Abstract
The pathogenesis of sepsis is characterized by an overwhelming systemic inflammatory response that can lead to multiple organ failure. Considering that we have recently demonstrated that mitochondrial respiratory chain and creatine kinase (CK) are altered in the brain of rats after cecal ligation and perforation (CLP) and that a combination of N-acetylcysteine/deferoxamine (NAC/DFX), taurine and RC-3095 were shown to be an effective treatment of sepsis, we investigated whether the alterations of these enzymes may be reversed by these drugs. The results demonstrated that CLP inhibited complexes I and II, and that all the treatments were able to reverse this inhibition in all brain areas studied in the present work. On the other hand, complexes III and IV were not affected by sepsis neither by any of the treatments. An increase in CK activity in brain of rats 12 h after CLP was also verified; the administration of NAC/DFX and taurine reversed the increase in CK activity in hippocampus, cerebral cortex, cerebellum and striatum. On the other hand, RC-3095 significantly decreased CK activity, when compared to sham group in all brain areas studied. This is a preliminary study which showed beneficial effects of the treatments we proposed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cell death within the central nervous system during sepsis has been described in animal models and humans [1, 2]. It has been demonstrated that several inflammatory mediators released during sepsis development [cytokines and nitric oxide (NO)] are potentially neurotoxic [3, 4]. In this context, recent findings demonstrated that sepsis is associated with long-term neurologic deficits [5–7]. However, the mechanisms responsible for the development of brain dysfunction during sepsis are not well understood.
We have previously demonstrated that oxidative damage occurs early in the course of sepsis development in the cecal ligation and perforation (CLP) animal model [6]. Increased generation of free radicals in rat brain 2 h after lipopolysaccharide (LPS) administration [8] has also been reported. In this context, treatments that reduce the generation or prevent/reverse the effects of reactive oxygen species (ROS) exert beneficial effects in a variety of animal models of endotoxemia and septic shock [9–13]. Among the antioxidant therapeutic interventions we find N-acetylcysteine (NAC) [9, 11, 12, 14, 15], deferoxamine (DFX) [13] and taurine [16]. In this regard, we have recently demonstrated that the combination of NAC and DFX is an effective treatment of severe sepsis in a rodent animal model [16], and in other models of inflammatory disease [15, 17–19]. Taurine is a naturally occurring free amino acid that has been shown to have neurotrophic and neuroprotective properties [20, 21]. It has been demonstrated that taurine has protective function against glutamate-induced neuronal injury [20].
The pathogenesis of sepsis is also characterized by an overwhelming systemic inflammatory response that can lead to lethal multiple organ failure [22]. To date, anti-inflammatory strategies produced modest clinical effects in critically ill patients [23], and new therapeutic strategies were described for the treatment of sepsis and its consequences [16, 24]. Bombesin/gastrin releasing peptide (GRP) receptor pathways were shown to participate in the control of central nervous and gastrointestinal systems functions [25–27], cancer growth [28], and immune cell regulation [29, 30]. Thus, these pathways are probably implicated in the pathogenesis of inflammatory diseases [31, 32]. We have previously shown that a selective GRP receptor antagonist, [D-Tpi6, Leu13 psi[CH2NH]-Leu14] bombesin (RC-3095), attenuates the release of pro-inflammatory cytokines in vitro and in vivo, and improved survival after CLP [33].
It has been well described that metabolism impairment is implicated in the pathogenesis of multiple organ dysfunction syndrome (MODS) and a wide variety of disease states [22, 34, 35]. Cytopathic hypoxia hypothesis postulates that a diminution in mitochondrial oxidative phosphorylation reduces aerobic adenosine triphosphate (ATP) production and induces MODS [36]. In this matter, some works reported deficiencies within the mitochondrial respiratory chain in sepsis [36, 37]. Mitochondrial-mediated apoptosis in rat brain early in sepsis development was also demonstrated [1]. We have also previously found that mitochondrial respiratory chain is inhibited 6 and 12 h after CLP. On the other hand, we verified that creatine kinase (CK) activity was increased at the same time. In that work, we hypothesized that an increase in ATP-regenerating capacity via CK reaction might be related to a delay in ATP depletion and, thereby, protecting the brain from damage [38].
Considering that we have recently demonstrated that mitochondrial respiratory chain and CK are altered in the brain of rats after CLP and that a combination of NAC/DFX, taurine and RC-3095 were shown to be an effective treatment of sepsis, we investigated whether the alterations of these enzymes may be reversed by these drugs.
Experimental Procedure
Animals
Adult and male Wistar rats were obtained from the Central Animal House of Universidade do Extremo Sul Catarinense. They were caged in groups of five with free access to food and water and were maintained on a 12-h light–dark cycle [lights on 7:00 am], at a room temperature of 22 ± 1°C. All experimental procedures were carried out in accordance with the National Institutes of Health Guide for Care and Use of Laboratory Animals, with the approval of Ethics Committee from Universidade do Extremo Sul Catarinense.
Cecal Ligation and Puncture (CLP) Model
Male Wistar rats 2–3 months old, subjected to CLP as previously described [39], were used in this study. Rats were anesthetized with a mixture of ketamine (80 mg/kg) and xylazine (10 mg/kg), given intraperitoneally. Under aseptic conditions, a 3-cm midline laparotomy was performed to allow exposure of the cecum with adjoining intestine. The cecum was tightly ligated with a 3.0 silk suture at its base, below the ileocecal valve, and was perforated once with a 14-gauge needle. The cecum was then gently squeezed to extrude a small amount of feces from the perforation site. The cecum was then returned to the peritoneal cavity and the laparotomy was closed with 4.0 silk sutures. All animals received isotonic saline solution (50 ml/kg s.c.) immediately after CLP. All animals were returned to their cages with free access to food and water. Septic rats in this model become bacteremic with Gram-negative enteric organisms. In the sham-operated group the rats were submitted to all surgical procedures and received isotonic saline solution (50 ml/kg s.c.) immediately after surgical procedure but the cecum was neither ligated nor perforated.
Experimental Protocols
Thirty animals were randomly divided into five groups: group 1, sham-operated; group 2, isotonic saline solution (50 ml/kg, s.c.) immediately after CLP; group 3, same as group 2 plus NAC (20 mg/kg, s.c.) and DFX (20 mg/kg, s.c.) 3 h after CLP; group 4, same as group 2 plus taurine (50 mg/kg, s.c.) immediately after CLP; group 5, same as group 2 plus RC-3095 (3 mg/kg, s.c.) immediately after CLP. These doses were in accordance to previous works [16, 33].
Homogenate Preparation
Twelve hours after CLP, the rats were killed by decapitation, the brain was removed and cerebral cortex, hippocampus, striatum, and cerebellum were isolated. Brain structures were homogenized (1:10, w/v) in SETH buffer (250 mM sucrose, 2 mM EDTA, 10 mM Trizma base, 50 IU/ml heparin, pH 7.4). The homogenates were centrifuged at 800g for 10 min and the supernatants were used for determination of mitochondrial respiratory chain enzymes and creatine kinase activities. The maximal period between homogenate preparation and enzyme analysis was always less than 5 days. Protein content was determined by the method described by Lowry et al. [40] using bovine serum albumin as standard.
Activities of Mitochondrial Respiratory Chain Enzymes
NADH dehydrogenase (complex I) was evaluated by the method described by Cassina and Radi [41] by the rate of NADH-dependent ferricyanide reduction at 420 nm. The activities of succinate-2,6-dichloroindophenol [DCIP]-oxidoreductase (complex II) and succinate: cytochrome c oxidoreductase (complex II–III) were determined by the method described by Fischer et al. [42]. Complex II activity was measured by following the decrease in absorbance due to the reduction of 2,6-DCIP at 600 nm. Complex II–III activity was measured by cytochrome c reduction from succinate at 550 nm. The activity of cytochrome c oxidase (complex IV) was assayed according to the method described by Rustin et al. [43], measured by following the decrease in absorbance due to the oxidation of previously reduced cytochrome c at 550 nm. The activities of the mitochondrial respiratory chain complexes were calculated as nmol/min mg protein.
Creatine Kinase (CK) Activity
CK activity was measured in brain homogenates pre-treated with 0.625 mM lauryl maltoside according to the colorimetric method of Hughes [44] and the results were expressed as units/min × mg protein.
Statistical Analysis
Data were analyzed by one-way analysis of variance (ANOVA) followed by the Tukey test when F was significant and are expressed as mean ± standard deviation. All analyses were performed using the Statistical Package for the Social Science software. Differences were considered significant when P < 0.05.
Results
In the present study we measured mitochondrial respiratory chain complexes I, II, III and IV and CK activity in the brain of rats 12 h after CLP. The animals also received isotonic saline solution and NAC/DFX, taurine or RC-3095.
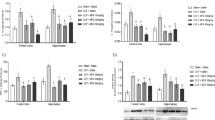
As seen in Figs. 1 and 2, we verified that CLP inhibited complexes I and II. All the treatments were able to reverse the inhibition of both enzymes in all brain areas evaluated. On the other hand, complexes III and IV were not affected by CLP neither by any of the treatments (Tables 1 and 2, respectively).
Mitochondrial respiratory chain complex I activity in hippocampus, cerebral cortex, cerebellum and striatum of rats 12 h after CLP. Rats were submitted to CLP and treated with isotonic saline solution [SAL], NAC/DFX [ATX], taurine [TAU] or RC-3095 [RC]. Results are expressed as mean ± SD [n = 6]. For more details, see “Material and Methods”. * Different from sham-operated group [P < 0.05]; # Different from CLP-SAL group [P < 0.05]
Mitochondrial respiratory chain complex II activity in hippocampus, cerebral cortex, cerebellum and striatum of rats 12 h after CLP. Rats were submitted to CLP and treated with isotonic saline solution [SAL], NAC/DFX [ATX], taurine [TAU] or RC-3095 [RC]. Results are expressed as mean ± SD [n = 6]. For more details, see “Material and Methods”. * Different from sham-operated group [P < 0.05]; # Different from CLP-SAL group [P < 0.05]
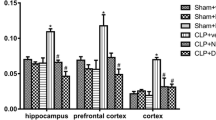
We also verified an increase in CK activity in brain of rats 12 h after CLP (Fig. 3). The administration of NAC/DFX and taurine reversed the increase in CK activity in hippocampus, cerebral cortex, cerebellum and striatum. On the other hand, RC-3095 significantly decreased CK activity, when compared to sham (control) group in all brain areas studied in the present work.
Creatine kinase activity in hippocampus, cerebral cortex, cerebellum and striatum of rats 12 h after CLP. Rats were submitted to CLP and treated with isotonic saline solution [SAL], NAC/DFX [ATX], taurine [TAU] or RC-3095 [RC]. Results are expressed as mean ± SD [n = 6]. For more details, see “Material and Methods”. * Different from sham-operated group [P < 0.05]; # Different from CLP-SAL group [P < 0.05]
Discussion
The results obtained in this study demonstrated that sepsis inhibited the activity of respiratory chain complexes I and II in the brain of rats, but had no effect on complexes III and IV. This inhibition was reversed by the treatments administered, NAC/DFX, taurine or RC-3095. We also demonstrated a substantial increase in CK activity in all brain areas. The alteration in CK activity was reversed by most of the treatments proposed; RC-3095 had an unexpected effect.
These data reinforce the hypothesis of mitochondrial injury during sepsis as being the primary target in systemic organs during acute phase of sepsis [45], since our treatment ameliorated the mitochondrial function. Damage/inhibition of respiratory chain complexes could decrease mitochondrial capacity of energy production to generate ATP, activating another source of energy, such as CK pathway. This enzyme plays a crucial role in cell metabolism by maintaining energy homeostasis at sites where energy turnover is high, such as muscle fibers, spermatozoa and neuronal cells, by catalyzing the following reversible reaction: phosphocreatine + MgADP + H+ ↔ creatine + MgATP, providing rapid regeneration of ATP in cells [46, 47]. An increase in its activity, as seen in our results, may be related to the augmented necessity of ATP production during sepsis due to decreased activity of mitochondrial respiratory chain.
Our present results showed that the use of antioxidants re-established CK and complexes I and II activities to sham-operated levels, contributing to a positive effect of in sepsis by diminishing the deleterious effects of ROS. It has been reported that the combination of NAC/DFX have reduced systemic inflammation, organic oxidative stress and mitochondrial dysfunction, resulting in survival improvement [16].
Taurine also reversed inhibition of complexes I and II and the increase of CK, perhaps by its antioxidant effects and by maintaining the vascular tone through vasodilatation action, thus ameliorating blood flow and O2 delivery [48]. It has been reported that taurine has a neuroprotective effect on the brain after ischemia/reperfusion injury [49]. Previous studies reported several functions of taurine, including membrane stabilization [50], neuroprotection, neuromodulation, and also an important factor on the development of central nervous system [20, 21, 51].
The use of RC-3095 also reversed the inhibition of respiratory chain complexes I and II. Dal-Pizzol et al. [33] have described the beneficial effects of the selective bombesin/GRP receptor antagonist, RC-3095, in a well-established model for experimental sepsis and acute lung injury, leading to a diminution of inflammatory infiltration and organ dysfunction, thus improving mortality in a clinically relevant model of sepsis. It was also indicated a memory retention improvement after systemic administration of GRPR in rodent models [52, 53].
RC-3095 also decreased CK activity in all brain areas studied, rising up questions about the beneficial effects of this antagonist in central nervous system in sepsis. Decrease of CK activity, as shown in several studies, is associated with neurodegenerative pathway resulting in neuronal loss and cell death [54, 55]. Furthermore, Dantas et al. [56] showed dose-dependent beneficial or deleterious effects on memory formation after the infusion of RC-3095 into the dorsal hippocampus in rats.
Brain injury seems to occur during sepsis development and several proposed mechanisms for the CNS dysfunction induced by sepsis include alterations in the blood-brain barrier (BBB), amino acid disruption, and brain ischemia resulting from a global or regional reduction in the cerebral blood flow. In animal models of sepsis, acute encephalopathy occurs, and survivors present cognitive impairment. Moreover, septic patients which survive from critical care units may have persistent brain-related morbidity, including neurocognitive deficits, and development of psychiatric disorders [57, 58]. We hypothesize that mitochondrial dysfunction may be related to CNS damage in sepsis.
References
Messaris E, Memos N, Chatzigianni E et al (2004) Time-dependent mitochondrial-mediated programmed neuronal cell death prolongs survival in sepsis. Crit Care Med 32:1764–1770
Semmler A, Frisch C, Debeir T et al (2007) Long-term cognitive impairment, neuronal loss and reduced cortical cholinergic innervation after recovery from sepsis in a rodent model. Exp Neurol 204:733–740
Faraco G, Fossati S, Bianchi ME et al (2007) High mobility group box 1 protein is released by neural cells upon different stresses and worsens ischemic neurodegeneration in vitro and in vivo. J Neurochem 103:590–603
Hunter RL, Dragicevic N, Seifert K et al (2007) Inflammation induces mitochondrial dysfunction and dopaminergic neurodegeneration in the nigrostriatal system. J Neurochem 100:1375–1386
Barichello T, Martins MR, Reinke A et al (2005) Cognitive impairment in sepsis survivors from cecal ligation and perforation. Crit Care Med 33:221–223
Barichello T, Fortunato JJ, Vitali AM et al (2006) Oxidative variables in the rat brain after sepsis induced by cecal ligation and perforation. Crit Care Med 34:886–889
Heyland DK, Hopman W, Coo H et al (2000) Long-term health-related quality of life in survivors of sepsis. Short Form 36: a valid and reliable measure of health-related quality of life. Crit Care Med 28:3599–3605
Abd El-Gawad HM, Khalifa AE (2001) Quercetin, coenzyme Q10, and L-canavanine as protective agents against lipid peroxidation and nitric oxide generation in endotoxin-induced shock in rat brain. Pharmacol Res 43:257–263
Peristeris P, Clark BD, Gatti S et al (1992) N-acetylcysteine and glutathione as inhibitors of tumor necrosis factor production. Cell Immunol 140:390–399
Salvemini D, Cuzzocrea S (2003) Therapeutic potential of superoxide dismutase mimetics as therapeutic agents in critical care medicine. Crit Care Med 31:S29–S38 [Suppl]
Sprong RC, Winkelhuyzen-Janssen AML, Aarsman CJM et al (1998) Low-dose N-acetylcysteine protects rats against endotoxin-mediated oxidative stress, but high-dose increases mortality. Am J Respir Crit Care Med 157:1283–1293
Villa P, Ghezzi P (1995) Effect of N-acetyl-l-cysteine on sepsis in mice. Eur J Pharmacol 292:341–344
Vulcano M, Meiss RP, Isturiz MA (2000) Deferoxamine reduces tissue injury and lethality in LPS-treated mice. Int J Immunopharmacol 22:635–644
Kozlov AV, Szalay L, Umar F et al (2003) EPR analysis reveals three tissues responding to endotoxin by increased formation of reactive oxygen and nitrogen species. Free Radic Biol Med 34:1555–1562
Erdamar H, Türközkan N, Ekremoğlu M et al (2007) The effect of taurine on polymorphonuclear leukocyte functions in endotoxemia. Amino Acids 33:581–585
Ritter C, Andrades ME, Reinke A et al (2004) Treatment with N-acetylcysteine plus deferoxamine protects rats against oxidative stress and improves survival in sepsis. Crit Care Med 32:342–349
Damiani CR, Benetton CA, Stoffel C et al (2007) Oxidative stress and metabolism in animal model of colitis induced by dextran sulfate sodium. J Gastroenterol Hepatol 22:1846–1851
Ritter C, Reinke A, Andrades M et al (2004) Protective effect of N-acetylcysteine and deferoxamine on carbon tetrachloride-induced acute hepatic failure in rats. Crit Care Med 32:2079–2084
Ritter C, Cunha AA, Echer IC et al (2006) Effects of N-acetylcysteine plus deferoxamine in lipopolysaccharide-induced acute lung injury in the rat. Crit Care Med 34:471–477
Wu JY, Wu H, Jin Y et al (2009) Mechanism of neuroprotective function of taurine. Adv Exp Med Biol 643:169–179
Chen K, Zhang Q, Wang J et al (2009) Taurine protects transformed rat retinal ganglion cells from hypoxia-induced apoptosis by preventing mitochondrial dysfunction. Brain Res 1279:131–138
Hotchkiss RS, Karl IE (2003) The pathophysiology and treatment of sepsis. N Engl J Med 348:138–150
Eichacker PQ, Parent C, Kalil A et al (2002) Risk and the efficacy of antiinflammatory agents: retrospective and confirmatory studies of sepsis. Am J Respir Crit Care Med 166:1197–1205
Giacometti A, Cirioni O, Ghiselli R et al (2004) Cathelicidin peptide sheep myeloid antimicrobial peptide-29 prevents endotoxin-induced mortality in rat models of septic shock. Am J Respir Crit Care Med 169:187–194
Meller CA, Henriques JAP, Schwartsmann G et al (2004) The bombesin/gastrin releasing peptide receptor antagonist RC-3095 blocks apomorphine but not MK-801-induced stereotypy in mice. Peptides 25:585–588
Roesler R, Henriques JA, Schwartsmann G (2004) Neuropeptides and anxiety disorders: bombesin receptors as novel therapeutic targets. Trends Pharmacol Sci 25:241–242
Yamada K, Santo-Yamada Y, Wada E et al (2002) Role of bombesin [BN]-like peptides/receptors in emotional behavior by comparison of three strains of BN-like peptide receptor knockout mice. Mol Psychiatry 7:113–117
Schwartsmann G (2004) Dexamethasone and gastrin-releasing peptide receptors in human lung cells. Lung Cancer 46:129
Genton L, Kudsk KA (2003) Interactions between the enteric nervous system and the immune system: role of neuropeptides and nutrition. Am J Surg 186:253–258
Medina S, Rio MD, De la Cuadra B et al (1999) Age-related changes in the modulatory action of gastrin-releasing peptide, neuropeptide Y and sulfated cholecystokinin octapeptide in the proliferation of murine lymphocytes. Neuropeptides 33:173–179
Grimsholm O, Rantapaa-Dahlqvist S, Forsgren S (2005) Levels of gastrin releasing peptide and substance P in synovial fluid and serum correlate with levels of cytokines in rheumatoid arthritis. Arthritis Res Ther 7:R416–R426
Subramaniam M, Sugiyama K, Coy DH et al (2003) Bombesin-like peptides and mast cell responses: relevance to bronchopulmonary dysplasia? Am. J Respir Crit Care Med 168:601–611
Dal-Pizzol F, Di Leone LP, Ritter C et al (2006) Gastrin-releasing peptide receptor antagonist effects on an animal model of sepsis. Am J Respir Crit Care Med 173:84–90
Streck EL, Matté C, Vieira PS et al (2003) Impairment of energy metabolism in hippocampus of rats subjected to chemically-induced hyperhomocysteinemia. Biochim Biophys Acta 1637:187–192
Wallace DC (2005) A mitochondrial paradigm of metabolic and degenerative diseases, aging, and cancer: a dawn for evolutionary medicine. Annu Rev Genet 39:359–407
Fink MP (2002) Cytopathic hypoxia. Is oxygen use impaired in sepsis as a result of an acquired intrinsic derangement in cellular respiration? Crit Care Clin 18:165–175
Crouser ED, Julian MW, Dorinsky PM (1999) Ileal VO[2]-O[2] alterations induced by endotoxin correlate with severity of mitochondrial injury. Am J Respir Crit Care Med 160:1347–1353
Comim CM, Rezin GT, Scaini G et al (2008) Mitochondrial respiratory chain and creatine kinase activities in rat brain after sepsis induced by cecal ligation and perforation. Mitochondrion 8:313–318
Hollenberg SM, Dumasius A, Easington C et al (2001) Characterization of a hyperdynamic murine model of resuscitated sepsis using echocardiography. Am J Respir Crit Care Med 164:891–895
Lowry OH, Rosebrough NJ, Farr AL et al (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Cassina A, Radi R (1996) Differential inhibitory action of nitric oxide and peroxynitrite on mitochondrial electron transport. Arch Biochem Biophys 328:309–316
Fischer JC, Ruitenbeek W, Berden JA et al (1985) Differential investigation of the capacity of succinate oxidation in human skeletal muscle. Clin Chim Acta 153:23–36
Rustin P, Chretien D, Bourgeron T et al (1994) Biochemical and molecular investigations in respiratory chain deficiencies. Clin Chim Acta 228:35–51
Hughes BP (1962) A method for the estimation of serum creatine kinase and its use in comparing creatine kinase and aldolase activity in normal and pathological sera. Clin Chim Acta 7:597–603
Crouser ED (2004) Mitochondrial dysfunction in septic shock and multiple organ dysfunction syndrome. Mitochondrion 4:729–741
Almeda FQ, Calvin JE, Parrillo JE (2001) Prevalence of angiographically significant stenosis in patients with chest pain and an elevated troponin I level and normal creatine kinase and creatine kinase-MB levels. Am J Cardiol 87:1286–1289
Ellington WR (2001) Evolution and physiological roles of phosphagen systems. Annu Rev Physiol 63:289–325
Schurr A, Rigor BM (1987) The mechanism of neuronal resistance and adaptation to hypoxia. FEBS Lett 224:4–8
Wang GH, Jiang ZL, Fan XJ et al (2007) Neuroprotective effect of taurine against focal cerebral ischemia in rats possibly mediated by activation of both GABAA and glycine receptors. Neuropharmacology 52:1199–1209
Richards DA, Lemos T, Whitton PS et al (1995) Extracellular GABA in the ventrolateral thalamus of rats exhibiting spontaneous absence epilepsy: a microdialysis study. J Neurochem 65:1674–1680
El Idrissi A, Trenkner E (1999) Growth factors and taurine protect against excitotoxicity by stabilizing calcium homeostasis and energy metabolism. J Neurosci 19:9459–9468
Flood JF, Morley JE (1988) Effects of bombesin and gastrin-releasing peptide on memory processing. Brain Res 460:314–322
Rashidy-Pour A, Razvani ME (1998) Unilateral reversible inactivations of the nucleus tractus solitarius and amygdala attenuate the effects of bombesin on memory storage. Brain Res 814:127–132
Aksenov M, Aksenova M, Butterfield DA et al (2000) Oxidative modification of creatine kinase BB in Alzheimer’s disease brain. J Neurochem 74:2520–2527
David S, Shoemaker M, Haley BE (1998) Abnormal properties of creatine kinase in Alzheimer’s disease brain: correlation of reduced enzyme activity and active site photolabeling with aberrant cytosol-membrane partitioning. Brain Res Mol Brain Res 54:276–287
Dantas AS, Luft T, Henriques JAP et al (2006) Opposite effect of low and high doses of the gastrin-releasing peptide receptor antagonist RC-3095 on memory consolidation in hippocampus: possible involvement of the GABAergic system. Peptides 27:2307–2312
Dal-Pizzol F, Ritter C, Cassol Jr OJ et al (2009) Oxidative mechanisms of brain dysfunction during sepsis. Neurochem Res. doi:10.1007/s11064-009-0043-4
Streck EL, Comim CM, Barichello T et al (2008) The septic brain. Neurochem Res 33:2171–2177
Acknowledgments
This work was supported by grants from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Fundação de Apoio à Pesquisa Científica e Tecnológica do Estado de Santa Catarina (FAPESC) and Universidade do Extremo Sul Catarinense (UNESC).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cassol, O.J., Rezin, G.T., Petronilho, F.C. et al. Effects of N-Acetylcysteine/Deferoxamine, Taurine and RC-3095 on Respiratory Chain Complexes and Creatine Kinase Activities in Rat Brain After Sepsis. Neurochem Res 35, 515–521 (2010). https://doi.org/10.1007/s11064-009-0089-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-009-0089-3