Abstract
Neuroinflammation has been reported to be involved in the pathogenesis of Parkinson’s disease (PD). Inhibition of microglia-mediated neuroinflammation might be a potential strategy for PD treatment. Biochanin A, is an O-methylated isoflavone, classified as a kind of phytoestrogens due to its chemical structure that is similar to mammalian estrogens. It has been found to possess antifibrotic, antiapoptotic, and antioxidant effects. In the present study, we investigated the neuroprotective effects of biochanin A on lipopolysaccharide (LPS)-induced dopaminergic neurons damage both in vivo and in vitro and the related molecular mechanisms. The results showed that biochanin A treatment for 21 days significantly attenuated the behavioral dysfunction of PD rats, prevented dopaminergic neurons damage, and inhibited activation of microglia in the LPS-induced PD rats. Furthermore, biochanin A decreased the levels of interleukin-1β (IL-1β), interleukin-6 (IL-6), and tumor necrosis factor-α (TNF-α) in the serum, and inhibited the phosphorylation of ERK, JNK, p38 in the substantia nigra of PD rats. In vitro test, biochanin A also inhibited primary microglial activation and protected dopaminergic neurons, decreased the content of nitric oxide, IL-1β, and TNF-α in supernatants, and inhibited the reactive oxygen species production. Taken together, these results suggest that biochanin A exerts protective effects on LPS-induced PD rats, and the mechanisms may be associated with the inhibition of inflammatory response and the MAPK signaling pathway.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The pathological feature of Parkinson’s disease (PD) is selective and progressive degeneration of dopaminergic neurons, which decreased dopamine (DA) content in nigrostriatal system (Jankovic 2008). Recent findings have suggested that the overactivated microglia-mediated neuroinflammation may be a key player in DA neuron degenerative process (Gao and Hong 2008; McGeer et al. 1988). Microglia are the resident immune cells in the brain and serve the role of immune surveillance. The presence of active microglia in the substantia nigra of patients was first demonstrated by McGeer et al.(1988). Since then, evidence has revealed that inflammation can exacerbate the pathogenic events associated with PD and intensify symptoms (Frank-Cannon et al. 2009; Tansey and Goldberg 2010; Collins et al. 2012). Microglia, the resident immune cells in the brain, play a role in immune surveillance and host defense against infectious agents under normal condition. However,in response to injury, infection, or inflammation, microglia become readily activated. Activated microglia secret proinflammatory factors, such as chemokines, cytokines, reactive oxygen species (ROS). Uncontrolled overactivated microglia is a major component of neuroinflammation, and the inflammatory factors contribute to neuronal damage (Gao and Hong 2008). Therefore, compounds that inhibit microglia activation and neuroinflammation may show neuroprotective effect.
Biochanin A (biochA) (C16H12O5), an O-methylated isoflavone, isolated from red clover, chickpea, or other legumes, is classified as a phytoestrogen. It has been reported to possess a variety of biological activities, including antifibrotic, antiapoptotic, and antioxidative effects (Breikaa et al. 2013; Tan et al. 2013). Also, biochanin A has been scientifically proven for its antiinflammatory response and treatment of cerebral ischemia in rats (Wang et al. 2015; Wu et al. 2015; Chen et al. 2007). Our previous studies have demonstrated that biochanin A can inhibit LPS-induced BV2 cell activation and decrease the content of IL-1β and TNF-α (Wu et al. 2015; Chen et al. 2007). However, little is known about the protective effects of biochanin A on PD rats.
Therefore, in the present study, we used the inflammation-induced PD model in vivo (LPS injection to substantia nigra) and in vitro (LPS incubation with rat mesencephalic neuron–glia cultures) to further investigate the neuroprotective activities of biochanin A, along with the underlying mechanisms.
Materials and Methods
Reagents
Biochanin A (molecular weight: 284.26), lipopolysaccharides from Escherichia coli O111:B4(LPS), dimethylsulfoxide (DMSO), and apomorphine were purchased from Sigma-Aldrich (St. Louis, MO, USA). Biochanin A was dissolved in DMSO to make a stock solution and stored at −20 °C for further use. The antibodies (rabbit antirat) of JNK, p-JNK, ERK, p-ERK, p38, p-p38 were purchased from Bioworld Technology (St. Louis Park, MN, USA). Primary antibody specific for neuronal Tyrosine hydroxylase (TH) and OX-42 were obtained from Abcam (Cambridge, MA, USA). Primary antibody against microglial marker ionized calcium-binding adapter molecule 1 (Iba-1) was obtained from Wako Pure Chemical Industries, Ltd. (Osaka, Japan). Streptavidin–biotin complex kit and diaminobenzidine (DAB) staining kit were purchased from Boster Biological Engineering Company (Wuhan, China). All other chemicals used were of the highest grade commercially available.
Animals
Male Sprague–Dawley (SD) rats (GradeII, Certificate No. SCXK 2005-001) weighting 220–250 g, were supplied by the center of laboratory animal of Anhui Province (China). All the experimental procedures were performed according to the National Institute of Health Guide for the Care and Use of Laboratory Animals. The protocol was approved by Animal Ethics Committee of Anhui Medical University.
Neurosurgery and Treatment of Biochanin A
All surgical procedures were operated under the aseptic conditions and 10 % chloral hydrate (3 mL/kg, i.p., Sigma) anesthesia. Rats were mounted in the stereotaxic apparatus (RWD Life Science Co. Ltd, Shenzhen, China) with the nose oriented 11° below the horizontal zero plane. Right-unilateral lesions of the dopaminergic neurons located in the substantia nigra were induced by LPS (Zhou et al. 2005). LPS (10 µg), dissolved in 4 µL physiological saline solution, was administrated through a Hamilton syringe (Hamilton Co., Reno, NV) at a rate of 1 µL/min. After each injection, the needle was left in situ for 5 min before complete retraction. The sham-operated rats were injected with saline solution. The following coordinates were used: 5.2 mm posterior to bregma, 1.7 mm lateral to the midline, 7.8 mm ventral to the surface of the cortex.
The rats were randomly divided into five groups: sham group, LPS-treated group, and three biochanin A-treated groups (12.5, 25, 50 mg/kg). Biochanin A was dissolved in dimethyl sulfoxide (DMSO) and administered intraperitoneally (i.p. 0.1 mL/100 g/day) to different groups of rats at three different concentrations of 12.5, 25, 50 mg/kg. After LPS injection, the rats were continuously treated with biochanin A for 21 days. The sham and LPS-treated group received equivalent volumes of DMSO.
Apomorphine-Induced Circling Behavior
The animals were tested for rotational behavior by apomorphine on the second day after the final biochanin A injection. The rats were allowed to adapt to the testing environment for 10 min, then were injected hypodermically with 0.5 mg/kg apomorphine dissolved in physiological saline solution. 5 min later, the measurement of rotational behavior began and lasted for 30 min under minimal external stimuli. The net number of turns performed during the entire 30 min testing period was counted.
Open-Field Test
The open-field tests were performed in a black box (60 × 60 × 60 cm), which was located in an experimental room with a 50-dB background noise and a white light bulb (15 W) located 60 cm high above the center of the open field. The bottom of the black box is divided into nine compartments (20 × 20 cm). The rat was put in the center of the open field; locomotion frequency (the number of squares crossed) and rearing frequency (the number of times the animal stood on their hind paws) were recorded by ANY-maze Video tracking system (Stoelting Company, USA). The analysis of motor activity lasted for 3 min after preadaption for 2 min.
Tumor Necrosis Factor (TNF)-α, Interleukin (IL)-1β, and Interleukin (IL)-6 Assays
After the apomorphine-induced circling behavior test, the rats were anesthetized and the blood was drawn through the abdominal aorta. Plasma was collected for measuring the levels of TNF-α, IL-1β, and IL-6 with the enzyme-linked immunosorbent assay (ELISA) kits (Boster, Wuhan, China).
Immunofluorescence Method
The brains of rats were removed, and then fixed in 4 % paraformaldehyde solution and embedded in paraffin. We examined serial sections located in areas between −4.8 and −5.8 mm from the bregma in the rostrocaudal direction. The brains were sliced into 5 μm using a section cutter (Leica, Germany). Paraffin sections were cut at 5 μm and affixed to slides to ensure adhesion. The sections of the substantia nigra (bregma −4.8 and −5.8 mm) were stained with primary antibody against neuronal TH (rabbit antirat, 1:500). Adjacent sections were immunostained for the detection of the microglial marker Iba-1 (rabbit antirat, 1:500). The sections were incubated with diluted hydrogen peroxide (3 %) for 10 min and blocked with nonimmune goat serum for 30 min. In all cases, the primary antibody was left to react at 4 °C overnight.
Then, the antibody was detected with a fluorescent antirabbit secondary antibody. The sections were incubated with 25 mm Hoechst 33258 (Sigma, USA) at 37 °C for 15 min, washed with PBS, mounted onto slides with antifade mounting medium (Beyotime Institute of Biotechnology, Shanghai, China).
The positive neurons were showed green influorescence and taken pictures under the fluorescence microscope (Ex/Em: 352/461 nm) (Olympus IX71, Japan). Eight sections per group and the three high-power fields of SN per section with the same magnification were used for quantitative analysis. Using Image-Pro Plus 6.0 analysis system, the mean fluorescence intensity of positive neurons in each section was measured. All sections were coded and examined blindly.
Western Blotting
The substantia nigra of rats were separated and prepared in an ice cold lysis buffer. The lysates were centrifuged at 12000 rpm and 4 °C for 10 min, the protein concentrations were measured by Bio-Rad assay kit. Protein extracts were separated on 12 % sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto PVDF membranes (Millipore Corp., Billerica, MA, USA), which blocked with 5 % skim milk in TBST. Membranes were further incubated sequentially with primary antibodies at 4 °C overnight. After three washes, the blots were incubated with a horseradish peroxidase (HRP)-conjugated secondary antibody at room temperature for 2 h, and then specific protein bands were detected using the ECL chemiluminescent kit (Thermo Scientific). The density of each band was quantified using image analysis software (National Institutes of Health, USA). The phosphor-JNK, phosphor-ERK, phosphor-p38 protein levels were normalized to total JNK, ERK, p38, respectively.
Primary Microglia Cultures
Rat primary microglia from rat mesencephalon was cultured to more than 95 % purity using a modified procedure as previously described (Zhou et al. 2005; Zhang et al. 2011). Briefly, cerebral cortices were isolated from 1-day-old pups, minced in Hank’s buffer, dissociated by trituration, and digested in the presence of 0.2 % trypsin at 37 °C for 15 min. Cells were resuspended in culture medium, which consisted of Ham F12 mixed medium 1:1, 10 % FBS, 100 μg/mL penicillin-streptomycin combination. Cells were then seeded in 20 μg/mL poly-l-lysine coated flasks. The cultures were maintained at 37 °C in a humidified atmosphere of 5 % CO2 and 95 % air. Culture medium was changed every 4 days. After cells grew and confluence for 14 days, microglia were removed by 2 h shaking at 250 revolutions per minute on a rotary shaker. Isolated microglia were plated on precoated 6- or 24-well plates in culture medium as mentioned above. The purified microglia were found that 95 % of them were immunoreactive to the microglia-specific marker OX-42.
Microglia Cell Viability Assay
The viability of primary microglia was determined by MTT assay. Briefly, microglia were incubated in 96-well plates for 24 h, then treated with various concentrations of biochanin A (1.25, 2.5, 5, 10, 15, 20, 25, 30 μM) and LPS (0.01, 0.1, 1, 10, 100 μg/mL) for 36 h, followed by incubation with 0.5 mg/mL MTT solution. After incubation for 4 h, the medium was removed, and the cells were lysed with 200 μL DMSO and shaken for 10 min. The optical density (OD) values at 570 nm were measured using an ELISA reader.
Determination of Nitric Oxide Production
The microglial cells were pretreated with various concentrations of biochanin A (1.25, 2.5, 5 μM) for 30 min, then treated with LPS (100 ng/mL) for additional 36 h. The level of nitric oxide (NO) was measured by the Griess reaction. Briefly, after treatment with LPS and various concentrations of biochanian A for 36 h, 50 μL of culture supernatant from each sample was mixed with an equal volume of the Griess reagent (0.1 % N-(1-naphthyl)-ethylenediamine dihydrochloride, 1 % sulfanilamide, 2.5 % phosphoric acid). Following incubation for 15 min, the absorbance values were read at 540 nm using an ELISA microplate reader. The nitrite content was calculated by comparison with that of standard concentration of sodium nitrite dissolved in DMEM.
Measurement of TNF-α and IL-1β Production
The microglial cells were treated with different concentrations of biochanin A and stimulated with LPS for 36 h. The levels of TNF-α and IL-1β in the supernatants were determined with commercial enzyme-linked immunosorbent assay (ELISA) kits (Genzyme Diagnostics, Cambridge, MA) according to the manufacturer’s instructions.
Measurement of Intracellular ROS by 2′,7′-Dichlorofluorescein Diacetate Assay (Zhang et al. 2011)
The intracellular formation of ROS was assessed with a reactive oxygen species assay kit (Beyotime Institute of Biotechnology, Shanghai, China). Nonfluorescent probe 2′,7′-dichlorofluorescein diacetate (DCFH-DA) passively diffuses into cells and is deacetylated by esterases to form nonfluorescent 2′,7′-dichlorofluorescein (DCFH). DCFH reacts with ROS to form the fluorescent product DCF, which is trapped inside the cells. The microglial cells were seeded at a density of 1 × 105/well in a 96-plate and pretreated with different concentrations of biochanin A for 30 min, then treated with LPS (1 μg/mL) for an additional 36 h. The culture medium was first removed and the cells were washed with PBS three times. DCFH-DA, diluted to a final concentration of 10 μM with DMEM/F12, was added to the culture medium and incubated at 37 °C for 20 min in the dark. After washing the cells three times with serum-free medium, the cells were visualized using an inverted fluorescence microscope (Olympus Opticals, Tokyo, Japan; excitation (Ex)/emission (Em): 352/461 nm). Six adjacent wells in each group were used for quantitative analysis. Using Image-Pro Plus 6.0 analysis system, fluorescence intensity in each group was measured to indicate the production of ROS.
Rat Mesencephalic Neuron–Glia Cultures
Primary mesencephalic neuron–glia cultures were prepared from the brains of embryonic day 14/15 rats according to the described protocol (Liu et al. 2000; Gao et al. 2003). Briefly, the ventral mesencephalic tissues were dissected on the ice, dissociated by a mild mechanical trituration, and digested in the presence of 0.2 % trypsin at 37 °C for 20 min. The dissociated cells were seeded at 5 × 105/well to 24-well culture plates precoated with poly-d-lysine (20 μg/mL) and maintained at 37 °C in a humidified atmosphere of 5 % CO2 and 95 % air in 0.5 mL/well maintenance medium. The medium consisted of minimum essential medium containing 10 % heat-inactivated fetal bovine serum and 10 % heat-inactivated horse serum, 1 g/L glucose, 2 mM l-glutamine, 1 mM sodium pyruvate, 100 μM nonessential amino acids, 50 U/mL penicillin, and 50 μg/mL streptomycin. After the initial seeding for 3 days, the cultures were replenished with 0.5 mL of fresh maintenance medium.
Immunocytochemical Staining
Immunostaining was performed as previously described. DA neurons were recognized with an anti-TH antibody, and microglia were detected with anti-OX-42 antibody. In brief, the cells were fixed with 3.7 % paraformaldehyde for 30 min, and then treated with 3 % hydrogen peroxide for 10 min. After incubation with blocking solution for 30 min, the cells were incubated with primary anti-TH (1:1000) and anti-OX-42 (1:200) antibodies at 4 °C overnight. Afterward, the cells were incubated with biotinylated secondary antibody for 1 h followed by incubation with horseradish peroxidase streptavidin (HRP-SA) avidin–biotin complex reagents for 30 min, and the color was developed with DAB. For morphological analysis, the images were recorded with a charge-coupled device camera and operated with the Meta Morph software (Molecular Devices, Sunnyvale, CA).
Statistical analysis
Data were expressed as mean ± standard deviation. Results were analyzed by one way analysis of variance (ANOVA) and Student’s t-test. Calculations were performed using the SPSS 13.0 statistical package (SPSS, Chicago, IL). A value of P < 0.05 was considered to be statistically significant.
Results
Biochanin A Treatment Improves the Behavioral Dysfunction of LPS-Induced PD Model Rats
Behavioral tests could be used to evaluate the effect of biochanin A treatment on motor dysfunction of animal models of PD. Three weeks after LPS injection, we observed the behavioral function. Apomorphine, which is a DA-receptor agonist, caused the animals to turn contralaterally; this is attributed to the direct stimulation of supersensitive postsynaptic receptors on the lesioned side (Hefti et al. 1980). The apomorphine-induced rotation test is a classic method to evaluate behavioral dysfunction of PD rats, which is used to detect dopaminergic system damage.
Three weeks after LPS injection, the rats exhibited rotational behavior towards the side of the lesion after apomorphine administration. As shown in Fig. 1, no rotation (0 turns per 30 min) was observed in the sham group of the rats. Compared with the sham group, LPS injection significantly increased the number of apoporphine-induced rotations of the rats (P < 0.01). However, biochanin A (25, 50 mg/kg) treatment dramatically decreased the number of apomorphine-induced rotation (Fig. 1a, P < 0.05 or P < 0.01) compared with the LPS-treated group.
Biochanin A treatment improves the behavioral dysfunction in LPS-induced PD model rats. Biochanin A was administrated once daily for 3 weeks after LPS injection, behavioral tests was conducted to assess the motor dysfunction. The number of apomorphine-induced rotation in PD rats after 3 weeks of biochanin A treatment (a). The number of squares crossed in the open-field test (b). The number of rearing was recorded in the open-field test (c). Moving distance was recorded in the open-field test (d). Results are expressed as mean ± SD (n = 16). ## P < 0.05 versus sham group, *P < 0.05, **P < 0.01 versus LPS group
Open-field test is also a behavioral test to evaluate the spontaneous behaviors of rats. Biochanin A (25, 50 mg/kg) treatment significantly increased the number of square crossed, rearing, and moving distance of PD rats during 3 min period in the open field (Fig. 1b–d, P < 0.05 or P < 0.01). The results indicated that biochanin A could improve the behavioral dysfunction of LPS-induced PD rats.
Biochanin A Treatment Inhibits Microglial Activation and Increases the Number of TH-Positive Cells in the Substantia Nigra of LPS-Induced PD Model Rats
Immunofluorescence of Iba-1 was used to evaluate microglial activation and morphological changes. Consistent with other reports, microglia transformed from resting cells to activated, large cells with long processes 3 weeks after LPS injection. As shown in Fig. 2, the cells in the substantia nigra of sham animals were ramified resting microglia with two or three fine processes. In the LPS-treated group, activated microglia was readily identified throughout the substantia nigra by their thicker processes and more rounded cell bodies. However, the activation of microglia was significantly suppressed by biochanin A treatment in a dose-dependent manner. The relative fluorescence unit was determined to represent the Iba-1 expression level in the substantia nigra. As shown in Fig. 3, compared with the sham group, the Iba-1 content significantly increased in the LPS-treated group (P < 0.01). However, biochanin A (25, 50 mg/kg) treatment for 21 days can reduce the Iba-1 expression level (P < 0.05).
Biochanin A inhibited the microglia activation in substantia nigra of PD rats (immunofluorescence, ×400). a Effects of biochanin A on microglial activation and morphological changes in SN induced by LPS in PD rats (immunofluorescence). a1, a2, a3 sham group; b1, b2, b3 LPS group; c1, c2, c3 biochanin A (12.5 mg/kg) group; d1, d2, d3 biochanin A (25 mg/kg) group; e1, e2, e3 biochanin A (50 mg/kg) group. The bar is 50 μm. b Quantitative analysis of Iba1 positive expression in SNpc. Results are expressed as mean ± SD (n = 8). # P < 0.05, ## P < 0.01 compared with sham group, **P < 0.01 compared with LPS group
Biochanin A increased the number of TH-positive cells in substantia nigra of PD rats (immunofluorescence, ×400). a Effects of biochanin A on dopaminergic neurons (TH-immunoreactive cells) loss in SN induced by LPS in PD rats (immunofluorescence). a1, a2, a3 sham group; b1, b2, b3 LPS group; c1, c2, c3 biochanin A (12.5 mg/kg) group; d1, d2, d3 biochanin A (25 mg/kg) group; e1, e2, e3 biochanin A (50 mg/kg) group. The bar is 50 μm. b Quantitative analysis of TH-positive expression in SN. Results are expressed as mean ± SD (n = 8). # P < 0.05, ## P < 0.01 compared with sham group, **P < 0.01 compared with LPS group
To further investigate the protective effect of biochanin A on dopaminergic neurons, we observed the TH expression in the substantia nigra. The results showed that the number of TH-positive neurons decreased in the substantia nigra of LPS-treated group (P < 0.01), while biochanin A (50 mg/kg) treatment markedly attenuated the loss of TH-positive neurons (P < 0.05), which indicates that biochanin A protects dopaminergic neuronal damage.
Biochanin A Inhibits Inflammatory Responses in LPS-Induced PD Model Rats
The levels of IL-1β, IL-6, and TNF-α in the serum of PD model rats were measured by ELISA. As shown in Fig. 4, compared with sham group, LPS injection caused increase of IL-1β, IL-6, and TNF-α levels in the serum. However, after biochanin A (50 mg/kg) treatment for 21 days, the contents of IL-1β, IL-6, and TNF-α significantly decreased. The results indicated that biochanin A might inhibit LPS-induced inflammatory response.
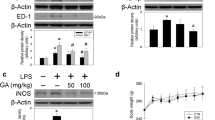
We further detected the expression of IL-1β and TNF-α in the SNpc by western blot. As shown in Fig. 5, compared with sham group, the expression of IL-1β and TNF-α were significantly increased in LPS-treated rats. Compared with LPS group, biochanin A (50 mg/kg) treatment decreased the expression of IL-1β and TNF-α.
Effects of biochanin A on expression of IL-1β and TNF-α of brain in PD rats. Proteins were extracted from the SNs of rats. The IL-1β and TNF-α were determined by western blot assay. a The expression of IL-1β and TNF-α in brain. b The relative density for expression of IL-1β and TNF-α relative to β-actin. Results are expressed as mean ± SD (n = 8). ## P < 0.05 versus sham group, *P < 0.05 versus LPS group
We further explored the role of biochanin A in regulating the phosphorylation of JNK, ERK, and p38 in LPS-induced PD rats. As shown in Fig. 6, LPS injection dramatically increased the phosphorylation of JNK, ERK, and p38. Compared with the LPS-treated group, biochanin A (25, 50 mg/kg) treatment markedly inhibited the phosphorylation of ERK, and biochanin A (50 mg/kg) treatment inhibited the phosphorylation of JNK and p38. For this classic signaling pathway, LPS strongly phosphorylated JNK, ERK, P38, and biochanin A showed inhibitory effect on phosphorylation.
Effects of biochanin A on expression of p-JNK, p-ERK, p-P38 of brain in PD rats. Proteins were extracted from the SNs of rats. The p-JNK, p-ERK, p-P38 were determined by western blot assay. a The expression of p-JNK, p-ERK, p-P38 in brain. b The relative density for expression of p-JNK, p-ERK, p-P38 relative to JNK, ERK, P38. Results are expressed as mean ± SD (n = 8). ## P < 0.05 versus sham group, *P < 0.05, **P < 0.01 versus LPS group
Cytotoxic Effect of Biochanin A and LPS on Primary Microglial Cells
Primary microglia was used in the present study to observe the antiinflammatory effect of biochanin A. To exclude the cytotoxicity of biochanin A used by MTT assay in the present study, the data showed that biochanin A (1.25, 2.5, 5, 10 μM) exhibited no cytotoxicity on cells, while biochanin A (15, 20, 25, 30 μM) decreased the activity of microglial cells (Fig. 7a), Therefore, in order to avoid the cytotoxicity of biochanin A, we choose the biochanin A (1.25, 2.5, 5 μM) for further study.
Cell viability of primary microglia cells. a Cytotoxicity of biochanin A in primary microglia cells was assessed by MTT assay. b Following treatment with various concentrations of lipopolysaccharide (LPS) for 36 h, the viability of the primary microglia cells was assessed by MTT assay. Results are the mean ± SD of at least 3 independent experiments. # P < 0.05, ## P < 0.01 versus control group
Furthermore, because we will use LPS to induce microglial inflammatory response, it is necessary to ascertain nontoxic concentration of LPS in primary microglial cells. Treatment with LPS (0.01, 0.1, 1 μg/mL) did not affect the cell viability (P > 0.05) (Fig. 7b), in addition, our preliminary experiment results suggested that 1 μg/mL LPS obviously induced proinflammatory cytokines production (data not shown). Therefore, we selected 1 μg/mL LPS as stimulated concentration for further study.
Biochanin A Treatment Inhibits LPS-Induced Inflammatory Responses in Primary Microglia
As shown in Fig. 8, microglia exposure to LPS increased the production of NO, while biochanin A inhibited the NO production, suggesting the biochanin A’s antiinflammatory effect in microglia. We further studied the effect of biochanin A on IL-1β and TNF-α in microglia, LPS treatment obviously increased the content of IL-1β and TNF-α in supernatant, while biochanin A decreased the supernatant’s content of IL-1β and TNF-α.
Biochanin A Treatment Inhibits LPS-Induced ROS Production in Primary Microglia
ROS lie the upstream of many cytokines related to signaling pathways, it was associated with various neurodegenerative diseases, except the direct neurotoxicity. So, we further observed the effect of biochanin A on ROS production, using the DCFH-DA as the fluorescent probe and observed the fluorescence intensity by the fluorescence microscope. As shown in Fig. 9, LPS treatment induced the production of ROS, while biochanin A (1.25, 2.5, 5 μM) pretreatment decreased the fluorescence intensity, indicating that biochanin A inhibited the ROS production in concentration-dependent manner.
Effects of biochanin A on reactive oxygen species (ROS) production in primary microglial cells. Primary microglia were preincubated with or without biochanin A for 30 min and then stimulated with lipopolysaccharide (LPS; 1 µg/mL) for 36 h. a control group; b LPS group; c biochanin A (12.5 μM) group; d biochanin A (25 μM) group; e biochanin A (50 μM) group. The bar is 20 μm. a Images presented are from one experiment and are representative of at least 3 independent experiments. b The fluorescence intensity is presented as the mean ± SD of at least 3 independent experiments. ## P < 0.01 compared with the control group; *P < 0.05, **P < 0.01 compared with LPS-treated group
Biochanin A Attenuated LPS-Induced Microglial Activation and Protected DA Neurons Against LPS-Induced Neurotoxicity in Mesencephalic Neuron–Glia Cultures
We investigated whether biochanin A inhibited the microglial activation in primary mesencephalic neuron–glia cultures. Rat primary mesencephalic neuron–glia cultures were pretreated with biochanin A for 30 min before LPS stimulation. As shown in Fig. 10, the activation of microglia was observed by immunocytochemical staining. Activated microglia exhibited an enlarged cell body and irregular shapes, changed from resting round and small cells to the highly activated amoeboid. In the LPS-treated group, the microglia activation was obvious, while biochanian A pretreatment attenuated the LPS-induced microglia activation.
Biochanin A attenuated LPS-induced microglia activation in mesencephalic neuron–glia cultures. Rat primary mesencephalic neuron–glia cultures were pretreated with biochanin A for 30 min then stimulated with lipopolysaccharide (LPS; 1 µg/mL) for 36 h. Morphological assessment of Iba immunoreactive primary microglia. a control group; b LPS group; c biochanin A (12.5 μM) group; d biochanin A (25 μM) group; e biochanin A (50 μM) group. The bar is 20 μm
Mesencephalic neuron–glia cultures were pretreated with biochanin A for 30 min and then stimulated with LPS. Three days later, the degeneration of DA neurons was assessed by immunocytochemical staining. As shown in Fig. 11, morphologically, the neuritis of the TH-positive neurons became shorter compared with the control cultures. In biochanin A-treated cultures, the more DA neurons survived, and the less neuritis of neurons affected compared with LPS- treated group.
Biochanin A produced neuroprotection against LPS-induced neurotoxicity in mesencephalic neuron–glia cultures. Rat primary mesencephalic neuron–glia cultures were pretreated with biochanin A for 30 min then stimulated with lipopolysaccharide (LPS; 1 µg/mL) for 36 h. Morphological assessment of TH-immunoreactive DA neurons. a control group; b LPS group; c biochanin A (12.5 μM) group; d biochanin A (25 μM) group; e biochanin A (50 μM) group. The bar is 20 μm
Discussion
Since neuroinflammation was thought as a pathogenic process in PD, researchers tried to build the suitable models in order to better understand the inflammation-mediated neuronal damage in the condition. LPS is extensively used as glial activator, intranigral injection of LPS to rats caused microglial activation and degeneration of nigrostriatal dopaminergic neurons (Liu and Bing 2011; Hoban et al. 2013). LPS-induced PD models both in vitro and in vivo have been widely used in drug discovery and to evaluate compound’s potentially neuroprotective activities. Previous studies demonstrated that naloxone, an antagonist of opioid receptors, provided the protective effects on dopaminergic neurons against LPS-induced damage (Liu et al. 2000; Lu et al. 2000). In the present study, we observed the neuroprotective effects of biochanin A on the LPS-induced PD model rats both in vitro and in vivo.
The motor dysfunction of PD rats was observed using apomorphine-induced rotation and open-field test. LPS was injected to right side of substantia nigra, and apomorphine-induced rotation to the lesioned side was used to evaluate the damage degree of dopaminergic system (Iancu et al. 2005; Tai et al. 2013). Rotation numbers significantly increased in PD model rats, while biochanin A treatment decreased the apomorphine-induced rotation numbers, and increased the squares crossed, rearing numbers, and moving distance in open-field test; biochanin A showed protective effect on the behavioral dysfunction of PD rats. Immunofluorescence was used to evaluate microglial activation and morphological changes in dopaminergic neurons. In vitro and in vivo tests, our results showed that biochanin A inhibited microglial activation and attenuated dopaminergic neuronal damage, suggesting that biochanin A exerted protective effects on DA neurons by inhibiting activation of microglia.
Microglia are the resident immune cells in the brain and play a major role in immune surveillance and host defense against infectious agents under normal condition. However, in response to injury, infection, or inflammation, microglia become readily activated (Kreutzberg 1996). Activated microglia produce a variety of proinflammatory factors, including cytokines such as IL-1β and TNF-α, ROS, and reactive nitrogen species such as NO. Accumulation of these factors is deleterious to neurons (Jeohn et al. 1998). Dopaminergic neurons in the substantia nigra (SN) are especially vulnerable to oxidative insults due to their reduced antioxidative capacity and potential defect in mitochondrial function. Because the midbrain that encompasses the SN is particularly rich in microglia (Kim et al. 2000), activation of microglia and release of proinflammatory factors are believed to contribute to the degeneration of dopaminergic neurons in PD (Le et al. 2001). In the present study, we have demonstrated that the production of IL-1β, IL-6, TNF-α, NO, and ROS by LPS-activated microglia is significantly inhibited in a dose-dependent manner by biochanin A pretreatment. Furthermore, the inhibition of the production of these proinflammatory factors confers significant protection to dopaminergic neurons against inflammation-mediated degeneration. In addition, previous in vitro studies using primary mesencephalic cultures have demonstrated that both genistein and biochanin A protected dopaminergic neurons against LPS-induced damage through inhibition of microglia activation and proinflammatory generation (Chen et al. 2007; Wang et al. 2005). Moreover, inhibition of microglial activation and suppression of proinflammatory factors have been reported to attenuate or delay the disease progression in both in vitro and in vivo model of PD (Carta et al. 2011; Qian et al. 2010).
Mitogen-activated protein kinase (MAPK), a family of serine/threonine kinases, involved in the response to various stimuli. In addition, MAPK family members play a critical role in the control of cellular responses to cytokines and stresses (Dou et al. 2014). Phosphorylation of MAPKs has been shown to be involved in the regulation of proinflammatory cytokines in LPS-stimulated microglia, such as IL-1β, IL-6, and TNF-α (Li et al. 2015). Inflammatory cytokines and oxidative stress activated MAP kinase kinases, leading to the phosphorylation of ERK1/2, JNK, and p38. In the present study, we found that biochanin A significantly inhibited the LPS-induced phosphorylation of ERK, JNK, and p38 in in vivo model rats of PD. We also found that biochanin A significantly inhibited the production of proinflammatory factors including IL-1β, IL-6, and TNF-α in the primary microglia, the mechanism of which at least in part, may involve the inhibition of MAPKs. Taken together, those results suggest that the inhibition of the MAPKs pathway may be a molecular mechanism underlying the antiinflammatory effects of biochanin A in the in vivo model of PD.
In conclusion, our results show that biochanin A has neuroprotective effect on dopaminergic neurons through inhibiting microglia activation in LPS-induced PD model both in vitro and in vivo. The neuroprotective mechanism of biochanin A may be related to the decreased the levels of proinflammatory factors such as TNF-α, IL-1β, NO, and ROS, and inhibited the activation of MAPK signaling pathway. These results suggest that biochanin A may be used as a pharmacological agent in the prevention of PD.
References
Breikaa RM, Algandaby MM, El-Demerdash E, Abdel-Naim AB (2013) Multimechanistic antifibrotic effect of biochanin A in rats: implications of proinflammatory and profibrogenic mediators. PLoS One 8(7):e69276
Carta AR, Frau L, Pisanu A, Wardas J, Spiga S, Carboni E (2011) Rosiglitazone decreases peroxisome proliferator receptor-gamma levels in microglia and inhibits TNF-alpha production: new evidences on neuroprotection in a progressive Parkinson’s disease model. Neuroscience 194:250–261
Chen HQ, Jin ZY, Li GH (2007) Biochanin A protects dopaminergic neurons against lipopolysaccharide- induced damage through inhibition of microglia activation and proinflammatory factors generation. Neurosci Lett 417(2):112–117
Collins LM, Toulouse A, Connor TJ, Nolan YM (2012) Contributions of central and systemic inflammation to the pathophysiology of Parkinson’s disease. Neuropharmacology 62(7):2154–2168
Dou W, Zhang J, Ren G, Ding L, Sun A, Deng C, Wu X, Wei X, Mani S, Wang Z (2014) Mangiferin attenuates the symptoms of dextran sulfate sodium-induced colitis in mice via NF-κB and MAPK signaling inactivation. Int Immunopharmacol 23(1):170–178
Frank-Cannon TC, Alto LT, McAlpine FE, Tansey MG (2009) Does neuroinflammation fan the flame in neurodegenerative diseases? Mol Neurodegener 16(4):47
Gao HM, Hong JS (2008) Why neurodegenerative diseases are progressive: uncontrolled inflammation drives disease progression. Trends Immunol 29(8):357–365
Gao HM, Liu B, Zhang W, Hong JS (2003) Critical role of microglial NADPH oxidase derived free radicals in the in vitro MPTP model of Parkinson’s disease. FASEB J 17:1954–1956
Hefti F, Melamed E, Sahakian BJ, Wurtman RJ (1980) Circling behavior in rats with partial, unilateral nigro-striatal lesions: effect of amphetamine, apomorphine, and DOPA. Pharmacol Biochem Behav 12(2):185–188
Hoban DB, Connaughton E, Connaughton C, Hogan G, Thornton C, Mulcahy P, Moloney TC, Dowd E (2013) Further characterisation of the LPS model of Parkinson’s disease: a comparison of intra-nigral and intra-striatal lipopolysaccharide administration on motor function, microgliosis and nigrostriatal neurodegeneration in the rat. Brain Behav Immun 27(1):91–100
Iancu R, Mohapel P, Brundin P, Paul G (2005) Behavioral characterization of a unilateral 6-OHDA-lesion model of Parkinson’s disease in mice. Behav Brain Res 162(1):1–10
Jankovic J (2008) Parkinson’s disease: clinical features and diagnosis. J Neurol Neurosurg Psychiatry 79(4):368–376
Jeohn GH, Kong LY, Wilson B, Hudson P, Hong JS (1998) Synergistic neurotoxic effects of combined treatments with cytokines in murine primary mixed neuron/glia cultures. J Neuroimmunol 85(1):1–10
Kim WG, Mohney RP, Wilson B, Jeohn GH, Liu B, Hong JS (2000) Regional difference in the susceptibility to lipopolysaccharide-induced neurotoxicity in the rat brain: role of microglia. J Neurosci 20(16):6309–6316
Kreutzberg GW (1996) Microglia: a sensor for pathological events in the CNS. Trends Neurosci 19(8):312–318
Le W, Rowe D, Xie W, Ortiz I, He Y, Appel SH (2001) Microglial activation and dopaminergic cell injury: an in vitro model relevant to Parkinson’s disease. J Neurosci 21(21):8447–8455
Li YY, Huang SS, Lee MM, Deng JS, Huang GJ (2015) Anti-inflammatory activities of cardamonin from Alpinia katsumadai through heme oxygenase-1 induction and inhibition of NF-κB and MAPK signaling pathway in the carrageenan-induced paw edema. Int Immunopharmacol 25(2):332–339
Liu B, Du L, Hong JS (2000a) Naloxone protects rat dopaminergic neurons against inflammatory damage through inhibition of microglia activation and superoxide generation. J Pharmacol Exp Ther 293(2):607–617
Liu M, Bing G (2011) Lipopolysaccharide animal models for Parkinson’s disease. Parkinsons Dis 2011:327089
Liu B, Jiang JW, Wilson BC, Du L, Yang SN, Wang JY, Wu GC, Cao XD, Hong JS (2000b) Systemic infusion of naloxone reduces degeneration of rat substantia nigral dopaminergic neurons induced by intranigral injection of lipopolysaccharide. J Pharmacol Exp Ther 295(1):125–132
Lu X, Bing G, Hagg T (2000) Naloxone prevents microglia-induced degeneration of dopaminergic substantia nigra neurons in adult rats. Neuroscience 97(2):285–291
McGeer PL, Itagaki S, Boyes BE, McGeer EG (1988) Reactive microglia are positive for HLA-DR in the substantia nigra of Parkinson’s and Alzheimer’s disease brains. Neurology 38(8):1285–1291
Qian L, Flood PM, Hong JS (2010) Neuroinflammation is a key player in Parkinson’s disease and a prime target for therapy. J Neural Transm. 117(8):971–979
Tai W, Ye X, Bao X, Zhao B, Wang X, Zhang D (2013) Inhibition of Src tyrosine kinase activity by squamosamide derivative FLZ attenuates neuroinflammation in both in vivo and in vitro Parkinson’s disease models. Neuropharmacology 75:201–212
Tan JW, Tham CL, Israf DA, Lee SH, Kim MK (2013) Neuroprotective effects of biochanin A against glutamate-induced cytotoxicity in PC12 cells via apoptosis inhibition. Neurochem Res 38(3):512–518
Tansey MG, Goldberg MS (2010) Neuroinflammation in Parkinson’s disease: its role in neuronal death and implications for therapeutic intervention. Neurobiol Dis 37(3):510–518
Wang X, Chen S, Ma G, Ye M, Lu G (2005) Genistein protects dopaminergic neurons by inhibiting microglial activation. NeuroReport 16(3):267–270
Wang W, Tang L, Li Y, Wang Y (2015) Biochanin A protects against focal cerebral ischemia/reperfusion in rats via inhibition of p38-mediated inflammatory responses. J Neurol Sci 348(1–2):121–125
Wu WY, Wu YY, Huang H, He C, Li WZ, Wang HL, Chen HQ, Yin YY (2015) Biochanin A attenuates LPS-induced pro-inflammatory responses and inhibits the activation of the MAPK pathway in BV2 microglial cells. Int J Mol Med 35(2):391–398
Zhang Y, Guo J, Zeng L, Zhang J, Hui Y, Liu J, Qing X, Sun X, Guo G (2011) Tert-butyl-2(4,5-dihydrogen-4,4,5,5-tetramethyl-3-O-1H-imidazole-3-cationic-1-oxyl-2-pyrrolidine-1-carboxylic ester displays novel cytotoxicity through reactive oxygen species-mediated oxidative damage in MCF-7 and MDA-MB-231 cells. Chem Biol Interact 192(3):287–297
Zhou HF, Liu XY, Niu DB, Li FQ, He QH, Wang XM (2005) Triptolide protects dopaminergic neurons from inflammation-mediated damage induced by lipopolysaccharide intranigral injection. Neurobiol Dis 18(3):441–449
Acknowledgments
This work was supported by the National Natural Science Foundation of China (NSFC) fund (Grant No. 31171650) and the Natural Science Foundation of Anhui Province Education Department (Grant No. KJ2014A115) and the Natural Science Foundation of Anhui Province (Grant No. 1508085MH186).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
J. Wang and W.-Y. Wu these authors contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wang, J., Wu, WY., Huang, H. et al. Biochanin A Protects Against Lipopolysaccharide-Induced Damage of Dopaminergic Neurons Both In Vivo and In Vitro via Inhibition of Microglial Activation. Neurotox Res 30, 486–498 (2016). https://doi.org/10.1007/s12640-016-9648-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12640-016-9648-y