Abstract
l-Glutamate plays a crucial role in neuronal cell death, which is known to be associated with various neurodegenerative diseases, such as Alzheimer’s, Parkinson’s, and Huntington’s diseases. In this study, we investigated the protective effects of biochanin A, a phytoestrogen compound found mainly in Trifolium pratense, against l-glutamate-induced cytotoxicity in a PC12 cell line. Exposure of the cells to 10 mM l-glutamate was found to significantly increase cell viability loss and apoptosis, whereas pretreatment with various concentrations of biochanin A attenuated the cytotoxic effects of l-glutamate. Specifically, the pretreatment led to not only decreases in the release of lactate dehydrogenase, the number of apoptotic cells, and the activity of caspase-3 but also an increase in the total glutathione level in the l-glutamate-treated PC12 cells. These results indicate that biochanin A may be able to exert neuroprotective effects against l-glutamate-induced cytotoxicity. Furthermore, our findings also imply that biochanin A may act as an antiapoptotic agent in order to perform its protective function.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Human neurodegenerative disease is a general term used to describe a wide range of conditions, for example, Alzheimer’s disease (AD) and Parkinson’s disease, wherein the primary neurons in the brain are affected. To date, the etiological factors of neurodegenerative diseases remain poorly understood. People with decreased estradiol levels, especially postmenopausal women, have been suggested to be more prone to such diseases [1]. A substantial number of studies have also reported that estrogen therapy can not only reduce the risk of AD but also enhance the memory of postmenopausal women with AD [2, 3]. Moreover, estrogen has been shown to attenuate cell death caused by various neurotoxins in vitro [4]. However, long-term hormone replacement therapy (HRT) may result in increased risks of breast cancer and various other cancers [5].
Glutamate, one of the primary neurotransmitters in the central nervous system (CNS), is an amino acid that mediates excitatory synaptic responses and neuronal development via glutamate receptor activation [6, 7]. However, excessive amounts of extracellular glutamate in the brain can lead to the possibility of acute neural cell damage. In fact, glutamate-induced neuronal cell death is thought to be closely associated with various CNS disorders, including cerebral ischemia, hypoxia, alcoholism, autoimmune encephalomyelitis, as well as Alzheimer’s, Huntington’s, and Parkinson’s diseases [8–10].
Two different mechanisms have been proposed for glutamate-induced neuronal cell death. The first mechanism proposed is that the excitotoxicity of glutamate is mediated by 3 types of excitatory amino acid receptors [11], resulting in a massive influx of extracellular Ca2+ [12]. The second possible mechanism is that glutamate-induced oxytosis, caused by competitive inhibition of cystine uptake [13, 14], results in decreased antioxidant defense against oxidative stress due to a marked decrease in the cellular glutathione (GSH) level and the activation of calcium-dependent enzymes [15]. The combined effects of both oxidative stress and intracellular Ca2+ levels have been suggested to cause apoptosis and necrosis in the neuronal cells [16].
Recently, much more attention has been paid toward discovering natural resources for human health research. Studies are currently underway on finding alternatives to replace HRT due to its various adverse effects. One alternative, for example, is based on the studies of phytoestrogen compounds and their neuroprotective abilities. Biochanin A, an O-methylated isoflavone, is a natural organic compound classified under the phytoestrogen group. It can be found predominantly in legume plants such as Trifolium pratense and soy. A number of studies have shown that biochanin A could provide beneficial effects for human health, including prevention of cancers, heart disease, menopausal symptoms, and osteoporosis [17]. However, little is known about the effect of this isoflavone compound on the CNS. Therefore, in the current study, we investigated the neuroprotective effects of biochanin A and found that it could reduce l-glutamate cytotoxicity by inhibiting apoptosis in a PC12 rat adrenal pheochromocytoma cell line.
Materials and Methods
Cell Culture and Reagents
PC12 cells were purchased from American Type Culture Collection (CRL-1721; Manassas, VA, USA) and maintained in Dulbecco’s modified Eagle medium (Hyclone, Utah, USA), supplemented with 10 % (v/v) of heat-inactivated fetal bovine serum as well as 1 % (v/v) of both penicillin and streptomycin (i-DNA Biotechnology, Singapore). The cells were grown in an incubator with optimal culture conditions of 37 °C and 5 % CO2, and the medium was routinely replaced every 2–3 days. Biochanin A, Hoechst 33342 (purity ≥98 %, by HPLC and TLC), and l-glutamate (purity ≥99 %, by TLC) were purchased from Sigma Aldrich (Saint Louis, MO, USA). MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) and DMSO (dimethyl sulfoxide) were purchased from Amresco (Solon, OH, USA).
l-Glutamate and Biochanin A Stock Preparation
l-Glutamate (1 M) was prepared in deionized water and stored at −20 °C. Biochanin A (100 mM) dissolved in DMSO was kept at room temperature. Solutions with desired concentrations were freshly prepared immediately before use from the stock solutions of l-glutamate and biochanin A.
Cell Viability Assay
PC12 cell viability after various treatments was assessed by the MTT assay. PC12 cells (1 × 105 cells/ml) were seeded into a collagen type I-precoated 96-well plate and grown in the incubator overnight. Cells pretreated for 2 h with different concentrations of biochanin A (1, 10, 50, and 100 μM) were exposed to l-glutamate (final concentration of 10 mM) for another 24 h. 10 mM of l-glutamate, based on our preliminary data of glutamate cytotoxicity test (Data not shown),was selected to induce the cytotoxicity in PC12 cells. Following the treatment, 20 μl of MTT (5 mg/ml) was added into each well. After 3 h of incubation at 37 °C, the medium containing MTT was slowly removed, and 100 μl of DMSO was added into each well to dissolve the purple MTT formazan. Absorbances were read at a wavelength of 570 nm by a microplate reader, and cell viability was expressed as the percentage of viable cells in the treated groups compared to that in the untreated control group.
Lactate Dehydrogenase Release Assay
PC12 cells (2 × 105 cells/ml) seeded into a collagen type I-precoated 96-well culture plate were subjected to various treatments as described in the MTT cell viability assay. Lactate dehydrogenase (LDH) leakage into the media, an indicator of cell injury, was detected using the lactate dehydrogenase activity assay kit (BioVision, California, USA), according to the manufacturer’s protocols. Briefly, the media from PC12 cells was collected and centrifuged at 10,000×g; 50 μl of the supernatant was then mixed with an equal volume of reaction mixture to initiate the LDH reaction. The absorbance of each sample was read at 450 nm by a microplate reader. The data were normalized to the LDH release activity of the control group.
Total GSH Assay
The total GSH (GSH/GSSG) level was determined using the ApoGSH™ Glutathione Colorimetric Detection Kit (BioVision, California, USA), according to the manufacturer’s protocols. Briefly, PC12 cells (3 × 105 cells/ml) were seeded into a collagen type I-precoated 12-well plate and were treated with biochanin A and l-glutamate as described earlier. The cells were collected, washed once with ice-cold PBS, and centrifuged at 700×g for 5 min. The supernatant was discarded, and the cell pellet was resuspended in ice-cold GSH buffer for 10 min; 5 % sulfosalicylic acid was then added to the lysed cells, which was followed by centrifugation at 8,000×g for 10 min. Twenty microliters of the supernatant was subsequently mixed with a reaction mixture consisting of NADPH-generating mix, GSH reductase, and GSH reaction buffer. The absorbance of each sample was read at 405 nm by a microplate reader. Data were normalized to the total GSH level detected in the control group.
Flow Cytometry Analysis
Apoptosis rate was measured by using the Fluorescein Isothiocyanate (FITC)-Annexin V Apoptosis Detection Kit (Becton, Dickinson and Company, NJ, USA), according to the manufacturer’s protocol. Briefly, PC12 cells (1 × 106 cells/ml) treated with l-glutamate and/or biochanin A were harvested by centrifugation, washed twice with cold PBS, and then resuspended in binding buffer at the original cell density. Subsequently, 5 μl each of FITC-Annexin V and propidium iodide (PI) were added to the cells, which were then incubated for 15 min in the dark. The apoptosis levels of the stained cells were then quantitatively analyzed by flow cytometry (BD FACSCalibur, California, USA).
Hoechst 33342 Staining Assay
The morphological features of apoptotic cells were observed using the Hoechst 33342 fluorescent dye. The treated PC12 cells were fixed with 4 % paraformaldehyde, stained with 10 μg/ml of Hoechst 33342 for 15 min, and then visualized using a fluorescence microscope (Leica, IL, USA).
Caspase-3 Activity Measurement
Caspase-3 activity was measured using the Caspase-3 Colorimetric Activity Assay Kit (Millipore, Billerica, MA, USA), following the manufacturer’s protocol. Briefly, PC12 cells (1 × 106 cells/ml) were exposed to l-glutamate, with or without biochanin A pretreatment. The cells were then harvested, washed with PBS, and incubated on ice for 10 min with the cell lysis buffer provided. The lysed cells were centrifuged and the supernatant was collected. A reaction mixture (100 μl in assay buffer) containing 40 μl of the supernatant and 10 μl of caspase-3 substrate (Ac-DEVD-pNA; final concentration of 0.3 mg/ml) was added into a 96-well plate and incubated at 37 °C for 1 h. The absorbance of each sample was measured at 405 nm by a microplate reader. Data were expressed as the percentage of caspase-3 activity in the treated groups relative to that in the untreated control group.
Statistical Analysis
The data were expressed as mean ± SEM. values and evaluated by one-way ANOVA followed by Fisher’s Least Significant Difference (LSD) test as a post hoc test. P values less than 0.05 were considered statistically significant.
Results
Effects of Biochanin A on Glutamate-Induced Cytotoxicity in PC12 Cells
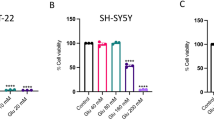
Glutamate-induced cytotoxicity was evaluated by the MTT cell viability and LDH release assays. Our preliminary data indicated that biochanin A at a high concentration (100 μM) did not have any toxic effect on the PC12 cells (Data not shown). On the other hand, as shown in Fig. 1a, the cells that were exposed to 10 mM l-glutamate showed a significant decrease in cell viability (66.5 ± 0.48 %, relative to the untreated control group). However, pretreatment with biochanin A (1, 10, 50, and 100 μM) for 2 h before l-glutamate exposure restored cell viability in a dose-dependent manner (72.9 ± 2.21 %, 80.1± 0.55 %, 85.9± 1.50 %, and 92.7± 1.88 %, respectively). To further quantify the protection provided by biochanin A against the l-glutamate-induce cytotoxicity, the LDH leakage into the medium was measured and compared to that for the control group. A similar pattern of results was obtained in both cases (Fig. 1b). The l-glutamate-treated PC12 cells released more LDH into the surroundings than the control untreated cells did (215.96± 18.29 %, relative to the control group), whereas the cells pretreated with biochanin A (1, 10, 50, and 100 μM) released lesser LDH (176.99± 16.91 %, 167.27± 12.95 %, 150.34± 7.49 %, and 106.23± 4.43 %, respectively).
Effects of biochanin A on l-glutamate-induced cytotoxicity and LDH leakage. a Cell viability, as assessed by the MTT assay. The PC12 cells were exposed to 10 mM l-glutamate for 24 h in the presence or absence of biochanin A (1, 10, 50, and 100 μM). b The PC12 cells were either untreated (control) or treated with 10 mM l-glutamate, with or without biochanin A. After 24 h, the release of LDH into extracellular surroundings was measured by using the LDH assay. Each value represents the mean ± SEM. value from 4 independent experiments. *P < 0.05 and **P < 0.01, compared to the group treated with l-glutamate alone; ## P < 0.01, compared to the control group
Effects of Biochanin A on Total GSH Levels in PC12 Cells
As shown in Fig. 2, when the cells were exposed to 10 mM l-glutamate alone, the total GSH level was 9.17± 3.69 % relative to the control group. In contrast, pretreatment for 2 h with biochanin A (1, 10, 50, and 100 μM) before l-glutamate exposure could significantly increase the levels in a dose-dependent manner (40.76 ± 8.45 %, 74.31 ± 11.15 %, 84.19 ± 9.28 %, and 86.78 ± 8.59 %, respectively).
Effects of biochanin A on l-glutamate-induced depletion of cellular glutathione. The PC12 cells cultured for 24 h were exposed to 10 mM l-glutamate in the presence or absence of biochanin A (1, 10, 50, and 100 μM). The intracellular glutathione levels were measured as described in Materials and Methods and compared to those in the control cells. Each value represents the mean ± SEM. value from 4 independent experiments. *P < 0.05 and **P < 0.01, compared to the group treated with l-glutamate alone; ## P < 0.01, compared to the control group
Effects of Biochanin A on Apoptosis in PC12 Cells
The apoptosis level was assessed by flow cytometry based on the detection of Annexin V and PI intake into the PC12 cells. As shown in Fig. 3, the untreated cells showed very little apoptosis (4.28 ± 0.37 %), whereas the level increased (33.02 ± 1.22 %) when the cells were treated with 10 mM l-glutamate alone. Pretreatment with 50 μM of biochanin A, the lowest concentration more effective than EC50 in our treatments, before l-glutamate exposure was found to significantly decrease apoptosis (14.22 ± 1.06 %). To further justify our findings, Hoechst 33342 staining was performed on these cells. Typical apoptotic characteristics, such as cell shrinkage, nuclear condensation, and fragmented fluorescent nuclei, were identified in the glutamate-treated cells (Fig. 4).
Protective effects of biochanin A against l-glutamate-induced apoptosis. The PC12 cells cultured for 24 h were either untreated (control) or were treated with 10 mM l-glutamate, with or without biochanin A. The cells were harvested and stained with a combination of annexin V-FITC and PI. The data shown are representative flow cytometry quadrant plots. a Control cells, b 10 mM l-glutamate treatment alone, c 50 μM biochanin A with 10 mM l-glutamate, and d live and apoptotic cells. All the data are presented as mean ± SEM. values from 4 independent experiments. **P < 0.01, compared to the l-glutamate-treated group; ## P < 0.01, compared to the control group
Effects of biochanin A on l-glutamate-induced nuclear condensation. The PC12 cells were treated with 10 mM l-glutamate for 24 h in the presence or absence of biochanin A (50 μM). The cell nuclei were stained with Hoechst 33342 and observed by fluorescence microscopy. a Control cells, b cells treated with l-glutamate alone, and c cells treated with both l-glutamate and biochanin A (50 μM). The arrows indicate the apoptotic cells
Effects of Biochanin A on the Caspase-3 Activity of the Treated PC12 Cells
The activity of caspase-3, an apoptosis marker, increased in 10 mM l-glutamate-treated PC12 cells (Fig. 5; 12.17 ± 0.27 μmol Pna min−1 ml−1) and was more than that in the untreated control group (4.75 ± 0.21 μmol pNA min−1 ml−1). On the other hand, pretreatment with increasing concentrations of biochanin A (1, 10, 50, and 100 μM) could reduce the caspase-3 activity in a dose-dependent manner (11.03 ± 0.08, 9.92 ± 0.28, 8.81 ± 0.65, and 8.32 ± 0.44 μmol pNA min−1 ml−1, respectively).
Effects of biochanin A against l-glutamate-induced caspase-3 activity. The PC12 cells cultured for 24 h were either untreated (control) or were treated with 10 mM l-glutamate, with or without biochanin A (1, 10, 50, and 100 μM). The cells were harvested and subjected to the caspase-3 activity assay. All the data are presented as the mean ± SEM. values from 3 independent experiments. *P < 0.05 and **P < 0.01, compared to the group treated with l-glutamate alone; ## P < 0.01, compared to the control group
Discussion
Patients with various pathological disorders such as Parkinson’s disease, AD, and stroke exhibit elevated l-glutamate levels [18]. While not many studies have explored the benefits of biochanin A in the case of the CNS, a previous report demonstrated that biochanin A protects dopaminergic neurons against lipopolysaccharide-induced damage through inhibition of microglial activation and proinflammatory factor generation [17]. Therefore, we believe that there is still much more to be discovered about this phytoestrogen compound and its potential effects. In the current study, we investigated the protective effects of biochanin A against l-glutamate-induced cell injury and apoptosis in PC12 cells. Our results showed that pretreatment with biochanin A not only inhibited LDH release and caspase-3 activity but also increased intracellular GSH levels, all in a dose-dependent manner, supporting the theory that l-glutamate-induced cytotoxicity could be inhibited by biochanin A.
Walton and Dodd [19] reported that glutamate is the major excitatory neurotransmitter in our nervous system and plays an important role in synaptic transmission, formation of the neuronal circuit, and neuronal development. High glutamate levels due to excessive glutamate release or uptake disorder could cause extensive neuronal damage and cell loss in the brain tissue. Our results showed that exposure of the PC12 cells to l-glutamate led to increases in the amount of LDH release and also the number of apoptotic cells. To confirm whether the morphological characteristics of apoptosis were present in the l-glutamate-treated cells, Hoechst staining was performed. Microscopic examination demonstrated the presence of Hoechst-stained positive cells with apoptotic-like structures such as condensed nuclei and cell shrinkage. These observations indicate that l-glutamate can induce cytotoxicity and thereby cause cell injury and apoptotic cell death. In addition, our data showed that biochanin A inhibited the glutamate-induced apoptosis in the PC12 cells in contrast with inducing apoptosis in some cancer cells. However, both longer treatment time and higher concentration of biochanin A were required for apoptosis induction in the cancer cells [20, 21].
Oxidative stress, defined as a disturbance between the antioxidant defense systems and the production of reactive oxygen species (ROS), may contribute to neuronal injury induced by glutamate [22, 23]. Previous in vitro and in vivo studies have reported that glutamate treatment caused a significant increase in the ROS level [22, 23] and that glutamate may be involved in the apoptotic mechanism [24], which could contribute to various neurodegenerative diseases. Glutamate-induced disruption of antioxidant defense systems by ROS can cause mismatched redox equilibrium, which in turn leads to neuronal disorders [25, 26]. To counteract this particular disruption, cells activate antioxidant defense systems to maintain balance in ROS production and oxidative conditions. Intracellular GSH, considered as a vital antioxidant defense system in the cell, plays an important role in antioxidant defense and redox regulation. It was reported that when GSH is exhausted, the ROS level increases and the redox balance is disturbed [27].
Although the PC12 cells do express NMDA receptors, the cytotoxicity caused by l-glutamate cannot be solely attributed to the presence of these receptors [28]. Apart from the activation of glutamate receptors [13], inhibition of cystine uptake that leads to decreased intracellular GSH levels can also contribute to l-glutamate cytotoxicity [28]. Since several researchers have reported that elevation of intracellular GSH levels can rescue neuronal cells from glutamate-induced cell death [29], we decided to examine the GSH levels in our treated cells. Consistent with the previously published data, our results showed that l-glutamate could rapidly deplete the intracellular GSH level, preceding the actual onset of cell death [28]. However, preincubation of the PC12 cells with biochanin A attenuated the effects of l-glutamate by restoring the intracellular GSH level, suggesting that the neuroprotective effects of biochanin A may be attributed to its antioxidant ability.
Several studies have also reported that a mitochondrion-dependent pathway may be involved in glutamate-induced apoptosis [30–32]. The ratio of the proapoptotic and antiapoptotic proteins of the Bcl-2 family is the main key factor that controls the activation of various caspases and therefore regulates the entire apoptosis cascade process [32, 33]. Caspases, the molecular machinery that drives apoptosis, are responsible for the morphological and biochemical characteristics of apoptotic cells [34]. Caspase-3 is one of the main executioner caspases and plays a vital role in activating the apoptosis process. Since l-glutamate exposure could lead to apoptosis activation, we also determined the caspase-3 activity in the current study. As expected, our results showed that pretreatment of the PC12 cells with biochanin A reduced the caspase-3 activity to a level lower than that observed in the l-glutamate-treated cells.
In summary, to our knowledge, our study is the first to show that biochanin A can provide protection against glutamate-induced apoptotic cell death in PC12 cells. The protective effects of biochanin A may be mediated by antioxidant recovery and apoptosis inhibition. However, more studies are required to determine whether the inhibition of apoptosis by biochanin A involves extrinsic (death ligand) or intrinsic (mitochondrial) pathways. Considering these protective effects, biochanin A may be a potential neuroprotective compound for treating neurodegenerative disorders such as Alzheimer’s and Parkinson’s diseases, where apoptosis and oxidative stress (depleted GSH levels) seem to occur in certain regions of the brain. However, further studies are required to determine explicit neuroprotective mechanism and in vivo animal model study before definite conclusions can be drawn.
References
Coffey CE, Lucke JF, Saxton JA, Ratcliff G, Unitas LJ, Billig B, Bryan RN (1998) Sex differences in brain aging: a quantitative magnetic resonance imaging study. Arch Neurol 55:169–179
Asthana S, Baker LD, Craft S, Stanczyk FZ, Veith RC, Raskind MA, Plymate SR (2001) High-dose estradiol improves cognition for women with AD: results of a randomized study. Neurology 57(4):605–612
Llera DA, Ferreiro EA, Chowena JA, Argente J, Jim′enez LP, Frago LM, Barrios V (2007) 17β-Estradiol protects depletion of rat temporal cortex somatostatinergic system by β-amyloid. Neurobiol Aging 28:1396–1409
Bhavnani BR, Berco M, Binkley J (2003) Equine estrogens differentially prevent cell death induced by glutamate. J Soc Gynecol Investig 10:302–308
Mueck AO, Seeger H, Lippert TH (2002) Estradiol metabolism and malignant disease-review. Maturitas 43:1–10
Jin R, Horning M, Mayer ML, Gouaux E (2002) Mechanism of activation and selectivity in a ligand-gated ion channel: structural and functional studies of GluR2 and quisqualate. Biochemistry 41:15635–15643
Molnar E, Isaac JT (2002) Developmental and activity dependent regulation of ionotropic glutamate receptors at synapses. The Scientific World J 2:27–47
van Os S, Ruitenbeek W, Hopman J, van de Bor M (2006) Excitatory amino acid release and electrocortical brain activity after hypoxemia in near-term lambs. Brain Dev 28:380–388
Camins A, Pallas M, Silvestre JS (2008) Apoptotic mechanisms involved in neurodegenerative diseases: experimental and therapeutic approaches. Methods Find Exp Clin Pharmacol 30:43–65
Benveniste H (2009) Glutamate, microdialysis, and cerebral ischemia: lost in translation? Anesthesiology 110:422–425
Monaghan DT, Bridges RJ, Cotman CW (1989) The excitatory amino acid receptors: their classes, pharmacology, and distinct properties in the function of the central nervous system. Annu Rev Pharmacol Toxicol 29:365–402
Bleich S, Romer K, Wiltfang J, Kornhuber J (2003) Glutamate and the glutamate receptor system: a target for drug action. Int J Geriatr Psych 18:S33–S40
Choi DW (1988) Glutamate neurotoxicity and diseases of the nervous system. Neuron 1:623–634
Murphy TH, Miyamoto M, Sastre A, Schnaar RL, Coyle JT (1989) Glutamate toxicity in a neuronal cell line involves inhibition of cystine transport leading to oxidative stress. Neuron 2:1547–1558
Zablocka A, Janusz M (2008) The two faces of reactive oxygen species. Postep Hig Med Dosw 62:118–124
Tan S, Schubert D, Maher P (2001) Oxytosis: a novel form of programmed cell death. Curr Top Med Chem 1:497–506
Chen HQ, Jin ZY, Li GH (2007) Biochanin A protects dopaminergic neurons against lipopolysaccharide-induced damage through inhibition of microglia activation and proinflammatory factors generation. Neurosci Lett 417:112–117
Beal MF (1992) Mechanisms of excitotoxicity in neurologic diseases. FASEB J 6:3338–3344
Walton HS, Dodd PR (2007) Glutamate-glutamine cycling in Alzheimer’s disease. Neurochem Int 50:1052–1066
Su SJ, Chow NH, Kung ML, Hung TC, Chang KL (2003) Effects of soy isoflavones on apoptosis induction and G2-M arrest in human hepatoma cells involvement of Caspase-3 activation, Bcl-2 and Bcl-XL downregulation, and Cdc2 kinase activity. Nutr Cancer 45(1):113–123
Szliszka E, Czuba ZP, Mertas A, Paradysz A, Krol W. 2011. The dietary isoflavone biochanin-A sensitizes prostate cancer cells to TRAIL-induced apoptosis. Urol Oncol-Semin Ori. http://www.urologiconcology.org/article/S1078-1439(11)00043-3/abstract
Penugonda S, Mare S, Goldstein G, Banks WA, Ercal N (2005) Effects of N-acetylcysteine amide (NACA), a novel thiol antioxidant against glutamate-induced cytotoxicity in neuronal cell line PC12. Brain Res 1056:132–138
Penugonda S, Mare S, Lutz P, Banks WA, Ercal N (2006) Potentiation of lead-induced cell death in PC12 cells by glutamate: protection by N-acetylcysteine amide (NACA), a novel thiol antioxidant. Toxicol Appl Pharmacol 216:197–205
Di Monte D, Sandy MS, Ekstrom G, Smith MT (1986) Comparative studies on the mechanisms of paraquat and 1-methyl-4-phenylpyridine (MPP+) cytotoxicity. Biochem Bioph Res Co 137:303–309
Kume T, Katsuki H, Akaike A (2004) Endogenous factors regulating neuronal death induced by radical stress. Biol Pharm Bull 27:964–967
Parfenova H, Basuroy S, Bhattacharya S, Tcheranova D, Qu Y, Regan RF, Leffler CW (2006) Glutamate induces oxidative stress and apoptosis in cerebral vascular endothelial cells: contributions of HO-1 and HO-2 to cytoprotection. Am J Physiol Cell Physiol 290:C1399–C1410
Mao YR, Jiang L, Duan YL, An LJ, Jiang B (2007) Efficacy of catalpol as protectant against oxidative stress and mitochondrial dysfunction on rotenone-induced toxicity in mice brain. Environ Toxicol Phar 23:314–318
Froissard P, Monrocq H, Duval D (1997) Role of glutathione metabolism in the glutamate-induced programmed cell death of neuronal-like PC12 cells. Eur J Pharmacol 326:93–99
Muller WE, Romero FJ, Perovic S, Pergande G, Pialoglou P (1997) Protection of flupirtine on beta-amyloid-induced apoptosis in neuronal cells in vitro: prevention of amyloid-induced glutathione depletion. J Neurochem 68:2371–2377
Leon R, Wu H, Jin Y, Wei J, Buddhala C, Prentice H, Wu JY (2009) Protective function of taurine in glutamate-induced apoptosis in cultured neurons. J Neurosci Res 87(5):1185–1194
Wang X, Zhu G, Yang S, Wang X, Cheng H, Wang F, Li X, Li Q (2011) Paeonol prevents excitotoxicity in rat pheochromocytoma PC12 cells via downregulation of ERK activation and inhibition of apoptosis. Planta Med 77(15):1695–1701
Ma SW, Liu HX, Jiao HY, Wang LY, Chen LY, Liang J, Zhao M, Zhang XT (2012) Neuroprotective effect of ginkgolide K on glutamate-induced cytotoxicity in PC12 cells via inhibition of ROS generation and Ca2+ influx. Neurotoxicology 33(1):59–69
Cory S, Adams JM (2002) The Bcl-2 family: regulators of the cellular life-or-death switch. Nat Rev Cancer 2:647–656
Liu WB, Zhou J, Qu Y, Li X, Lu CT, Xie KL, Sun XL, Fei Z (2010) Neuroprotective effect of osthole on MPP+-induced cytotoxicity in PC12 cells via inhibition of mitochondrial dysfunction and ROS production. Neurochem Int 57:206–215
Acknowledgments
This research was supported by Research University Grant Scheme (RUGS 05-02-12-1860RU), Universiti Putra Malaysia (UPM). Ji Wei Tan is a recipient of Graduate research fellowship from UPM.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tan, J.W., Tham, C.L., Israf, D.A. et al. Neuroprotective Effects of Biochanin A Against Glutamate-Induced Cytotoxicity in PC12 Cells Via Apoptosis Inhibition. Neurochem Res 38, 512–518 (2013). https://doi.org/10.1007/s11064-012-0943-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-012-0943-6