Abstract
Purpose
To evaluate the effects of sepsis on brain microvasculature leukocyte rolling and adherence, myeloperoxidase (MPO) activity, cytokine and chemokine concentrations, and behavioral screening 6, 12, and 24 h after sepsis induction.
Methods
C57BL/6 mice or Wistar rats underwent cecal ligation and perforation (CLP) or sham operation. At 6, 12, and 24 h after sepsis induction, intravital microscopy was performed in the mice brain microvasculature to evaluate leukocyte rolling and adherence. Animals were killed and had the brain removed to determine MPO activity and the levels of cytokines and chemokines. A behavioral screening was also performed in a separate cohort of animals. Blood–brain barrier (BBB) permeability and cytokines and chemokines were determined in different brain regions in Wistar rats.
Results
There was a decrease in circulating leukocyte levels at 6, 12, and 24 h, an increase in rolling and adhesion of leukocytes in the brain microvasculature, followed by an increase in brain MPO activity. In addition, there was an increase in both brain cytokines and chemokines at different times. There was a decrease in the neuropsychiatric state muscle tone and strength only at 6 h, and a decrease in the autonomous function at 6 and 12 h. The pattern of brain cytokines and chemokines, and BBB permeability between the analyzed regions seemed to be similar with minor differences.
Conclusions
During sepsis the brain’s production of cytokines and chemokines is an early event and it seemed to participate both in central nervous system (CNS) dysfunction and BBB permeability alterations, reinforcing the role of brain inflammatory response in the acute CNS dysfunction associated with sepsis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sepsis is a frequent condition accounting for approximately 750,000 cases per year in North America [1]. An essential feature of sepsis is the production of cytokines, chemokines, reactive oxygen species (ROS), and nitric oxide (NO) [2]. The potential neurotoxic effects of these pro-inflammatory mediators have been documented [3]. Indeed, the brain is one of the first organs affected during sepsis development, but the mechanisms associated with septic encephalopathy (SE) are not well known [4]. In addition, sepsis was associated not only with acute brain dysfunction but also with long-term cognitive deficits [5, 6].
During the early stages of brain dysfunction, blood–brain barrier (BBB) alterations were found in a rodent model of sepsis [7]; these alterations potentially caused infiltration of inflammatory cells and exposed the brain to toxins. It is supposed, as occurs in several other organs, that in the course of sepsis leukocytes are activated, adhere to the blood vessel, and move into the brain, but no clear demonstration of this is described [8]. Thus, we supposed that during sepsis, brain cytokines and chemokines drive alterations in the BBB permeability that lead to an increase in the flux of inflammatory cells and toxic mediators into the brain that contributes to injury.
To date there is no clear evidence of the time course of brain inflammatory response during sepsis development or if this response is similar to the systemic response. In addition, it is not known if there is any brain region that is more susceptible to the alterations induced by sepsis. Thus, in the present study, we aimed to evaluate: (1) leukocyte rolling and adherence in the brain microvasculature and BBB permeability; (2) brain levels of cytokines (IL-1β, IL-10, and TNF-α), chemokines (CXCL1/Kc, CXCL9/MIG, CCL2/MCP-1, CCL3/MIP-1α, and CCL5/RANTES) and myeloperoxidase (MPO) activity; and (3) animal behavior in the cecal ligation and perforation (CLP) model of sepsis.
Materials and methods
Animals
Male C57BL/6 mice (6–9 weeks, 20–25 g) were obtained from our breeding colony (Universidade do Extremo Sul Catarinense, UNESC). The animals were housed five to a cage with food and water available ad libitum, and were maintained on a 12-h light/dark cycle (lights on at 7:00 a.m.). In some subsets of experiments, adult male Wistar rats (220–300 g) were used. They were housed five to a cage with food and water available ad libitum and were maintained on a 12-h light/dark cycle (lights on at 7:00 a.m.).
All experimental procedures involving animals were performed in accordance with the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals and the Brazilian Society for Neuroscience and Behavior (SBNeC) recommendations for animal care.
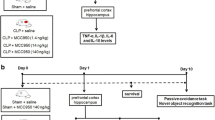
Cecal ligation and perforation in C57BL/6 mice
Mice were subjected to CLP as previously described [9]. Briefly, a midline laparotomy was performed to allow exposure of the cecum with the adjoining intestine. The cecum was tightly ligated with a 3.0 silk suture at its base, below the ileocecal valve, and perforated seven times with a 21-gauge needle. The cecum was then gently squeezed to extrude a small amount of feces from the perforation site and then returned into the peritoneal cavity; the laparotomy was closed with 4.0 silk sutures. The sham-operated mice were submitted to all surgical procedures, but the cecum was neither ligated nor perforated. After the surgery, all the groups received 50 mL/kg saline (subcutaneously) immediately and 12 h after CLP. Then, the mice were divided into six groups: sham (6, 12, and 24 h) and CLP (6, 12, and 24 h) (16 animals per group: 8 animals for SHIRPA analyses, and 8 animals for biochemical analyses).
Cecal ligation and perforation in Wistar rats
Animals were subjected to CLP as described [9] with adaptations [10]. Under aseptic conditions, a 3-cm midline laparotomy was performed to allow exposure of the cecum with the adjoining intestine. The cecum was tightly ligated with a 3.0 silk suture at its base, below the ileocecal valve, and was perforated once with a 14-gauge needle. The cecum was then gently squeezed to extrude a small amount of feces from the perforation site and then returned to the peritoneal cavity; the laparotomy was closed with 4.0 silk sutures. Animals were resuscitated with normal saline (50 mL/kg subcutaneously) immediately and 12 h after CLP. In the sham-operated group, the rats were submitted to all surgical procedures but the cecum was neither ligated nor perforated. Then, the rats were divided into six groups: sham (6, 12, and 24 h) and CLP (6, 12, and 24 h).
Intravital microscopy
At 6, 12, and 24 h post-infection intravital microscopy of the mice brain microvasculature was performed [11]. Briefly, a craniotomy was performed using a high-speed drill and the dura mater was removed to expose the underlying pial vasculature. Throughout the experiment, the mouse was maintained at 37°C with a heating pad, and the exposed brain was continuously superfused with artificial cerebrospinal fluid buffer. Leukocytes were fluorescently labeled by intravenous administration of Rhodamine 6G (0.5 mg/kg of body weight; Sigma) and were observed using a microscope (Olympus B201, ×20 objective lens, corresponding to 100 μm2 of area) outfitted with a fluorescent light source (epi-illumination at 510–560 nm, using a 590-nm emission filter) in the temporo-pariteal cortex. The number of rolling and adherent leukocytes was determined offline during video playback analyses. Leukocytes were considered adherent to the venular endothelium if they remained stationary for a minimum of 30 s. Rolling leukocytes were defined as white cells moving at a velocity lower than that of erythrocytes within a given vessel.
ELISA analyses
One fragment of the brain was homogenized in extraction solution containing aprotinin. The concentrations of cytokines (IL-1β, IL-10, and TNF-α) and chemokines (CXCL1/Kc, CXCL9/MIG, CCL2/MCP-1, CCL3/MIP-1α, and CCL5/RANTES) were determined by ELISA (R&D Systems, Minneapolis, MN, USA). In addition, plasma was obtained for the determination of cytokines (IL-10, and TNF-α) and chemokines (CXCL1/Kc, CCL5/RANTES).
In rats, several times after CLP or sham operation, animals were killed by decapitation, and the brain structures (hippocampus, striatum, cortex, and pre-frontal cortex) were immediately isolated and used for the determination of cytokines (IL-1β, IL-10, and TNF-α) and chemokines (CXCL1, CCL2/MCP-1) by ELISA (R&D Systems, Minneapolis, MN, USA).
Myeloperoxidase activity
As an index of neutrophil infiltration we measured MPO activity as follows. Briefly, brain extracts were homogenized (50 mg/mL) in 0.5% hexadecyltrimethylammonium bromide and centrifuged at 15,000g for 40 min. An aliquot of supernatant was mixed with a solution of 1.6 mM tetramethylbenzidine and 1 mM H2O2. The activity was measured spectrophotometrically as the change in absorbance at 650 nm at 37°C at the same times points described for brain cytokine determinations.
Blood–brain barrier permeability
A 2% solution of Evans blue in saline was injected into the tail vein 1 h before harvesting of brain tissues and allowed to circulate for 60 min. Subsequently, the chest was surgically opened under anesthesia and the intravascular dye was removed by saline perfusion (40–50 mL) through the left heart ventricle. The brain was then removed and quantitative evaluation of BBB disruption was achieved by measuring the Evans blue content in the brain regions [12]. In brief, rat brain was homogenized in 50% wt/vol trichloroacetic acid, after centrifugation the supernatant was diluted fourfold with ethanol, and fluorescence intensity (ng/mL) was measured on a microplate fluorescence reader. The total Evans blue content (ng) in each sample was derived from the concentrations of external standards.
Histopathological analyses
For histopathologic analyses after fixation, the brain tissues from rats were embedded in paraffin and then routinely stained with hematoxylin and eosin. An experienced pathologist performed blinded histopathologic analyses.
SHIRPA test
The SmithKline/Harwell/Imperial College/Royal Hospital/Phenotype Assessment (SHIRPA) was conceived as a multi-test battery used for longitudinal studies with standardized guidelines and materials [13]. The SHIRPA primary screen consists of a series of observations of reflexes and basic sensorimotor functions, and provides a behavioral and functional profile by observational assessment of individual performance.
The SHIRPA protocol was used to evaluate behavioral changes during the course of sepsis. For the purpose of analysis, the individual parameters assessed by SHIRPA were grouped into five functional categories (neuropsychiatric state; motor behavior; autonomic function; muscle tone and strength; and reflex and sensory function) according to Lackner et al. [14], determining an overall score and five domain scores. The reflex and sensory domain involves visual placing, pinna reflex, corneal reflex, toe pinch, and righting reflex. The neuropsychiatric state involves spontaneous activity, transfer arousal, touch escape, positional passivity, fear, biting, irritability and vocals. The motor behavior involves locomotor activity, body position, shivering, gait, pelvic elevation, tail elevation, trunk curl, limb grasping, wire maneuver, and negative geotaxis. Autonomic function involves respiration rate, palpebral closure, ruffled fur, skin color, heart rate, tears, and salivation. Muscle tone and strength involves grip strength, body tone, limb tone, and abdominal tone.
Statistical analyses
The data are presented as mean and standard error of the mean (SEM). Data of leukocyte counts and SHIRPA were analyzed by two-way analysis of variance (ANOVA), and multiple comparisons were performed by Dunnett’s test. Data of the intravital microscopy, ELISA assay (cytokine levels, chemokine levels, and MPO activity), and BBB permeability were analyzed by two-way ANOVA, and multiple comparisons were performed by Newman–Keuls correction. Differential blood cell count data were analyzed by two-way ANOVA, and multiple comparisons were performed by Tukey test. Values of p < 0.05 were considered to be significant.
Results
Figure 1 illustrates the leukocyte–endothelium interaction in the brain microvasculature after CLP in the mice. Rolling of leukocytes in the sepsis group was increased at 24 h and leukocytes adherence was increased as early as 6 h in the CLP group when compared to the sham group. Figure 2 shows cytokine (IL-1β, IL-10, and TNF-α) and chemokine (CXCL1/Kc, CXCL9/MIG, CCL2/MCP-1, CCL3/MIP-1α, and CCL5/RANTES) levels in brain extracts after sepsis induction in the mice. There was an increase in IL-1β levels at 12 and 24 h, IL-10 at 6, 12, and 24 h, and TNF-α at 6, 12, and 24 h when compared to the sham group. In addition, there was an increase in CXCL1/Kc levels at 12 h, CCL3/MIP-1α at 6, 12, and 24 h, and CCL5/RANTES at 6, 12, and 24 h, but not CXCL9/MIG and CCL2/MCP-1 levels, when compared to the sham group. The kinetics of plasma cytokines and chemokines were quite different when compared to the brain. Plasma IL-10 concentration in CLP and sham animals was not significantly different when compared at all analyzed time points (Fig. 1, supplementary material), and TNF-α increases significantly only after 12 h (Fig. 1, supplementary material), suggesting that brain cytokines increase earlier when compared to plasma. This pattern was also observed for CCL5/RANTES which only increases in plasma 24 h after CLP (Fig. 1, supplementary material). In contrast, CXCL1/Kc increases earlier in plasma (6 h) when compared to the brain. A significant increase in MPO activity was found at 6 h, but a large increase was observed only 24 h after CLP in the mice (Fig. 2A, supplementary material).
Leukocyte–endothelium interaction in the brain microvasculature after CLP in the mice. Sepsis was induced in animals and after 6, 12, and 24 h intravital microscopy was used to assess rolling and adherence of leukocytes in the brain microvasculature. Data indicate mean ± SEM of cells per minute and 100 μm, eight mice per group. Leukocytes count in CLP mice was statistically different when compared to sham mice. One-way ANOVA with Newman–Keuls correction, *p < 0.05, **p < 0.01, and ***p < 0.001
Cytokine and chemokine levels in the brain after CLP in the mice. Sepsis was induced and after 6, 12, and 24 h cytokine (IL-1β, IL-10, and TNF-α) and chemokine (CXCL1/Kc, CXCL9/MIG, CCL2/MCP-1, CCL3/MIP-1α, and CCL5/RANTES) levels were measured in brain extracts from CLP and sham groups using ELISA kits. Data indicate mean ± SEM, eight mice per group. One-way ANOVA with Newman–Keuls correction, *p < 0.05, **p < 0.01, and ***p < 0.001
Figure 3 illustrates the performance of C57BL/6 mice at 6, 12, and 24 h after sepsis induction and sham-operated mice in the five distinct functional categories: reflex and sensory function, neuropsychiatric state, motor behavior, autonomous function, and muscle tone and strength. The sum of the scores of all functional categories is shown in the “total” graph. There were no significant differences between the groups in the reflex and sensory function and motor behavior. However, there was a decrease in the neuropsychiatric state and muscle tone and strength at 6 h. There was a decrease in the autonomous function at 6 and 12 h. Finally, there was a decrease in the sum of the scores of all functional categories at 6 and 12 h when compared with the sham group.
Behavioral impairment after sepsis induced by CLP in the mice. Sepsis was induced and after 6, 12, and 24 h behavioral impairment was measured in the five distinct functional categories: reflex and sensory function, neuropsychiatric state, motor behavior, autonomous function, and muscle tone and strength using the SHIRPA protocol. The sum of the scores of all functional categories was shown as a “total” score (bottom right). Data are presented as mean ± SEM, eight mice in CLP group and four mice in sham group. One-way ANOVA with Dunnett’s multiple comparison test correction, *p < 0.05, **p < 0.01, and ***p < 0.001
To determine if there is any specific response in some brain regions we used a CLP model in the rat that allows a better discrimination of the brain structures. An increase in the permeability of the BBB was observed only 24 h after CLP in all analyzed regions (Fig. 4). The pattern of brain cytokines and chemokines between these regions seemed to be similar, with minor differences (Fig. 3, supplementary material). In the analyzed brain structures cytokines and chemokines increase 6 or 12 h after CLP induction, and their levels remained higher when compared to sham animals until 24 h, similar to the situation observed in mice. In the rat model all analyzed structures presented an increase of the MPO activity only 24 h after CLP (Fig. 2B, supplementary material). Mild to moderate focal edema was observed with minimal inflammatory response and necrosis in the brain of CLP animals. These alterations were similar when different brain regions were compared at corresponding analysis times (Fig. 4, supplementary material).
BBB permeability in different brain regions after CLP in the rat. Sepsis was induced and after 6, 12, and 24 h BBB permeability was measured in the hippocampus, cerebellum, cortex and pre-frontal cortex using the Evans blue dye. Data indicate mean ± SEM, eight rats per group. One-way ANOVA with Newman–Keuls correction, *p < 0.05, **p < 0.01, and ***p < 0.001
Discussion
In this study we demonstrated, both in mice and rats, that production of brain cytokines and chemokines is an early event during sepsis and was followed by increased BBB permeability and brain accumulation of inflammatory cells. Regardless of BBB permeability histopathological analyses demonstrated mild to moderate focal edema, suggesting a mechanism leading to brain edema that is independent of BBB permeability. In addition, mice presented several impairments in brain function determined by the SHIRPA protocol, suggesting that encephalopathy is present in these animals.
In a variety of neurological (inflammatory, infectious, neoplasic, and neurodegenerative) diseases BBB dysfunction has been described not only as a late event, but as a putative mechanism involved in the early steps of disease progression [15]. In fact, under physiological conditions leukocyte entry into the healthy central nervous system (CNS) across the BBB is kept at a low level. Consequently, the interaction between circulating leukocytes and the endothelium of the BBB is a crucial step in diverse pathologic processes [16, 17]. In this study, we demonstrated an increase in the rolling and adherence of leukocytes in brain microcirculation that was followed by an increase of neutrophil accumulation in the brain in an animal model of severe sepsis. The increase in MPO activity is temporally related to the alteration in the BBB permeability, and occurs at later time points when compared to the increase of brain cytokines and chemokines, suggesting a role of brain cytokines and chemokines in the genesis of this process. This was also true for brain edema which is an early event when compared to the increase of BBB permeability and did not change in magnitude after BBB breakdown. It is also possible that the systemic inflammation increased BBB permeability leading to brain inflammation, but our data suggest the opposite because brain cytokine and chemokine production seemed to increase earlier (or at least at the same time) when compared to serum, with a significant magnitude, and before any detectable alteration in BBB. In addition, three main systems are tightly interconnected to orchestrate homeostasis in stressful conditions such as sepsis and include the limbic system, the hypothalamic–pituitary axis, and the locus coeruleus. Cytokines such as IL-1 can signal the CNS through stimulation of the vagus nerve and activation of brainstem regions such as the nucleus of the tractus solitarius. The sympathetic and parasympathetic systems are thought to modulate the inflammatory responses. Thus, the several alterations demonstrated here could be related to acute brain dysfunction during sepsis but also could contribute to homeostatic and immune alterations that occur during sepsis.
Using the SHIRPA protocol that determines several different CNS functions we demonstrated that septic animals presented, as early as 6 h, impairment in the neuropsychiatric state, muscle tone and strength, and autonomous function. Some of these dysfunctions are maintained until 12 h, but at 24 h when BBB permeability was increased and there was a higher MPO activity in the brain we could not find any alteration of CNS function assessed by the SHIRPA protocol. Thus, before any detectable dysfunction of the BBB and MPO activity increase, animals presented CNS dysfunction suggesting that brain-produced cytokines could participate in this process. Whereas leukocyte invasion may be delayed in response to acute insults, activation of brain microglia and release of inflammatory mediators are rapid, occurring within minutes or hours. Cytokines produced by the brain can modulate the activity of neurons [18–20]. However, it is unlikely that cytokines act directly on neurons because cytokine receptors are sparsely expressed in neuronal cell types [21]. This suggests the involvement of intermediate(s) that could mediate cytokine actions on neuronal activities. Following stimulation by cytokines and many other inflammatory factors (such as substance P, ATP, glutamate, NO, prostaglandins, and/or heat shock proteins), glial and endothelial cells locally release factors such as NO, arachidonic acid, prostaglandins, ROS, peroxynitrite, and excitatory amino acids (mainly glutamate) which can modify the activity of neurons [22–24].
Some limitations of our study must be mentioned. First, we could not make a clear cause–effect relation, but only a mechanistic suggestion based on a time-dependent analyses of several factors associated with brain inflammation during sepsis. Second, despite the SHIRPA protocol being a standardized tool to study CNS dysfunction in mice it did not determine essential characteristics of SE observed in humans (i.e., attention). In fact, to date no animal model resembles the clinical picture associated with SE, and this is a limitation of all rodent studies performed to determine the mechanisms associated with SE. Third, MPO is not specific to neutrophil but is also seen in macrophages, thus we could not ascertain that, besides neutrophils, monocytes had also been activated in the brain tissue after sepsis induction.
In conclusion, during sepsis the brain’s production of cytokines and chemokines is an early event and it seemed to participate both in CNS dysfunction and BBB permeability alterations, reinforcing the role of brain inflammatory response in the acute CNS dysfunction associated with sepsis.
References
Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR (2001) Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med 29:1303–1310
Andrades ME, Ritter C, Dal-Pizzol F (2009) The role of free radicals in sepsis development. Front Biosci 1:277–287
Chao CC, Hu S, Peterson PK (1995) Glia, cytokines, and neurotoxicity. Crit Rev Neurobiol 9:189–205
Streck EL, Comim CM, Barichello T, Quevedo J (2008) The septic brain. Neurochem Res 33:2171–2177
Barichello T, Martins MR, Reinke A, Feier G, Ritter C, Quevedo J, Dal-Pizzol F (2005) Cognitive impairment in sepsis survivors from cecal ligation and perforation. Crit Care Med 33:221–223
Tuon L, Comim CM, Petronilho F, Barichello T, Izquierdo I, Quevedo J, Dal-Pizzol F (2008) Time-dependent behavioral recovery after sepsis in rats. Intensive Care Med 34:1724–1731
Nishioku T, Dohgu S, Takata F, Eto T, Ishikawa N, Kodama KB, Nakagawa S, Yamauchi A, Kataoka Y (2009) Detachment of brain pericytes from the basal lamina is involved in disruption of the blood–brain barrier caused by lipopolysaccharide-induced sepsis in mice. Cell Mol Neurobiol 29:309–316
Ransohoff RM, Liu L, Cardona AE (2007) Chemokines and chemokine receptors: multipurpose players in neuroinflammation. Int Rev Neurobiol 82:187–204
Fink MP, Heard SO (1990) Laboratory models of sepsis and septic shock. J Surg Res 49:186–196
Ritter C, Andrades M, Frota Júnior ML, Bonatto F, Pinho RA, Polydoro M, Klamt F, Pinheiro CT, Menna-Barreto SS, Moreira JC, Dal-Pizzol F (2003) Oxidative parameters and mortality in sepsis induced by cecal ligation and perforation. Intensive Care Med 29:1782–1789
Vilela MC, Mansur DS, Lacerda-Queiroz N, Rodrigues DH, Arantes RM, Kroon EG, Campos MA, Teixeira MM, Teixeira AL (2008) Traffic of leukocytes in the central nervous system is associated with chemokine up-regulation in a severe model of herpes simplex encephalitis: an intravital microscopy study. Neurosci Lett 445:18–22
Liu W, Hendren J, Qin XJ, Shen J, Liu KJ (2009) Normobaric hyperoxia attenuates early blood–brain barrier disruption by inhibiting MMP-9-mediated occludin degradation in focal cerebral ischemia. J Neurochem 108:811–820
Rogers DC, Fisher EM, Brown SD, Peters J, Hunter AJ, Martin JE (1997) Behavioral and functional analysis of mouse phenotype: SHIRPA, a proposed protocol for comprehensive phenotype assessment. Mamm Genome 8:711–713
Lackner P, Beer R, Heussler V, Goebel G, Rudzki D, Helbok R, Tannich E, Schmutzhard E (2006) Behavioural and histopathological alterations in mice with cerebral malaria. Neuropathol Appl Neurobiol 32:177–188
Weiss N, Miller F, Cazaubon S, Couraud PO (2009) The blood–brain barrier in brain homeostasis and neurological diseases. Biochim Biophys Acta 1788:842–857
Hendriks JJ, Alblas J, van der Pol SM, van Tol EA, Dijkstra CD, de Vries HE (2004) Flavonoids influence monocytic GTPase activity and are protective in experimental allergic encephalitis. J Exp Med 2004:1667–1672
Teleshova N, Pashenkov M, Huang YM, Söderström M, Kivisäkk P, Kostulas V, Haglund M, Link H (2002) Multiple sclerosis and optic neuritis: CCR5 and CXCR3 expressing T cells are augmented in blood and cerebrospinal fluid. J Neurol 249:723–729
Pringle AK, Gardner CR, Walker RJ (1996) Reduction of cerebellar GABA responses by interleukin-1 (IL-1) through an indomethacin insensitive mechanism. Neuropharmacology 35:147–152
Ferri CC, Ferguson AV (2003) Interleukin-1 beta depolarizes paraventricular nucleus parvocellular neurones. J Neuroendocrinol 15:126–133
Houzen H, Kikuchi S, Kanno M, Shinpo K, Tashiro K (1997) Tumor necrosis factor enhancement of transient outward potassium currents in cultured rat cortical neurons. J Neurosci Res 50:990–999
John GR, Lee SC, Brosnan CF (2003) Cytokines: powerful regulators of glial cell activation. Neuroscientist 9:10–22
Bezzi P, Gundersen V, Galbete JL, Seifert G, Steinhäuser C, Pilati E, Volterra A (2004) Astrocytes contain a vesicular compartment that is competent for regulated exocytosis of glutamate. Nat Neurosci 7:613–620
Block ML, Hong JS (2005) Microglia and inflammation-mediated neurodegeneration: multiple triggers with a common mechanism. Prog Neurobiol 76:77–98
Jourdain P, Bergersen LH, Bhaukaurally K, Bezzi P, Santello M, Domercq M, Matute C, Tonello F, Gundersen V, Volterra A (2007) Glutamate exocytosis from astrocytes controls synaptic strength. Nat Neurosci 10:331–339
Acknowledgments
This research was supported by grants from CNPq (JQ and FD-P), FAPESC (JQ and FD-P), UNESC (JQ and FD-P) and Rede Instituto Brasileiro de Neurociência (ALT). ALT, JQ, and FD-P are CNPq Research Fellows. CMC, MCV, and FP are holders of CNPq Studentships, LSC is holder of a FAPESC studentship, and DHR and NLQ are holders of CAPES studentships.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
134_2011_2151_MOESM1_ESM.tif
Figure 1. Plasma cytokines and chemokines levels after CLP in the mice. Sepsis was induced and after 6, 12, and 24 h cytokine (IL-10, TNF-α) and chemokine (CXCL1/Kc, CCL5/RANTES) levels were measured in the plasma from CLP and sham groups using ELISA kits. Data indicate mean ± SEM, 8 mice per group. One-way ANOVA with Newman–Keuls correction, *p < 0.05, **p < 0.01, and ***p < 0.001. (TIFF 445 kb)
134_2011_2151_MOESM2_ESM.doc
Figure 2. Myeloperoxidase activity in the brain after CLP. Sepsis was induced and after 6, 12, and 24 h brain myeloperoxidase activity was measured spectrophotometrically in brain extracts from CLP and sham groups in both mice (A) and rat (B). Data indicate mean ± SEM, 8 animals per group. One-way ANOVA with Newman–Keuls correction, *p < 0.05, **p < 0.01, and ***p < 0.001. (DOC 101 kb)
134_2011_2151_MOESM3_ESM.doc
Figure 3. Cytokine and chemokine levels in different brain regions after CLP in the rat. Sepsis was induced and after 6, 12, and 24 h cytokine (IL-1β, IL-10, TNF-α) and chemokine (CXCL1, CCL2/MCP-1) levels were measured in the hippocampus, cerebellum, cortex, and pre-frontal cortex using ELISA kits. Data indicate mean ± SEM, 8 rats per group. One-way ANOVA with Newman–Keuls correction, *p < 0.05, **p < 0.01, and ***p < 0.001. (DOC 164 kb)
134_2011_2151_MOESM4_ESM.doc
Figure 4. Brain histopathological alterations in an animal model of sepsis. Sepsis was induced and after A 6, B 12, C 24 h brain histopathological alterations were determined by a blinded pathologist. Representative illustrations from the cortex (n = 12). Hematoxylin and eosin; original magnification, ×400. (DOC 23451 kb)
Rights and permissions
About this article
Cite this article
Comim, C.M., Vilela, M.C., Constantino, L.S. et al. Traffic of leukocytes and cytokine up-regulation in the central nervous system in sepsis. Intensive Care Med 37, 711–718 (2011). https://doi.org/10.1007/s00134-011-2151-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-011-2151-2