Abstract
Sepsis is caused by a dysregulated host response to infection, often associated with acute central nervous system (CNS) dysfunction, which results in long-term cognitive impairment. Dimethyl fumarate (DMF) is an important agent against inflammatory response and reactive species in CNS disorders. Evaluate the effect of DMF on acute and long-term brain dysfunction after experimental sepsis in rats. Male Wistar rats were submitted to the cecal ligation and puncture (CLP) model. The groups were divided into sham (control) + vehicle, sham + NAC, sham + DMF, CLP + vehicle, CLP + NAC, and CLP + DMF. The animals were treated with DMF (15 mg/kg at 0 and 12 h after CLP, per gavage) and the administration of n-acetylcysteine (NAC) (20 mg/kg; 3, 6, and 12 h after CLP, subcutaneously) was used as positive control. Twenty-four hours after CLP, cytokines, myeloperoxidase (MPO), nitrite/nitrate (N/N), oxidative damage to lipids and proteins, and antioxidant enzymes were evaluated in the hippocampus, total cortex, and prefrontal cortex. At 10 days after sepsis induction, behavioral tests were performed to assess cognitive damage. We observed an increase in cytokine levels, MPO activity, N/N concentration, and oxidative damage, a reduction in SOD and GPx activity in the brain structures, and cognitive damage in CLP rats. DMF treatment was effective in reversing these parameters. DMF reduces sepsis-induced neuroinflammation, oxidative stress, and cognitive impairment in rats subjected to the CLP model.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sepsis is the most frequent cause of death in intensive care units (ICU) (Mayr et al. 2014), and it is determined by a systemic inflammatory response associated with an infection (Singer et al. 2016). The central nervous system (CNS) is rapidly damaged during sepsis, causing sepsis-associated encephalopathy (SAE), which is clinically characterized by disorientation, delirium, or coma (Wassmer et al. 2006). Also, long-term memory disturbance and impaired learning ability frequently affect sepsis survivors (Iwashyna et al. 2010).
The pathophysiological mechanisms associated with SAE development comprise the production of proinflammatory mediators, oxidative stress, and mitochondrial dysfunction (Dal-Pizzol et al. 2014). Proinflammatory cytokines, including tumor necrosis factor-α (TNF-α), interleukin (IL)-1β, and IL-6, activate target cells and stimulate the production of other cytokines, chemokines, reactive oxygen species (ROS), reactive nitrogen species (RNS), eicosanoids, and proteolytic enzymes (Doyle and O’Neill 2006). In fact, the production of inflammatory mediators is exacerbated in sepsis, leading to microcirculation dysfunction, tissue damage, and multiple organ failure (Comim et al. 2011b).
Sepsis survivors usually display an increase in IL-6 levels and hippocampal hypotrophy (Wiersinga et al. 2014); similar findings are observed in preclinical studies using rodent model of sepsis, in which TNF-α, IL-1β, IL-6, and IL-8 levels in the CNS increase within a few hours after sepsis onset (Mina et al. 2014; Michels et al. 2015a). As the inflammatory scenario persists, several deleterious consequences take place, e.g., increased blood-brain barrier (BBB) permeability, oxidative damage, energy metabolism alterations, microglial activation and glutamate excitotoxicity, aggravating SAE, and contributing to neuronal dysfunction, degeneration, and cognitive impairment (Petronilho et al. 2012; Michels et al. 2014, 2015b; Mina et al. 2014).
Oxidative stress in sepsis favors systemic inflammation and multiple organ failure due to the excessive production of reactive species and the deterioration of antioxidant defenses (Chelkeba et al. 2015). In response, neutrophils are activated and recruited to the infectious site, thus stimulating ROS and RNS production (Andrades et al. 2011; Comim et al. 2011b; Lange et al. 2012; Zheng et al. 2015). At high concentrations, such species disrupt the oxidation-antioxidant axis and generate oxidative stress, i.e., a deleterious cellular process to membrane lipids, proteins, and DNA (Singer 2014).
Considering that neuroinflammation and oxidative stress lead to cerebral changes in sepsis, therapeutic alternatives are necessary to prevent SAE or minimize its consequences (Zampieri et al. 2011). In this sense, dimethyl fumarate (DMF), a fumaric acid ester, is an important target for CNS changes due to both processes (Albrecht et al. 2012; Parodi et al. 2017). Studies involving multiple sclerosis and psoriasis show that DMF suppresses the inflammatory response, oxidative stress, neuronal injury, and microglial activation (Wilms et al. 2010; Albrecht et al. 2012; Gill et al. 2014; Parodi et al. 2015).
Dimethyl fumarate acts in two main pathways: (i) it increases nuclear factor erythroid 2-related factor 2 (Nrf2) activation, which enhances the endogenous levels of antioxidant enzymes, such as glutathione peroxidase (GPx) (Kobayashi and Yamamoto 2006; Linker et al. 2011), and (ii) it controls the immunomodulatory response by inhibiting nuclear factor-κB (NF-κB). Studies showed an antiinflammatory effect of DMF, represented by a reduced cytokine production in response of NF-κB inhibition (Wierinckx et al. 2005; Scannevin et al. 2012).
In view of all these aspects, the lack of studies evaluating DMF on brain damage after sepsis and considering that neuroinflammation and oxidative stress are intrinsically related to this condition, we hypothesize that DMF may have protective effect against such changes. To test our hypothesis, we measured neutrophil infiltrate, TNF-α, IL-6, and cytokine-induced neutrophil chemoattractant-1 (CINC-1) levels; nitrite and nitrate concentration; oxidative damage to lipids and proteins; the antioxidant activity of SOD, CAT, and GPx; and long-term cognitive damage in the hippocampus, prefrontal cortex, and total cortex of rats submitted to polymicrobial sepsis.
Materials and Methods
Animals
Male Wistar rats (60 days, 250–300 g) were used in this study. Animals were housed in groups of four per cage with food and water ad libitum, on a 12-h light/dark cycle (lights on at 7:00 a.m.). All experimental procedures were approved by the Animal Care and Experimentation Committee of UNISUL (protocol number 15.009.4.03.IV), Brazil.
Sepsis Induction—CLP Model
Sepsis was induced by cecal ligation and puncture (CLP), as previously described (Hubbard et al. 2005). Briefly, animals were anesthetized intraperitoneally (i.p.) with a mixture of ketamine (80 mg/kg) and xylazine (10 mg/kg). Under aseptic conditions, a 3-cm midline laparotomy was performed to expose the cecum and adjoining intestine. The cecum was tightly ligated with a 3.0 silk suture in the middle of its length (below the ileocecal valve), perforated once with a 14-gauge needle, squeezed gently to extrude a small amount of feces through the perforation site, and returned to the peritoneal cavity, and the laparotomy was closed with 4.0 silk sutures. Animals were resuscitated with regular saline (50 mL/kg) and ceftriaxone (30 mg/kg) subcutaneously (s.c.) immediately after CLP and 12 h after. All animals were returned to their cages with free access to food and water. In the sham-operated group, the rats were submitted to all surgical procedures, but the cecum was neither ligated nor perforated. To minimize variability between different experiments, the same investigator always performed the CLP procedure.
Experimental Groups and Sample Obtention
Animals were randomly divided into six groups: sham + vehicle, sham + NAC, sham + DMF, CLP + vehicle, CLP + NAC, or CLP + DMF. Considering a 40% mortality rate, for biochemical analysis, 7 animals per sham group and 12 animals per CLP group were used, and for behavior analysis, 12 animals per sham group and 18 per CLP group were used (Carvalho et al. 2008). The animals were treated with DMF (15 mg/kg dissolved in 0.08% dimethylsulfoxide at 0 h and 12 h after CLP, per gavage) or n-acetylcysteine (NAC) (20 mg/kg, at 3, 6, and 12 h after CLP, s.c.) as control (Barichello et al. 2007; Reick et al. 2014). Twenty-four hours after sepsis induction, all animals designed for biochemical evaluations were subjected to a painless assisted death with thiopental overdose (0.5 g/kg, i.p.) followed by decapitation. The remaining animals designed for behavioral evaluation were subjected to the same euthanasia procedure at 10 days after sepsis induction. The brain structures prefrontal cortex, hippocampus, and “total cortex” (the remaining tissue after harvesting both previous structures) were quickly isolated and stored at − 80 °C until further analysis. Behavioral tests were performed 10 days after surgery procedure.
Biochemical Analysis
Neutrophil Infiltrate
Neutrophil infiltrate in the three brain structures was measured by MPO activity [22]. Brain tissues were homogenized (50 mg/mL) in 0.5% hexadecyltrimethylammonium bromide and centrifuged at 15,000×g for 40 min. An aliquot of supernatant was mixed with a solution of 1.6 mM tetramethylbenzidine and 1 mM H2O2. Activity was measured spectrophotometrically as the change in absorbance at 650 nm at 37 °C. Data were expressed as milliunits per milligram of protein (De Young et al. 1989).
Cytokine Measurement
Interleukin-6, TNF-α, and CINC-1 concentrations in the three brain structures were determined using commercially available enzyme-linked immunosorbent assay kits according to the manufacturer’s instructions (R&D Systems, Minneapolis, MN) and were expressed as picograms per milliliter.
Nitrite/Nitrate Concentration
Nitrite/nitrate (N/N) concentration was measured in the three brain structures by Griess reaction, by adding 100 μL of Griess reagent [0.1% (w/v) naphthylethylendiamide dihydrochloride in H2O and 1% (w/v) sulfanilamide in 5% (v/v) concentrated H3PO4], with vol. [1:1] to the 100 μL sample. After 1 h of incubation at room temperature, absorbance was recorded in a spectrophotometer at 550 nm (Green et al. 1982). Data were expressed as nanomoles of nitrite/nitrate concentration per milligram of protein.
Lipid Peroxidation
Lipid peroxidation was measured in the three brain structures by formation of thiobarbituric acid (TBA) reactive substances (Draper and Hadley 1990). After dissection, samples were washed with PBS, harvested, and lysed. Thiobarbituric reactive species, obtained by acid hydrolysis of 1,1,3,3-tetra-ethoxy-propane (TEP), were used as the standard for the quantification of TBARS. TBA 0.67% was added to each tube and vortexed. The reaction mixture was incubated at 100 °C for 20 min, and the reaction was stopped by placing samples on ice. The optical density of each solution was measured in a spectrophotometer at 535 nm. Data were expressed as nanomoles of malondialdehyde (MDA) equivalents per milligram of protein.
Carbonyl Protein Formation
Carbonyl protein content was measured in the three brain structures using 2,4-dinitrophenylhydrazine (DNPH) in a spectrophotometric assay (Levine et al. 1990). Briefly, sample tissues were sonicated in ice cold homogenization buffer containing phosphatase and protease inhibitors (200 nM calyculin, 10 μg/mL leupeptin, 2 μg/mL aprotinin, 1 mM sodium orthovanadate, and 1 μM microcystin-LR) and centrifuged at 1000×g for 15 min to sediment insoluble material. Three hundred-microliter aliquots of the supernatant containing 0.7–1.5 mg of protein were treated with 300 μL of 10 mM DNPH, dissolved in 2 M HCl, and compared with 2 M HCl alone (reagent blank). Samples were incubated for 1 h at room temperature in the dark, stirred every 10 min, precipitated with trichloroacetic acid (final concentration of 20%), and centrifuged at 16,000×g at 4 °C for 15 min. The pellet was washed three times with 1 mL of ethanol/ethyl acetate (1:1 v/v). Each time, the pellet was lightly vortexed and left exposed to the washing solution for 10 min before centrifugation (16,000×g for 5 min). The final pellet was dissolved in 1 mL of 6 M guanidine in 10 mM phosphate buffer-trifluoroacetic acid, pH 2.3, and the insoluble material was removed by centrifugation at 16,000×g for 5 min. Absorbance was recorded in a spectrophotometer at 370 nm for both DNPH-treated and HCl-treated samples. Carbonyl protein levels were expressed as nanomoles of carbonyl per milligram of protein.
Superoxide Dismutase Activity
Superoxide dismutase (SOD; EC 1.15.1.1) activity was determined in the three brain structures by a spectrophotometric assay based on superoxide-dependent oxidation of epinephrine to adrenochrome at 32 °C (Bannister and Calabrese 1987). Absorption was measured at 480 nm. SOD-specific activity was represented as milliunits per milligram of protein.
Catalase Activity
Catalase (CAT; EC 1.11.1.6) activity was determined in the three brain structures by the absorbance decrease at 240 nm in a reaction medium containing 20 mM H2O2, 0.1% Triton X-100, 10 mM potassium phosphate buffer, pH 7.0, and the supernatants containing 0.1–0.3 mg protein. mL-1 (Aebi 1984). The specific activity was expressed as milliunits per milligram of protein.
Glutathione Peroxidase Activity
Glutathione peroxidase (GPx) activity was determined in the three brain structures spectrophotometrically in a system containing GSH/NADPH/GR by H2O2 dismutation. In this assay, the enzyme activity is indirectly measured by NADPH decay at 340 nm. The enzymatic activity was expressed as nanomoles per minute per milligram of protein (Wendel 1981).
Protein Determination
All biochemical measures were normalized to the protein content with bovine serum albumin as standard (Lowry et al. 1951).
Behavioral Analyses
Habituation to the Open Field Task
The habituation to the open field task evaluates motor performance in the training section and non-associative memory in the retention test session. Habituation to an open field was carried out in a 40 × 60 cm open field surrounded by 50 cm high walls made of brown plywood with a frontal glass wall. The floor of the open field was divided into 12 equal rectangles by black lines. The animals were gently placed on the left rear quadrant and left to explore the arena for 5 min (training session). Immediately following this, the animals were taken back to their home cage and 24 h later submitted again to a similar open-field session (test session). The number of times the animal crossed the black lines and rearing behavior in both sessions was counted. The decrease in the number of crossings and rearings between the two sessions was taken as a measure of the retention of habituation (Vianna et al. 2000; de Lima et al. 2005).
Novel Object Recognition Task
This task evaluates non-aversive and non-spatial memory. The apparatus and procedures for the object recognition task are the same used for open-field task. All animals were submitted to a habituation session when they could freely explore the open field for 5 min. No objects were placed in the box during the habituation trial. Crossings of the black lines and rearings performed in this session were evaluated as locomotors and exploratory activity, respectively. Twenty-four hours after habituation, training was conducted by placing individually a rat for 5 min in the field, in which two identical objects (objects A1 and A2) were positioned in two adjacent corners, 10 cm from the walls. The short-term recognition memory test was performed 1.5 h after training, when the rats explored the open-field for 5 min in the presence of one familiar (A) and one novel (B) object. The long-term recognition memory test was performed 24 h after training following the same protocol. All objects had similar textures, colors, and sizes, but distinctive shapes. A recognition index was calculated for each animal reported as the ratio TB/(TA + TB) (TA = time spent exploring the familiar object A; TB = time spent exploring the novel object B). Exploration was defined as sniffing (exploring the object 3–5 cm away from it) or touching the object with the nose and/or forepaws (de Lima et al. 2005).
Data Analysis
Data was analyzed using Statistical Package for the Social Sciences software (SPSS, Chicago, IL, USA). Data is expressed as mean ± S.D., and differences among experimental groups were determined by one-way analysis of variance (ANOVA), followed by Tukey post-hoc for all inflammation and oxidative stress analysis. For behavior tests, data was expressed as median ± interquartile rage and differences among groups were determined by Mann-Whitney and Wilcoxon test. In all comparisons, statistical significance was set at p < 0.05.
Results
DMF Reduces Neuroinflammation in Sepsis
Figure 1 shows the results of MPO activity, i.e., indicative of activated neutrophil infiltrate, in the hippocampus, prefrontal cortex, and total cortex at 24 h after CLP procedure. CLP + vehicle animals presented higher levels of MPO, compared to sham + vehicle animals, in all brain structures. DMF treatment was effective in decreasing neutrophil infiltrate after sepsis in all brain structures.
MPO activity in the hippocampus, prefrontal cortex, and total cortex of rats subjected to polymicrobial sepsis and treated with DMF or NAC. Data is expressed as mean ± standard deviation, analyzed by one-way ANOVA with Tukey post-hoc test. *p < 0.05 compared to sham + vehicle group and #p < 0.05 compared to CLP + vehicle group
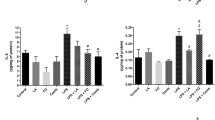
Figure 2 shows the results of cytokines. CINC-1 levels were initially elevated (Fig. 2a) in all brain structures of CLP + vehicle group, while DMF treatment significantly decreased CINC-1 levels only in the total cortex. Regarding TNF-α levels (Fig. 2b), it was increased in the hippocampus and prefrontal cortex at 24 h after sepsis induction, and DMF treatment was effective in decreasing this elevation only in the hippocampus. Concerning IL-6 levels (Fig. 2c), CLP + vehicle animals displayed enhanced levels in all brain structures, while DMF treatment was effective in decreasing it only in the hippocampus.
CINC-1 (a), TNF-α (b), and IL-6 (c) levels in the hippocampus, prefrontal cortex, and total cortex of rats subjected to polymicrobial sepsis and treated with DMF or NAC. Data is expressed as mean ± standard deviation, analyzed by one-way ANOVA with Tukey post-hoc test. *p < 0.05 compared to sham + vehicle group and #p < 0.05 compared to CLP + vehicle group
DMF Reduces the Brain Levels of Oxidative Stress After Sepsis
Figure 3 illustrates nitrite/nitrate (N/N) concentration at 24 h after CLP surgery. We observed an increase in N/N concentration in the hippocampus and total cortex of septic animals (CLP + vehicle group), while septic animals treated with DMF (CLP + DMF group) showed a decrease in N/N concentration in the hippocampus and total cortex. With regard to lipid peroxidation (Fig. 4a), there was an increase in MDA equivalent levels in all brain structures of rats subjected to sepsis, while DMF-treated animals exhibited decreased lipid peroxidation in the hippocampus and total cortex. Oxidative damage to protein is represented by protein carbonyls levels (Fig. 4b). All brain structures of CLP + vehicle group were significantly affected, and DMF treatment was effective in diminishing the levels of this oxidative marker in all structures.
Nitrite/nitrate concentration in the hippocampus, prefrontal cortex, and total cortex of rats subjected to polymicrobial sepsis and treated with DMF or NAC. Data is expressed as mean ± standard deviation, analyzed by one-way ANOVA with Tukey post-hoc test. *p < 0.05 compared to sham + vehicle group and #p < 0.05 compared to CLP + vehicle group
Lipid peroxidation (a) and protein carbonylation (b) in the hippocampus, prefrontal cortex, and total cortex of rats subjected to polymicrobial sepsis and treated with DMF or NAC. Data is expressed as mean ± standard deviation, analyzed by one-way ANOVA with Tukey post-hoc test. *p < 0.05 compared to sham + vehicle group and #p < 0.05 compared to CLP + vehicle group
SOD activity was decreased in the three brain structures of septic animals (Fig. 5a). DMF treatment was effective in increasing SOD activity only in the hippocampus. When CAT activity was analyzed (Fig. 5b), no changes were observed due to sepsis induction nor the treatments. On the other hand, there was a decrease in GPx activity (Fig. 5c) in all brain structures of CLP + vehicle rats, and the treatment with DMF prevented this alteration in the hippocampus and total cortex.
SOD (a), CAT (b), and GPx (c) activity in the hippocampus, prefrontal cortex, and total cortex of rats subjected to polymicrobial sepsis and treated with DMF or NAC. Data is expressed as mean ± standard deviation, analyzed by one-way ANOVA with Tukey post-hoc test. *p < 0.05 compared to sham + vehicle group and #p < 0.05 compared to CLP + vehicle group
DMF Prevents Memory Impairment Due to Sepsis
The open field habituation task results are represented in Fig. 6. We observed that all sham animals decreased the number of crossings between the training and test sessions, indicating an adequate memory retention (Fig. 6a). Animals subjected to sepsis (CLP + DMS group) did not present significant difference between both sessions, which demonstrate memory impairment. On the contrary, CLP animals treated with DMF or NAC showed a similar behavior to sham animals, indicating no memory deficit. Regarding the number of rearings (Fig. 6b), there was no difference between treated and untreated CLP groups. Concerning the novel object recognition task (Fig. 7), we noted that animals subjected to sepsis presented memory damage to recognize a new object in short-term (Fig. 7a) and long-term (Fig. 7b) evaluations. Treatment with DMF significantly prevented this alteration only in the short-term evaluation.
Discussion
The development of sepsis cerebral dysfunction contributes to its high mortality, especially because of the exacerbated inflammatory response, as well as the oxidative stress, which causes neurotransmitters and behavioral alterations that negatively impact quality of life (Sonneville et al. 2013). In the present study, we showed for the first time that DMF was effective in reversing neuroinflammation, oxidative brain damage, and cognitive impairment in an animal model of sepsis that is characterized by inducing cognitive impairment associated with deleterious effects in the brain tissue.
The systemic inflammatory response associated with sepsis pathophysiology induces the activation of BBB endothelial cells, which result in the stimulation of proinflammatory mediators in the cerebral parenchyma (Sonneville et al. 2013). Besides, proteins such as matrix metalloproteinases (MMP)-2 and 9 are incite the degradation of tight junctions that guarantee the BBB integrity and its functions (Vafadari et al. 2016). Also, cytokines can activate these MMPs, propitiating the alteration in BBB permeability. Previous research verified that BBB permeability is negatively altered within 24 h after sepsis onset (Dal-Pizzol et al. 2013), being accompanied by increased neutrophil migration to cerebral structures, such as the hippocampus (Comim et al. 2011b). In the present study, we observed an increased neutrophil infiltration, represented by MPO activity, in all brain structures, and the administration of DMF was effective in attenuating this alteration.
DMF, along with its active metabolite monomethyl fumarate (MMF), has been tested to treat inflammatory- and oxidative stress-related diseases (Gill and Kolson 2013). After oral administration, DMF is metabolized to MMF, reaching the CNS and having a direct effect on resident cells (Wilms et al. 2010). DMF is considered a potent oral immunomodulatory treatment for patients with relapsing-remitting multiple sclerosis due to its ability to cross the BBB, thus exerting neuroprotection by modulating microglial activation (Wilms et al. 2010). Activated microglia is a critical element of the neuroinflammatory cascade, as this cell starts to release proinflammatory cytokines and reactive species that leads to neurodegeneration and altered synaptic transmission, as occurs in neurodegenerative diseases such as multiple sclerosis (Wilms et al. 2010). Consequently, DMF can modulate neuroinflammation by diminishing microglial activation and proinflammatory cytokines levels, with less attraction of immune cells to the region (Lin et al. 2016).
A study of multiple sclerosis (MS) using the experimental model of encephalomyelitis showed that neutrophils infiltrated the CNS, and DMF treatment was able to reverse it through hydrocarboxylic acid activation, a membrane receptor coupled to the G protein. Hydrocarboxylic acid activates the protein extracellular signal-regulated kinase (ERK), which is able to decrease neutrophils migration to brain endothelial cells (Chen et al. 2014). Of note, DMF has been tested in patients with relapsing-remitting MS in clinical trials and the current findings demonstrate significant benefits, including improved quality of life, reductions in relapses, number of new lesions, and lesion volume (Kita et al. 2014; Miller et al. 2015).
Rats subjected to an ischemic stroke model not only presented reduced neutrophil infiltrate with DMF treatment, but also displayed a smaller number of activated microglia in the cerebral ischemic region and decreased levels of proinflammatory cytokines (Lin et al. 2016). Chemokines such as CINC-1, the mouse homolog for interleukin-8 (IL-8) in humans, play a key role in neutrophil chemotaxis and allows the release of other proinflammatory cytokines, e.g. TNF-α and IL-6 (Reddy et al. 2008). When glial cells recognize these molecules, it activates different intracellular pathways, including the transcription factor NF-κB in microglia, with consequent elevation of proinflammatory cytokines (Parodi et al. 2015). In our study, CINC-1, TNF-α, and IL-6 levels were elevated and followed the profile of increased MPO activity in the brain structures. Particularly, studies showed the presence of constitutive cytokine (IL-1β, IL-6, TNF-α) and chemokine receptors around the brain (Cunningham et al. 2002; Otero and Merrill 1994). It appears that in adult mice, a majority of these receptors are located in the hippocampus (Banisadr et al. 2002; Dopp et al. 1997). This may explain the reason for the vulnerability of these regions to the systemic inflammatory response and oxidative stress in sepsis and the effectiveness of DMF treatment.
When activated by bacterial toxins or inflammatory mediators, NF-κB stimulates genes that encode proinflammatory cytokines (Leibowitz and Yan 2016). After microglial activation by lipopolysaccharide (LPS), DMF decreased the release of proinflammatory cytokines through a reduction in NF-κB activation (Michels et al. 2015b), and this antiinflammatory role played by DMF has been demonstrated in several studies (Gerdes et al. 2007; Ghoreschi et al. 2011; Gillard et al. 2015; Kastrati et al. 2016). However, NF-κB inhibition occurs because DMF activates another transcription factor, the Nrf2. Studies using Nrf2−/− knockout mice showed increased instigation of NF-κB and synthesis of cytokines and chemokines after an inflammatory insult by LPS (Wakabayashi et al. 2010) and after a traumatic brain injury (Jin et al. 2008).
Sepsis is associated with increased ROS/RNS production, decreased antioxidant capacity, and the occurrence of oxidative damage (Petronilho et al. 2012; Annane and Sharshar 2014; Vieira et al. 2015). Thus, the inflammatory response in sepsis is characterized by the excessive release of cytokines, associated with increased oxidative stress, leading to mitochondrial dysfunction with consequent cell death (Comim et al. 2011a).
The brain is often exposed to reactive species because of its high oxygen consumption, and during sepsis, this organ is rapidly affected (Comim et al. 2011a), since there is an expressive increase in superoxide (O2−) anion (Chuang et al. 2002), nitric oxide (NO), and peroxynitrite (ONOO-) production in the brain tissue after sepsis onset (Berg et al. 2011). As a consequence, mitochondrial degeneration and apoptosis have been observed in different brain regions of septic animals and also in human patients (Bozza et al. 2013; Kasahara and Inoue 2015). In our study, DMF regulated N/N levels, as indicative of NO production, in the hippocampus and prefrontal cortex. Wilms and colleagues have previously shown that DMF decreases NO synthesis in microglia and astrocytes cell culture stimulated with LPS and such response depends on Nrf2 activation (Wilms et al. 2010). Our results demonstrate that the decrease of N/N due to DMF reflected in the reduced oxidative damage to lipids and proteins.
Antioxidant compounds usually act by scavenging reactive species, thus protecting the cells from oxidative damage and reducing neurological conditions related to oxidative stress (Sitar et al. 2016). In sepsis, the production of antioxidant enzymes such as SOD, CAT, and GPx, is disrupted, and our results corroborate the literature, as SOD and GPx activities were decreased in the brain of CLP + vehicle animals. Also, the endogenous machinery is normally satisfactory to restore redox balance and an adequate cellular function; however, sepsis promotes a very intense inflammatory and oxidative response that is not easily diminished by the body itself. This is well observed when we compare sham + vehicle and CLP + vehicle groups, as septic animals display a pronounced reduction in the antioxidant defense system.
When challenged with situations like inflammation and oxidative stress, the primary mechanism for maintenance of cellular redox balance and to reestablish homeostasis is the Nrf2 pathway. Usually, Nrf2 is removed in the cytoplasm by Kelch-like ECH-associated protein 1 (Keap1) and targeted for degradation. However, reactive species and electrophiles modify cysteine residues on Keap1 that lead to conformational changes and reduce the affinity of KEAP1 for Nrf2, thus allowing Nrf2 to initiate the transcription of many genes involved in the antioxidant and antiinflammatory responses (Itoh et al. 2003, 2004; Kobayashi and Yamamoto 2006; Giustina et al. 2017).
Importantly, DMF acts like an electrophile on Keap1, limiting Nrf2 degradation (Brennan et al. 2017). Thus, DMF contributes to reestablish the levels of antioxidant enzymes. Here we demonstrate that DMF treatment improved the antioxidant activity of SOD and GPx in the hippocampus and total cortex of septic rats. Similar findings were also observed by our group in peripheral organs of septic rats subjected to a DMF treatment (Giustina et al. 2017).
In addition, DMF also has cytoprotecting properties in glial cells, oligodendrocytes, and neurons (Bomprezzi 2015). A recent study showed that DMF, by activating Nrf2, attenuated neurological deficits and cerebral edema at 24 and 72 h after an experimental model of intracerebral hemorrhage (Iniaghe et al. 2015). Such positive cytoprotecting impact also influences cognitive function, which is normally disrupted in rodents subjected to the CLP model up to 30 days after surgery, even though they seem fully recovered from infection and motor alterations (Barichello et al. 2005; Tuon et al. 2008; Michels et al. 2015a). In our study, we found that DMF treatment was effective in reversing memory impairment evaluated in the habituation to an open field and novel object recognition tasks, and these findings corroborate with previous studies that evaluated different compounds with directly or indirectly antioxidant effect, e.g. α-lipoic acid (Della Giustina et al. 2017) and vitamin B6 (Danielski et al. 2017).
In the present research, we decided to use NAC as a positive control because it is a classical antioxidant precursor of amino acid cysteine, which is necessary to synthetize glutathione (Minarini et al. 2017). In sepsis, several studies have related the effectiveness of NAC in reducing oxidative damage, both in peripheral and in the CNS (Ritter et al. 2004; Cassol-Jr et al. 2010; Andrades et al. 2011). The use of DMF, on the other hand, seems relevant due to its multiple therapeutic targets beyond the evaluations performed in the present study. Sepsis suppresses the immune function, and one of DMF differentials is its potent immunomodulatory effect (Schulze-Topphoff et al. 2016).
Importantly, our results concerning neutrophil infiltrate have some limitations. Sepsis increases blood/serum MPO activity, and this may have contributed to the MPO activity observed in our results, as we used non-perfused brain tissue to perform the analysis. However, to our knowledge, this research provides novel information about DMF effects in the CNS of septic rats. Therefore, we demonstrated in the present study that DMF reduces sepsis-induced neuroinflammation, oxidative stress, and cognitive impairment in rats subjected with a polymicrobial model of sepsis.
References
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126
Albrecht P, Bouchachia I, Zimmermann C et al (2012) Effects of dimethyl fumarate on neuroprotection and immunomodulation. J Neuroinflammation 9:163. https://doi.org/10.1186/1742-2094-9-163
Andrades M, Ritter C, De Oliveira MR et al (2011) Antioxidant treatment reverses organ failure in rat model of sepsis: role of antioxidant enzymes imbalance, neutrophil infiltration, and oxidative stress. J Surg Res 167:e307–e313. https://doi.org/10.1016/j.jss.2009.08.005
Annane D, Sharshar T (2014) Cognitive decline after sepsis. Lancet Respir Med 3:61–69. https://doi.org/10.1016/S2213-2600(14)70246-2
Banisadr G, Quéraud-Lesaux F, Boutterin MC, Pélaprat D, Zalc B, Rostène W, Haour F, Parsadaniantz SM (2002) Distribution, cellular localization and functional role of CCR2 chemokine receptors in adult rat brain. J Neurochem 81(2):257–269
Bannister JV, Calabrese L (1987) Assays for superoxide dismutase. Methods Biochem Anal 32:279–312
Barichello T, Machado RA, Constantino L, Valvassori SS, Réus GZ, Martins MR, Petronilho F, Ritter C, Quevedo J, Dal-Pizzol F (2007) Antioxidant treatment prevented late memory impairment in an animal model of sepsis. Crit Care Med 35:2186–2190. https://doi.org/10.1097/01.CCM.0000281452.60683.96
Barichello T, Martins MR, Reinke A, Feier G, Ritter C, Quevedo J, Dal-Pizzol F (2005) Cognitive impairment in sepsis survivors from cecal ligation and perforation. Crit Care Med 33:221–223. https://doi.org/10.1097/01.CCM.0000150741.12906.BD
Berg RMG, Møller K, Bailey DM (2011) Neuro-oxidative-nitrosative stress in sepsis. J Cereb Blood Flow Metab 31:1532–1544. https://doi.org/10.1038/jcbfm.2011.48
Bomprezzi R (2015) Dimethyl fumarate in the treatment of relapsing-remitting multiple sclerosis: an overview. Ther Adv Neurol Disord 8:20–30. https://doi.org/10.1177/1756285614564152
Bozza FA, D’Avila JC, Ritter C et al (2013) Bioenergetics, mitochondrial dysfunction, and oxidative stress in the pathophysiology of septic encephalopathy. Shock 39(Suppl 1):10–16. https://doi.org/10.1097/SHK.0b013e31828fade1
Brennan MS, Matos MF, Richter KE, Li B, Scannevin RH (2017) The NRF2 transcriptional target, OSGIN1, contributes to monomethyl fumarate-mediated cytoprotection in human astrocytes. Sci Rep 7:42054. https://doi.org/10.1038/srep42054
Carvalho D, Petronilho F, Vuolo F, Machado RA, Constantino L, Guerrini R, Calo G, Gavioli EC, Streck EL, Dal-Pizzol F (2008) The nociceptin/orphanin FQ-NOP receptor antagonist effects on an animal model of sepsis. Intensive Care Med 34:2284–2290. https://doi.org/10.1007/s00134-008-1313-3
Cassol-Jr OJ, Rezin GT, Petronilho FC et al (2010) Effects of N-acetylcysteine/deferoxamine, taurine and RC-3095 on respiratory chain complexes and creatine kinase activities in rat brain after sepsis. Neurochem Res 35:515–521. https://doi.org/10.1007/s11064-009-0089-3
Chelkeba L, Ahmadi A, Abdollahi M, Najafi A, Ghadimi MH, Mosaed R, Mojtahedzadeh M (2015) The effect of parenteral selenium on outcomes of mechanically ventilated patients following sepsis: a prospective randomized clinical trial. Ann Intensive Care 5:29. https://doi.org/10.1186/s13613-015-0071-y
Chen H, Assmann JC, Krenz A, Rahman M, Grimm M, Karsten CM, Köhl J, Offermanns S, Wettschureck N, Schwaninger M (2014) Hydroxycarboxylic acid receptor 2 mediates dimethyl fumarate’s protective effect in EAE. J Clin Invest 124:2188–2192. https://doi.org/10.1172/JCI72151
Chuang Y-C, Tsai J-L, Chang AYW, Chan JYH, Liou CW, Chan SHH (2002) Dysfunction of the mitochondrial respiratory chain in the rostral ventrolateral medulla during experimental endotoxemia in the rat. J Biomed Sci 9:542–548. https://doi.org/10.1007/BF02254981
Comim CM, Cassol-Jr OJ, Constantino LS, Felisberto F, Petronilho F, Rezin GT, Scaini G, Daufenbach JF, Streck EL, Quevedo J, Dal-Pizzol F (2011a) Alterations in inflammatory mediators, oxidative stress parameters and energetic metabolism in the brain of sepsis survivor rats. Neurochem Res 36:304–311. https://doi.org/10.1007/s11064-010-0320-2
Comim CM, Vilela MC, Constantino LS, Petronilho F, Vuolo F, Lacerda-Queiroz N, Rodrigues DH, da Rocha JL, Teixeira AL, Quevedo J, Dal-Pizzol F (2011b) Traffic of leukocytes and cytokine up-regulation in the central nervous system in sepsis. Intensive Care Med 37:711–718. https://doi.org/10.1007/s00134-011-2151-2
Cunningham C, Konsman JP, Cartmell T. (2002) Cytokines and the ageing brain. Trends Neurosci 25(11):546–547
Dal-Pizzol F, Rojas HA, Dos Santos EM et al (2013) Matrix metalloproteinase-2 and metalloproteinase-9 activities are associated with blood-brain barrier dysfunction in an animal model of severe sepsis. Mol Neurobiol 48:62–70. https://doi.org/10.1007/s12035-013-8433-7
Dal-Pizzol F, Tomasi CD, Ritter C (2014) Septic encephalopathy: does inflammation drive the brain crazy? Rev Bras Psiquiatr 36:251–258. https://doi.org/10.1590/1516-4446-2013-1233
Danielski LG, Giustina AD, Goldim MP, et al (2017) Vitamin B6 reduces neurochemical and long-term cognitive alterations after polymicrobial sepsis: involvement of the kynurenine pathway modulation. Mol Neurobiol 1–14. doi: https://doi.org/10.1007/s12035-017-0706-0
de Lima MNM, Laranja DC, Caldana F, Bromberg E, Roesler R, Schröder N (2005) Reversal of age-related deficits in object recognition memory in rats with l-deprenyl. Exp Gerontol 40:506–511. https://doi.org/10.1016/j.exger.2005.03.004
De Young LM, Kheifets JB, Ballaron SJ, Young JM (1989) Edema and cell infiltration in the phorbol ester-treated mouse ear are temporally separate and can be differentially modulated by pharmacologic agents. Agents Actions 26:335–341
Della Giustina A, Goldim MP, Danielski LG, Florentino D, Mathias K, Garbossa L, Oliveira Junior AN, Fileti ME, Zarbato GF, da Rosa N, Martins Laurentino AO, Fortunato JJ, Mina F, Bellettini-Santos T, Budni J, Barichello T, Dal-Pizzol F, Petronilho F (2017) Alpha-lipoic acid attenuates acute neuroinflammation and long-term cognitive impairment after polymicrobial sepsis. Neurochem Int 108:436–447. https://doi.org/10.1016/j.neuint.2017.06.003
Dopp JM, Mackenzie-Graham A, Otero GC, Merrill JE (1997) Differential expression, cytokine modulation, and specific functions of type-1 and type-2 tumor necrosis factor receptors in rat glia. J Neuroimmunol 75(1-2):104–112
Doyle SL, O’Neill LAJ (2006) Toll-like receptors: from the discovery of NFKappaB to new insights into transcriptional regulations in innate immunity. Biochem Pharmacol 72:1102–1113. https://doi.org/10.1016/j.bcp.2006.07.010
Draper HH, Hadley M (1990) Malondialdehyde determination as index of lipid peroxidation. Methods Enzymol 186:421–431
Gerdes S, Shakery K, Mrowietz U (2007) Dimethylfumarate inhibits nuclear binding of nuclear factor κB but not of nuclear factor of activated T cells and CCAAT/enhancer binding protein β in activated human T cells. Br J Dermatol 156:838–842. https://doi.org/10.1111/j.1365-2133.2007.07779.x
Ghoreschi K, Bruck J, Kellerer C et al (2011) Fumarates improve psoriasis and multiple sclerosis by inducing type II dendritic cells. J Exp Med 208:2291–2303. https://doi.org/10.1084/jem.20100977
Gill AJ, Kolson DL (2013) Dimethyl fumarate modulation of immune and antioxidant responses: application to HIV therapy. Crit Rev Immunol 33:307–359. https://doi.org/10.1615/CritRevImmunol.2013007247
Gill AJ, Kovacsics CE, Cross SA, Vance PJ, Kolson LL, Jordan-Sciutto KL, Gelman BB, Kolson DL (2014) Heme oxygenase-1 deficiency accompanies neuropathogenesis of HIV-associated neurocognitive disorders. J Clin Invest 124:4459–4472. https://doi.org/10.1172/JCI72279
Gillard GO, Collette B, Anderson J, Chao J, Scannevin RH, Huss DJ, Fontenot JD (2015) DMF, but not other fumarates, inhibits NF-κB activity in vitro in an Nrf2-independent manner. J Neuroimmunol 283:74–85. https://doi.org/10.1016/j.jneuroim.2015.04.006
Giustina AD, Bonfante S, Zarbato GF et al (2017) Dimethyl fumarate modulates oxidative stress and inflammation in organs after sepsis in rats. Inflammation 41:1–13. https://doi.org/10.1007/s10753-017-0689-z
Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR (1982) Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal Biochem 126:131–138. https://doi.org/10.1016/0003-2697(82)90118-X
Hubbard WJ, Choudhry M, Schwacha MG, Kerby JD, Rue LW III, Bland KI, Chaudry IH (2005) Cecal ligation and puncture. Shock 24:52–57. https://doi.org/10.1097/01.shk.0000191414.94461.7e
Iniaghe LO, Krafft PR, Klebe DW, Omogbai EKI, Zhang JH, Tang J (2015) Dimethyl fumarate confers neuroprotection by casein kinase 2 phosphorylation of Nrf2 in murine intracerebral hemorrhage. Neurobiol Dis 82:349–358. https://doi.org/10.1016/j.nbd.2015.07.001
Itoh K, Tong KI, Yamamoto M (2004) Molecular mechanism activating Nrf2-Keap1 pathway in regulation of adaptive response to electrophiles. Free Radic Biol Med 36:1208–1213
Itoh K, Wakabayashi N, Katoh Y, Ishii T, O'Connor T, Yamamoto M (2003) Keap1 regulates both cytoplasmic-nuclear shuttling and degradation of Nrf2 in response to electrophiles. Genes Cells 8:379–391. https://doi.org/10.1046/j.1365-2443.2003.00640.x
Iwashyna TJ, Ely EW, Smith DM, Langa KM (2010) Long-term cognitive impairment and functional disability among survivors of severe sepsis. J Am Med Assoc 304:1787–1794. https://doi.org/10.1001/jama.2010.1553
Jin W, Zhu L, Guan Q et al (2008) Influence of Nrf2 genotype on pulmonary NF-κB activity and inflammatory response after traumatic brain injury. Ann Clin Lab Sci 38:221–227
Kasahara E, Inoue M (2015) Cross-talk between HPA-axis-increased glucocorticoids and mitochondrial stress determines immune responses and clinical manifestations of patients with sepsis. Redox Rep 20:1–10. https://doi.org/10.1179/1351000214Y.0000000107
Kastrati I, Siklos MI, Calderon-Gierszal EL, el-Shennawy L, Georgieva G, Thayer EN, Thatcher GRJ, Frasor J (2016) Dimethyl fumarate inhibits the nuclear factor κB pathway in breast cancer cells by covalent modification of p65 protein. J Biol Chem 291:3639–3647. https://doi.org/10.1074/jbc.M115.679704
Kita M, Fox RJ, Gold R, Giovannoni G, Phillips JT, Sarda SP, Kong J, Viglietta V, Sheikh SI, Okwuokenye M, Kappos L (2014) Effects of delayed-release dimethyl fumarate (DMF) on health-related quality of life in patients with relapsing-remitting multiple sclerosis: an integrated analysis of the phase 3 DEFINE and CONFIRM studies. Clin Ther 36:1958–1971. https://doi.org/10.1016/j.clinthera.2014.08.013
Kobayashi M, Yamamoto M (2006) Nrf2-Keap1 regulation of cellular defense mechanisms against electrophiles and reactive oxygen species. Adv Enzym Regul 46:113–140. https://doi.org/10.1016/j.advenzreg.2006.01.007
Lange M, Szabo C, Traber DL, Horvath E, Hamahata A, Nakano Y, Traber LD, Cox RA, Schmalstieg FC, Herndon DN, Enkhbaatar P (2012) Time profile of oxidative stress and neutrophil activation in ovine acute lung injury and Sepsis. Shock 37:468–472. https://doi.org/10.1097/SHK.0b013e31824b1793
Leibowitz SM, Yan J (2016) NF-κB pathways in the pathogenesis of multiple sclerosis and the therapeutic implications. Front Mol Neurosci 9:84. https://doi.org/10.3389/fnmol.2016.00084
Levine RL, Garland D, Oliver CN et al (1990) Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol 186:464–478
Lin R, Cai J, Kostuk EW, Rosenwasser R, Iacovitti L (2016) Fumarate modulates the immune/inflammatory response and rescues nerve cells and neurological function after stroke in rats. J Neuroinflammation 13:269. https://doi.org/10.1186/s12974-016-0733-1
Linker RA, Lee DH, Ryan S, van Dam AM, Conrad R, Bista P, Zeng W, Hronowsky X, Buko A, Chollate S, Ellrichmann G, Brück W, Dawson K, Goelz S, Wiese S, Scannevin RH, Lukashev M, Gold R (2011) Fumaric acid esters exert neuroprotective effects in neuroinflammation via activation of the Nrf2 antioxidant pathway. Brain 134:678–692. https://doi.org/10.1093/brain/awq386
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Mayr FB, Yende S, Angus DC (2014) Epidemiology of severe sepsis. Virulence 5:4–11. https://doi.org/10.4161/viru.27372
Michels M, Danielski LG, Dal-Pizzol F, Petronilho F (2014) Neuroinflammation: microglial activation during sepsis. Curr Neurovasc Res 11:262–270
Michels M, Danieslki LG, Vieira A et al (2015a) CD40-CD40 ligand pathway is a major component of acute neuroinflammation and contributes to long-term cognitive dysfunction after sepsis. Mol Med 21:219–226. https://doi.org/10.2119/molmed.2015.00070
Michels M, Vieira AS, Vuolo F, Zapelini HG, Mendonça B, Mina F, Dominguini D, Steckert A, Schuck PF, Quevedo J, Petronilho F, Dal-Pizzol F (2015b) The role of microglia activation in the development of sepsis-induced long-term cognitive impairment. Brain Behav Immun 43:54–59. https://doi.org/10.1016/j.bbi.2014.07.002
Miller DH, Fox RJ, Phillips JT, Hutchinson M, Havrdova E, Kita M, Wheeler-Kingshott CAM, Tozer DJ, MacManus DG, Yousry TA, Goodsell M, Yang M, Zhang R, Viglietta V, Dawson KT, For the CONFIRM study investigators (2015) Effects of delayed-release dimethyl fumarate on MRI measures in the phase 3 CONFIRM study. Neurology 84:1145–1152. https://doi.org/10.1212/WNL.0000000000001360
Mina F, Comim CM, Dominguini D, Cassol-Jr OJ, Dall’Igna DM, Ferreira GK, Silva MC, Galant LS, Streck EL, Quevedo J, Dal-Pizzol F (2014) Il1-β involvement in cognitive impairment after sepsis. Mol Neurobiol 49:1069–1076. https://doi.org/10.1007/s12035-013-8581-9
Minarini A, Ferrari S, Galletti M, Giambalvo N, Perrone D, Rioli G, Galeazzi GM (2017) N-acetylcysteine in the treatment of psychiatric disorders: current status and future prospects. Expert Opin Drug Metab Toxicol 13:279–292. https://doi.org/10.1080/17425255.2017.1251580
Otero GC, Merrill JE. (1994) Cytokine receptors on glial cells. Glia 11(2):117–128
Parodi B, Cordano C, Morando S, Bragoni A, Giunti D, Usai C, Centonze D, de Rosbo NK, Scannevin RH, Uccelli A (2017) Monomethyl fumarate inhibits the NFkB pathway and pro-inflammatory cytokine expression in microglia through HCA2 signaling via the AMPK/Sirt axis. J Neuroimmunol 275:167–168. https://doi.org/10.1016/j.jneuroim.2014.08.450
Parodi B, Rossi S, Morando S, Cordano C, Bragoni A, Motta C, Usai C, Wipke BT, Scannevin RH, Mancardi GL, Centonze D, Kerlero de Rosbo N, Uccelli A (2015) Fumarates modulate microglia activation through a novel HCAR2 signaling pathway and rescue synaptic dysregulation in inflamed CNS. Acta Neuropathol 130:279–295. https://doi.org/10.1007/s00401-015-1422-3
Petronilho F, Périco SR, Vuolo F, Mina F, Constantino L, Comim CM, Quevedo J, Souza DO, Dal-Pizzol F (2012) Protective effects of guanosine against sepsis-induced damage in rat brain and cognitive impairment. Brain Behav Immun 26:904–910. https://doi.org/10.1016/j.bbi.2012.03.007
Reddy RC, Narala VR, Keshamouni VG, Milam JE, Newstead MW, Standiford TJ (2008) Sepsis-induced inhibition of neutrophil chemotaxis is mediated by activation of peroxisome proliferator-activated receptor-{gamma}. Blood 112:4250–4258. https://doi.org/10.1182/blood-2007-12-128967
Reick C, Ellrichmann G, Thone J et al (2014) Neuroprotective dimethyl fumarate synergizes with immunomodulatory interferon beta to provide enhanced axon protection in autoimmune neuroinflammation. Exp Neurol 257:50–56. https://doi.org/10.1016/j.expneurol.2014.04.003
Ritter C, Andrades ME, Reinke A, Menna-Barreto S, Moreira JCF, Dal-Pizzol F (2004) Treatment with N-acetylcysteine plus deferoxamine protects rats against oxidative stress and improves survival in sepsis. Crit Care Med 32:342–349. https://doi.org/10.1097/01.CCM.0000109454.13145.CA
Scannevin RH, Chollate S, Jung M-Y, Shackett M, Patel H, Bista P, Zeng W, Ryan S, Yamamoto M, Lukashev M, Rhodes KJ (2012) Fumarates promote cytoprotection of central nervous system cells against oxidative stress via the nuclear factor (erythroid-derived 2)-like 2 pathway. J Pharmacol Exp Ther 341:274–284. https://doi.org/10.1124/jpet.111.190132
Schulze-Topphoff U, Varrin-Doyer M, Pekarek K, Spencer CM, Shetty A, Sagan SA, Cree BAC, Sobel RA, Wipke BT, Steinman L, Scannevin RH, Zamvil SS (2016) Dimethyl fumarate treatment induces adaptive and innate immune modulation independent of Nrf2. Proc Natl Acad Sci 113:4777–4782. https://doi.org/10.1073/pnas.1603907113
Singer M (2014) The role of mitochondrial dysfunction in sepsis-induced multi-organ failure. Virulence 5:66–72. https://doi.org/10.4161/viru.26907
Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche JD, Coopersmith CM, Hotchkiss RS, Levy MM, Marshall JC, Martin GS, Opal SM, Rubenfeld GD, van der Poll T, Vincent JL, Angus DC (2016) The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA 315:801–810. https://doi.org/10.1001/jama.2016.0287
Sitar G, Kucuk M, Erinc Sitar M, Yasar O, Aydin S, Yanar K, Cakatay U, Buyukpınarbasili N (2016) Crucial roles of systemic and tissue lipid peroxidation levels and anti-oxidant defences following contrast agent application. Iran Red Crescent Med J 18:e37331. https://doi.org/10.5812/ircmj.37331
Sonneville R, Verdonk F, Rauturier C, Klein IF, Wolff M, Annane D, Chretien F, Sharshar T (2013) Understanding brain dysfunction in sepsis. Ann Intensive Care 3:1–11. https://doi.org/10.1186/2110-5820-3-15
Tuon L, Comim CM, Petronilho F, Barichello T, Izquierdo I, Quevedo J, Dal-Pizzol F (2008) Time-dependent behavioral recovery after sepsis in rats. Intensive Care Med 34:1724–1731. https://doi.org/10.1007/s00134-008-1129-1
Vafadari B, Salamian A, Kaczmarek L (2016) MMP-9 in translation: from molecule to brain physiology, pathology, and therapy. J Neurochem 139:91–114
Vianna MR, Alonso M, Viola H, Quevedo J, de Paris F, Furman M, de Stein ML, Medina JH, Izquierdo I (2000) Role of hippocampal signaling pathways in long-term memory formation of a nonassociative learning task in the rat. Learn Mem 7:333–340
Vieira AA, Michels M, Florentino D, Nascimento D, Rezin G, Leffa D, Fortunato J, Dal-Pizzol F, Barichello T, Quevedo J, Petronilho F (2015) Obesity promotes oxidative stress and exacerbates sepsis-induced brain damage. Curr Neurovasc Res 12:147–154
Wakabayashi N, Slocum SL, Skoko JJ, Shin S, Kensler TW (2010) When NRF2 talks, who’s listening? Antioxid Redox Signal 13:1649–1663. https://doi.org/10.1089/ars.2010.3216
Wassmer SC, Combes V, Candal FJ, Juhan-Vague I, Grau GE (2006) Platelets potentiate brain endothelial alterations induced by Plasmodium falciparum. Infect Immun 74:645–653. https://doi.org/10.1128/IAI.74.1.645-653.2006
Wendel A (1981) Glutathione peroxidase. Methods Enzymol 77:325–333
Wierinckx A, Brevé J, Mercier D et al (2005) Detoxication enzyme inducers modify cytokine production in rat mixed glial cells. J Neuroimmunol 166:132–143. https://doi.org/10.1016/j.jneuroim.2005.05.013
Wiersinga WJ, Leopold SJ, Cranendonk DR, van der Poll T (2014) Host innate immune responses to sepsis. Virulence 5:36–44. https://doi.org/10.4161/viru.25436
Wilms H, Sievers J, Rickert U, Rostami-Yazdi M, Mrowietz U, Lucius R (2010) Dimethylfumarate inhibits microglial and astrocytic inflammation by suppressing the synthesis of nitric oxide, IL-1beta, TNF-alpha and IL-6 in an in-vitro model of brain inflammation. J Neuroinflammation 7:30. https://doi.org/10.1186/1742-2094-7-30
Zampieri FG, IIM P, Fabio III, Park M, Machado FS, Azevedo LCP (2011) Sepsis-associated encephalopathy: not just delirium. Clinics 66:1825–1831. https://doi.org/10.1590/S1807-59322011001000024
Zheng G, Lyu J, Huang J et al (2015) Experimental treatments for mitochondrial dysfunction in sepsis: a narrative review. J Res Med Sci 20:185–195
Funding
This research was supported by grants from the National Council for Scientific and Technological Development (CNPq) and the Coordination for the Improvement of Higher Education Personnel (CAPES) (FP). FP, PFS, and TB are CNPq Research Fellows. The funding sources were not involved in the conduction of the research, preparation of the article nor in the decision to submit the article for publication.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All experimental procedures were approved by the Animal Care and Experimentation Committee of UNISUL (protocol number 15.009.4.03.IV), Brazil.
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Zarbato, G.F., de Souza Goldim, M.P., Giustina, A.D. et al. Dimethyl Fumarate Limits Neuroinflammation and Oxidative Stress and Improves Cognitive Impairment After Polymicrobial Sepsis. Neurotox Res 34, 418–430 (2018). https://doi.org/10.1007/s12640-018-9900-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12640-018-9900-8