://doi.org/10.1111/j.1469-0691.2009.02750.x
Li L, Zhang W, Cheng S, Cao D, Parent M (2012) Isoprenoids and related pharmacological interventions: potential application in Alzheimer’s disease. Mol Neurobiol 46(1):64–77. https://doi.org/10.1007/s12035-012-8253-1
Liddelow SA, Barres BA (2017) Reactive astrocytes: production, function, and therapeutic potential. Immunity 46(6):957–967. https://doi.org/10.1016/j.immuni.2017.06.006
Liddelow SA, Guttenplan KA, Clarke LE, Bennett FC, Bohlen CJ, Schirmer L, Bennett ML, Münch AE, Chung WS, Peterson TC, Wilton DK, Frouin A, Napier BA, Panicker N, Kumar M, Buckwalter MS, Rowitch DH, Dawson VL, Dawson TM, Stevens B, Barres BA (2017) Neurotoxic reactive astrocytes are induced by activated microglia. Nature 541(7638):481–487. https://doi.org/10.1038/nature21029
Liu H, Yang J, Wang K, Niu T, Huang D (2019) Moderate- and low-dose of atorvastatin alleviate cognition impairment induced by high-fat diet via Sirt1 activation. Neurochem Res 44(5):1065–1078. https://doi.org/10.1007/s11064-019-02738-z
Lu D, Shen L, Mai H, Zang J, Liu Y, Tsang CK, Li K, Xu A (2019) HMG-CoA reductase inhibitors attenuate neuronal damage by suppressing oxygen glucose deprivation-induced activated microglial cells. Neural Plast 2019:7675496–7675415. https://doi.org/10.1155/2019/7675496
Maramattom BV (2007) Sepsis associated encephalopathy. Neurol Res 29(7):643–646. https://doi.org/10.1179/016164107X240233
Martin CP, Talbert RL, Burgess DS, Peters JI (2007) Effectiveness of statins in reducing the rate of severe sepsis: a retrospective evaluation. Pharmacotherapy 27(1):20–26. https://doi.org/10.1592/phco.27.1.20
Matsuzaki H, Tamatani M, Mitsuda N, Namikawa K, Kiyama H, Miyake S, Tohyama M (1999) Activation of Akt kinase inhibits apoptosis and changes in Bcl-2 and Bax expression induced by nitric oxide in primary hippocampal neurons. J Neurochem 73(5):2037–2046
Mawuenyega KG, Sigurdson W, Ovod V, Munsell L, Kasten T, Morris JC, Yarasheski KE, Bateman RJ (2010) Decreased clearance of CNS beta-amyloid in Alzheimer’s disease. Science 330(6012):1774. https://doi.org/10.1126/science.1197623
McManus RM, Heneka MT (2017) Role of neuroinflammation in neurodegeneration: new insights. Alzheimers Res Ther 9(1):14. https://doi.org/10.1186/s13195-017-0241-2
McQuade A, Blurton-Jones M (2019) Microglia in Alzheimer’s disease: exploring how genetics and phenotype influence risk. J Mol Biol 431(9):1805–1817. https://doi.org/10.1016/j.jmb.2019.01.045
Mehl A, Harthug S, Lydersen S, Paulsen J, Åsvold BO, Solligård E, Damås JK, Edna TH (2015) Prior statin use and 90-day mortality in Gram-negative and Gram-positive bloodstream infection: a prospective observational study. Eur J Clin Microbiol Infect Dis 34(3):609–617. https://doi.org/10.1007/s10096-014-2269-6
Michels M, Vieira AS, Vuolo F, Zapelini HG, Mendonça B, Mina F, Dominguini D, Steckert A, Schuck PF, Quevedo J, Petronilho F, Dal-Pizzol F (2015) The role of microglia activation in the development of sepsis-induced long-term cognitive impairment. Brain Behav Immun 43:54–59. https://doi.org/10.1016/j.bbi.2014.07.002
Mina F, Comim CM, Dominguini D, Cassol-Jr OJ, Dall Igna DM et al (2014) Il1-β involvement in cognitive impairment after sepsis. Mol Neurobiol 49(2):1069–1076. https://doi.org/10.1007/s12035-013-8581-9
Naiki H, Higuchi K, Hosokawa M, Takeda T (1989) Fluorometric determination of amyloid fibrils in vitro using the fluorescent dye, thioflavin T1. Anal Biochem 177(2):244–249
Nemzek JA, Xiao HY, Minard AE, Bolgos GL, Remick DG (2004) Humane endpoints in shock research. Shock 21(1):17–25. https://doi.org/10.1097/01.shk.0000101667.49265.fd
Oliveira-Pelegrin GR, Basso PJ, Rocha MJ (2014) Cellular bioenergetics changes in magnocellular neurons may affect copeptin expression in the late phase of sepsis. J Neuroimmunol 267(1–2):28–34. https://doi.org/10.1016/j.jneuroim.2013.12.006
Oliveira-Pelegrin GR, Basso PJ, Soares AS, Martinez MR, Riester KD, Rocha MJA (2013) Cleaved caspase-3 expression in hypothalamic magnocellular neurons may affect vasopressin secretion during experimental polymicrobial sepsis. J Neuroimmunol 258:10–16
Olivieri R, Michels M, Pescador B, Ávila P, Abatti M, Cucker L, Burger H, Dominguini D, Quevedo J, Dal-Pizzol F (2018) The additive effect of aging on sepsis-induced cognitive impairment and neuroinflammation. J Neuroimmunol 314:1–7. https://doi.org/10.1016/j.jneuroim.2017.11.014
Ostrowski SM, Johnson K, Siefert M, Shank S, Sironi L, Wolozin B, Landreth GE, Ziady AG (2016) Simvastatin inhibits protein isoprenylation in the brain. Neuroscience 329:264–274. https://doi.org/10.1016/j.neuroscience.2016.04.053
Pan S, Wu Y, Pei L, Li S, Song L, Xia H, Wang Y, Yu Y, Yang X, Shu H, Zhang J, Yuan S, Shang Y (2018) BML-111 reduces neuroinflammation and cognitive impairment in mice with sepsis via the SIRT1/NF-κB signaling pathway. Front Cell Neurosci 12:267. https://doi.org/10.3389/fncel.2018.00267
Paxinos G, Watson C (2005) The rat brain in stereotaxic coordinates. Elsevier Academic Press, Amsterdam
Pekny M, Pekna M, Messing A, Steinhäuser C, Lee JM, Parpura V, Hol EM, Sofroniew MV, Verkhratsky A (2016) Astrocytes: a central element in neurological diseases. Acta Neuropathol 131(3):323–345. https://doi.org/10.1007/s00401-015-1513-1
Piechota M, Barylski M, Hannam S, Piechota-Urbańska M, Banach M (2013) Rationale of statin therapy in septic patients. Curr Vasc Pharmacol 11(5):795–800
Posada-Duque RA, Velasquez-Carvajal D, Eckert GP, Cardona-Gomez GP (2013) Atorvastatin requires geranylgeranyl transferase-I and Rac1 activation to exert neuronal protection and induce plasticity. Neurochem Int 62(4):433–445. https://doi.org/10.1016/j.neuint.2013.01.026
Qin L, Xie X, Fang P, Lin J (2019) Prophylactic simvastatin treatment modulates the immune response and increases survival of mice following induction of lethal sepsis. J Int Med Res 300060519858508. https://doi.org/10.1177/0300060519858508
Ransohoff RM (2016) How neuroinflammation contributes to neurodegeneration. Science 353(6301):777–783. https://doi.org/10.1126/science.aag2590
Reis PA, Alexandre PC, D’Avila JC, Siqueira LD, Antunes B et al (2017) Statins prevent cognitive impairment after sepsis by reverting neuroinflammation, and microcirculatory/endothelial dysfunction. Brain Behav Immun 60:293–303. https://doi.org/10.1016/j.bbi.2016.11.006
Saheki A, Terasaki T, Tamai I, Tsuji A (1994) In vivo and in vitro blood-brain barrier transport of 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors. Pharm Res 11(2):305–311
Santos-Junior NN, Catalão CH, Costa LH, Rossignoli BB, Dos-Santos RC et al (2018) Alterations in hypothalamic synaptophysin and death markers may be associated with vasopressin impairment in sepsis survivor rats. J Neuroendocrinol:e12604. https://doi.org/10.1111/jne.12604
Sastre M, Dewachter I, Landreth GE, Willson TM, Klockgether T, van Leuven F, Heneka MT (2003) Nonsteroidal anti-inflammatory drugs and peroxisome proliferator-activated receptor-gamma agonists modulate immunostimulated processing of amyloid precursor protein through regulation of beta-secretase. J Neurosci 23(30):9796–9804
Satou T, Cummings BJ, Cotman CW (1995) Immunoreactivity for Bcl-2 protein within neurons in the Alzheimer’s disease brain increases with disease severity. Brain Res 697(1–2):35–43. https://doi.org/10.1016/0006-8993(95)00748-f
Schwalm MT, Pasquali M, Miguel SP, Dos Santos JP, Vuolo F et al (2014) Acute brain inflammation and oxidative damage are related to long-term cognitive deficits and markers of neurodegeneration in sepsis-survivor rats. Mol Neurobiol 49(1):380–385. https://doi.org/10.1007/s12035-013-8526-3
Semmler A, Okulla T, Sastre M, Dumitrescu-Ozimek L, Heneka MT (2005) Systemic inflammation induces apoptosis with variable vulnerability of different brain regions. J Chem Neuroanat 30(2–3):144–157. https://doi.org/10.1016/j.jchemneu.2005.07.003
Semmler A, Frisch C, Debeir T, Ramanathan M, Okulla T, Klockgether T, Heneka MT (2007) Long-term cognitive impairment, neuronal loss and reduced cortical cholinergic innervation after recovery from sepsis in a rodent model. Exp Neurol 204(2):733–740. https://doi.org/10.1016/j.expneurol.2007.01.003
Semmler A, Hermann S, Mormann F, Weberpals M, Paxian SA, Okulla T, Schäfers M, Kummer MP, Klockgether T, Heneka MT (2008) Sepsis causes neuroinflammation and concomitant decrease of cerebral metabolism. J Neuroinflammation 5:38. https://doi.org/10.1186/1742-2094-5-38
Semmler A, Widmann CN, Okulla T, Urbach H, Kaiser M, Widman G, Mormann F, Weide J, Fliessbach K, Hoeft A, Jessen F, Putensen C, Heneka MT (2013) Persistent cognitive impairment, hippocampal atrophy and EEG changes in sepsis survivors. J Neurol Neurosurg Psychiatry 84(1):62–69. https://doi.org/10.1136/jnnp-2012-302883
Shankar GM, Walsh DM (2009) Alzheimer’s disease: synaptic dysfunction and Aβ. Mol Neurodegener 4(1):48
Shetty AK, Upadhya R, Madhu LN, Kodali M (2019) Novel insights on systemic and brain aging, stroke, amyotrophic lateral sclerosis, and Alzheimer’s disease. Aging Dis 10(2):470–482. https://doi.org/10.14336/AD.2019.0330
Sonneville R, Verdonk F, Rauturier C, Klein IF, Wolff M, Annane D, Chretien F, Sharshar T (2013) Understanding brain dysfunction in sepsis. Ann Intensive Care 3(1):15. https://doi.org/10.1186/2110-5820-3-15
Sprung CL, Peduzzi PN, Shatney CH, Schein RM, Wilson MF et al (1990) Impact of encephalopathy on mortality in the sepsis syndrome. The Veterans Administration Systemic Sepsis Cooperative Study Group. Crit Care Med 18(8):801–806
Steckert AV, Comim CM, Igna DM, Dominguini D, Mendonça BP et al (2015) Effects of sodium butyrate on aversive memory in rats submitted to sepsis. Neurosci Lett 595:134–138. https://doi.org/10.1016/j.neulet.2015.04.019
Stein A, Stroobants S, Gieselmann V, D’Hooge R, Matzner U (2015) Anti-inflammatory therapy with simvastatin improves neuroinflammation and CNS function in a mouse model of metachromatic leukodystrophy. Mol Ther 23(7):1160–1168. https://doi.org/10.1038/mt.2015.69
Taccone FS, Castanares-Zapatero D, Peres-Bota D, Vincent JL, Berre’ J et al (2010) Cerebral autoregulation is influenced by carbon dioxide levels in patients with septic shock. Neurocrit Care 12(1):35–42. https://doi.org/10.1007/s12028-009-9289-6
Tamatani M, Ogawa S, Niitsu Y, Tohyama M (1998) Involvement of Bcl-2 family and caspase-3-like protease in NO-mediated neuronal apoptosis. J Neurochem 71(4):1588–1596. https://doi.org/10.1046/j.1471-4159.1998.71041588.x
Tian M, Qingzhen L, Zhiyang Y, Chunlong C, Jiao D, Zhang L, Li W (2019) Attractylone attenuates sepsis-associated encephalopathy and cognitive dysfunction by inhibiting microglial activation and neuroinflammation. J Cell Biochem 120:7101–7108. https://doi.org/10.1002/jcb.27983
van Gool WA, van de Beek D, Eikelenboom P (2010) Systemic infection and delirium: when cytokines and acetylcholine collide. Lancet 375(9716):773–775. https://doi.org/10.1016/S0140-6736(09)61158-2
Vasudevan AR, Hamirani YS, Jones PH (2005) Safety of statins: effects on muscle and the liver. Cleve Clin J Med 72(11):990–993, 996-1001. https://doi.org/10.3949/ccjm.72.11.990
Vianna MR, Alonso M, Viola H, Quevedo J, de Paris F, Furman M, de Stein ML, Medina JH, Izquierdo I (2000) Role of hippocampal signaling pathways in long-term memory formation of a nonassociative learning task in the rat. Learn Mem 7(5):333–340
Viatour P, Bentires-Alj M, Chariot A, Deregowski V, de Leval L, Merville MP, Bours V (2003) NF- kappa B2/p100 induces Bcl-2 expression. Leukemia 17(7):1349–1356. https://doi.org/10.1038/sj.leu.2402982
Vickers S, Duncan CA, Vyas KP, Kari PH, Arison B, Prakash SR, Ramjit HG, Pitzenberger SM, Stokker G, Duggan DE (1990) In vitro and in vivo biotransformation of simvastatin, an inhibitor of HMG CoA reductase. Drug Metab Dispos 18(4):476–483
Vuletic S, Riekse RG, Marcovina SM, Peskind ER, Hazzard WR, Albers JJ (2006) Statins of different brain penetrability differentially affect CSF PLTP activity. Dement Geriatr Cogn Disord 22(5–6):392–398. https://doi.org/10.1159/000095679
Wahab F, Santos-Junior NN, de Almeida Rodrigues RP, Costa LHA, Catalão CHR, Rocha MJA (2016) Interleukin-1 receptor antagonist decreases hypothalamic oxidative stress during experimental sepsis. Mol Neurobiol 53(6):3992–3998. https://doi.org/10.1007/s12035-015-9338-4
Wan YD, Sun TW, Kan QC, Guan FX, Zhang SG (2014) Effect of statin therapy on mortality from infection and sepsis: a meta-analysis of randomized and observational studies. Crit Care 18(2):R71. https://doi.org/10.1186/cc13828
Weberpals M, Hermes M, Hermann S, Kummer MP, Terwel D, Semmler A, Berger M, Schafers M, Heneka MT (2009) NOS2 gene deficiency protects from sepsis-induced long-term cognitive deficits. J Neurosci 29(45):14177–14184. https://doi.org/10.1523/JNEUROSCI.3238-09.2009
Widmann CN, Heneka MT (2014) Long-term cerebral consequences of sepsis. Lancet Neurol 13(6):630–636. https://doi.org/10.1016/S1474-4422(14)70017-1
Wood WG, Igbavboa U, Muller WE, Eckert GP (2013) Statins, Bcl-2, and apoptosis: cell death or cell protection? Mol Neurobiol 48(2):308–314. https://doi.org/10.1007/s12035-013-8496-5
Xiong H, McCabe L, Costello J, Anderson E, Weber G, Ikezu T (2004) Activation of NR1a/NR2B receptors by soluble factors from APP-stimulated monocyte-derived macrophages: implications for the pathogenesis of Alzheimer’s disease. Neurobiol Aging 25(7):905–911. https://doi.org/10.1016/j.neurobiolaging.2003.09.007
Xu Y, Yan J, Zhou P, Li J, Gao H, Xia Y, Wang Q (2012) Neurotransmitter receptors and cognitive dysfunction in Alzheimer’s disease and Parkinson’s disease. Prog Neurobiol 97(1):1–13. https://doi.org/10.1016/j.pneurobio.2012.02.002
Xue C, Lin TY, Chang D, Guo Z (2017) Thioflavin T as an amyloid dye: fibril quantification, optimal concentration and effect on aggregation. R Soc Open Sci 4(1):160696. https://doi.org/10.1098/rsos.160696
Yang CH, Kao MC, Shih PC, Li KY, Tsai PS, Huang CJ (2015) Simvastatin attenuates sepsis-induced blood-brain barrier integrity loss. J Surg Res 194(2):591–598. https://doi.org/10.1016/j.jss.2014.11.030
Young GB, Bolton CF, Austin TW, Archibald YM, Gonder J, Wells GA (1990) The encephalopathy associated with septic illness. Clin Invest Med 13(6):297–304
Zheng X, Liao Y, Wang J, Hu S, Rudramurthy GR, Swamy MK, Rohit KC, Wang Y (2018) The antineuroinflammatory effect of simvastatin on lipopolysaccharide activated microglial cells. Evid Based Complement Alternat Med 2018:9691085–9691089. https://doi.org/10.1155/2018/9691085
Zhuo Y, Zhang S, Li C, Yang L, Gao H, Wang X (2018) Resolvin D1 promotes SIRT1 expression to counteract the activation of STAT3 and NF-κB in mice with septic-associated lung injury. Inflammation 41(5):1762–1771. https://doi.org/10.1007/s10753-018-0819-2
Acknowledgments
The authors thank Marcelo Batalhão for the use of the Nitric Oxide Analyzer (Sievers Instruments Nitric Oxide Analyzer). Technical support by Nadir Fernandes and Renato Meireles is gratefully acknowledged. The authors thank Dr. Klaus Hartfelder for his assistance with English language.
Funding
This work was supported in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES) - Finance Code 001 and by the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP, grants 2015/12152, 2017/12462-0, 2018/02854-0 and 2018/10089-2).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This study involves the use of rats. All animal experiments in this study were carried out according to an Institutional Ethics Committee approved protocol (CEUA protocol number: 2019.1.51.58.6).
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Catalão, C.H.R., Santos-Junior, N.N., da Costa, L.H.A. et al. Simvastatin Prevents Long-Term Cognitive Deficits in Sepsis Survivor Rats by Reducing Neuroinflammation and Neurodegeneration. Neurotox Res 38, 871–886 (2020). https://doi.org/10.1007/s12640-020-00222-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12640-020-00222-z