Abstract
Chronic inflammation in the brain plays a critical role in major neurodegenerative diseases such as Alzheimer’s disease (AD), Parkinson’s disease (PD), and multiple sclerosis (MS). Microglia, the resident macrophages and intrinsic components of the central nervous system (CNS), appear to be the main effectors in this pathological process. Magnesium lithospermate B (MLB) is one of the major bioactive components of Radix Salviae miltiorrhizae, which has been documented to protect neurons against multiple types of neuronal injury. However, its functions on microglia and the related neuroinflammation remain unknown. In the present study, BV2 microglial cells were used to assess the anti-neuroinflammatory capacity of MLB. Our data show that treatment with MLB could not only suppress lipopolysaccharide (LPS)-induced proliferation and morphological changes, but also interfere with cell cycle progression in BV2 cells. More strikingly, it attenuated the production of the inflammatory mediator nitric oxide (NO) and a panel of pro-inflammatory cytokine in LPS-stimulated BV2 cells, including tumor necrosis factor-α (TNF-α), interleukin (IL)-1α, IL-1β, and IL-6, and also promoted a phenotypic switch from the M1 to the M2 phenotype. Additionally, an in vivo study showed that the administration of MLB could ameliorate lipopolysaccharide-induced neurodegeneration and microglial activation in the hippocampus of adult mice. Mechanistically, MLB blocked the activation of the NF-κB pathway upon LPS stimulation, indicating that the effects of MLB on microglia may be mediated by the NK-κB pathway. These results suggest the therapeutic potential of MLB as a novel anti-inflammatory and microglia-modulating drug for neurodegenerative diseases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Microglia are highly specialized macrophages that reside in the brain and play critical roles in neural development, homeostasis, and neuroinflammation (Salter and Stevens 2017). Under normal physiological conditions, microglia exist in a quiescent state and dynamically survey the surrounding environment through their ramified processes (Benarroch 2013). Under pathological conditions, however, microglia are activated in response to numerous detrimental signals, such as neuronal injury and infection, ischemia, and oxidative stress (H et al. 2017). The activated microglia migrate towards the lesion site; proliferate; produce neurotoxic factors such as pro-inflammatory cytokines and chemokines, phagocytosing cell debris, or damaged neurons; and modulate neuronal regeneration (Lynch 2009).
Recently, accumulating evidence has documented that chronic microglial activation and related neuroinflammation are closely associated with the pathogeneses of a variety of neurodegenerative diseases, including Alzheimer’s disease (AD), Parkinson’s disease (PD), Huntington’s disease (HD), multiple sclerosis (MS), and amyotrophic lateral sclerosis (ALS) (Xu et al. 2016). Generally, activated microglia have two heterogeneous phenotypes that can be categorized as the classic pro-inflammatory/cytotoxic M1 type or the anti-inflammatory M2 type (Song and Suk 2017). M1 microglial activation is considered a key pathogenic event in the progression of neurodegenerative diseases, which is modeled by exposure to inflammatory stimuli, such as pathogenic misfolded proteins and reactive oxygen species (ROS), and consequently causes neuronal loss in specific regions of the central nervous system (CNS) through the release of pro-inflammatory factors and various neurotoxic mediators (Orihuela et al. 2016). In contrast, the M2 phenotype is triggered through coordinated regulation of a variety of anti-inflammatory cytokines, which inhibits M1 phenotype-related neuroinflammation (Varnum and Ikezu 2012). In addition, M2 microglia can facilitate the clearance of misfolded proteins and cell debris, promote tissue repair and wound healing, and release neurotrophic factors to support neuronal survival (Varnum and Ikezu 2012). During the chronic inflammatory progression of neurodegenerative diseases, however, the normal homeostatic balance between the M1 and the M2 phenotypes is impaired, which leads to prolonged and predominant M1 polarization that can further exacerbate neuronal damage in these diseases (Boche et al. 2013). Therefore, inhibition of microglial activation and interference with M1 polarization processes may provide a promising therapeutic strategy against the development and progression of neurodegenerative diseases.
Taking this strategy into consideration, in recent years, many researchers and institutions have focused their interest on natural plant compounds with potential neuroprotective and anti-neuroinflammatory effects; a number of natural neuroprotective compounds have demonstrated robust effects on the pro-inflammatory responses and polarization of microglia in in vitro and/or in vivo studies (Choi et al. 2011; Suk and Ock 2012). Magnesium lithospermate B (MLB), one of the major bioactive components of Radix Salviae miltiorrhizae, has been demonstrated to have a protective effect against multiple types of neuronal injury in neurons (Jiang et al. 2015; Xiao et al. 2013). More importantly, the neuroprotective activities of MLB are reported to be mediated through the inhibition of the NF-κB pathway, which is essential for toxicity induced by microglial activation in neurodegenerative diseases (Popiolek-Barczyk and Mika 2016). Based on these findings, we hypothesize that MLB has an inhibitory effect on microglial activation and can interfere with microglial polarization and neuro-inflammatory processes. In order to test this hypothesis, in the current study, we examined the anti-neuroinflammatory and microglia-modulating effects of MLB in BV2 microglial cell line and investigated its efficacy against LPS-induced neurodegeneration in LPS-injected mice.
Materials and Methods
Reagents and Animals
MLB (purity = 99.9%) was purchased from Hangzhou Asure Biotech Co., Ltd. (Hangzhou, China). C57BL/6 mice were from the Shanghai Laboratory Animal Center of the Chinese Academy of Sciences (Shanghai, China). Lipopolysaccharide (LPS) was purchased from Sigma-Aldrich (St. Louis, MO, USA). Propidium iodide (PI), the nitric oxide (NO) assay kit, and the enzyme-linked immunosorbent assay (ELISA) kits for mouse tumor necrosis factor alpha (TNF-α), interleukin (IL)-1α, IL-1β, and IL-6 were obtained from Thermo Fisher Scientific Inc. (Waltham, MA, USA). Cell counting kit-8 (CCK-8) was provided by Dojindo Laboratories (Kumamoto, Japan).
Cell Culture
Mouse microglial BV2 cells were purchased from the Cell Culture Center at the Institute of Basic Medical Sciences (IBMS) of the Chinese Academy of Medical Sciences (Beijing, China); maintained in DMEM enriched with 10% heat-inactivated fetal bovine serum (FBS), 100 μg/mL streptomycin, and 100 U/mL penicillin; and incubated in a humidified atmosphere of 5% CO2 at 37 °C.
Cell Proliferation Assay
CCK-8 was used to determine cell proliferation under different treatment conditions. In brief, BV2 cells were seeded into 96-well plates at a density of 103 cells per well. Prior to exposure to 100 ng/mL LPS for 24 h, cells were pre-treated for 6 h with varying concentrations of MLB (0, 5, 20, and 50 μg/mL). The concentrations of MLB were chosen according to previous studies (Jiang et al. 2015; Xiao et al. 2013). After exposure to LPS for the indicated time periods, CCK-8 reagent was added to each well and incubated at 37 °C for 1 h. Absorbance was measured at 450 nm in a spectrophotometer (Model 680, Bio-Rad, Hercules, CA, USA). Each experiment was performed in sextuplicate and repeated three times.
Immunocytochemistry
Immunocytochemistry was performed as described previously (Lim et al. 2013). Briefly, cells were fixed with 4% paraformaldehyde and permeabilized by 0.1% Triton X-100. After blocking with 10% donkey serum, fixed cells were incubated for 2 h with an anti-Iba1 antibody (WAKO Chemicals; No. 019-19741, 1:1000) followed by a fluorochrome-conjugated secondary antibody (Alexa Fluor 555, 1:200, Thermo Fisher Scientific Inc.). Nuclei were counterstained with 4′,6-diamidino-2-phenylindole (DAPI). Fluorescence images were acquired using a confocal laser microscope (LSM 700; Carl Zeiss AG, Oberkochen, Germany). To analyze the morphological changes of BV2 cells under different treatment conditions, we calculated the percentage of amoeboid BV2 cells over the total BV2 cells from six randomly selected fields (250 × 250 μm) in triplicates for each experimental group by using ImageJ software (https://imagej.nih.gov/ij/).
Flow Cytometry Assay
BV2 cells were plated in six-well dishes and allowed to attach overnight. After the indicated treatments, the cells were washed with PBS and trypsinized. Then, the cells were pelleted and adjusted to a concentration of 1–2 × 106 cells/mL and fixed by adding 50–150 mL of cell suspension into 70% ethanol dropwise with continuous mixing. After fixation overnight at − 20 °C, the cells were pelleted and gently resuspended in ice-cold PBS. After two washes, cells were suspended in PI staining solution (10 μg/mL PI, 100 μg/mL RNase, 0.1% (v/v) Triton-X) for 15 min at 37 °C in the dark. The cell cycle was analyzed using a flow cytometer (FACScan Flow Cytometry System; BD Bioscience, San Jose, CA, USA). All experiments were conducted three times independently.
Measurement of Nitric Oxide and Cytokine Levels
Production of NO, TNF-α, IL-1α, IL-1β, and IL-6 in BV2 microglial cells was measured using an ELISA or colorimetric kits (Thermo Fisher Scientific Inc.). Briefly, after collecting the supernatants of BV2 cells exposed to different treatment conditions, the concentrations of NO and four cytokines were detected using the respective kits according to the manufacturer’s instructions. All experiments were conducted three times independently.
Quantitative Real-Time Polymerase Chain Reaction
After exposure to the various treatments, total RNA was extracted from BV2 cells using the TRIzol® plus RNA purification kit (Thermo Fisher Scientific Inc.) following the manufacturer’s instruction. The cDNAs were synthesized using a high-capacity cDNA reverse transcription kit (Thermo Fisher Scientific Inc.). The primers for inducible nitric oxide synthase (iNOS) and arginase-1 were synthesized according to previous literature (Song et al. 2000; Xiang et al. 2009). Quantitative real-time PCR (qRT-PCR) was carried out using a Power SYBR Green Master Mix kit (Thermo Fisher Scientific Inc.). Messenger RNA (mRNA) levels were calculated using the delta–delta cycle threshold (ΔΔCt) method (Schmittgen and Livak 2008). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) served as an internal control. All experiments were conducted three times independently.
Western Blot
BV2 microglial cells under various treatment conditions were harvested by centrifugation at 1500 rpm for 5 min and lysed with RIPA buffer. The homogenates were centrifuged at 10,000×g for 10 min. The supernatant was then subjected to Western blot analysis according to routine procedures. Preparation of the nuclear and cytoplasmic extracts was performed according to the methods described in previous literature (Abmayr et al. 2006). Immunoblots were performed with the following antibodies: Inhibitor κBα (IκBα, ab32518), phosphorylated IκBα (p-IκBα at Ser32/36, ab12135), IκB kinase α (IKKα, ab54628), phosphorylated IKKα (p-IKKα at Ser176, ab138426), iNOS (ab3523) (Abcam, Cambridge, MA, USA), arginase-1 (no. 9819), and NF-κB P65 (no. 8242) (Cell Signaling; Danvers, MA, USA). Antibodies against β-actin (Santa Cruz Biotechnology, sc-47,778) and histone H3 (Abcam, ab1791) were used as loading controls. Proteins were detected using Pierce ECL Western blotting substrate (Thermo Fisher Scientific Inc.). The bands were quantified using ImageJ software.
LPS-Injected Mouse Model and MLB Treatment
Twenty-four 8-week-old male C57BL/6 mice were divided into four groups: vehicle-treated group (vehicle, n = 6), MLB-treated group (50 mg/kg MLB, n = 6), LPS-injected group (LPS, n = 6), and LPS treatment group (LPS + 50 mg/kg MLB, n = 6). The animal study protocol was approved by the animal ethics committee of the corresponding author’s institution. All animal care and use were performed in accordance with institutional guidelines. Before the study, all mice were housed under a 12-h light–dark cycle and had free access to food and water.
The drugs and doses for all experimental groups were as follows: vehicle group (intraperitoneal (i.p.) saline once daily for 4 weeks), LPS group (i.p. 250 μg/kg once daily for 4 weeks), MLB (i.p. 50 mg/kg, once daily for 4 weeks), and LPS + MLB (i.p. 250 μg/kg LPS + 50 mg/kg MLB once daily for 4 weeks). The dosages for LPS and MLB administrations were based on previous literature (Badshah et al. 2016; Xiao et al. 2013).
Nissl Staining
By the end of the animal experiment, mice were deeply anesthetized with chloral hydrate and then flush-perfused transcardially with PBS. Brains were removed and fixed for 48 h in 4% paraformaldehyde and equilibrated in 30% sucrose for histological processing. Thirty-micrometer-thick coronal sections were cut using a microtome under frozen condition. Afterwards, every 12th section (360-μm interval) was processed for cresyl violet staining to assess cell loss. The Nissl-stained neurons in the cornu ammonis (CA) 1 and CA3 regions were quantified using ImageJ software. All images were background-corrected by subtracting the mean intensity of background regions for quantification.
Immunohistochemistry
In order to evaluate the effect of MLB on microglial activation, we detected the expression of CD68, a marker of activated microglia, in the hippocampi of animals using immunohistochemistry. In brief, the obtained sections were treated with 10 mM sodium citrate for 30 min at 90 °C and then blocked with 5% bovine serum albumin (BSA) for 1 h. Afterwards, sections were incubated with an anti-CD68 antibody (Thermo Fisher Scientific Inc., MA5-16674) at a 1:200 dilution overnight at 4 °C followed by incubation with peroxidase-conjugated secondary antibody (1:1000; Thermo Fisher Scientific Inc.) for 1 h at room temperature. Immunopositive cells were visualized using a 3,3′-diaminobenzidine (DAB) staining kit (Vector Laboratories, Burlingame, CA, USA) and quantified using ImageJ software.
Statistical Analysis
All statistical analyses were carried out in GraphPad Prism 6.01 software (GraphPad Software, La Jolla, CA, USA). The Shapiro–Wilk normality test was used to test the normality of data. Data with normal distribution are presented as means ± standard deviation (SD) or means ± standard error of the mean (SEM). One-way analysis of variance (ANOVA) with the Tukey post hoc test (for multiple comparisons) was used to compare means among groups. A P value less than 0.05 was considered statistically significant.
Results
MLB Suppresses LPS-Induced Proliferation and Morphological Changes in BV2 Cells
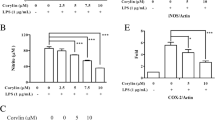
First, we examined the effects of MLB on LPS-induced proliferation and morphological changes in BV2 microglial cells. As indicated in Fig. 1a, MLB did not produce any cytotoxicity in BV2 cells at concentrations ranging from 5 to 50 μg/mL for 24 h. After exposure to 100 ng/mL LPS for 24 h, however, BV2 cells showed a significant increase in cell proliferation as compared to vehicle-treated controls. When the cells were incubated with varying doses of MLB for 6 h prior to LPS treatment, the LPS-induced proliferation was significantly suppressed in a dose-dependent manner, and the most potent effect was observed at 50 μg/mL of MLB (Fig. 1b). By using confocal microscopy, the morphological changes were also examined in all experimental groups. It has been reported that activated BV2 cells upon LPS stimulation display an amoeboid morphology (Bozic et al. 2015; Dai et al. 2015). As shown in Fig. 1c, d, vehicle-treated BV2 cells displayed ramified cell morphology, while treatment with 100 ng/mL LPS for 24 h induced morphological changes from ramified to amoeboid, indicating the activation of BV2 cells in response to LPS treatment. Nevertheless, when cells were pre-incubated with MLB prior to LPS exposure, the morphological changes were inhibited, evidenced by the decreased percentage of amoeboid BV2 cells under this condition (Fig. 1d). Additionally, a considerable reduction in cell number was observed, which was in accordance with the proliferation data presented in Fig. 1b. Taken together, these results demonstrate the effects of MLB against LPS-induced proliferation and morphological changes in BV2 microglial cells. In subsequent in vitro experiments, to ensure adequate concentrations for efficacy, 20 and 50 μg/mL of MLB were used accordingly.
MLB suppresses LPS-induced proliferation and morphological changes in BV2 cells. a Treatment with up to 50 μg/mL of MLB for 24 h did not influence cell viability of BV2 cells. b BV2 cells were incubated with different doses of MLB for 6 h before exposure to 100 ng/mL LPS for an additional 24 h; LPS-induced proliferation was significantly attenuated by MLB. Cell proliferation was detected by CCK-8 assay. Data of proliferation rates (histograms) were from three independent experiments performed in sextuplicate and are presented as means ± SEM. c Representative confocal images of Iba1 staining (red) showing microglial morphology upon LPS treatment in the presence or absence of MLB (scale bar = 10 μm). Blue color indicates the DAPI staining. White arrows indicate representative amoeboid BV2 cells. d The percentage of amoeboid BV2 cells over the total BV2 cells for each experimental group. Data (histograms) were from six randomly selected sampling fields (250 × 250 μm) in triplicates for each group and are presented as means ± SEM. As compared with the vehicle group: ***P < 0.001; as compared with the group treated with LPS alone: #P < 0.05; ##P < 0.01; ###P < 0.001; ANOVA followed by Tukey’s post hoc test
MLB Interferes with Cell Cycle Progression of LPS-Stimulated BV2 Cells
Next, we conducted flow cytometry analysis to examine the effect of MLB on the cell cycle of BV2 microglial cells. As shown in Fig. 2a, b, as compared with the vehicle, the treatment with 100 ng/mL LPS significantly increased the percentage of BV2 cells in the G1 phase (78 vs. 65%, P < 0.05), indicating that LPS promotes the arrest of the BV2 cell cycle at the G1 phase, which was consistent with previous report (Kaneko et al. 2015; Yu et al. 2012). However, when cells were pre-treated with 20 or 50 μg/mL of MLB, a considerable decrease in the number of cells in the G1 phase was observed (58% at 20 μg/mL MLB, P < 0.01; 50% at 50 μg/mL MLB, P < 0.001; as compared to the LPS-treated group), along with a notable accumulation of cells in the G2/M phase. Thus, combined with the data shown in Fig. 1, these results suggest that MLB could attenuate LPS-treated proliferation and interfere with cell cycle progression in BV2 cells.
Inhibitory effect of MLB on LPS-induced cell cycle progression of BV2 cells. Cells were stained by PI and analyzed by flow cytometry. a Representative histograms of the cell cycle of BV2 cells under different treatment conditions. b Quantitative analysis of cell cycle phase distribution. Results represent means from three independent measurements. As compared with the vehicle group: *P < 0.05; as compared with the group treated with LPS alone: **P < 0.01; ##P < 0.01; ###P < 0.001; ANOVA followed by Tukey’s post hoc test
MLB Attenuates the Release of Nitric Oxide and Pro-inflammatory Cytokines in LPS-Treated BV2 Cells
It has been well-documented that LPS directly triggers cytokine release from microglia (Dutta et al. 2008). Therefore, we investigated whether MLB exerts an anti-inflammatory effect on LPS-treated BV2 microglial cells. As depicted in Fig. 3a–e, the production of NO, TNF-α, IL-1α, IL-1β, and IL-6 was significantly increased in LPS-treated BV2 cells as compared to those treated with the vehicle. When cells were pre-incubated with 20 or 50 μg/mL MLB, production of the five inflammatory indicators was considerably suppressed in a dose-dependent manner. Collectively, these results reveal that MLB could counteract LPS-triggered inflammatory responses in microglia.
MLB attenuates the release of NO and pro-inflammatory cytokines induced by LPS in BV2 cells. Cells were incubated with different doses of MLB for 6 h before exposure to 100 ng/mL LPS for an additional 24 h. The production of NO (a), TNF-α (b), IL-1α (c), IL-1β (d), and IL-6 (e) was measured by ELISA kits. All data (histograms) were from three independent experiments, and the bars indicate means ± SD. As compared with the vehicle group: ***P < 0.001; as compared with the group treated with LPS alone: #P < 0.05; ##P < 0.01; ###P < 0.001; ANOVA followed by Tukey’s post hoc test
MLB Promotes Phenotypic Switch of BV2 Cells from the M1 to the M2 Phenotypes
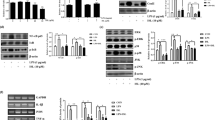
Although there is still some debate, it is generally accepted that once activated, microglia can adopt either a classical M1 phenotype (neurotoxic phenotype) or an alternatively anti-inflammatory M2 phenotype (neuroprotective phenotype) (Tang and Le 2016). Next, we examined whether MLB promotes phenotypic switch in BV2 microglia. As shown in Fig. 4a, b, LPS stimulation induced the mRNA and protein expression of iNOS, a M1 phenotype marker, in BV2 cells as well as downregulated the mRNA and protein production of Arg-1, a sensitive marker of immunosuppressive activity in the M2 phenotype. Nevertheless, this effect was counteracted by pre-incubation with either 20 or 50 μg/mL MLB, suggesting that MLB may have the potential to promote phenotypic switch of BV2 cells from the M1 to the M2 phenotype.
Effect of MLB on expression of iNOS and arginase-1. a mRNA levels of iNOS and arginase-1 assayed by qRT-PCR. b Expression levels of iNOS and arginase-1 detected by Western blot. All data were from three independent experiments and are presented as means ± SD. As compared with the vehicle group: ***P < 0.001; as compared with the group treated with LPS alone: ##P < 0.01; ###P < 0.001. ANOVA followed by Tukey’s post hoc test
MLB Suppresses LPS-Induced Activation of the NF-κB Pathway
LPS has been reported to activate NF-κB in microglia, while a previous study demonstrates that the neuroprotective effect of MLB is most likely mediated by the NK-κB pathway (Jiang et al. 2015). To test whether MLB can prevent LPS-induced activation of the NF-κB pathway in microglia, we detected the levels of cytoplasmic and nuclear NF-κB p65 subunits in all experimental groups by Western blot. We observed that the treatment with LPS resulted in a significantly higher level of nuclear p65 and a considerably lower level of cytoplasmic p65 in BV2 cells as compared to those in the vehicle (Fig. 5a). However, this effect was reversed by pre-incubation with 20 or 50 μg/mL MLB, indicating the inhibitory effects of MLB on LPS-induced NF-κB pathway activation in microglial cells.
MLB suppresses LPS-induced activation of NF-κB and phosphorylation of IKKα and IκBα in BV2 cells. The cells were pre-incubated with 20 or 50 μg/mL of MLB for 6 h prior to LPS treatment for another 1 h. Expression levels of cytoplasmic and nuclear NF-κB p65 subunit (a) and cytoplasmic phosphorylated IKKα and IκBα (b) were detected by Western blot and quantified with ImageJ software. All data (histograms) were from three independent experiments, and the bars indicate means ± SD. As compared with the vehicle group: *P < 0.05; **P < 0.01; ***P < 0.001; as compared with the group treated with LPS alone: #P < 0.05; ##P < 0.01; ###P < 0.001; ANOVA followed by Tukey’s post hoc test
In addition, activation of the NF-κB pathway involves phosphorylation of IKK and IκBα (Ma and Hottiger 2016). We, therefore, determined the expression of the phosphorylated forms of IKKα and IκBα in BV2 microglial cells in response to different treatments. As presented in Fig. 5b, Western blot analysis showed that treatment with 100 ng/mL LPS for 1 h induced significantly higher levels of phosphorylated IKKα and IκBα as compared to those in the vehicle, while these effects were attenuated by pre-treatment with 20 or 50 μg/mL MLB before exposure to LPS. These results are consistent with the data presented in Fig. 4, further demonstrating that MLB suppresses LPS-induced activation of the NF-κB pathway. Taken together, our findings suggest that the effect of MLB against LPS-induced microglial activation is most likely related to the NF-κB pathway.
MLB Attenuates LPS-Induced Neurodegeneration and Microglial Activation in the Hippocampus of Adult Mice
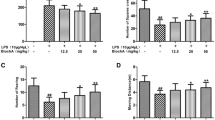
To further validate the therapeutic potential in vivo, we administered MLB in LPS-injected C57BL/6 mice and examined its effect on LPS-induced neurodegeneration. As expected, intraperitoneal administration of LPS at 250 μg/kg for 4 weeks caused neuronal loss in the hippocampus, especially in the CA1 and CA3 areas (Fig. 6a). However, simultaneous administration of 50 mg/kg MLB exhibited significant protection against LPS-induced neuronal loss, evidenced by the quantitative threshold analysis in Fig. 6b, c. These data demonstrated that MLB can attenuate neurodegeneration in the hippocampus of LPS-injected animals. Furthermore, by using IHC staining of CD68, we found that hippocampal samples from the LPS-injected group exhibited a considerably higher expressions of CD68 in the CA1 and CA3 areas than those from the control group (Fig. 7a), indicating the activation of microglia in response to LPS. Nevertheless, when animals received LPS and MLB simultaneously, the expression of CD68 was noticeably attenuated (Fig. 7b, c), suggesting that MLB has an inhibitory effect on microglial activation in the mouse brain following LPS injection.
MLB attenuates neurodegeneration in the hippocampus of LPS-injected mice. The hippocampal structure was stained with cresyl violet. a Representative images of stained brain sections exhibiting LPS-induced injury with or without MLB treatment (scale bar = 200 μm). The magnified views below indicate the CA1 and CA3 regions from the boxed areas of each image (scale bar = 50 μm). b Quantification of intensity of the CA1 region. c Quantification of the intensity of the CA3 region. Data are expressed as means ± SEM from 10 sections/mouse, 6 mice/group. ***P < 0.001 as compared with the vehicle-treated group; ##P < 0.01 as compared with the LPS-treated group; ANOVA followed by Tukey’s post hoc test
MLB attenuates LPS-induced microglial activation in the hippocampus of adult mice. a Representative IHC images of CD68 staining for all experimental groups (scale bar = 200 μm). The magnified views below indicate the CA1 and CA3 regions from the boxed areas of each image (scale bar = 50 μm). b Quantification of intensity of the CA1 region. c Quantification of the intensity of the CA3 region. Data are expressed as means ± SEM from 10 sections/mouse, 6 mice/group. As compared with the vehicle-treated group: ***P < 0.001; as compared with the LPS-treated group: ##P < 0.01; ###P < 0.01; ANOVA followed by Tukey’s post-hoc test
Discussion
In the current study, we evaluated the anti-neuroinflammatory and microglia-modulating effects of MLB by using LPS-treated BV2 microglial cells. We found that pre-treatment with MLB could suppress LPS-induced cell proliferation and morphological changes, interfere with cell cycle progression, attenuate the production of NO and pro-inflammatory cytokines, and promote phenotypic switch of BV2 cells from the M1 to the M2 phenotype. Mechanistically, these effects of MLB may be mediated by the NK-κB pathway. Moreover, an additional in vivo experiment showed that the administration of MLB could ameliorate LPS-induced neural loss and microglial activation in the hippocampus of adult mice, further suggesting the therapeutic potential of MLB as a novel anti-inflammatory and microglia-modulating drug for neurodegenerative diseases.
In recent years, accumulating evidence has suggested that microglia-mediated neuroinflammation is a hallmark shared by various neurodegenerative diseases (Xu et al. 2016). Under pathological or stress conditions, microglia in the CNS undergo changes in cell proliferation and morphology to adopt an activated state in order to adequately respond to the injury or stimulus (Song and Suk 2017). Based on their function, activated microglia can be divided into at least two distinct subtypes: M1 and M2, which are considered neurotoxic and neuroprotective, respectively (Orihuela et al. 2016). In the diseased brain, M1 microglia predominate in the milieu, promoting neurotoxicity via the production and release of several pro-inflammatory mediators and cytokines (Ramirez et al. 2017), while the M2 subtype is associated with neurogenesis and anti-inflammatory effects, although little is known about M2 activation in pathogenic processes (Song and Suk 2017). Based on this understanding, an innovative therapeutic strategy has been proposed to modulate microglial proliferation and activation and target the M1/M2 balance in patients with neurodegenerative diseases (Tang and Le 2016).
In this study, in vitro experiments revealed that in BV2 microglial cells and pre-treatment with MLB could suppress cell proliferation and morphological changes in response to LPS, as well as counteract LPS-induced cell cycle arrest at the G1 phase and production of pro-inflammatory mediators. These effects of MLB may be directly attributed to its intrinsic anti-inflammatory and microglia-modulating capabilities. MLB consists of magnesium ions and salvianolic acid B (Sal B) anions. As a known antagonist of some Ca2+ ion channels, magnesium has been demonstrated to reduce microglial neurotoxicity by inhibiting Ca2+ entry via purinergic channels and protect microglia against LPS-induced release of inflammatory mediators via inhibition of L-type calcium channels (Gao et al. 2013; Lee et al. 2011). A previous study demonstrates that Sal B can significantly reduce the production of NO, ROS, and a panel of pro-inflammatory cytokines in LPS-treated rat primary microglia (Wang et al. 2010; Zhang et al. 2017). In line with these previous observations, our results confirm the anti-inflammatory and microglia-modulating effects of MLB. Another striking finding of this study was that MLB could promote phenotypic switch of BV2 cells from the M1 to the M2 phenotype, which corresponds with the previous finding that Sal B can inhibit LPS-stimulated microglial M1 activation and promote microglial M2 polarization in vitro (Zhang et al. 2017). As stated above, the switch of the M1/M2 microglial phenotypes has been regarded as a novel therapeutic strategy for neurodegenerative diseases. Therefore, our data reported here provide evidence supporting the potential benefit of MLB in this setting. Further mechanistic study indicated that inhibition of the activated NF-κB signaling pathway by LPS may be an underlying mechanism.
Multiple molecular pathways have been implicated in controlling microglial activation and polarization, of which NF-κB signaling is crucial for regulating the phenotypic balance of microglia (Kopitar-Jerala 2015). Under normal physiological conditions, NF-κB is maintained in a latent form in the cytoplasm of microglia. However, in certain pathological situations, NF-κB is activated by exposure to a variety of inflammatory stimuli (e.g., LPS or the aggregated misfolded proteins seen in various neurodegenerative diseases) through degradation of the inhibitor IκBα by phosphorylated IKK (Ma and Hottiger 2016). The activated NF-κB translocates to the nucleus and binds to κB sites, consequently activating transcription of NF-κB-dependent genes for microglial function, reactivity, and phenotypic switch (Brown and Vilalta 2015). Therefore, targeting NF-κB activity in microglia has been regarded as a potential approach for modulating microglia-dependent neuroinflammation (Mattson 2005). For MLB, previous studies with different in vitro models have demonstrated that its anti-inflammatory and anti-oxidative effects are mediated, at least in part, by inhibition of NF-κB signaling pathways (Jiang et al. 2015; Jung et al. 2014; Quan et al. 2013). In line with these findings, in our study, MLB counteracted LPS-induced activation of the NF-κB pathway, manifested by the reduced nuclear translocation of the p65 subunit and the decreased expression of phosphorylated IKKα and IκBα when BV2 cells were pre-incubated with MLB prior to LPS treatment. Nevertheless, according to recent literature, other signaling pathways, such as PI3K/Akt/GSK-3β and miR-107/glutamate transporter 1, are documented to be involved in the neuroprotective effects of MLB (Xiao et al. 2013; Yang et al. 2015). We still do not know whether these pathways may be linked to the activity of MLB in influencing the activation and polarization of microglial cells, and thus, additional studies are required to elucidate this issue.
In the in vivo experiment portion of this study, we observed that the administration of MLB could ameliorate LPS-induced neural loss in the hippocampus of adult mice. This effect may, at least in part, be related to the inhibition of microglial activation upon MLM treatment, as evidenced by the decreased expression of microglial activation marker CD68 in the hippocampus of MLB-treated animals. These results provided basic evidence for the potential benefits of MLB against neurodegenerative diseases. Nevertheless, several limitations should be addressed here: (1) We did not detect the expression of M2 microglia markers, so we do not know whether MLB could improve the M1/M2 microglia balance towards a less pro-inflammatory state; (2) LPS-induced neural loss cannot fully mimic the pathological events/conditions found in various neurodegenerative diseases; thus, the therapeutic effects of MLB should be further evaluated in other animal models; and (3) the timing, stage, and severity of neurodegenerative diseases are critically associated with microglial activation, polarization, and cytotoxicity (Tang and Le 2016); so far, we still do not know the relationship and impacts of these factors with respect to the therapeutic effects of MLB, which will require further studies to be clarified.
In summary, this study suggests that MLB not only suppresses LPS-induced neuroinflammation and microglial activation in BV2 cells, but also attenuates neurodegeneration in LPS-injected mice. Mechanistically, the effects of MLB may be mediated by the NK-κB pathway. These findings support the therapeutic perspective of MLB as a novel anti-inflammatory and microglia-modulating drug for neurodegenerative diseases.
References
Abmayr SM, Yao T, Parmely T, and Workman JL (2006) Preparation of nuclear and cytoplasmic extracts from mammalian cells. Curr Protoc Mol Biol Chapter 12 Unit 12:11.
Badshah H, Ali T, Kim MO (2016) Osmotin attenuates LPS-induced neuroinflammation and memory impairments via the TLR4/NFkappaB signaling pathway. Sci Rep 6(1):24493. https://doi.org/10.1038/srep24493
Benarroch EE (2013) Microglia: multiple roles in surveillance, circuit shaping, and response to injury. Neurology 81(12):1079–1088. https://doi.org/10.1212/WNL.0b013e3182a4a577
Boche D, Perry VH, Nicoll JA (2013) Review: activation patterns of microglia and their identification in the human brain. Neuropathol Appl Neurobiol 39(1):3–18. https://doi.org/10.1111/nan.12011
Bozic I, Savic D, Laketa D, Bjelobaba I, Milenkovic I, Pekovic S, Nedeljkovic N, Lavrnja I (2015) Benfotiamine attenuates inflammatory response in LPS stimulated BV-2 microglia. PLoS One 10(2):e0118372. https://doi.org/10.1371/journal.pone.0118372
Brown GC, Vilalta A (2015) How microglia kill neurons. Brain Res 1628(Pt B):288–297
Choi DK, Koppula S, Suk K (2011) Inhibitors of microglial neurotoxicity: focus on natural products. Molecules 16(2):1021–1043. https://doi.org/10.3390/molecules16021021
Dai XJ, Li N, Yu L, Chen ZY, Hua R, Qin X et al (2015) Activation of BV2 microglia by lipopolysaccharide triggers an inflammatory reaction in PC12 cell apoptosis through a toll-like receptor 4-dependent pathway. Cell Stress Chaperones 20(2):321–331. https://doi.org/10.1007/s12192-014-0552-1
Dutta G, Zhang P, Liu B (2008) The lipopolysaccharide Parkinson’s disease animal model: mechanistic studies and drug discovery. Fundam Clin Pharmacol 22(5):453–464. https://doi.org/10.1111/j.1472-8206.2008.00616.x
Gao F, Ding B, Zhou L, Gao X, Guo H, Xu H (2013) Magnesium sulfate provides neuroprotection in lipopolysaccharide-activated primary microglia by inhibiting NF-kappaB pathway. J Surg Res 184(2):944–950. https://doi.org/10.1016/j.jss.2013.03.034
H EH, Noristani HN, Perrin FE (2017) Microglia responses in acute and chronic neurological diseases: what microglia-specific transcriptomic studies taught (and did not teach) us. Front Aging Neurosci 9:227
Jiang F, Mao Y, Liu H, Xu P, Zhang L, Qian X, Sun X (2015) Magnesium lithospermate B protects neurons against amyloid beta (1-42)-induced neurotoxicity through the NF-kappaB pathway. Neurochem Res 40(9):1954–1965. https://doi.org/10.1007/s11064-015-1691-1
Jung YR, Kim DH, Kim SR, An HJ, Lee EK, Tanaka T, Kim ND, Yokozawa T, Park JN, Chung HY (2014) Anti-wrinkle effect of magnesium lithospermate B from Salvia miltiorrhiza BUNGE: inhibition of MMPs via NF-kB signaling. PLoS One 9(8):e102689. https://doi.org/10.1371/journal.pone.0102689
Kaneko YS, Ota A, Nakashima A, Nagasaki H, Kodani Y, Mori K, Nagatsu T (2015) Lipopolysaccharide treatment arrests the cell cycle of BV-2 microglial cells in G(1) phase and protects them from UV light-induced apoptosis. J Neural Transm (Vienna) 122(2):187–199. https://doi.org/10.1007/s00702-014-1256-5
Kopitar-Jerala N (2015) Innate immune response in brain, NF-kappa B signaling and cystatins. Front Mol Neurosci 8:73. https://doi.org/10.3389/fnmol.2015.00073
Lee M, Jantaratnotai N, McGeer E, McLarnon JG, McGeer PL (2011) Mg2+ ions reduce microglial and THP-1 cell neurotoxicity by inhibiting Ca2+ entry through purinergic channels. Brain Res 1369:21–35. https://doi.org/10.1016/j.brainres.2010.10.084
Lim JY, Sul D, Hwang BY, Hwang KW, Yoo KY, Park SY (2013) Suppression of LPS-induced inflammatory responses by inflexanin B in BV2 microglial cells. Can J Physiol Pharmacol 91(2):141–148. https://doi.org/10.1139/cjpp-2012-0242
Lynch MA (2009) The multifaceted profile of activated microglia. Mol Neurobiol 40(2):139–156. https://doi.org/10.1007/s12035-009-8077-9
Ma B, Hottiger MO (2016) Crosstalk between Wnt/beta-catenin and NF-kappaB signaling pathway during inflammation. Front Immunol 7378
Mattson MP (2005) NF-kappaB in the survival and plasticity of neurons. Neurochem Res 30(6–7):883–893. https://doi.org/10.1007/s11064-005-6961-x
Orihuela R, McPherson CA, Harry GJ (2016) Microglial M1/M2 polarization and metabolic states. Br J Pharmacol 173(4):649–665. https://doi.org/10.1111/bph.13139
Popiolek-Barczyk K, Mika J (2016) Targeting the microglial signaling pathways: new insights in the modulation of neuropathic pain. Curr Med Chem 23(26):2908–2928. https://doi.org/10.2174/0929867323666160607120124
Quan W, Wei G, Zhou D, Zhu Y, Guo C, Wang Y, Weng Y, Xi M, Wen A (2013) Magnesium lithospermate B reduces myocardial ischemia/reperfusion injury in rats via regulating the inflammation response. Pharm Biol 51(11):1355–1362. https://doi.org/10.3109/13880209.2013.791324
Ramirez AI, de Hoz R, Salobrar-Garcia E, Salazar JJ, Rojas B, Ajoy D, López-Cuenca I, Rojas P, Triviño A, Ramírez JM (2017) The role of microglia in retinal neurodegeneration: Alzheimer’s disease, Parkinson, and glaucoma. Front Aging Neurosci 9:214. https://doi.org/10.3389/fnagi.2017.00214
Salter MW, Stevens B (2017) Microglia emerge as central players in brain disease. Nat Med 23(9):1018–1027. https://doi.org/10.1038/nm.4397
Schmittgen TD, Livak KJ (2008) Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc 3(6):1101–1108. https://doi.org/10.1038/nprot.2008.73
Song GJ, Suk K (2017) Pharmacological modulation of functional phenotypes of microglia in neurodegenerative diseases. Front Aging Neurosci 9:139. https://doi.org/10.3389/fnagi.2017.00139
Song W, Lu X, Feng Q (2000) Tumor necrosis factor-alpha induces apoptosis via inducible nitric oxide synthase in neonatal mouse cardiomyocytes. Cardiovasc Res 45(3):595–602. https://doi.org/10.1016/S0008-6363(99)00395-8
Suk K, Ock J (2012) Chemical genetics of neuroinflammation: natural and synthetic compounds as microglial inhibitors. Inflammopharmacology 20(3):151–158. https://doi.org/10.1007/s10787-011-0108-2
Tang Y, Le W (2016) Differential roles of M1 and M2 microglia in neurodegenerative diseases. Mol Neurobiol 53(2):1181–1194. https://doi.org/10.1007/s12035-014-9070-5
Varnum MM, Ikezu T (2012) The classification of microglial activation phenotypes on neurodegeneration and regeneration in Alzheimer’s disease brain. Arch Immunol Ther Exp 60(4):251–266. https://doi.org/10.1007/s00005-012-0181-2
Wang SX, LM H, Gao XM, Guo H, Fan GW (2010) Anti-inflammatory activity of salvianolic acid B in microglia contributes to its neuroprotective effect. Neurochem Res 35(7):1029–1037. https://doi.org/10.1007/s11064-010-0151-1
Xiang X, Poliakov A, Liu C, Liu Y, Deng ZB, Wang J et al (2009) Induction of myeloid-derived suppressor cells by tumor exosomes. Int J Cancer 124(11):2621–2633. https://doi.org/10.1002/ijc.24249
Xiao G, Hu W, Chen X (2013) Magnesium lithospermate B protects neurons from N-methyl-D-aspartic acid injury and attenuates kainic acid-induced neurodegeration in FVB mice. J Mol Neurosci 51(2):550–557. https://doi.org/10.1007/s12031-013-0023-7
Xu L, He D, Bai Y (2016) Microglia-mediated inflammation and neurodegenerative disease. Mol Neurobiol 53(10):6709–6715. https://doi.org/10.1007/s12035-015-9593-4
Yang ZB, Luo XJ, Ren KD, Peng JJ, Tan B, Liu B, Lou Z, Xiong XM, Zhang XJ, Ren X, Peng J (2015) Beneficial effect of magnesium lithospermate B on cerebral ischemia-reperfusion injury in rats involves the regulation of miR-107/glutamate transporter 1 pathway. Eur J Pharmacol 766:91–98. https://doi.org/10.1016/j.ejphar.2015.09.042
Yu HM, Zhao YM, Luo XG, Feng Y, Ren Y, Shang H et al (2012) Repeated lipopolysaccharide stimulation induces cellular senescence in BV2 cells. Neuroimmunomodulation 19(2):131–136
Zhang J, Xie X, Tang M, Zhang J, Zhang B, Zhao Q et al (2017) Salvianolic acid B promotes microglial M2-polarization and rescues neurogenesis in stress-exposed mice. Brain Behav Immun pii S0889-1591(17):30219–30212
Acknowledgements
All authors thank Dr. Jeffery Zheng, Aardvark Therapeutics Inc., and Ms. Rebekah A. Burdyshaw, Cleveland State University, for their critical proofreading of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The animal study protocol was approved by the animal ethics committee of the corresponding author’s institution. All animal care and use were performed in accordance with institutional guidelines.
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Tai, Y., Qiu, Y. & Bao, Z. Magnesium Lithospermate B Suppresses Lipopolysaccharide-Induced Neuroinflammation in BV2 Microglial Cells and Attenuates Neurodegeneration in Lipopolysaccharide-Injected Mice. J Mol Neurosci 64, 80–92 (2018). https://doi.org/10.1007/s12031-017-1007-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12031-017-1007-9