Abstract
Considering the significance of the gut microbiota on human health, there has been ever-growing research and commercial interest in various aspects of probiotic functional foods and drugs. A probiotic food requires cautious consideration in terms of strain selection, appropriate process and storage conditions, cell viability and functionality, and effective delivery at the targeted site. To address these challenges, several technologies have been explored and some of them have been adopted for industrial applicability. Encapsulation of probiotics has been recognized as an effective way to stabilize them in their dried form. By conferring a physical barrier to protect them from adverse conditions, the encapsulation approach renders direct benefits on stability, delivery, and functionality. Various techniques have been explored to encapsulate probiotics, but it is noteworthy that the encapsulation method itself influences surface morphology, viability, and survivability of probiotics. This review focuses on the need to encapsulate probiotics, trends in various encapsulation techniques, current research and challenges in targeted delivery, the market status of encapsulated probiotics, and future directions. Specific focus has been given on various in vitro methods that have been explored to better understand their delivery and performance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the early twentieth century, Elie Metchnikoff discovered the beneficial gut microbe which normalizes gut health and prolongs life, later it was termed as “probiotic” [1]. Probiotics refer to “live organisms which when administered in adequate amounts confer a health benefit to the host” [2]. Probiotics are health-promoting microorganisms and are also considered next-generation bio-therapeutics in the field of gut microbiomics [3]. Several bacterial species of Lactobacillus, Lactococcus, Bacillus, Streptococcus, Bifidobacterium, Pediococcus, and Propionibacterium are well-known probiotics [4]. Yeasts such as Saccharomyces cerevisiae, S. carisbergensis, and S. boulardii and fungi such as Aspergillus niger and A. oryzae are also considered as probiotics. However, the most common probiotic bacterial genera are Lactobacillus and Bifidobacterium [5]. Until March 2020, the Lactobacillus genus contained 261 genetically diverse species. Recently, it was reclassified into 25 genera by a group of scientists based on whole-genome sequencing [6]. The update of this current taxonomic classification may help in understanding the mechanisms of probiotics for health benefits.
Apart from modulating the gut functionality, probiotics have also been associated with various other health benefits such as brain functioning, boosting immunity, reducing cholesterol, and promoting metabolic homeostasis through their biological mechanisms in the body. Probiotics can produce short-chain fatty acids, vitamins, enzymes, organic acids, and antimicrobial peptides [7]. These compounds are involved in the physiological functions of the body. “Probiotic Supplements Market—Global Outlook and Forecast 2020–2025” reported that the growth in immune health concerns among people leads to an elevation in the market growth of the probiotic supplements during the COVID-19 pandemic [8]. The recent report on “Probiotics Market—Growth, Trends, and Forecast” forecasted the global probiotics market to reach USD 76.85 billion by 2024, registering a CAGR of 8.15% during the forecast period between 2020 and 2025 [9]. The report also indicated that the bacteria market would grow at the fastest CAGR, owing to the growing demands for prominent applications in fortifying foods with probiotics.
The science of probiotics covers aspects from the field of microbiology to food processing and has found applications in various fields such as nutraceuticals and functional foods, therapeutics in dental care, skincare, oncology, gastroenterology, immunology, and psychoneuroendocrinology [10]. In general, probiotics are orally administered and are commercially available in the forms of functional foods, dietary supplements, and drugs (medicinal probiotics). Usually, people prefer food over supplements/medicinal drugs considering the hedonistic aspects of food ingestion [11]. FAO/WHO 2002 reported that the viability of probiotics in food products must be in adequate amounts to confer a health benefit. Also, it has been reported that probiotic foods should have at least 106 CFU/g viability of live microorganisms [12,13,14]. However, the stability of probiotics is the most desirable concern for targeted colon delivery when ingested orally. It is necessary to maintain its viability during gastrointestinal (GI) transit to promote its efficacy. Probiotics require protection against various stress factors during processing, storage, and digestion. Different strains of probiotics show variations in their abilities such as functional properties, stability, and efficacy. The stability of probiotics can be improved by various strategies such as stress adaptations through pre-treatment, mutagenesis, selective pressure treatment, and genetic modifications through omics technologies [15]. However, some of these processes may alter the potential of probiotics, and also genetically modified strains are not well accepted for food applications [16, 17]. Encapsulation is proved to be the best way for the protection of probiotics to ensure their stability without any change in native strain properties.
“Food Encapsulation: Global Market Analysis, Trends, and Forecasts” report has highlighted the encapsulation market outlook (2019–2024) on microencapsulation of probiotics to drive the growth of this sector [18]. The recent report on “Food encapsulation market by shell material, technology, application, method, core phase, and the region” forecasted the food encapsulation market to reach USD 14.1 billion by 2025, registering a CAGR of 7.5% during the forecast period between 2020 and 2025. The functional food segment accounted for the largest market share in 2020, also the probiotics market is projected to record the highest CAGR during the forecast period [19].
Encapsulation of probiotics provides the protection made up of encapsulating materials that stabilize the probiotics during processing, storage, and at the site of action by enhancing stress resistance [20]. Thereby, the encapsulation process also imparts targeted delivery. The formulation is one of the critical considerations for a targeted delivery system and can be developed by proper designing of the process. Many approaches are available for the delivery of probiotics to the lower intestinal tract toward the colon. Lee et al. [21] reviewed various formulation approaches for colon targeted delivery such as polymeric/lipid-coated, pH-controlled, magnetic/enzyme-triggered, and ligand-receptor-based delivery systems. Milk, yogurt, cheese, ice cream, honey, chocolate, and fermented foods (rice/fruit/vegetables) are natural delivery systems of probiotics. Besides, prebiotics are nondigestible food ingredients that support the delivery of probiotics to the colon (as encapsulation agents) and in all cases, nourish probiotic microbes [22, 23]. Natural prebiotics includes raw banana, onion, garlic, sugarcane, artichokes, and the roots of chicory and yacon [24, 25], and are commercially available as resistant starch, inulin, lactulose, lactitol, lactosucrose, fructooligosaccharide (FOS), xylooligosaccharides, and galactooligosaccharides [26, 27]. Probiotics along with prebiotics are termed “synbiotics” that improve the survivability of probiotics, whilst stimulating the growth of specific native strains present in the GI tract through a synergistic approach [28, 29]. Synbiotics in which prebiotics are considered as a substrate is selectively utilized by the co-administered probiotics (synergistic synbiotic) or by the endogenous micro-organisms (complementary synbiotic) [30]. Generally, the viability of the microbes highly depends on various factors such as matrix, storage temperature, moisture, pH, and oxygen level [28, 31, 32]. The encapsulation matrix improves the stress tolerance of probiotics [33]. Thus, the synergistic synbiotic approach ensures the stability of the co-administered probiotics when both are combined in a matrix form. Also, this helps to maintain the potential of probiotics throughout the shelf-life.
Some of the recent reviews have explained the selection of probiotics, factors affecting the viability of probiotics, different encapsulation methods, encapsulating materials, and targeted delivery systems [28, 34,35,36]. There exists a gap particularly in terms of critical issues with non-encapsulated probiotics, the efficacy of encapsulated probiotics, in vitro and in vivo methods to confirm the targeted delivery of probiotics, market status, and feasibility of encapsulated probiotics at the industrial level for commercialization. Thus, the focus of this review is too ambitious to aim for a complete understanding of the need for encapsulating the probiotics, the prominence of the encapsulation process, their impacts on targeted delivery, in vitro and in vivo methods to confirm the targeted action, market status of encapsulated probiotics and economic feasibility for commercialization.
Biological Mechanisms and the Role of Probiotics in Human Health
Probiotics provide various health benefits through their biological mechanisms in the body. The “human microbiome project” has reported that the number of other bacterial cells in the human body is about 10 times the number of human cells [37]. These bacteria include beneficial microbes as well that play a vital role in the sustenance of human health. The exact mechanism of action is not fully known, but probiotic bacteria produce postbiotics such as short-chain fatty acids, enzymes, lactic acid, and also secrete antimicrobial peptides that kill pathogenic bacterial strains [7]. These bacteriocins are considered to be “natural preservatives” [38]. Probiotics have also been associated with nutrients to compete with pathogens, thereby inhibit/block pathogenic bacterial adhesion in the colonic lumen, and thereby improve mucus production, which in turn enhances the intestinal epithelial barrier for stimulation of the immune system [39, 40].
Probiotics reduce toxins through bile salt hydrolase [41] and increase the bioavailability of nutrients in the body through other enzymatic activities [42, 43]. Some probiotics even have the potential to secrete specific anticarcinogenic and antioxidant metabolites that help in disease treatment [44]. Endogenous supplementation of probiotics can help in the replenishment of the gut microbiome after antibiotic treatment. Consumption of probiotics along with antibiotics or after antibiotic treatment can prevent antibiotics-associated diarrhea [45]. Researchers have also found that specific mechanisms involved in probiotics-linked signaling of nerve functions in the central nervous system can promote potential therapeutic actions on neuropsychiatric disorders and stress-related diseases [46, 47].
Apart from enhancing digestive health, probiotics also relate to brain functioning [48,49,50], help to treat irritable bowel syndrome [51], and reduce the level of low-density lipoprotein in the blood [52, 53], prevent vaginal and urinary tract infections (yeast/ bacterial) in women [54,55,56], prevent pancreatitis and improve pancreatic health [57], contribute to respiratory tract health [58,59,60], inhibit tumorigenesis [61], regulate immune responses [7, 62], and contribute to metabolic homeostasis [63, 64]. Moreover, probiotics aid in the treatment of metabolic disorders such as diabetes, non-alcoholic fatty liver disease and cardiovascular diseases [65,66,67,68], cancer [52, 69,70,71], oral Candida infections, and periodontitis [72, 73]. As different strains provide specific health effects, the selection of appropriate strains and genus of probiotics is a key for distinct applications and it must be done carefully [74, 75].
The biological effects of probiotics can be explained based on their metabolic processes, describing the significance of probiotics in the human body, i.e., the physiologic effects of probiotics. Such effects are categorized below:
Modulation
Probiotics involve metabolic modulations through direct or indirect influences on the signaling pathways that either suppress or activate the regulation of pathways [50, 76]. The ability of probiotics to adhere to the intestinal mucosa for colonization and their interaction with innate immune responses can modulate the barrier functions of intestinal epithelial cells, thereby conferring health benefits to the host [77, 78]. These modulatory mechanisms assist in antagonism against pathogens, thereby improving immunity (immunomodulation), increase the anti-oxidant potential, improve intestinal transit, enhance nervous reflexes (neuromodulation), regulate vascular endothelial function and blood pressure, reduce cholesterol level, and maintain the dynamic balance of healthy and damaged cells [79,80,81,82,83,84,85].
Synthesis
Synthesis of bioactive metabolites through their mechanism of action is a key aspect of probiotics. For instance, synthesis of short-chain fatty acids (acetic, butyric, and propionic acids) through non-specific mechanism [86], vitamins by species-specific mechanism (Vit-B2 and Vit-K2 by B. subtilis, and Vit-B12 by B. megaterium) [87], signaling molecules (strain-dependent release of cytokines by LDR0723, BNL1059, RGS1746, and CRL1528 strains of L. salivarius) by strain-specific mechanism [62].
Absorption
Probiotics can enhance the bioavailability of micronutrients and their metabolism [88]. Iron absorption in Fe2+ form is significant; however, in the intestinal mucosa, iron binds with apoferritin which converts Fe2+ to Fe3+ (ferritin) [89]. Probiotics present in the gut microbiome can help in the reduction of Fe3+ to Fe2+, facilitating an increased duodenal absorption of iron [42]. Hoppe et al. [90] reported that the intake of lactic acid bacteria increases iron absorption rates and the relative iron bioavailability through extracellular enzymes. Similarly, Dubey and Patel [43] found that probiotics play a significant role in the enhancement of calcium uptake, and the improvement of its absorption through colonic fermentation. In another study, Costanzo et al. [91] revealed that probiotics are involved in vitamin D synthesis and absorption. These examples explain the role of probiotics in improving the bioavailability of vitamins and minerals in the human body.
Prophylaxis
The prophylaxis mechanism of probiotics helps to prevent diseases and to reduce the risk of infections, type 1 allergies, viral infections, and cancers [92,93,94]. Monteagudo-Mera et al. [39] have explained the role of probiotic prophylaxis in the treatment of bacterial vaginosis. Importantly, several interventional clinical trials are being conducted to understand the effectiveness of probiotic prophylaxis. Panigrahi et al. [95] conducted the randomized clinical trials registered under NIH clinicaltrials.gov (NCT01214473 and NCT00518596) to study the efficacy of synbiotic supplement (L. plantarum with FOS) against neonatal sepsis and reported significant reductions in both culture-positive and culture-negative sepsis. Some other ongoing clinical trials are probiotic prophylaxis on microbiome modulation and prevention of severe infections like pneumonia in multi-trauma patients (NCT03074552) and the effect of probiotic oral mixture Labinic® on preventing sepsis infection by the colonization of extended-spectrum beta-lactamase (ESBL) caused by Enterobacteriaceae (NCT04172012).
Encapsulation of Probiotics: Need and Critical Considerations
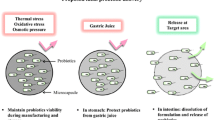
Probiotics ingested through the oral route encounter several stress environments in the alimentary pathway. For instance, the human digestive system has variable pH levels. Approximately, the mouth has a pH of 6–7, the stomach has a lower pH of 1–3, and the pH ranges from 6 to 7 in the small and large intestines [96]. Owing to the low pH conditions in the stomach and the high bile salt content in the small intestine, maintaining the viability of probiotic microorganisms that reach the large intestine (colon-target site of action) is a challenge.
Accordingly, appropriate protection of probiotics is vital during the development of any functional probiotic food product. Apart from the conditions of the human body, other factors such as processing temperature, pH of encapsulating/matrix material, the oxygen level in the product, presence of other competing bacteria, and toxicity of metabolites have implications on probiotic viability [28]. In this context, during storage, the temperature and the moisture content of the products are major factors to be considered. Moreover, the rehydration and solubility behavior of dried probiotics are related to their survival and revival [97].
Various technologies have been developed to improve the external stress tolerance by probiotics, thereby enhancing colonization of gut microbiota through food matrix modifications and by engaging process engineering approaches. In this regard, food matrix selection and formulation are critical considerations in terms of technological performance and probiotic stability [98,99,100].
The encapsulation of probiotics as powder formulations can protect these live microorganisms and improve their stability and offer benefits in terms of targeted delivery [20]. Importantly, in the process of optimizing an encapsulation methodology for probiotics, microbial stability, functionality, safety, efficacy, and targeting ability must be established before and after the encapsulation process [28]. Figure 1 explains various screening sections involved in the evaluation of probiotics for food use as per FAO/WHO guidelines. To maintain the characteristic features of probiotics, an encapsulation approach should consider the following aspects.
-
i)
Stability of probiotics: assurance for improved retention of the viability of encapsulated probiotic bacteria
-
ii)
The functionality of probiotics: functional aspects such as resistance to gastric acids, bile salts and digestive enzymes, antimicrobial activity against potential pathogens, adhesion to mucus, aggregation, and other potential characteristics should be retained after encapsulation
-
iii)
Safety and efficacy: the probiotic strain should be safe, free from contamination, non-toxicogenic, retention of therapeutic efficiency after encapsulation
-
iv)
Targeting ability: improved tolerance towards environmental stress and the ability to target the colon for the enhancement of gut microbiota and beneficial health effects
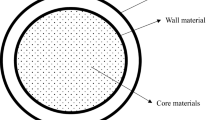
Various techniques have been explored for the encapsulation of probiotics (Fig. 2). Though the focus of all such methods is to protect probiotic viability/stability, the concept of each technique is unique and has direct implications on the product quality. Processing conditions are considered as the major factors that are responsible for the retention of quality and viability of encapsulated probiotics. Further, different wall materials have been explored as protecting/coating agents for the encapsulation of probiotics. They are sourced from dietary fibers, polysaccharides, proteins, and synthetic polymers. Typically, the selection depends on their functionality, film-forming ability, stability, solubility, digestibility, and releasing properties. To attain the desired properties, a combination of wall materials or the addition of emulsifier/ filling agents can also be employed. However, most established encapsulation approaches for probiotics have considered only the survivability of probiotics, and not the functionality or characteristics of probiotics. During encapsulation/drying of probiotics, the surface properties of the cell and their functionality can be impacted. This can have effects on probiotic characteristics such as aggregation (autoaggregation and co-aggregation) property, intestinal mucus adhesion ability, antagonistic activity, and bile salt hydrolase activity. Hence, future research needs to explore the functional characteristics of encapsulated probiotics in addition to the survivability of encapsulated probiotics, providing a better focus on the health benefits of probiotics.
Critical Issues with Non-encapsulated Probiotics
Non-encapsulated probiotics (free cells) lose their viability at high temperatures, high operational pressures, shear stresses, and under low gastric pH, which in turn results in the depletion of probiotic cell count and performance [28, 101, 102]. The encapsulation process can protect probiotics by entrapping them in a protective wall material/matrix [28, 103,104,105]. Prebiotic materials such as FOS, inulin, and filler materials such as maltodextrin (MD), whey protein (WP), whey protein isolate (WPI), whey protein concentrate (WPC), alginate, gelatin, etc. have been reportedly used to encapsulate probiotics and have shown a significant role in improving the stability of probiotics in different external environments [106,107,108,109]. Different wall materials reportedly used for encapsulating probiotics have been detailed in Fig. 3.
Several studies have confirmed that the encapsulated form of probiotics performs better than their non-encapsulated counterparts. As an example of the former, Rajam et al. [110] reported that the synergistic effect of DWPI (denatured whey protein isolate) and sodium alginate could provide better protection during high-temperature spray drying. Praepanitchai et al. [101] have encapsulated probiotics (L. plantarum) in hydrogel beads made up of alginate-soy protein isolate and studied the survivability of probiotics at different pH and temperature in pasteurized mango juice. Encapsulated probiotics showed enhanced survival during the thermal processing of mango juice. The viability of encapsulated probiotics was retained; whereas, non-encapsulated free cells lost their viability within 3 h when exposed to strong acidic conditions (pH 2). In another study, it was observed that non-encapsulated L. acidophilus showed a drastic loss in its viability in harsh environmental conditions such as high thermal pasteurization and acidic gastric conditions, whereas encapsulated probiotics in hydrogel beads made up of WPI and alginate showed reverse trends [111].
During transit through adverse and fluctuating conditions of the GI, encapsulation can provide significant protection to probiotics. For example, Del Piano et al. [112] observed intestinal colonization of microencapsulated probiotics and compared it with non-encapsulated probiotics. They explained that microencapsulated probiotic bacterial species showed improved gastric and bile tolerance owing to the protective coating, apart from enhanced probiotic activity. Interestingly, five-fold higher efficiency in colonization effect was observed as compared with non-encapsulated probiotics. It was reported that layer-by-layer encapsulation of probiotics with alginate and chitosan can improve the stability of probiotics and enhance their mucoadhesive properties [113].
Encapsulation contributes significantly to the storage stability and post-storage performance of probiotics. Prasanna and Charalampopoulos [114] conducted a study on the encapsulation of B. animalis subsp. lactis BB-12 using alginate–goats’ milk–inulin as the matrix. A significant loss in viability of non-encapsulated probiotics (reduction in 3.67 log10 CFU/g) compared to encapsulated probiotics was reported during the storage period. In another study, polysaccharides (sodium alginate and carrageenan) were used as encapsulating material for L. acidophilus and the encapsulated probiotics were incorporated into ice cream. Storage studies were conducted at −20 °C for 120 days. Results revealed that free cells showed 3 to 4 log reductions, while only 1 and 1.5 log reduction was observed in the sodium alginate and carrageenan encapsulated probiotics, respectively [115]. Hossain et al. [102] studied the survival of encapsulated and non-encapsulated antibiotics-resistant lactic acid bacteria in orange juice. They reported a significant reduction in viability of non-encapsulated probiotics at both 37 °C and 4 °C temperatures as compared with encapsulated probiotics; by the fifth week, almost no viable cells remained in the non-encapsulated form. Moreover, it is established that the post-acidification process is slower in encapsulated probiotics than in non-encapsulated probiotics [116].
A study on the effect of co-administration of antibiotics along with encapsulated probiotics (antibiotic-susceptible probiotics) revealed the improved therapeutic efficacy of encapsulated probiotics against antibiotic-resistant pathogens [117]. Further, antibiotic treatments can kill beneficial bacteria apart from the targeted pathogens; hence, to nourish the gut microbiome, probiotic supplementation is necessary. Encapsulated probiotics have a protective shell made up of alginate or another suitable biocompatible material that protects against antibiotics. Oral delivery of probiotics requires such coatings. Qi et al. [118] studied the microencapsulation of S. boulardii and E. faecium in microbeads using sodium alginate through the emulsion and internal gelation techniques. Survival of bacteria was studied under high temperatures, extreme humidity, gastric, and intestinal conditions. Encapsulated probiotics have better survival rates as compared to their non-encapsulated forms. In another study, Cao et al. [119] reported that the biocompatible lipid coating provides bio-interfacial supramolecular self-assembly of gut microbes for enhanced oral delivery and treatment .
Techniques Used for Encapsulation of Probiotics
Different methods of encapsulation have been explored by the researchers and the industry for encapsulation of different ingredients of interest. As well-established merit, encapsulated probiotics can offer better scope for targeted delivery. This section explains the concept of each technique as well as recent applications in the context of encapsulation efficiency, viability, and stability of probiotics. Table 1 represents the summary of different encapsulation techniques.
Conventional Approaches
Spray Drying
The spray drying technique helps in producing probiotic powders with desired powder properties such as bulk density, flowability, uniform spherical shape, and size distribution [120]. In the spray drying process, probiotics along with the encapsulating wall material (liquid feed) are atomized into the hot gas drying chamber, the wet droplets transit through high temperature. In a short span, crust formation occurs, leading to the formation of dried solid particles. The encapsulated powder can be separated from the drying air using a cyclone separator. This technique is known for its merits in terms of the flexibility to have high production volumes, high reproducibility, low production cost, and the convenience to scale-up [121,122,123]. The major drawback of this technique is the use of high temperatures as it can severely affect encapsulation efficiency [124].
The selection of wall material is a key aspect in the science of encapsulation as it directly links to encapsulation efficiency, stability, and release. Rajam and Anandharamakrishnan [106] studied the encapsulation of L. plantarum (MTCC 5422) using FOS along with WPI and DWPI as wall materials at a constant air inlet temperature of 110 ± 2 °C and outlet temperature of 55 ± 3 °C. These researchers reported encapsulation efficiency ranging between 70.77 and 72.82% in formulations with FOS. Higher values were noted when FOS was used with WPI or DWPI as they provide combined protection to the cell wall and in the prevention of cellular destruction, thereby contributing to the retention of cell viability. During storage at 4 °C for 60 days, loss of cell viability was found to be higher in FOS formulations. These values were reduced in formulations with WPI owing to the high moisture retaining capacity of FOS. FOS with DWPI can be an effective wall material for the encapsulation of probiotics as it has proven benefits during the spray drying process, storage, and digestion.
Inlet air temperature and concentration of the wall material also influence the encapsulation efficiency. Nunes et al. [108] explained the effect of an inlet air temperature of 130 °C and outlet temperature of 76 ± 5 °C on L. acidophilus La-5 with inulin, Hi-maize, and trehalose as thermal protectants. They reported encapsulation efficiencies of 93.12%, 94.26%, and 90.34%, respectively. Under GI conditions, better survivability was found in Hi-maize encapsulated probiotics. Behboudi-Jobbehdar et al. [125] also reported that an increase in inlet temperature from 120 to 160 °C could reduce the total viable count of L. acidophilus from 9.02 to 7.20 log10 CFU/g when MD, WPC, and D-glucose (60:20:20 w/w) were used as wall materials. However, using feed flow rates from 6 to 7.5 mL/min would decrease viability loss caused by the effects of temperature.
Burns et al. [126] evaluated the impact of the spray drying process on the stability and immunomodulation capacities of breast milk-derived B. lactis INL1 and compared it with commercial strain B. lactis BB12. The bacterial cells were re-suspended in 20% (w/v) skim milk and then spray-dried at an inlet temperature of 137.5 ± 3.5 °C, and outlet temperature of 82.5 ± 7.8 °C. The study resulted in the retention of viability and functionality of spray-dried probiotics.
In another study, Agudelo et al. [107] studied the encapsulation efficiency of L. rhamnosus with WPI and MD as encapsulating materials using an inlet temperature of 160 °C and outlet temperature of 62–65 °C. They reported that there was no significant loss in cell viability in the process. Avila-Reyes et al. [127] reported the effect of different inlet temperatures (135, 145, and 155 °C) on L. rhamnosus using native rice starch and inulin as wall materials. Probiotic viabilities of around 74% and 54% were observed with rice starch and inulin, respectively, at 145 °C.
Yoha et al. [128] studied the effects of encapsulation methods on the stability of probiotics and reported the encapsulation efficiency of 89.21% using FOS:WP:MD (2:0.5:0.5) as prebiotic encapsulating material in the spray drying process; and also highlighted 0.95-fold increased viability retention achieved by spray drying as compared with spray-freeze-drying during storage. Ma et al. [129] reported that an increase in feed flow rate (550 mL/h, 650 mL/h, and 750 mL/h) can increase the viability (83.57%, 82.24%, and 84.73%) of Bacillus subtilis (B99-2); however, these changes were not very significant.
Freeze-drying
Freeze-drying is a very effective and widely used method for the encapsulation of probiotics [130]. Freeze-drying occurs in three steps: freezing, primary drying, and secondary drying. Probiotics along with the encapsulating material (liquid feed) first undergo freezing in which the water is converted into ice at sub-zero temperatures. The process is dependent on several aspects such as the initial amount of solute present in the feed solution, freezing temperature, and freezing rate. During the freezing stage, the product temperature is decreased, resulting in the formation of ice through effects associated with nucleation and latent heat of crystallization. In the primary drying step, frozen water is removed by sublimation under vacuum, and in the secondary drying step, unfrozen water is removed by the desorption process, resulting in the dried end-product. To protect live organisms from adverse stress conditions, cryoprotectants (like sugars) are used as they can, in turn, alter the state of the material and contribute to retention of cell viability [131,132,133]. Nevertheless, longer drying time and higher power consumption are the major drawbacks associated with the freeze-drying process [134].
Rajam et al. [110] studied the effect of encapsulation of L. plantarum (MTCC 5422) with FOS and WP using freeze-drying and its subsequent addition to noodles [135]. The encapsulation efficiency was reported above 98%, attributed to the usage of low temperatures (−40 to 30 °C). Survivability was also high when stored for 60 days, 9% cell viability loss was observed when only FOS was used as wall material. However, when FOS was combined with WPI or DWPI only 2% loss occurred and this was attributed to the fact that WP contributes to low oxygen and water vapor permeability, thereby restricting the movement of substances across the wall, in turn improving storability [136]. Another comparative study conducted by Barbosa et al. [137] also reported that a higher number of viable cells can be obtained using freeze-drying, based on studies conducted on L. plantarum (299v) and P. acidilactici (HA-6111–2). The addition of cryoprotectants could increase the viability of cells during the freeze-drying process.
The freeze-drying process gives a more porous structure to encapsulated probiotics, resulting in poor barrier effects [138]. With a large air–solid interface area, the faster death rate of probiotics has been reported during storage [139]. However, the addition of nanoparticles during the freeze-drying process is known to increase the viability of encapsulated probiotics. Yao et al. [140] reported enhancement in the encapsulation efficiency of Pediococcus pentosaceus (Li05) when incorporated with magnesium oxide (MgO) nanoparticles. Encapsulated probiotics showed a lower reduction in viable cells (2 log10 CFU/g) unlike the free cells (5 log10 CFU/g) under gastric conditions. Increased encapsulation efficiency was observed in all conditions (long-term storage in an aerobic environment, heat treatment, and GI transit) when doped with nanoparticles owing to the infilling of pores in the microgel matrix, in turn protecting the cells during various stress conditions.
Sakai et al. [141] evaluated the survivability of encapsulated freeze-dried probiotics on the International Space Station for a storage period of one month under the temperature range 20–24.5 °C and reported that the genetic and functional characteristics of freeze-dried probiotics were well-retained during storage. Thomas et al. [142] explained a layer-by-layer coating approach for freeze-drying in which S. boulardii was encapsulated using layers of chitosan and dextran sulfate. The coated cells were subsequently frozen in liquid nitrogen before freeze-drying. Enhanced viability was observed in encapsulated S. boulardii and the permeability of the encapsulated cells was studied using confocal imaging. Permeation of dyes confirmed the presence of pores on the surface of encapsulated cells. Moreover, it was observed that the low molecular weight fluorescence dye (DAPI (4′,6-diamidino-2-phenylindole)) showed blue fluorescence inside the cells unlike the high molecular weight fluorescence dye (dextran-FITC (fluorescein isothiocyanate)/dextran-TRITC (tetramethylrhodamine isothiocyanate)) which showed green/red fluorescence in the encapsulated wall, indicating selective permeability of pores in the surface of the encapsulated cells.
The study by Bora et al. [143] reported an encapsulation efficiency of 98% in studies conducted on the microencapsulation of L. acidophilus and L. casei using freeze-drying with a combination of WPI and FOS as wall materials. WPI and FOS used as the sole wall materials provided 86% and 90% encapsulation efficiency, respectively. A high number of viable cells were attributed to the use of low temperatures. After incorporation of these probiotics with WPI + FOS into banana powder, a higher survival rate was observed in samples stored at 4 °C, as compared with those at 25 °C, since lower temperatures can significantly contribute to the survivability of bacteria [144]. Higher viability can also be linked to the use of WPI that is known to give improved protection in buffered pH [145], apart from the role of FOS in providing ATP during the glycolysis process [146].
Moayyedi et al. [147] reported higher cell viability in the freeze-dried L. rhamnosus (ATCC 7469) encapsulated with WPI, inulin, and Persian gum, in comparison with spray drying and electrospraying methods. Viability during a storage time of 24 weeks at 25 °C was reported as 10.44–10.78 log10 CFU/g of freeze-dried samples, indicating higher values compared to the other two techniques. Similarly, higher survivability was observed under digestive conditions. Halim et al. [148] reported that encapsulation of P. acidilactici (ATCC 8042) with calcium alginate and incorporation of cryoprotectant (10% w/w skim milk) can increase cell viability as skim milk provides sufficient barrier to the cell walls, thus protecting harsh conditions, apart from behaving as a buffer in the system [149].
Extrusion Technique
The extrusion process is easy to perform, cost-effective, and provides good cell viability. In the extrusion process, the feed solution is dripped or sprayed through a nozzle at high pressure, to form encapsulated gel beads. This process is accompanied by vibration or pulsation technology it is explained as a prilling or vibrational jet that can provide standardized production of capsules without affecting the viability of the microflora [150]. When the probiotic solution is passed through the nozzle at a defined flow rate, the laminar jet breaks up at different positions due to applied stress and surface tension which results in the formation of spherical droplets or capsules as it free-falls into the hardening agent. Morphology of the droplets plays a crucial role as it impacts the stability and viability of the microflora being encapsulated. However, the process is slow, offering very low production capacities, limiting its usage at the industry-scale [151].
The study reported by Poletto et al. [152] used prebiotic dietary fibers and observed encapsulation efficiencies of 96.75%, 94.87%, 94.10%, and 76.17% for the extrusion-encapsulation of L. acidophilus with inulin, rice bran, alginate, and Hi-maize treatment, respectively. Storage studies conducted over 120 days at three different temperatures (25 °C, −18 °C, and 7 °C) concluded that alginate, rice bran, and Hi-maize encapsulated L. acidophilus maintained the viability at 25 °C. In the case of inulin encapsulates, storage at −18 °C could only retain viability; other storage conditions resulted in a significant loss in cell viability, even below 6 log10 CFU/g. Such variations explicit the influence of storage conditions on the viability of probiotics. Jantarathin et al. [153] also used the extrusion approach for encapsulation of L. acidophilus TISTR 133 using artichoke and inulin (separately) in sodium alginate (gelling agent). Following this, chitosan coating was performed and results indicated 88.19 to 90.40% encapsulation efficiencies. Eckert et al. [150] observed minimal losses (~ 0.07 to 0.74 log10 CFU/mL) in encapsulates obtained through vibrational extrusion technique in studies on encapsulation of probiotics (Lactiplantibacillus plantarum ATCC8014, Lacticaseibacillus paracasei ML33, and Lactiplantibacillus pentosus ML82) using whey-alginate-pectin (WAP) and whey permeate-alginate-pectin (WPAP) wall materials. During the storage period of three months (at 4 °C), cell viability was found to be higher than 6 log10 CFU/mL. Among the different species, L. plantarum ATCC8014 and L. pentosus ML82 showed the highest encapsulation efficiencies during storage.
In another study, Silva et al. [154] performed extrusion and co-extrusion for the encapsulation of L. acidophilus LA3 with alginate and a mixture of alginate shellac as wall materials. They reported viability ranges of probiotics co-extrusion-encapsulated with alginate or alginate-shellac of 6.2 and 7.2 log10 CFU/g, respectively. During the storage period (60 days at 25 °C), these values were found to be around 5.3 and 6.2 log10 CFU/g in encapsulates prepared using the extrusion technique. It is well-explained that the wall material has significant implications on the survival of microorganisms. A different study involving the use of alginate, Gul and Dervisoglu [155] reported wall material concentration-dependent encapsulation efficiencies ranging from 95.92–99.75% for L. casei Shirota.
Emulsion Technique
Emulsification is a chemical process that involves the interaction between a continuous phase and a discontinuous phase with the addition of an emulsifier. In this technique, microcapsules are formed after the formation of a water-in-oil emulsion, usually stabilized by surfactants like Tween 80/Span 20/glycerol, and gelled by external gelation by the addition of calcium chloride solution. Small capsules (size ≤ 100 μm) are produced using this technique and the size of beads/ capsules can be controlled by varying the process parameters [156, 157]. However, variable distribution of particle size and low yields obtained against the amount of material used in this process are key limitations of the technique [118]. Further, the viability of probiotics also gets affected when during the intermediate high-shear processes [158].
The double emulsion process of L. plantarum was studied by de Almeida Paula et al. [109] and reported an encapsulation efficiency of 97.78% with gelatin and gum arabic following a coacervation process. During coacervation, one phase becomes slightly richer in the solvent and the other in both polymers [159]. High viability of cells can be obtained because of the dual protection offered by the water–oil interface and the wall material; these act as a barrier for the probiotics and do not allow cells to move out easily. In another study conducted by Eratte et al. [160], encapsulation efficiency of 84.95% was observed by microencapsulating probiotic (L. casei 431) and omega-3, followed by a coacervation process with WPI and gum arabic. Gul and Dervisoglu [155] studied the encapsulation of L. casei Shirota with different sodium alginate concentrations (0.5–3%) using the emulsion method. They reported an increase in encapsulation efficiency from 86.71 to 95.25% with an increase in alginate concentration. With the increment in the alginate concentration, the viscosity of the solution also increases and results in the formation of a protective layer over the cells/beads, in turn improving cell viability [161]. Qi et al. [118] varied conditions of the emulsion process by modifying temperature and humidity values for the encapsulation of S. boulardii and Enterococcus faecium, and reported encapsulation efficiencies of 25% and 40%, respectively.
Multi-layer encapsulation studied by Zhang et al. [162] reported the encapsulation efficiencies ranging from 77 to 90% for spray-dried L. salivarius (NRRL B-30514) when encapsulated in primary (milk fat/WPI/sodium caseinate) and secondary (milk fat/WPI/sodium caseinate/pectin) emulsions. The highest encapsulation efficiency of 90% was observed in the secondary emulsion because of the thicker interface it provides, in turn improving the stability and viability of probiotics [163]. Holkem et al. [164] conducted a study using Bifidobacterium (BB-12) by emulsification using sodium alginate as wall material and reported encapsulation efficiency of ~ 89.71%. A similar study was reported by Martín et al. [165] using the same wall material, with an encapsulation efficiency of 80–98%, indicating that emulsion-encapsulation is an effective approach for encapsulation of probiotics.
Emerging Approaches
Spray-freeze-drying
Spray-freeze-drying possesses advantages of the spray drying as well as those of freeze-drying. Spray-freeze-drying is carried out in three stages, namely atomization, freezing, and freeze-drying. In this process, probiotics along with the encapsulating wall material (liquid feed) are atomized, forming fine droplets with a high interfacial area. This is then allowed to get in contact with a cryogenic medium such as liquid nitrogen at very low temperatures. This freezes the probiotic cell in the wall matrix with the formation of frozen droplets. These frozen droplets are further subjected to drying in a freeze dryer. Particle size can be easily controlled in the spray-freeze-drying process and these particles have a larger specific area than spray-dried particles [165, 166]. The spray-freeze-drying method produces highly porous particles with excellent reconstitution capacity. However, the method is time-consuming, and handling cryogens remains a major concern, particularly to convert the technology to industry-scale. Further, energy consumption is higher, and overall process cost is reportedly 30–50 times higher than the spray-drying process [138].
The effect of different wall materials on the encapsulation of L. plantarum (MTCC 5422) using spray-freeze-drying was studied by Rajam and Anandharamakrishnan [166] and reported the encapsulation efficiencies of around 87.92–94.86% with different combinations of wall material including WPI + sodium alginate (SA), WPI + FOS, DWPI + SA, and DWPI + FOS. Among that, DWPI + FOS gave maximum viability and stability during the storage period. However, encapsulation efficiency was around 3.16–6.73% lesser as compared with those obtained through freeze-drying. This reduction is because of the effect of atomization pressure and freezing conditions on cell viability. Semyonov et al. [167] studied spray-freeze-drying of L. paracasei (LMG P-21380) with MD and trehalose as encapsulating materials and obtained encapsulation efficiency of ~ 60%. During the spraying stage, cell viability was not affected; however, the osmotic pressure created by the solute concentration is known to affect cell viability during the freezing stage [168].
An increase in the concentration of the solute can result in the improvement of probiotic viability owing to the formation of a glassy-state that would act as a protectant [28, 169]. A variant, the vacuum spray-freeze-drying method is known to have reduced effects of oxidation and lesser drying temperature, both of which are key essentials to prevent loss of probiotic cell viability. Cao et al. [170] reported encapsulation efficiencies of 56.58–74.79% for S. cerevisiae in different skimmed milk powder concentrations (wall materials) produced through vacuum spray-freeze-drying. In a recent study, Yoha et al. [128] reported the encapsulation efficiency of 96.16% using spray-freeze-drying when encapsulating with the prebiotic FOS:WP:MD (2:0.5:0.5) and observed 1.3-fold increased cell viability in spray-freeze-dried synbiotics as compared with spray-dried synbiotics.
Refractance Window Drying
Refractance window (RW) drying and its variant, conductive hydro drying are emerging drying techniques for the encapsulation of probiotics. A typical RW dryer consists of a hot water reservoir above which a food-grade infrared transparent polyester film is placed. The homogenized mixture of probiotics and encapsulating material is spread over the film and is allowed to dry. All three modes of heat transfer occur, resulting in enhanced drying rates and reduced drying times. Raghavi et al. [171] have clearly explained the mechanism of the drying approach. Once the product gets dried, with a mismatch in the refractive index between dried product and hot water, the “window” is said to get closed at a Mylar film and deflecting the incident radiation back into the hot water [172]. The product temperature is much lower than the temperature of the hot water, and the effects of over-drying can be eliminated. The approach has been successfully used for the drying of several products such as slurries, purees [173], slices of fruits and vegetables [174], and meat powders [175]. The high-quality flakes or films can be effectively obtained using this low-cost (operational) technique [172]. Importantly, the drying method is considered to be a nonthermal drying approach [176] and works very well for heat-sensitive food and bioactive ingredients.
Not many studies report the use of RW drying for the production of probiotic powders. In recent work, Yoha et al. [177] reported the encapsulation of L. plantarum (NCIM 2083) with different combinations of prebiotics such as FOS, WP, and MD using RW drying technique. Better viability of probiotics was achieved at 40 °C using the RW drying and encapsulation using FOS:WP:MD (2:0.5:0.5) exhibited higher viability. Drying temperature and wall material composition affect the encapsulation efficiency. For example, it was observed that the FOS:WP:MD combination showed higher viability than the other two wall material combinations. The encapsulation technique had a significant impact on the surface morphology of the synbiotic flakes. RW dried synbiotic flakes had porous structures owing to rapid surface drying before the expansion of a thin layer surface that results in crust formation on the top layer, which in turn restricts capillary forces that are responsible for the collapse. Moisture content was found to be lesser in RW dried samples (5.25–6.51%) as compared with freeze-dried samples (5.84–6.75%), contributing to extended shelf-life. The encapsulation efficiency of RW dried synbiotic flakes was lesser (88.05–93.29%) than that of freeze-dried (89.62–95.74%) counterparts. However, being a very cost-effective and rapid method, RW drying was explained to be an alternative to freeze-drying for the commercial production of probiotic powders.
Electrohydrodynamic Processes
Electrohydrodynamic processes such as electrospinning and electrospraying involve the use of electrostatic force to produce polymeric materials in the form of fibers (electrospinning) or powders (electrospraying). A typical electrohydrodynamic system consists of a spinneret needle, syringe pump, and collector, connected to a high voltage power supply. Feed solution in the spinneret needle is induced by electrostatic forces (repulsion and Coulombic) to form a Taylor cone jet during the spinning/spraying process. This electrostatic force is created by the applied high electric field at a particular critical voltage that overcomes the surface tension of the feed solution, with ejection from the tip of the Taylor cone. Increasing the voltage and decreasing the polymer concentration leads to electrospraying. In this encapsulation approach, the encapsulation efficiency of probiotics can be enhanced by optimizing the operating parameters and the consistency of the feed solution.
Electrohydrodynamic methods of probiotic encapsulation with different biopolymers and prebiotic materials are considered a promising method to protect microbial cells under various stress conditions. Coghetto et al. [35] have electrosprayed L. plantarum using sodium alginate and pectin as encapsulating materials. Storage studies of electrosprayed probiotics showed retention in cell viability of 9 log10 CFU mL−1 over 21 days under refrigeration. Librán et al. [178] developed a new encapsulation method (electrospray coating atomization) for the encapsulation of probiotics using WP-MD-polyvinylpyrrolidone. Storage studies on encapsulated probiotics were performed at different conditions and significant differences were observed under stress conditions. In short, the results explained that the electrospray coating improved the survivability of probiotics. Feng et al. [179] reported the synbiotic effect of probiotics and prebiotics in their study on L. plantarum by electrospinning it with polyvinyl alcohol and FOS. Results confirmed that the addition of FOS enhanced cell viability and the thermal stability of probiotics.
Some studies have also reported that high voltage electrospraying can be injurious to cells and can affect cell viability. Moayyedi et al. [147] have comparatively studied electrospraying, freeze-drying, and spray-drying. Their results indicate that electrosprayed probiotics were perfectly spherical compared to the other methods. However, in terms of viability, electrosprayed probiotics showed a higher loss in viability due to cell damage. Whereas, Gomez-Mascaraque et al. [180] reported that there was no significant loss in viability during electrospraying and also explained that electrospraying of L. plantarum could offer enhanced protection to cells in gastric conditions as well as during storage under high humid conditions. Škrlec et al. [181] produced L. plantarum loaded polyethylene oxide (PEO) fibers and reported that the concentration of probiotic cells and lyoprotectants have a significant impact on cell viability. Also, they studied the release kinetics of probiotic-PEO fibers and concluded that almost all cells get released in 30 min, indicative of its potential for local administration.
Coaxial electrospraying of probiotics was conducted by Gómez-Mascaraque et al. [182] using gelatin-WP wall material. Increased loss in viability was reported and was explained to have occurred because of the acidic gelatin solution and the effect of acetic acid (low pH) on a drastic reduction of cell viability during storage. In a different approach, Zaeim et al. [183] performed double-layer co-encapsulation of probiotics along with inulin (prebiotic) using electrospraying. They reported better encapsulation yield and GI survival rates of 5.9 log10 CFU/g and 7.2 log10 CFU/g for L. plantarum and B. lactis, respectively. Amna et al. [184] encapsulated L. gasseri in polymeric nanofibers using electrospinning and concluded that there was no significant reduction in cell viability. However, loss in cell viability was reported during the storage period. López-Rubio et al. [185] encapsulated Bifidobacterium strains using electrospinning with WPC and pullulan. These researchers reported retention in the viability of probiotic fibers even at high humidity conditions. Liu et al. [186] fabricated L. rhamnosus GG loaded polysaccharide fibrous mats using pectin and pullulan. The fibrous mat was then post-processed with calcium chloride for improving its structural integrity through cross-linking. Around 90% of cell viability was achieved concluding that the exposure to calcium chloride had an insignificant impact on the viability of probiotics (reduction in cell viability from 7.4 log10 CFU/g to 7.18 log10 CFU/g). Lancuški et al. [187] developed probiotic fibers by coaxial electrospinning. L. paracasei was electrospun with starch-formate/glycerol and storage viability was monitored over 21 days.
In an interesting study, Akbar et al. [188] explained the synergistic effect of probiotics in encapsulating fibers. B. animalis subsp. Lactis Bb12 and a combination of three probiotics (Streptococcus thermophilus (TH-4®), L. paracasei 431®, and Bb-12) were encapsulated with polyvinyl alcohol by electrospinning. High encapsulation efficiency (90.09%) was obtained in the synergistic probiotic fibers than B. animalis subsp. LactisBb12 fiber (84.07%).
Other Emerging Techniques
3D Printing
3D printing is an emerging technology and has promising applications for the food industry [189]. Apart from unmatched customization in terms of shape and designs of the product, 3D food printing can also facilitate customization and nutrient levels, providing new insights for personalized nutrition. Different researchers have studied the printability of different nutrient-rich food ingredients [190]. Recent reports also explain the scope of printing high-fiber foods [191], focusing on developing a novel range of healthy food products using 3D food printing. Different nutraceuticals and functional food ingredients can also be added to the printing material supply. For example, Liu et al. [192] reported the printability of probiotics incorporated in mashed potatoes and observed the influence of variables such as nozzle diameter and printing temperature on the viability of probiotic cells. Also, Zhang et al. [193] 3D printed food structures incorporated with probiotics and reported cell viability of > 106 CFU/g even after the baking process. Food 3D printing can also be integrated with encapsulation and electrohydrodynamic processes to load the printed constructs with probiotics. Importantly, apart from understanding printability, it is important to understand the effect of post-printing conditions on the stability and viability of probiotic cells. Recently, Yoha et al. [271] studied the combined effect of encapsulation and 3D printing process on the viability of probiotics and reported that the printing process has no adverse effect on the viability of probiotic cells. They concluded that the spray-freeze dried encapsulation approach followed by the freeze drying post-processing method has the highest cell viability of 8.18±0.16 log10 CFU/g. Further, they observed better survival rates of 6.43±0.17 log10 CFU/ml and 7.98±0.48 log10 CFU/g under static in vitro digestion conditions and during 35 days of storage, respectively.
Microfluidics
Recently, microfluidic approaches have been used for the cultivation of individual bacteria inside microfluidic droplets that are double-layered emulsions. The microfluidic double water-in-oil-in-water emulsion (MDE) is considered a “deep functional profiling” technique, with the advantages of providing a single-cell functional characterization of microbes [194]. Chen et al. [195] used droplet-based microfluidics for lactic acid bacterial strain improvement, performing cell encapsulation through a droplet generator (T-junction on a chip). The applied cell concentration in the disperse phase and its distribution patterns can influence droplet formation. Accordingly, cell density determines the ratio of cells in a droplet (single-cell/multi-cell). This high-throughput droplet technology can provide monodisperse and highly stable microbeads. In another study, the combined effect of microfluidics and molecular techniques have been reported for developing the “MicDrop” platform for isolation of individual gut bacteria, explaining the suitability of this approach for personalized microbiota-directed therapies in the future [196].
Methods to Confirm the Targeted Delivery and Viability of Encapsulated Probiotics
Encapsulation of probiotics can protect cell viability until it reaches the colon, allowing improved effectiveness of probiotic functions after reaching the distal part of the intestine (lower GI tract). Confirmation of the delivery of probiotics can be done initially by in vitro studies using simulated static in vitro digestion or dynamic in vitro digestion system (simulator). These can provide a proof-of-concept for targeted delivery. Further, in vivo studies on the delivery of probiotics are needed to have a realistic view of the delivery and the need for extended research on health effects, efficacy, and safety.
Static In vitro Digestion Studies
In vitro digestion studies can be performed using simulated digestion fluids such as simulated salivary fluid (SSF), simulated gastric fluid (SGF), and simulated intestinal fluid (SIF) under controlled temperature, pH, and continuous shaking conditions; nevertheless, these sparingly mimic the digestion process (Fig. 4).
The efficiency of dual-process microencapsulation of L. plantarum on the survival of probiotics under simulated GI conditions was evaluated by de Almeida Paula et al. [109] who reported that the cell viability was observed to be higher (80.4%) in microencapsulated L. plantarum as compared with free cells (25%). The survival of encapsulated L. plantarum (MTCC 5422) was evaluated by Rajam et al. [110] under SGF and SIF conditions, separately. In both fluids, denatured whey protein isolate (DWPI) encapsulated cells showed better stability than whey protein isolate (WPI). Also, it was reported that the synergistic effect of DWPI and sodium alginate wall matrix could deliver probiotics with high cell survival rates. In another study, FOS was used along with WPI and DWPI for the encapsulation of L. plantarum (MTCC 5422), resulting in more viable cells under simulated gastric conditions [106]. This is because of the barrier properties offered by whey protein (WP), which slow-down the diffusion of the gastric medium into the cells, thereby retaining viable cell counts.
Afzaal et al. [115] developed the encapsulated probiotic beads using an encapsulator. They have explored the effect of encapsulating materials (sodium alginate and carrageenan) on the stability of probiotics (L. acidophilus) under simulated GI conditions and observed that non-encapsulated probiotics had 7 log reductions as compared with 3 log reductions in the cell viability of the encapsulated form. In a different study with L. acidophilus and L. casei, the microencapsulated probiotics were incorporated into freeze-dried banana powder and its survivability under simulated GI conditions (for 90 min) was found to be 7.05 ± 0.1 log10CFU/g and 5.48 ± 0.1 log10 CFU/g for L. acidophilus and L. casei, respectively. Whereas, the unencapsulated probiotics loss their viability even under SGF conditions was found to be 4.69 log10 CFU/g and 5.64 log10 CFU/g for L. acidophilus and L. casei, respectively [143]. In another report, Eckert et al. [150] concluded that encapsulation with whey-alginate-pectin (WAP) was more effective than whey permeate-alginate-pectin (WPAP) based on observations made on the stability of probiotics under GI conditions. They have studied the simulated gastric digestion under three different pH (2, 2.5, and 3) of SGF and reported that the unencapsulated cells were not survived at pH of 2 and the encapsulated cells showed 3 log reductions still maintained the viability 6 log10 CFU/ml. Further, it showed 3 and 2 log reductions from initial cell count at pH of 2.5 and 3, respectively, under simulated gastric conditions. Also, they have studied the simulated intestinal digestion under two different conditions (with and without bile salts) and reported that there is no significant difference in survival rate between unencapsulated and encapsulated cells under SIF without bile salts. Whereas, significant log reductions (4 log reductions) were reported under SIF with bile salts at a concentration of 0.5% (w/v). Silva et al. [154] reported that alginate-shellac encapsulating material provided better survival of L. acidophilus LA3 in simulated GI fluids because of the resinous nature of shellac. Similarly, Poletto et al. [152] have studied the influence of different prebiotic materials (inulin, rice bran, alginate, and Hi-maize) on the survival of encapsulated L. acidophilus and reported that all these prebiotic materials provided improved protection and enhanced stability of probiotics under simulated GI conditions.
The efficiency of solid/oil/water emulsions prepared with sugar beet pectin as a delivery system for spray-dried L. salivarius (NRRL B-30514) was evaluated and observed the increase in cell viability in simulated GI conditions when the secondary emulsion was cross-linked with calcium [197]. Recently, Yoha et al. [128] studied the effect of encapsulation methods (spray drying and spray-freeze drying) on the survivability of L. plantarum NCIM 2083 under simulated oral-GI conditions. They concluded that free cells lose their viability, whereas encapsulated probiotics are retained. In another study, Yoha et al. [177] evaluated the stability of RW dried and freeze-dried synbiotics under oral-GI conditions. Their results indicated that RW dried synbiotics showed similar efficiency in cell survivability with freeze-dried synbiotics.
Dynamic In vitro Digestion Studies
Static models can only represent the biochemical processes in the GI tract, but fail to provide the dynamic environment such as constant physical forces (axial and shear) on the stomach, gastric emptying, synchronized contractions of involuntary muscles for the intestinal peristaltic movement, feedback mechanisms, and the effects of meal and resident microflora. To address these challenges dynamic in vitro digestion models have been developed.
Mainville’s Model (IViDiS)
A dynamic model of the human upper GI tract mimics the events of the upper GI tract transit. This model consists of the stomach (gastric) and duodenum reactors which can be used for validation of the survival of probiotic bacteria isolated from humans, animals, and fermented dairy products. This model considering the pH variations in the presence of meal in the stomach and bile salt in the small intestine. Especially, it demonstrates the impact of the meal as well as encapsulation matrix on the viability of probiotics [198].
Reid et al. [199] evaluated the stability of extrusion-based encapsulated probiotics using this model. In this study, L. rhamnosus was entrapped with Ca2+ induced WPI gels and subjected to freeze-drying. The viability of micro-entrapped cells was measured and compared with the viability results of free cells freeze-dried in a milk-based cryo-protective solution and free cells freeze-dried in a pre-denatured WPI solution. Viability was measured by the plate count method after 30, 60, and 90 min from the gastric and the duodenum reactors. They have reported that there was no significant difference in viability among the samples between 60 and 90 min in the gastric reactor. At the end of 90 min under GI conditions in the stomach-gastric reactor, free cells significantly lose their viability (~ 4 log reductions), whereas micro-entrapped cells maintain their viability even after 90 min. The study concluded that the Ca2+ induced WPI gelation could protect the cells in the upper GI tract and deliver towards the colon. Following this study, Tompkins et al. [200] studied the impact of meals on a probiotic during transit through the same model. They observed that the milk with 1% milk fat and oatmeal-milk gruel showed better survival of probiotics than apple juice or spring water and concluded that the protein content of the meal was probably not very important for the survival of the bacteria, unlike the fat content. Further, the study highlighted that non-enteric coated probiotics should be taken with or just before a meal containing some fats. More recently, Aragón-Rojas et al. [201] investigated the effect of the carrier material (culture medium and culture medium with 0.6:0.4 ratio of maltodextrin (MD):sweet whey), drying technology (spray-drying and freeze-drying), and dissolution media (water and milk) on the viability of L. fermentum K73 during simulated GI transit in this model. They observed that the viability of probiotics was not dependent on the drying technology used and the culture medium as a carrier material provided the highest viability with water or milk (both dissolution media) than the culture medium with MD and sweet whey. Further, cell survival was found to increase when digested with milk as the medium. Though this model is more realistic for the validation of probiotics viability, it is not suitable to evaluate the functional properties such as mucus adhesion, aggregation effect, and the impact of resident microflora in the gut [198].
SHIME®
The Simulated Human Intestinal Microbial Ecosystem (SHIME®) is a computer-controlled simulated human GI model. This model consists of five reactors that simulate from the stomach to the descending colon of the large intestine. The stomach reactor mimics the acidic environment with pepsin digestion, the small intestine reactor provides the bile conditions for digestive processes and the three reactors of the large intestine mimic the differential regions (ascending, transverse, and descending) of the colon which helps to study the microbial processes. Urbanska et al. [202] evaluated the targeted delivery of alginate-chitosan microencapsulated L. acidophilus using SHIME® and observed that the cell viability was retained at about 8.37 log10 CFU/mL and 7.96 log10 CFU/mL after the exposure of gastric (2 h) and intestinal (6 h) conditions, respectively. Thereby, they confirmed the successful delivery of probiotics at the target site. Pham and Mohajeri [203] highlighted the applications of the SHIME® model for the screening of probiotics and prebiotics. Later, Patrignani et al. [204] studied the potential of L. crispatus BC4 along with squacquerone cheese for the prevention of gynecological infections in women. They have evaluated GI stability using SHIME® model and the presence of L. crispatus BC4 was verified using genomic DNA extraction followed by sequencing of the 16S rRNA region. The study confirmed that the viability of probiotic strain was more significantly affected by the low pH of the stomach, whereas the strain was observed to be resistant toward bile salts and pancreatic juices.
SIMGI®
SIMulator of the Gastro-Intestinal tract (SIMGI®) is a multi-compartmental GI model designed to simulate the digestion process in the stomach, small intestine, and large intestine (with ascending, transverse, and descending colon compartments). Yao et al. [205] highlighted that the SIMGI® system could handle the proliferation of colonic microbiota. Gil-Sánchez et al. [206] studied the impact of L. plantarum CLC17 supplementation in polyphenol metabolism. They used the SIMGI® model for simulated GI digestion and the microbiota composition was quantified by qPCR (quantitative polymerase chain reaction) and 16S rRNA gene sequence analyses. The results inferred that the strain could be successfully delivered in compartments of the colon region. Cueva et al. [207] studied the impact of silver nanoparticles on human gut microbiota using the SIMGI® model and reported that nanoparticles do not affect the metabolic activity of human intestinal microbiota.
ARCOL
The Artificial Colon (ARCOL) is a mono-compartmental model that simulates the colon (large intestine) digestion. It can be employed with any other dynamic upper GI system. The composition of intestinal microbiota and its activity can be studied using the ARCOL model. Moreover, the dialysis fibers present in this model mimics the passive absorption of microbial metabolites [208].
The TIM
The TNO Gastro-Intestinal Model (TNO) system has two models TIM-1 (consists of stomach, duodenum, jejunum, and ileum) and TIM-2 (large intestine). Marteau et al. [209] first studied the survival of lactic acid bacteria using TIM-1 and reported that it can be used for the validation of probiotics viability during GI transit. Blanquet-Diot et al. [210] investigated the influence of biopharmaceutical compounds on the survival of probiotic yeast using TIM -1 with ARCOL and observed a two-fold increase in strain survival because of food-matrix intake along with yeast-HPMC capsule, which delayed the release of yeast. Cordonnier et al. [211] used TIM -1 with ARCOL dynamic simulators to study the survival kinetics of S. cerevisiae CNCM I-3856 and its influence on intestinal microbiota. Results showed a high survival rate of probiotic strain in the upper GI tract whereas the strain was more sensitive to colonic conditions. Venema et al. [212] studied the survival of multilayer-coated probiotic strains (L. gasseri PA 16/8, B. longum SP 07/3, and B. bifidum MF 20/5) using the TNO GI model (TIM-1 system)—the in vitro model of the stomach and small intestine. They have reported that the gastric survival percentage of Bifidobacteria and L. gasseri at 72% and 53%, respectively, were delivered to the small intestine. Thus, the enteric coating of probiotic strains offered 20–40-fold increased delivery of viable cells as compared with uncoated probiotic strains.
GITS
Gastrointestinal tract simulator (GITS) is a single bioreactor that simulates human GI tract conditions. Sumeri et al. [213] studied the survival of different probiotic bacteria (L. acidophilus La-5, L. johnsonii NCC533, L. casei strain Shirota, and L. rhamnosus GG) using GITS and reported that L. acidophilus La-5 and L. johnsonii NCC533 exhibit more resistance to bile salts, whereas L. casei strain Shirota and L. rhamnosus GG showed 6 log reductions in their viability.
GIDS
Gastrointestinal digestive simulator (GIDS)—a semi-dynamic in vitro GI model, its mimics “fasted-mode,” i.e., digestion in an empty stomach—it did not contain digestive fluid before sample addition. The salivary fluid is manually added outside before feeding into the stomach reactor to mimic oral digestion. Adouard et al. [214] have developed this model specifically for probiotics and established it to assess probiotic viability throughout the human GI tract. This system was initially validated only for fermented milk products and further used for assessing the species (both inter and intra) variability among 16 different bacterial strains of B. animalis, B. breve, and L. paracasei species. After simulated digestion, the digesta has to be subjected to qPCR and flow cytometry for the quantification of specific viable strains. Adouard et al. [214] reported that the GIDS system provides valid insights for the identification of targeted bacterial strains to reach the colon.
Customized Dynamic In vitro Digestion Systems
The GI tolerance of encapsulated lactic acid bacteria was studied by Moumita et al. [215] using a customized in vitro GI model. Free and encapsulated forms of lactic acid bacteria were subjected to the digestion process and observed that the encapsulated L. acidophilus NCIM 2660 showed increased resistance as compared with its free form. Whereas, L. bulgaricus NCIM 2056 and L. fermentum NCIM 2156 did not show any significant difference in resistance between encapsulated and free cells. These results concluded that the resistance/ tolerance against stress conditions are strain-dependent apart from the encapsulation. Gbassi et al. [216] investigated the study on the release of L. plantarum strains encapsulated/immobilized in the alginate-WP beads. In vitro GI model was designed to find the release and the viability of probiotic strains with an incubation period of 10.25 h (consists of 5 compartments—2 h stomach, 0.25 h duodenum, 3 h jejunum, 4 h ileum, and 1 h caecum) and observed that the release of L. plantarum was pH-dependent. The probiotic strains were released in the SGIF (simulated gastrointestinal fluid) at a range of pH (6.0–6.5), and therefore, the viable probiotics were found in the jejunum compartment of the small intestine.
Other Dynamic In vitro Digestion Systems
Parthasarathi et al. [217] developed an engineered small intestinal system to study the intestinal absorption and perfusion processes with the interference of the mucosal layer. It consists of a perfusion chamber connected with a peristaltic pump, pH meter, donor, receiver, and buffer circulation compartments. Small intestine from small animals (rat/chicken) can be fitted with this model, which mimics the exact in vivo model. Parthasarathi et al. [217] used rat small intestine in this engineered small intestinal system to study the intestinal permeability of bioactive compounds and reported that the developed system is well-fitted for passive diffusion of intestinal permeability. This approach was used by Jayan et al. [218] to study the bioavailability of nano-encapsulated zein-resveratrol and a modified approach can be extended to probiotics. Several other systems such as the M.I.D.A. (Model of an Infant Digestive Apparatus), Dynamic Gastric Model (DGM), Human Gastric Simulator (HGS), DIDGI® system, and Engineered Stomach and Small Intestinal (ESIN) have been used to evaluate food digestibility, dissolution property, absorption and bioaccessibility of nutrients, including pharmaceutical applications.
In vivo Studies on Probiotics Delivery
Targeted delivery of layer-by-layer encapsulated Bacillus coagulans was evaluated in BALB/c mice and reported that layer-by-layer coatings provided enhanced survival of probiotics [113]. Further, they have conducted mucoadhesion and intestinal colonization studies using porcine small intestine and observed that probiotics were well-adhered with the intestinal tissue at a short time-points through bioluminescence imaging using In Vivo Imaging Systems (IVIS) software. Sharma et al. [219] evaluated the in vivo targeting efficacy of spray-dried probiotics-embedded 5-fluorouracil microparticles using X-ray transmission radiographic technique in Wistar rats. In this study, the radiopaque marker (barium sulfate) was incorporated along with the formulation for tracking the position of the microparticles at various time points during the in vivo movement in the GI tract. Further, this study proved the colon targeted delivery of probiotics.
In another study, Dinoto et al. [220] investigated the effects of administration of gelatin-encapsulated B. breve JCM 1192 T cells in the male WKAH/HkmSlc rats. After the study period, the rats were sacrificed and their cecal contents were analyzed using fluorescence in situ hybridization (FISH) and terminal restriction fragment length polymorphism (T-RFLP) for the identification of the specific bacterial strain. B. breve cells were detected only in the group treated with gelatin-encapsulated B. breve JCM 1192 T and it was quantified about 6.3% of the total cells using FISH analysis. Coelho-Rocha et al. [221] investigated the survival of encapsulated and non-encapsulated lactic acid bacteria in C57BL/6 mice by oral administration and the intestinal sections of mice were analyzed by confocal microscopy and qRT-PCR (quantitative reverse transcription–polymerase chain reaction). The results ensured the presence of viable lactic acid bacteria at different sections of the intestine. Researchers have observed that both the encapsulated and non-encapsulated lactic acid bacteria have a higher relative expression in the duodenum and jejunum sections, whereas in ileum and colon sections the relative expression was only observed in the encapsulated lactic acid bacteria.
Probiotic Pearls™ Acidophilus consists of L. acidophilus NCFM and B. longum BB536 encapsulated in a bi-layer of gelatin and pectin. Mai et al. [222] investigated the recovery of these encapsulated probiotic strains (Probiotic Pearls™ Acidophilus) in the fecal sample of human volunteers. They have confirmed the presence of encapsulated probiotic strains using strain-specific PCR (polymerase chain reaction), and the differential effects on overall microbiota composition were identified by 16S rRNA gene sequencing. Arioli et al. [223] evaluated the effect of GI transit on the quantitative recovery of viable L. paracasei CNCM I-1572 (L. casei DG®) in healthy adults. Fecal samples were analyzed and confirmed the presence of viable L. paracasei CNCM I-1572 using two molecular techniques (strain-specific colony PCR used for phenotype identification and qPCR used for genotype identification and quantification). The above examples of in vivo studies can confirm the delivery of probiotics in the target site of action; still, proofs are needed for the evidence of their health claims/efficacy/destination for their wholesome applications.
Evidence for the Efficacy of Encapsulated Probiotics
Several studies have been corroborated the efficacy, safety, and tolerability of encapsulated probiotics. Spray-dried L. plantarum HM47 was incorporated into milk chocolate and its effect on acute oral toxicity was evaluated in Swiss albino mice [224]. Enhanced intestinal lactic acid bacteria count and diminished enteric pathogen count were observed in this study, indicative of colonization of encapsulated probiotics in the colon region. Moreover, the safety aspects of probiotic consumption in mice were studied and results confirmed that there was no adverse effect or mortality. Histopathology studies of mice ileum confirmed the absence of necrosis, or inflammation, or any deteriorative changes in the intestinal epithelial cells. In another study, Ayyanna et al. [225] reported the anti-inflammatory and antioxidant potentials of sodium alginate coated probiotic beads (L. mucosae AN1 and L. fermentum SNR1) in both acute and chronic animal models (Wistar rats). The study by Wang et al. [226] explained the effect of microencapsulated probiotics along with prebiotics in the growth performance, antioxidant potential, and immune functions of broiler chickens. They have observed improvement in serum immunoglobulins/interleukin levels apart from increased cecal lactic acid bacteria count in treated broiler chickens.
Commercial Probiotic Fortified Products
Most probiotic food products available in the market contain free cells, whereas their encapsulated forms come in supplements such as tablets, pills, and capsules. Table 2 lists examples of commercially available encapsulated probiotic fortified products. Though the techniques involved are not clear in most cases, consumers are provided with information on specific ingredients in small-font texts on the product label [227]. Most of these probiotic products contain multistrain rather than a single strain. For example, the products such as PRO15 Probiotics, ProBio-40, Bifa-15™ contain a combination of Lactobacillus and Bifidobacterium strains, ProbioFerm contains Lactobacillus, Bifidobacterium, and Pediococcus strains. Table 3 lists recent patents on encapsulated probiotics and/or their incorporation into the products. It is to be understood that a thorough understanding of the effects of such products needs to be explored through research studies, failing which, consumers would lack clarity on selection and usage, particularly for specific health conditions. However, some organizations have systematically reviewed the available evidence and developed recommendations on specific information including appropriate product, dose, and formulation [228].
The World Gastroenterology Organization (WGO) explains that the optimal dose of probiotics depends on the strains present in the product. The organization recommends that clinicians/physicians who advise their patients to use probiotics must specify probiotic strains, doses, duration, and benefits of usage. It is also suggested that consumers check the labels of probiotic supplements for clear guidelines on storage conditions and usage [229]. ISAPP advises manufacturers to list the “number of CFUs” of viable cells at the time of manufacture as well as a minimum number of viable cells at the end of the product’s shelf-life, also including “expiration/use by date” on the product label. It is important that consumers check product labels for the number of CFU at the end of the product’s shelf life and not at the time of manufacture. This is because significant losses in cell viability may occur during the storage period [230]. Earlier, FDA labeling regulations “21 CFR 101.36” mentioned that dietary supplements containing live microbes must provide information on the quantity in terms of weight of the microorganisms on the label; this cell mass consists of both live and dead microorganisms. Later in 2018, these regulations were amended to consider “the quantitative amount of probiotic ingredients in a dietary supplement to be presented in terms of colony-forming units (CFUs) instead of by weight.” This also explains that the quantity in CFUs measures only the live micro-organisms and must not include inactive/dead/non-viable cells [231].
Challenges in Shelf-stability of Probiotics
The range of encapsulation techniques provides shelf-stable probiotics. However, regulatory issues arise with the shelf-stability of probiotics at ambient conditions during storage [32, 232, 233]. Most of the probiotic products in the market require refrigeration. Encapsulation techniques are well documented on enhanced stability and few reports suggested the ambient storage of encapsulated probiotics [28, 33, 234]. But the fact that even in encapsulated form, it should be refrigerated at least after opening the package to maintain the probiotic potency, because the humid conditions of the atmosphere cause metabolic fermentation or degradation [235]. Especially in probiotic fruit juices, acidic stress is a challenge due to high organic acid content requires refrigeration [28]. This challenge can be overcome by selecting the specific resilient genotypic probiotics (like spore-forming probiotics). The encapsulation approach together with the selective strain of probiotics will result in improved shelf-life even under non-refrigerated conditions.
Future Scope
The encapsulation of probiotics is well-studied by many researchers using various methods with different encapsulating materials. Polysaccharides or proteins dominate the field. Lipids are important and occasionally edible fats are added as additives (co-encapsulating materials) for the encapsulation of probiotics in emulsification/electrospraying/spray drying. Also, the self-assembly phenomenon of lipids offers a new encapsulation system called “liposomes.” Liposomal delivery systems can also be used as pH-responsive delivery of drug/vitamin/enzyme/antibody/antigen/gene, etc. It remains a challenge to encapsulate probiotics using the liposome-approach because of the large size (microscale) of probiotics [36]. However, considering their prospective benefits, liposomal delivery of probiotics and its implications need to be investigated.
On an application basis, probiotics play a vital role in the treatment of various diseases as discussed in earlier sections of this article. Interestingly, the role of probiotics in viral infections– “anti-viral probiotics” is a new concept in medical sciences. Recently many studies have focused on the use of probiotics for the treatment of acute respiratory tract infections, considering the science involved in intestinal–pulmonary cross-talks through the gut-lung axis [236, 237]. The immunomodulation and prophylaxis mechanisms of probiotics can be used in the treatment/prevention of viral infections. It is known that gut probiotics-mediated immunomodulation up-regulates the respiratory mucosal immunity by the secretion of cytokines and thereby prevents respiratory viral infections [238]. It is established that the probiotic strain E. faecium NCIMB 10415 has antiviral effects against enteropathogenic coronavirus [239]. There are several interventional clinical trials reported on the effect of probiotics against respiratory tract infections (NCT01782755, NCT03449459, NCT03636191, and NCT03683927).
Recently, probiotics are being recommended by China’s National Health Commission for patients with COVID-19 infection to maintain the balance of intestinal microbiota and prevent secondary bacterial infection [240]. Further, clinical trials have been registered under the Chinese clinical trial registry (ChiCTR2000029974) to evaluate the efficacy and safety of live Clostridium butyricum capsules and live Bacillus coagulans tablets for the treatment of patients affected with the novel coronavirus pneumonia and to study its action mechanism. ISAPP highlights the importance of dealing with harmful microorganisms using other microbes, explaining the concept as “germ warfare” (direct antagonism/non-specific immune effects/metabolic products). Growing awareness of probiotics and their novel findings may pave the way for solutions to better human health, and the role of encapsulation remains critical.
Conclusion
This comprehensive review explains the role of probiotics in human health and the need for encapsulating probiotics, to achieve the desired benefits. The need for encapsulation is explained by providing an understanding of the complex pathway and the series of stress-environments of the human oral-GI tract. The encapsulation of probiotics has proven potential in protecting probiotics and facilitating their target delivery. Encapsulation techniques have significant implications on probiotics and some of them have been explored only at the lab-level to date. A summary report on available commercial products and technologies involving probiotic foods is presented. Probiotics encapsulation, methods of detection of the targeted delivery, and the effects of encapsulation on the probiotics have been explained with key findings from recent studies, providing an up-to-date resource for researchers and industry professionals in this field. All of these include aspects of challenges in research that must be addressed for taking the technology from the lab to the industry.
References
Ansari JM, Colasacco C, Emmanouil E et al (2019) Strain-level diversity of commercial probiotic isolates of Bacillus, Lactobacillus, and Saccharomyces species illustrated by molecular identification and phenotypic profiling. PLoS One 14(3):e0213841. https://doi.org/10.1371/journal.pone.0213841
FAO/WHO (2006) Probiotics in food: health and nutritional properties and guidelines for evaluation. FAO Food and Nutrition Paper. http://www.fao.org/3/a0512e/a0512e.pdf. Accessed 8 Jul 2020
Singh BN, Raghubanshi AS, Koffas M, Gupta VK (2019) Microbial engineering biotechnologies. Biotechnol Adv 37:107399. https://doi.org/10.1016/j.biotechadv.2019.05.005
Kerry RG, Pradhan P, Samal D et al (2018) Probiotics: the ultimate nutritional supplement. In: Patra J, Das G, Shin HS (eds) Microbial Biotechnology. Springer, Singapore, pp 141–152. https://doi.org/10.1007/978-981-10-7140-9_7
Matejčeková Z, Vlková E, Liptáková D, Valík Ľ (2019) Preliminary screening of growth and viability of 10 strains of Bifidobacterium spp.: effect of media composition. Fermentation 5:38. https://doi.org/10.3390/fermentation5020038
Zheng J, Wittouck S, Salvetti E et al (2020) A taxonomic note on the genus Lactobacillus: Description of 23 novel genera, emended description of the genus Lactobacillus Beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae. Int J Syst Evol Microbiol 70:2782–2858. https://doi.org/10.1099/ijsem.0.004107
Pickard JM, Zeng MY, Caruso R, Núñez G (2017) Gut microbiota: role in pathogen colonization, immune responses, and inflammatory disease. Immunol Rev 279:70–89. https://doi.org/10.1111/imr.12567
Probiotic supplements market - global outlook and forecast 2020–2025. Market Research. https://www.marketresearch.com/Arizton-v4150/Probiotic-Supplements-Global-Outlook-Forecast-13297891/. Accessed 8 Jul 2020
Mordor Intelligence LLP (2019) Probiotics market - growth, trends, and forecast (2019 - 2024). https://www.mordorintelligence.com/industry-reports/global-probiotics-market-industry. Accessed 8 Jul 2020
Singh B, Mal G, Marotta F (2017) Designer probiotics: paving the way to living therapeutics. Trends Biotechnol 35:679–682. https://doi.org/10.1016/j.tibtech.2017.04.001
Meybodi N, Mortazavian A (2017) Probiotic supplements and food products: a comparative approach. Biochem Pharmacol 6:2. https://doi.org/10.4172/2167-0501.1000227
Mantzourani I, Chondrou P, Bontsidis C et al (2019) Assessment of the probiotic potential of lactic acid bacteria isolated from kefir grains: evaluation of adhesion and antiproliferative properties in vitro experimental systems. Ann Microbiol 69:751–763. https://doi.org/10.1007/s13213-019-01467-6
Kechagia M, Basoulis D, Konstantopoulou S et al (2013) Health benefit of probiotic a review. ISRN Nutr 2013:481651. https://doi.org/10.5402/2013/481651
Aspri M, Papademas P, Tsaltas D (2020) Review on non-dairy probiotics and their use in non-dairy based products. Ferment 6:30. https://doi.org/10.3390/fermentation6010030
Gueimonde M, Sánchez B (2012) Enhancing probiotic stability in industrial processes. Microb Ecol Health Dis 23. https://doi.org/10.3402/mehd.v23i0.18562
Mathipa MG, Thantsha MS (2017) Probiotic engineering: towards development of robust probiotic strains with enhanced functional properties and for targeted control of enteric pathogens. Gut Pathog 9:28. https://doi.org/10.1186/s13099-017-0178-9
Mazhar SF, Afzal M, Almatroudi A et al (2020) The prospects for the therapeutic implications of genetically engineered probiotics. J Food Qual 2020:9676452. https://doi.org/10.1155/2020/9676452
Global food encapsulation market outlook 2019–2024 - Micro-encapsulation of probiotics to drive growth - ResearchAndMarkets.com. Business Wire. https://www.businesswire.com/news/home/20190613005360/en/Global-Food-Encapsulation-Market-Outlook-2019-2024--. Accessed 8 Jul 2020
Food encapsulation market by shell material, technology, application, method, core phase and region - Global forecast to 2025. https://www.reportlinker.com/p03984708/Food-Encapsulation-Market-by-Shell-Material-Core-Phase-Technology-by-Region-Global-Forecasts-to.html?utm_source=PRN. Accessed 26 Jul 2020
Douillard FP, de Vos WM (2019) Biotechnology of health-promoting bacteria. Biotechnol Adv 37:107369. https://doi.org/10.1016/j.biotechadv.2019.03.008
Lee SH, Bajracharya R, Min JY et al (2020) Strategic approaches for colon targeted drug delivery: an overview of recent advancements. Pharmaceutics 12:68. https://doi.org/10.3390/pharmaceutics12010068
Kerry RG, Patra JK, Gouda S et al (2018) Benefaction of probiotics for human health: a review. J Food Drug Anal 26:927–939. https://doi.org/10.1016/j.jfda.2018.01.002
Carlson JL, Erickson JM, Lloyd BB, Slavin JL (2018) Health effects and sources of prebiotic dietary fiber. Curr Dev Nutr 2:nzy005. https://doi.org/10.1093/cdn/nzy005
Martins GN, Ureta MM, Tymczyszyn EE et al (2019) Technological aspects of the production of fructo and enzymatic synthesis and hydrolysis. Front Nutr 6:78. https://doi.org/10.3389/fnut.2019.00078
Sridharan S, Das KMS (2019) A study on suitable non dairy food matrix for probiotic bacteria–a systematic review. Curr Res Nutr Food Sci J 7:5–16. https://doi.org/10.12944/CRNFSJ.7.1.02
Ashwini A, Ramya HN, Ramkumar C et al (2019) Reactive mechanism and the applications of bioactive prebiotics for human health: review. J Microbiol Methods 159:128–137. https://doi.org/10.1016/j.mimet.2019.02.019
Delzenne NM, Olivares M, Neyrinck AM et al (2020) Nutritional interest of dietary fiber and prebiotics in obesity : lessons from the MyNewGut consortium. Clin Nutr 39:414–424. https://doi.org/10.1016/j.clnu.2019.03.002
Terpou A, Papadaki A, Lappa IK et al (2019) Probiotics in food systems: significance and emerging strategies towards improved viability and delivery of enhanced beneficial value. Nutrients 11:1591. https://doi.org/10.3390/nu11071591
Yang B, Lu P, Li M et al (2019) A meta-analysis of the effects of probiotics and synbiotics in children with acute diarrhea. Med (Baltimore) 98:e16618. https://doi.org/10.1097/MD.0000000000016618
Swanson KS, Gibson GR, Hutkins R et al (2020) The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of synbiotics. Nat Rev Gastroenterol Hepatol 17:687–701. https://doi.org/10.1038/s41575-020-0344-2
Vesterlund S, Salminen K, Salminen S (2012) Water activity in dry foods containing live probiotic bacteria should be carefully considered: a case study with Lactobacillus rhamnosus GG in flaxseed. Int J Food Microbiol 157:319–321. https://doi.org/10.1016/j.ijfoodmicro.2012.05.016
Cabello-Olmo M, Oneca M, Torre P et al (2020) Influence of storage temperature and packaging on bacteria and yeast viability in a plant-based fermented food. Foods 9:302
Weinbreck F, Bodnár I, Marco ML (2010) Can encapsulation lengthen the shelf-life of probiotic bacteria in dry products? Int J Food Microbiol 136:364–367. https://doi.org/10.1016/j.ijfoodmicro.2009.11.004
Šipailienė A, Petraitytė S (2018) Encapsulation of probiotics: proper selection of the probiotic strain and the influence of encapsulation technology and materials on the viability of encapsulated microorganisms. Probiotics Antimicrob Proteins 10:1–10. https://doi.org/10.1007/s12602-017-9347-x
Coghetto CC, Brinques GB, Ayub MAZ (2016) Probiotics production and alternative encapsulation methodologies to improve their viabilities under adverse environmental conditions. Int J Food Sci Nutr 67:929–943. https://doi.org/10.1080/09637486.2016.1211995
Sarao LK, Arora M (2015) Probiotics, prebiotics and microencapsulation–a review. Crit Rev Food Sci Nutr 57:344–371. https://doi.org/10.1080/10408398.2014.887055
NIH Human Microbiome Project defines normal bacterial makeup of the body. National Institutes of Health (NIH). In: Turn Discov into Heal. https://www.nih.gov/news-events/news-releases/nih-human-microbiome-project-defines-normal-bacterial-makeup-body. Accessed 30 Apr 2020
Juturu V, Wu JC (2018) Microbial production of bacteriocins: latest research development and applications. Biotechnol Adv 36:2187–2200. https://doi.org/10.1016/j.biotechadv.2018.10.007
Monteagudo-Mera A, Rastall RA, Gibson GR et al (2019) Adhesion mechanisms mediated by probiotics and prebiotics and their potential impact on human health. Appl Microbiol Biotechnol 103:6463–6472. https://doi.org/10.1007/s00253-019-09978-7
Molska M, Reguła J (2019) Potential mechanisms of probiotics action in the prevention and treatment of colorectal cancer. Nutrients 11:2453. https://doi.org/10.3390/nu11102453
Allain T, Chaouch S, Thomas M et al (2018) Bile-salt-hydrolases from the probiotic strain Lactobacillus johnsonii La1 mediate anti-giardial activity in vitro and in vivo. Front Microbiol 8:2707. https://doi.org/10.3389/fmicb.2017.02707
Skrypnik K, Bogdański P, Schmidt M, Suliburska J (2019) The effect of multispecies probiotic supplementation on iron status in rats. Biol Trace Elem Res 192:234–243. https://doi.org/10.1007/s12011-019-1658-1
Dubey MR, Patel VP (2018) Probiotics: a promising tool for calcium absorption. Open Nutr J 12:59–69. https://doi.org/10.2174/1874288201812010059
Gulzar N, Saleem IM, Rafiq S, Nadeem M (2019) Therapeutic potential of probiotics and prebiotics. In: Razzagh Mahmoudi (ed) Oral health by using probiotic products. Intech Open. https://doi.org/10.5772/intechopen.86762
Rodgers B, Kirley K, Mounsey A (2013) Prescribing an antibiotic? Pair it with probiotics. J Fam Pract 62:148–150 (PMID: 23520586)
Sudo N (2019) Role of gut microbiota in brain function and stress-related pathology. Biosci Microbiota, Food Heal 38:75–80. https://doi.org/10.12938/bmfh.19-006
Spencer NJ, Hu H (2020) Enteric nervous system: sensory transduction, neural circuits and gastrointestinal motility. Nat Rev Gastroenterol Hepatol 17:338–351. https://doi.org/10.1038/s41575-020-0271-2
Bermúdez-Humarán LG, Salinas E, Ortiz GG et al (2019) From probiotics to psychobiotics: live beneficial bacteria which act on the brain-gut axis. Nutrients 11:890. https://doi.org/10.3390/nu11040890
Kim CS, Shin DM (2019) Probiotic food consumption is associated with lower severity and prevalence of depression : a nationwide cross-sectional study. Nutrition 63:169–174. https://doi.org/10.1016/j.nut.2019.02.007
Dalile B, Van Oudenhove L, Vervliet B, Verbeke K (2019) The role of short-chain fatty acids in microbiota–gut–brain communication. Nat Rev Gastroenterol Hepatol 16:461–478. https://doi.org/10.1038/s41575-019-0157-3
Lavelle A, Sokol H (2020) Gut microbiota-derived metabolites as key actors in inflammatory bowel disease. Nat Rev Gastroenterol Hepatol 17:223–237. https://doi.org/10.1038/s41575-019-0258-z
Nazir Y, Hussain SA, Abdul Hamid A, Song Y (2018) Probiotics and their potential preventive and therapeutic role for cancer, high serum cholesterol, and allergic and HIV diseases. Biomed Res Int 2018:3428437. https://doi.org/10.1155/2018/3428437
Yan S, Tian Z, Li M et al (2019) Effects of probiotic supplementation on the regulation of blood lipid levels in overweight or obese subjects: a meta-analysis. Food Funct 10:1747–1759. https://doi.org/10.1039/C8FO02163E
Akgül T, Karakan T (2018) The role of probiotics in women with recurrent urinary tract infections. Turkish J Urol 44:377–383. https://doi.org/10.5152/tud.2018.48742
Atassi F, Ahn PV, Diane L, Moal LL (2019) Diverse expression of antimicrobial activities against bacterial vaginosis and urinary tract infection pathogens by cervicovaginal microbiota strains of Lactobacillus gasseri and Lactobacillus crispatus. Front Microbiol 10:2900. https://doi.org/10.3389/fmicb.2019.02900
van de Wijgert JHHM, Verwijs MC (2019) Lactobacilli-containing vaginal probiotics to cure or prevent bacterial or fungal vaginal dysbiosis : a systematic review and recommendations for future trial designs. BJOG An Int J Obstet Gynaecol 127:287–299. https://doi.org/10.1111/1471-0528.15870
Thomas RM, Jobin C (2019) Microbiota in pancreatic health and disease: the next frontier in microbiome research. Nat Rev Gastroenterol Hepatol 17:53–64. https://doi.org/10.1038/s41575-019-0242-7
Eguchi K, Fujitani N, Nakagawa H, Miyazaki T (2019) Prevention of respiratory syncytial virus infection with probiotic lactic acid bacterium Lactobacillus gasseri SBT2055. Sci Rep 9:4812. https://doi.org/10.1038/s41598-019-39602-7
Clua P, Kanmani P, Zelaya H et al (2017) Peptidoglycan from immunobiotic Lactobacillus rhamnosus improves resistance of infant mice to respiratory syncytial viral infection and secondary Pneumococcal Pneumonia. Front Immunol 8:948. https://doi.org/10.3389/fimmu.2017.00948
Percopo CM, Rice TA, Brenner TA et al (2015) Immunobiotic Lactobacillus administered post-exposure averts the lethal sequelae of respiratory virus infection. Antiviral Res 121:109–119. https://doi.org/10.1016/j.antiviral.2015.07.001
Konishi H, Fujiya M, Tanaka H et al (2016) Probiotic-derived ferrichrome inhibits colon cancer progression via JNK-mediated apoptosis. Nat Commun 7:12365. https://doi.org/10.1038/ncomms12365
Drago L, Nicola L, Iemoli E et al (2010) Strain-dependent release of cytokines modulated by Lactobacillus salivarius human isolates in an in vitro model. BMC Res Notes 3:44. https://doi.org/10.1186/1756-0500-3-44
Galdeano CM, Cazorla SI, Dumit JML et al (2019) Beneficial effects of probiotic consumption on the immune system. Ann Nutr Metab 74:115–124. https://doi.org/10.1159/000496426
Zhang Z, Tang H, Chen P et al (2019) Demystifying the manipulation of host immunity, metabolism, and extraintestinal tumors by the gut microbiome. Signal Transduct Target Ther 4:41. https://doi.org/10.1038/s41392-019-0074-5
Hendijani F, Akbari V (2018) Probiotic supplementation for management of cardiovascular risk factors in adults with type II diabetes: a systematic review and meta-analysis. Clin Nutr 37:532–541. https://doi.org/10.1016/j.clnu.2017.02.015
Mishra SP, Wang S, Nagpal R et al (2019) Probiotics and prebiotics for the amelioration of type 1 diabetes : present and future perspectives. Microorganisms 7:67. https://doi.org/10.3390/microorganisms7030067
Vasquez EC, Pereira TMC, Peotta VA et al (2019) Probiotics as beneficial dietary supplements to prevent and treat cardiovascular diseases : uncovering their impact on oxidative stress. Oxid Med Cell Longev 2019:3086270. https://doi.org/10.1155/2019/3086270
Aron-Wisnewsky J, Vigliotti C, Witjes J et al (2020) Gut microbiota and human NAFLD: disentangling microbial signatures from metabolic disorders. Nat Rev Gastroenterol Hepatol 17:279–297. https://doi.org/10.1038/s41575-020-0269-9
Górska A, Przystupski D, Niemczura MJ, Kulbacka J (2019) Probiotic bacteria : a promising tool in cancer prevention and therapy. Curr Microbiol 76:939–949. https://doi.org/10.1007/s00284-019-01679-8
Shu Z, Li P, Yu B et al (2020) The effectiveness of probiotics in prevention and treatment of cancer therapy-induced oral mucositis : A systematic review and meta-analysis. Oral Oncol 102:104559. https://doi.org/10.1016/j.oraloncology.2019.104559
Wong SH, Yu J (2019) Gut microbiota in colorectal cancer: mechanisms of action and clinical applications. Nat Rev Gastroenterol Hepatol 16:690–704. https://doi.org/10.1038/s41575-019-0209-8
Hu L, Zhou M, Young A et al (2019) In vivo effectiveness and safety of probiotics on prophylaxis and treatment of oral candidiasis: a systematic review and meta-analysis. BMC Oral Health 19:140. https://doi.org/10.1186/s12903-019-0841-2
Nyvad B, Takahashi N (2020) Integrated hypothesis of dental caries and periodontal diseases. J Oral Microbiol 12:1710953. https://doi.org/10.1080/20002297.2019.1710953
Aceti A, Beghetti I, Maggio L et al (2018) Filling the gaps: current research directions for a rational use of probiotics in preterm infants. Nutrients 10:1472. https://doi.org/10.3390/nu10101472
Sanders ME, Merenstein D, Merrifield CA, Hutkins R (2018) Probiotics for human use Nutr Bull 43:212–225. https://doi.org/10.1111/nbu.12334
Plaza-diaz J, Ruiz-ojeda FJ, Gil-campos M, Gil A (2019) Mechanisms of action of probiotics. Adv Nutr 10:S49–S66. https://doi.org/10.1093/advances/nmy063
Bron PA, Kleerebezem M, Brummer R et al (2017) Can probiotics modulate human disease by impacting intestinal barrier function? Br J Nutr 117:93–107. https://doi.org/10.1017/S0007114516004037
Suez J, Zmora N, Segal E, Elinav E (2019) The pros, cons, and many unknowns of probiotics. Nat Med 25:716–729. https://doi.org/10.1038/s41591-019-0439-x
Michael DR, Davies TS, Moss JWE et al (2017) The anti-cholesterolaemic effect of a consortium of probiotics: An acute study in C57BL/6J mice. Sci Rep 7:2883. https://doi.org/10.1038/s41598-017-02889-5
Hemarajata P, Versalovic J (2013) Therapeutic advances in gastroenterology effects of probiotics on gut microbiota : mechanisms of intestinal immunomodulation. Therap Adv Gastroenterol 6:39–51. https://doi.org/10.1177/1756283X12459294
Llewellyn A, Foey A (2017) Probiotic modulation of innate cell pathogen sensing and signaling events. Nutrients 9:1156. https://doi.org/10.3390/nu9101156
Luo J, Yang H, Song BL (2019) Mechanisms and regulation of cholesterol homeostasis. Nat Rev Mol Cell Biol 21:225–245. https://doi.org/10.1038/s41580-019-0190-7
Ma C, Zhang S, Lu J et al (2019) Screening for cholesterol-lowering probiotics from lactic acid bacteria isolated from corn silage based on three hypothesized pathways. Int J Mol Sci 20:2073. https://doi.org/10.3390/ijms20092073
Park S, Kang J, Choi S et al (2018) Cholesterol-lowering effect of Lactobacillus rhamnosus BFE5264 and its influence on the gut microbiome and propionate level in a murine model. PLoS One 13(8):e0203150. https://doi.org/10.1371/journal.pone.0203150
Vanhatalo A, Blackwell JR, Heureux L et al (2018) Nitrate-responsive oral microbiome modulates nitric oxide homeostasis and blood pressure in humans. Free Radic Biol Med 124:21–30. https://doi.org/10.1016/j.freeradbiomed.2018.05.078
Tungland B (2018) Short-chain fatty acid production and functional aspects on host metabolism. In: Tungland B (ed) Human microbiota in health and disease. Academic Press, Elsevier, pp 37–106. https://doi.org/10.1016/B978-0-12-814649-1.00002-8
Gu Q, Li P (2016) Biosynthesis of vitamins by probiotic bacteria. In: Rao V, Rao L (eds) Probiotics and prebiotics in human nutrition and health. Intech Open. https://doi.org/10.5772/63117
Ballini A, Gnoni A, De Vito D et al (2019) Effect of probiotics on the occurrence of nutrition absorption capacities in healthy children: a randomized double-blinded placebo-controlled pilot study. Eur Rev Med Pharmacol Sci 23:8645–8657. https://doi.org/10.26355/eurrev_201910_19182
Kumar S, Anukiruthika T, Dutta S et al (2020) Iron deficiency anemia: a comprehensive review on iron absorption, bioavailability and emerging food fortification approaches. Trends Food Sci Technol 99:58–75. https://doi.org/10.1016/j.tifs.2020.02.021
Hoppe M, Önning G, Berggren A, Hulthén L (2015) Probiotic strain Lactobacillus plantarum 299v increases iron absorption from an iron-supplemented fruit drink : a double-isotope cross-over single-blind study in women of reproductive age. Br J Nutr 114:1195–1202. https://doi.org/10.1017/S000711451500241X
Costanzo M, Cesi V, Palone F et al (2018) Krill oil, vitamin D and Lactobacillus reuteri cooperate to reduce gut inflammation. Benef Microbes 9:389–399. https://doi.org/10.3920/BM2017.0078
Sadeghi-Bojd S, Naghshizadian R, Mazaheri M et al (2019) Efficacy of probiotic prophylaxis after the first febrile urinary tract infection in children with normal urinary tracts. J Pediatric Infect Dis Soc 9:305–310. https://doi.org/10.1093/jpids/piz025
Chandel D, Sharma M, Chawla V et al (2019) Isolation, characterization and identification of antigenotoxic and anticancerous indigenous probiotics and their prophylactic potential in experimental colon carcinogenesis. Sci Rep 9:14769. https://doi.org/10.1038/s41598-019-51361-z
Wells JM, Mercenier A (2008) Mucosal delivery of therapeutic and prophylactic molecules using lactic acid bacteria. Nat Rev Microbiol 6:349–362. https://doi.org/10.1038/nrmicro1840
Panigrahi P, Parida S, Nanda NC et al (2017) A randomized synbiotic trial to prevent sepsis among infants in rural India. Nature 548:407–412. https://doi.org/10.1038/nature23480
Cieplak T, Soffer N, Sulakvelidze A, Nielsen DS (2018) A bacteriophage cocktail targeting Escherichia coli reduces E. coli in simulated gut conditions, while preserving a non-targeted representative commensal normal microbiota. Gut Microbes 9:391–399. https://doi.org/10.1080/19490976.2018.1447291
Broeckx G, Vandenheuvel D, Claes IJJ et al (2016) Drying techniques of probiotic bacteria as an important step towards the development of novel pharmabiotics. Int J Pharm 505:303–318. https://doi.org/10.1016/j.ijpharm.2016.04.002
Pop OL, Pop CR, Dufrechou M et al (2020) Edible films and coatings functionalization by probiotic incorporation : a review. Polymers (Basel) 12:12. https://doi.org/10.3390/polym12010012
Soares MB, Martinez RCR, Pereira EPR et al (2019) The resistance of Bacillus, Bifidobacterium, and Lactobacillus strains with claimed probiotic properties in different food matrices exposed to simulated gastrointestinal tract conditions. Food Res Int 125:108542. https://doi.org/10.1016/j.foodres.2019.108542
Sun X, Acquah C, Aluko RE, Udenigwe CC (2020) Considering food matrix and gastrointestinal effects in enhancing bioactive peptide absorption and bioavailability. J Funct Foods 64:103680. https://doi.org/10.1016/j.jff.2019.103680
Praepanitchai O-A, Noomhorm A, Anal AK (2019) Survival and behavior of encapsulated probiotics (Lactobacillus plantarum) in calcium-alginate-soy protein isolate-based hydrogel beads in different processing conditions (pH and temperature) and in pasteurized mango juice. Biomed Res Int 2019:9768152. https://doi.org/10.1155/2019/9768152
Hossain MS, Al-Bari MAA, Mahmud ZH, Wahed MII (2016) Antibiotic resistant microencapsulated probiotics synergistically preserved orange juice. BMC Nutr 2:59. https://doi.org/10.1186/s40795-016-0098-y
Corrêa-Filho LC, Moldão-Martins M, Alves VD (2019) Advances in the application of microcapsules as carriers of functional compounds for food products. Appl Sci 9:571. https://doi.org/10.3390/app9030571
El-Salam MHA, El-Shibiny S (2015) Preparation and properties of milk proteins-based encapsulated probiotics: a review. Dairy Sci Technol 95:393–412. https://doi.org/10.1007/s13594-015-0223-8
Dianawati D, Mishra V, Shah NP (2016) Survival of microencapsulated probiotic bacteria after processing and during storage: a review. Crit Rev Food Sci Nutr 56:1685–1716. https://doi.org/10.1080/10408398.2013.798779
Rajam R, Anandharamakrishnan C (2015) Microencapsulation of Lactobacillus plantarum (MTCC 5422) with fructooligosaccharide as wall material by spray drying. LWT 60:773–780. https://doi.org/10.1016/j.lwt.2014.09.062
Agudelo J, Cano A, González-Martínez C, Chiralt A (2017) Disaccharide incorporation to improve survival during storage of spray dried Lactobacillus rhamnosus in whey protein-maltodextrin carriers. J Funct Foods 37:416–423. https://doi.org/10.1016/j.jff.2017.08.014
Nunes GL, de Araújo EM, Cichoski AJ et al (2018) Inulin, hi-maize, and trehalose as thermal protectants for increasing viability of Lactobacillus acidophilus encapsulated by spray drying. LWT 89:128–133. https://doi.org/10.1016/j.lwt.2017.10.032
de Almeida PD, Martins EMF, de Almeida CN et al (2019) Use of gelatin and gum arabic for microencapsulation of probiotic cells from Lactobacillus plantarum by a dual process combining double emulsification followed by complex coacervation. Int J Biol Macromol 133:722–731. https://doi.org/10.1016/j.ijbiomac.2019.04.110
Rajam R, Karthik P, Parthasarathi S et al (2012) Effect of whey protein – alginate wall systems on survival of microencapsulated Lactobacillus plantarum in simulated gastrointestinal conditions. J Funct Foods 4:891–898. https://doi.org/10.1016/j.jff.2012.06.006
Fang Y, Kennedy B, Rivera T et al (2017) Encapsulation system for protection of probiotics during processing. U.S. Patent No. 9,788,563. 17 Oct 2017
Del Piano M, Carmagnola S, Andorno S et al (2010) Evaluation of the intestinal colonization by microencapsulated probiotic bacteria in comparison with the same uncoated strains. J Clin Gastroenterol 44:42–46. https://doi.org/10.1097/MCG.0b013e3181ed0e71
Anselmo AC, McHugh KJ, Webster J et al (2016) Layer-by-layer encapsulation of probiotics for delivery to the microbiome. Adv Mater 28:9486–9490. https://doi.org/10.1002/adma.201603270
Pradeep Prasanna PH, Charalampopoulos D (2019) Encapsulation in an alginate–goats’ milk–inulin matrix improves survival of probiotic Bifidobacterium in simulated gastrointestinal conditions and goats’ milk yoghurt. Int J Dairy Technol 72:132–141. https://doi.org/10.1111/1471-0307.12568
Afzaal M, Saeed F, Arshad MU et al (2019) The effect of encapsulation on the stability of probiotic bacteria in ice cream and simulated gastrointestinal conditions. Probiotics Antimicrob Proteins 11:1348–1354. https://doi.org/10.1007/s12602-018-9485-9
Muzzafar A, Sharma V (2018) Microencapsulation of probiotics for incorporation in cream biscuits. J Food Meas Charact 12:2193–2201. https://doi.org/10.1007/s11694-018-9835-z
Li Z, Behrens AM, Ginat N et al (2018) Biofilm-inspired encapsulation of probiotics for the treatment of complex infections. Adv Mater 30(51):e1803925. https://doi.org/10.1002/adma.201803925
Qi W, Liang X, Yun T, Guo W (2019) Growth and survival of microencapsulated probiotics prepared by emulsion and internal gelation. J Food Sci Technol 56:1398–1404. https://doi.org/10.1007/s13197-019-03616-w
Cao Z, Wang X, Pang Y et al (2019) Biointerfacial self-assembly generates lipid membrane coated bacteria for enhanced oral delivery and treatment. Nat Commun 10:5783. https://doi.org/10.1038/s41467-019-13727-9
Lavanya MN, Kathiravan T, Moses JA, Anandharamakrishnan C (2019) Influence of spray-drying conditions on microencapsulation of fish oil and chia oil. Dry Technol 38:279–292. https://doi.org/10.1080/07373937.2018.1553181
Anandharamakrishnan C, Padma Ishwarya S (2015) Spray drying techniques for food ingredient encapsulation. John Wiley & Sons, Canada
Gover Antoniraj M, Maria Leena M, Moses JA, Anandharamakrishnan C (2019) Cross-linked chitosan microparticles preparation by modified three fluid nozzle spray drying approach. Int J Biol Macromol 147:1268–1277. https://doi.org/10.1016/j.ijbiomac.2019.09.254
Maria Leena M, Gover Antoniraj M, Moses JA, Anandharamakrishnan C (2020) Three fluid nozzle spray drying for co-encapsulation of curcumin and resveratrol and controlled release. J Drug Deliv Sci Technol 57:101678. https://doi.org/10.1016/j.jddst.2020.101678
Anandharamakrishnan C, Rielly CD, Stapley AGF (2008) Loss of solubility of α-lactalbumin and β-lactoglobulin during the spray drying of whey proteins. LWT 41:270–277. https://doi.org/10.1016/j.lwt.2007.03.004
Behboudi-Jobbehdar S, Soukoulis C, Yonekura L, Fisk I (2013) Optimization of spray-drying process conditions for the production of maximally viable microencapsulated L. acidophilus NCIMB 701748. Dry Technol 31:1274–1283. https://doi.org/10.1080/07373937.2013.788509
Burns P, Alard J, Hrdỳ J et al (2017) Spray-drying process preserves the protective capacity of a breast milk-derived Bifidobacterium lactis strain on acute and chronic colitis in mice. Sci Rep 7:43211. https://doi.org/10.1038/srep43211
Avila-Reyes SV, Garcia-Suarez FJ, Jiménez MT et al (2014) Protection of L. rhamnosus by spray-drying using two prebiotics colloids to enhance the viability. Carbohydr Polym 102:423–430. https://doi.org/10.1016/j.carbpol.2013.11.033
Yoha KS, Moses JA, Anandharamakrishnan C (2020) Effect of encapsulation methods on the physicochemical properties and the stability of Lactobacillus plantarum (NCIM 2083) in synbiotic powders and in-vitro digestion conditions. J Food Eng 283:110033. https://doi.org/10.1016/j.jfoodeng.2020.110033
Ma X, Wang X, Cheng J et al (2015) Microencapsulation of Bacillus subtilis B99–2 and its biocontrol efficiency against Rhizoctonia solani in tomato. Biol Control 90:34–41. https://doi.org/10.1016/j.biocontrol.2015.05.013
Ceballos AM, Giraldo GI, Orrego CE (2012) Effect of freezing rate on quality parameters of freeze dried soursop fruit pulp. J Food Eng 111:360–365. https://doi.org/10.1016/j.jfoodeng.2012.02.010
Jesus S, Borchard G, Borges O (2013) Freeze dried chitosan/poly-ε-caprolactone and poly-ε-caprolactone nanoparticles: evaluation of their potential as DNA and antigen delivery systems. J Genet Syndr Gene Ther 4:1000164. https://doi.org/10.4172/2157-7412.1000164
Li B, Tian F, Liu X et al (2011) Effects of cryoprotectants on viability of Lactobacillus reuteri CICC6226. Appl Microbiol Biotechnol 92:609–616. https://doi.org/10.1007/s00253-011-3269-4
Marcial-Coba MS, Cieplak T, Cahú TB et al (2018) Viability of microencapsulated Akkermansia muciniphila and Lactobacillus plantarum during freeze-drying, storage andin vitro simulated upper gastrointestinal tract passage. Food Funct 9:5868–5879. https://doi.org/10.1039/C8FO01331D
Ray S, Raychaudhuri U, Chakraborty R (2016) An overview of encapsulation of active compounds used in food products by drying technology. Food Biosci 13:76–83. https://doi.org/10.1016/j.fbio.2015.12.009
Rajam R, Kumar SB, Prabhasankar P, Anandharamakrishnan C (2015) Microencapsulation of Lactobacillus plantarum MTCC 5422 in fructooligosaccharide and whey protein wall systems and its impact on noodle quality. J Food Sci Technol 52:4029–4041. https://doi.org/10.1007/s13197-014-1506-4
Perez-Gago MB, Krochta JM (2001) Denaturation time and temperature effects on solubility, tensile properties, and oxygen permeability of whey protein edible films. J Food Sci 66:705–710. https://doi.org/10.1111/j.1365-2621.2001.tb04625.x
Barbosa J, Borges S, Amorim M et al (2015) Comparison of spray drying, freeze drying and convective hot air drying for the production of a probiotic orange powder. J Funct Foods 17:340–351. https://doi.org/10.1016/j.jff.2015.06.001
Zuidam NJ, Shimoni E (2010) Overview of microencapsulates for use in food products or processes and methods to make them. In: Viktor N (ed) Zuidam NJ. Encapsulation technologies for active food ingredients and food processing. Springer-Verlag, New York, pp 3–29
Krasaekoopt W, Bhandari B (2012) Properties and applications of different probiotic delivery systems. In: Garti N, McClements DJ (eds) Encapsulation technologies and delivery systems for food ingredients and nutraceuticals. Woodhead Publishing, Elsevier, pp 541–594
Yao M, Li B, Ye H et al (2018) Enhanced viability of probiotics (Pediococcus pentosaceus Li05) by encapsulation in microgels doped with inorganic nanoparticles. Food Hydrocoll 83:246–252. https://doi.org/10.1016/j.foodhyd.2018.05.024
Sakai T, Moteki Y, Takahashi T et al (2018) Probiotics into outer space: feasibility assessments of encapsulated freeze-dried probiotics during 1 month’s storage on the International Space Station. Sci Rep 8:10687. https://doi.org/10.1038/s41598-018-29094-2
Ben TM, Vaidyanathan M, Radhakrishnan K, Raichur AM (2014) Enhanced viability of probiotic Saccharomyces boulardii encapsulated by layer-by-layer approach in pH responsive chitosan – dextran sulfate polyelectrolytes. J Food Eng 136:1–8. https://doi.org/10.1016/j.jfoodeng.2014.03.015
Bora AFM, Li X, Zhu Y, Du L (2018) Improved viability of microencapsulated probiotics in a freeze-dried banana powder during storage and under simulated gastrointestinal tract. Probiotics Antimicrob Proteins 11:1330–1339. https://doi.org/10.1007/s12602-018-9464-1
Heidebach T, Först P, Kulozik U (2010) Influence of casein-based microencapsulation on freeze-drying and storage of probiotic cells. J Food Eng 98:309–316. https://doi.org/10.1016/j.jfoodeng.2010.01.003
Zou Q, Zhao J, Liu X et al (2011) Microencapsulation of Bifidobacterium bifidum F-35 in reinforced alginate microspheres prepared by emulsification/internal gelation. Int J Food Sci Technol 46:1672–1678. https://doi.org/10.1111/j.1365-2621.2011.02685.x
Corcoran BM, Ross RP, Fitzgerald GF, Stanton C (2004) Comparative survival of probiotic Lactobacilli spray-dried in the presence of prebiotic substances. J Appl Microbiol 96:1024–1039. https://doi.org/10.1111/j.1365-2672.2004.02219.x
Moayyedi M, Eskandari MH, Rad AHE et al (2018) Effect of drying methods (electrospraying, freeze drying and spray drying) on survival and viability of microencapsulated Lactobacillus rhamnosus ATCC 7469. J Funct Foods 40:391–399. https://doi.org/10.1016/j.jff.2017.11.016
Halim M, Mustafa NAM, Othman M et al (2017) Effect of encapsulant and cryoprotectant on the viability of probiotic Pediococcus acidilactici ATCC 8042 during freeze-drying and exposure to high acidity, bile salts and heat. LWT 81:210–216. https://doi.org/10.1016/j.lwt.2017.04.009
Ming LC, Rahim RA, Wan HY, Ariff AB (2009) Formulation of protective agents for improvement of Lactobacillus salivarius I 24 survival rate subjected to freeze drying for production of live cells in powderized form. Food Bioprocess Technol 2:431. https://doi.org/10.1007/s11947-009-0184-0
Eckert C, Agnol WD, Dallé D et al (2018) Development of alginate-pectin microparticles with dairy whey using vibration technology: effects of matrix composition on the protection of Lactobacillus spp. from adverse conditions. Food Res Int 113:65–73. https://doi.org/10.1016/j.foodres.2018.07.001
Burgain J, Gaiani C, Linder SJ (2011) Encapsulation of probiotic living cells: from laboratory scale to industrial applications. J Food Eng 104:467–483. https://doi.org/10.1016/j.jfoodeng.2010.12.031
Poletto G, Raddatz GC, Cichoski AJ et al (2019) Study of viability and storage stability of Lactobacillus acidophillus when encapsulated with the prebiotics rice bran, inulin and Hi-maize. Food Hydrocoll 95:238–244. https://doi.org/10.1016/j.foodhyd.2019.04.049
Jantarathin S, Borompichaichartkul C, Sanguandeekul R (2017) Microencapsulation of probiotic and prebiotic in alginate-chitosan capsules and its effect on viability under heat process in shrimp feeding. Mater Today Proc 4:6166–6172. https://doi.org/10.1016/j.matpr.2017.06.111
Silva MP, Tulini FL, Martins E et al (2018) Comparison of extrusion and co-extrusion encapsulation techniques to protect Lactobacillus acidophilus LA3 in simulated gastrointestinal fluids. LWT 89:392–399. https://doi.org/10.1016/j.lwt.2017.11.008
Gul O, Dervisoglu M (2017) Application of multicriteria decision technique to determine optimum sodium alginate concentration for microencapsulation of Lactobacillus casei Shirota by extrusion and emulsification. J Food Process Eng 40:e12481. https://doi.org/10.1111/jfpe.12481
Heidebach T, Först P, Kulozik U (2009) Microencapsulation of probiotic cells by means of rennet-gelation of milk proteins. Food Hydrocoll 23:1670–1677. https://doi.org/10.1016/j.foodhyd.2009.01.006
Mokarram RR, Mortazavi SA, Najafi MBH, Shahidi F (2009) The influence of multi stage alginate coating on survivability of potential probiotic bacteria in simulated gastric and intestinal juice. Food Res Int 42:1040–1045. https://doi.org/10.1016/j.foodres.2009.04.023
Gbassi GK, Vandamme T (2012) Probiotic encapsulation technology: from microencapsulation to release into the gut. Pharmaceutics 4:149–163. https://doi.org/10.3390/pharmaceutics4010149
Eghbal N, Yarmand MS, Mousavi M et al (2016) Complex coacervation for the development of composite edible films based on LM pectin and sodium caseinate. Carbohydr Polym 151:947–956. https://doi.org/10.1016/j.carbpol.2016.06.052
Eratte D, Mcknight S, Gengenbach T et al (2015) Co-encapsulation and characterisation of omega-3 fatty acids and probiotic bacteria in whey protein isolate–gum Arabic complex coacervates. J Funct Foods 19:882–892. https://doi.org/10.1016/j.jff.2015.01.037
Lotfipour F, Maghsoodi M (2012) Evaluation of the effect of CaCl2 and alginate concentrations and hardening time on the characteristics of Lactobacillus acidophilus loaded alginate beads using response surface analysis. Adv Pharm Bull 2:71–78. https://doi.org/10.5681/apb.2012.010
Zhang Y, Lin J, Zhong Q (2015) The increased viability of probiotic Lactobacillus salivarius NRRL B-30514 encapsulated in emulsions with multiple lipid-protein-pectin layers. Food Res Int 71:9–15. https://doi.org/10.1016/j.foodres.2015.02.017
McClements DJ (2004) Protein-stabilized emulsions. Curr Opin Colloid Interface Sci 9:305–313. https://doi.org/10.1016/j.cocis.2004.09.003
Holkem AT, Raddatz GC, Nunes GL et al (2016) Development and characterization of alginate microcapsules containing Bifidobacterium BB-12 produced by emulsification/internal gelation followed by freeze drying. LWT 71:302–308. https://doi.org/10.1016/j.lwt.2016.04.012
Martín MJ, Lara-Villoslada F, Ruiz MA, Morales ME (2015) Microencapsulation of bacteria: a review of different technologies and their impact on the probiotic effects. Innov Food Sci Emerg Technol 27:15–25. https://doi.org/10.1016/j.ifset.2014.09.010
Rajam R, Anandharamakrishnan C (2015) Spray freeze drying method for microencapsulation of Lactobacillus plantarum. J Food Eng 166:95–103. https://doi.org/10.1016/j.jfoodeng.2015.05.029
Semyonov D, Ramon O, Kaplun Z et al (2010) Microencapsulation of Lactobacillus paracasei by spray freeze drying. Food Res Int 43:193–202. https://doi.org/10.1016/j.foodres.2009.09.028
Levine H (1992) Another view of trehalose for drying and stabilizing biological materials. BioPharm 36–40. NAID: 10021025728
Meng XC, Stanton C, Fitzgerald GF et al (2008) Anhydrobiotics: the challenges of drying probiotic cultures. Food Chem 106:1406–1416. https://doi.org/10.1016/j.foodchem.2007.04.076
Cao L, Xu Q, Xing Y et al (2019) Effect of skimmed milk powder concentrations on the biological characteristics of microencapsulated Saccharomyces cerevisiae by vacuum-spray-freeze-drying. Dry Technol 38:476–494. https://doi.org/10.1080/07373937.2019.1581797
Raghavi LM, Moses JA, Anandharamakrishnan C (2018) Refractance window drying of foods: a review. J Food Eng 222:267–275. https://doi.org/10.1016/j.jfoodeng.2017.11.032
Zotarelli MF, Carciofi BAM, Laurindo JB (2015) Effect of process variables on the drying rate of mango pulp by refractance window. Food Res Int 69:410–417. https://doi.org/10.1016/j.foodres.2015.01.013
Nindo CI, Feng H, Shen GQ et al (2003) Energy utilization and microbial reduction in a new film drying system. J Food Process Preserv 27:117–136. https://doi.org/10.1111/j.1745-4549.2003.tb00506.x
Ochoa-Martínez CI, Quintero PT, Ayala AA, Ortiz MJ (2012) Drying characteristics of mango slices using the refractance windowTM technique. J Food Eng 109:69–75. https://doi.org/10.1016/j.jfoodeng.2011.09.032
Rostami H, Dehnad D, Jafari SM, Tavakoli HR (2017) Evaluation of physical, rheological, microbial, and organoleptic properties of meat powder produced by refractance window drying. Dry Technol 36:1076–1085. https://doi.org/10.1080/07373937.2017.1377224
Forero DP, Orrego CE, Peterson DG, Osorio C (2015) Chemical and sensory comparison of fresh and dried lulo (Solanum quitoense Lam.) fruit aroma. Food Chem 169:85–91. https://doi.org/10.1016/j.foodchem.2014.07.111
Yoha KS, Moses JA, Anandharamakrishnan C (2020) Conductive hydro drying through refractance window drying – an alternative technique for drying of Lactobacillus plantarum (NCIM 2083). Dry Technol 38:610–620. https://doi.org/10.1080/07373937.2019.1624972
Librán CM, Castro S, Lagaron JM (2017) Encapsulation by electrospray coating atomization of probiotic strains. Innov Food Sci Emerg Technol 39:216–222. https://doi.org/10.1016/j.ifset.2016.12.013
Feng K, Zhai MY, Zhang Y et al (2018) Improved viability and thermal stability of the probiotics encapsulated in a novel electrospun fiber mat. J Agric Food Chem 66:10890–10897. https://doi.org/10.1021/acs.jafc.8b02644
Gomez-Mascaraque LG, Morfin RC, Pérez-Masiá R et al (2016) Optimization of electrospraying conditions for the microencapsulation of probiotics and evaluation of their resistance during storage and in-vitro digestion. LWT 69:438–446. https://doi.org/10.1016/j.lwt.2016.01.071
Škrlec K, Zupančič Š, Prpar Mihevc S et al (2019) Development of electrospun nanofibers that enable high loading and long-term viability of probiotics. Eur J Pharm Biopharm 136:108–119. https://doi.org/10.1016/j.ejpb.2019.01.013
Gómez-Mascaraque LG, Ambrosio-Martín J, Perez-Masiá R, Lopez-Rubio A (2017) Impact of acetic acid on the survival of L. plantarum upon microencapsulation by coaxial electrospraying. J Healthc Eng 2017:4698079. https://doi.org/10.1155/2017/4698079
Zaeim D, Sarabi-Jamab M, Ghorani B, Kadkhodaee R (2019) Double layer co-encapsulation of probiotics and prebiotics by electro-hydrodynamic atomization. LWT 110:102–109. https://doi.org/10.1016/j.lwt.2019.04.040
Amna T, Hassan M, shamshi, Pandeya D, et al (2013) Classy non-wovens based on animate L. gasseri – inanimate poly(vinyl alcohol): upstream application in food engineering. Appl Microbiol Biotechnol 97:4523–4531. https://doi.org/10.1007/s00253-012-4666-z
López-Rubio A, Sanchez E, Wilkanowicz S et al (2012) Electrospinning as a useful technique for the encapsulation of living Bifidobacteria in food hydrocolloid. Food Hydrocoll 28:159–167. https://doi.org/10.1016/j.foodhyd.2011.12.008
Liu S-C, Li R, Tomasula PM et al (2016) Electrospun Food-Grade Ultrafine Fibers from Pectin and Pullulan Blends. Food Nutr Sci 07:636–646. https://doi.org/10.4236/fns.2016.77065
Lancuški A, Abu Ammar A, Avrahami R et al (2017) Design of starch-formate compound fibers as encapsulation platform for biotherapeutics. Carbohydr Polym 158:68–76. https://doi.org/10.1016/j.carbpol.2016.12.003
Akbar Z, Zahoor T, Huma N et al (2018) Electrospun probiotics: an alternative for encapsulation. J Biol Regul Homeost Agents 32:1551–1556 (PMID: 30574764)
Nachal N, Moses JA, Karthik P, Anandharamakrishnan C (2019) Applications of 3D printing in food industry: a review. Food Eng Rev 11:123–141. https://doi.org/10.1007/s12393-019-09199-8
Anukiruthika T, Moses JA, Anandharamakrishnan C (2020) 3D printing of egg yolk and white with rice flour blends. J Food Eng 265:109691. https://doi.org/10.1016/j.jfoodeng.2019.109691
Krishnaraj P, Anukiruthika T, Choudhary P et al (2019) 3D extrusion printing and post-processing of fibre-rich snack from indigenous composite flour. Food Bioprocess Technol 12:1776–1786. https://doi.org/10.1007/s11947-019-02336-5
Liu Z, Bhandari B, Zhang M (2020) Incorporation of probiotics (Bifidobacterium animalis subsp. Lactis) into 3D printed mashed potatoes: effects of variables on the viability. Food Res Int 128:108795. https://doi.org/10.1016/j.foodres.2019.108795
Zhang L, Lou Y, Schutyser MAI (2018) 3D printing of cereal-based food structures containing probiotics. Food Struct 18:14–22. https://doi.org/10.1016/j.foostr.2018.10.002
Terekhov SS, Smirnov IV, Malakhova MV et al (2018) Ultrahigh-throughput functional profiling of microbiota communities. Proc Natl Acad Sci 115:9551–9556. https://doi.org/10.1073/pnas.1811250115
Chen J, Vestergaard M, Shen J et al (2018) Droplet-based microfluidics as a future tool for strain improvement in lactic acid bacteria. FEMS Microbiol Lett 365:fny258. https://doi.org/10.1093/femsle/fny258
Villa M, Bloom RJ, Silverman JD et al (2019) High-throughput isolation and culture of human gut bacteria with droplet microfluidics. bioRxiv 630822. https://doi.org/10.1101/630822
Zhang Y, Lin J, Zhong Q (2016) S/O/W emulsions prepared with sugar beet pectin to enhance the viability of probiotic Lactobacillus salivarius NRRL B-30514. Food Hydrocoll 52:804–810. https://doi.org/10.1016/j.foodhyd.2015.08.020
Mainville I, Arcand Y, Farnworth ER (2005) A dynamic model that simulates the human upper gastrointestinal tract for the study of probiotics. Int J Food Microbiol 99:287–296. https://doi.org/10.1016/j.ijfoodmicro.2004.08.020
Ainsley Reid A, Vuillemard JC, Britten M et al (2005) Microentrapment of probiotic bacteria in a Ca2+ - induced whey protein gel and effects on their viability in a dynamic gastro-intestinal model. J Microencapsul 22:603–619. https://doi.org/10.1080/02652040500162840
Tompkins T, Mainville I, Arcand Y (2011) The impact of meals on a probiotic during transit through a model of the human upper gastrointestinal tract. Benef Microbes 2:295–303. https://doi.org/10.3920/BM2011.0022
Aragón-Rojas S, Hernández-Álvarez AJ, Mainville I et al (2020) Effect of the carrier material, drying technology and dissolution media on the viability of Lactobacillus fermentum K73 during simulated gastrointestinal transit. Food Funct 11:2339–2348. https://doi.org/10.1039/c9fo01091b
Urbanska AM, Bhathena J, Prakash S (2007) Live encapsulated Lactobacillus acidophilus cells in yogurt for therapeutic oral delivery: preparation and in vitro analysis of alginate–chitosan microcapsules. Can J Physiol Pharmacol 85:884–893. https://doi.org/10.1139/Y07-057
Pham VT, Mohajeri MH (2018) The application of in vitro human intestinal models on the screening and development of pre-and probiotics. Benef Microbes 9:725–742. https://doi.org/10.3920/BM2017.0164
Patrignani F, Siroli L, Parolin C et al (2019) Use of Lactobacillus crispatus to produce a probiotic cheese as potential gender food for preventing gynaecological infections. PLoS ONE 14:e0208906. https://doi.org/10.1371/journal.pone.0208906
Yao M, Xie J, Du H et al (2020) Progress in microencapsulation of probiotics: a review. Compr Rev Food Sci Food Saf 19(2):857–874. https://doi.org/10.1111/1541-4337.12532
Gil-Sánchez I, Cueva C, Tamargo A et al (2020) Application of the dynamic gastrointestinal simulator (simgi®) to assess the impact of probiotic supplementation in the metabolism of grape polyphenols. Food Res Int 129:108790. https://doi.org/10.1016/j.foodres.2019.108790
Cueva C, Gil-Sánchez I, Tamargo A et al (2019) Gastrointestinal digestion of food-use silver nanoparticles in the dynamic simulator of the gastrointestinal tract (simgi®). Impact on human gut microbiota. Food Chem Toxicol 132:110657. https://doi.org/10.1016/j.fct.2019.110657
Dupont D, Alric M, Blanquet-Diot S et al (2019) Can dynamic in vitro digestion systems mimic the physiological reality? Crit Rev Food Sci Nutr 59:1546–1562. https://doi.org/10.1080/10408398.2017.1421900
Marteau PM, Minekus M, Havenaar R, Huis JHJ (1997) Survival of lactic acid bacteria in a dynamic model of the stomach and small intestine: validation and the effects of bile. J Dairy Sci 80:1031–1037. https://doi.org/10.3168/jds.S0022-0302(97)76027-2
Blanquet-Diot S, Denis S, Chalancon S et al (2012) Use of artificial digestive systems to investigate the biopharmaceutical factors influencing the survival of probiotic yeast during gastrointestinal transit in humans. Pharm Res 29:1444–1453. https://doi.org/10.1007/s11095-011-0620-5
Cordonnier C, Thévenot J, Etienne-Mesmin L et al (2015) Dynamic in vitro models of the human gastrointestinal tract as relevant tools to assess the survival of probiotic strains and their interactions with gut microbiota. Microorganisms 3:725–745. https://doi.org/10.3390/microorganisms3040725
Venema K, Verhoeven J, Verbruggen S et al (2019) Probiotic survival during a multi-layered tablet development as tested in a dynamic, computer-controlled in vitro model of the stomach and small intestine (TIM-1). Lett Appl Microbiol 69:325–332. https://doi.org/10.1111/lam.13211
Sumeri I, Arike L, Adamberg K, Paalme T (2008) Single bioreactor gastrointestinal tract simulator for study of survival of probiotic bacteria. Appl Microbiol Biotechnol 80:317–324. https://doi.org/10.1007/s00253-008-1553-8
Adouard N, Fujimoto J, Oki K et al (2019) Toward an accessible and robust in vitro approach to evaluate bacterial viability in the upper gastro-intestinal tract: a gastro-intestinal digestive simulator (GIDS) combined with alternative methods to plating. J Funct Foods 59:30–39. https://doi.org/10.1016/j.jff.2019.05.026
Moumita S, Goderska K, Johnson EM et al (2017) Evaluation of the viability of free and encapsulated lactic acid bacteria using in-vitro gastro intestinal model and survivability studies of synbiotic microcapsules in dry food matrix during storage. LWT 77:460–467. https://doi.org/10.1016/j.lwt.2016.11.079
Gbassi GK, Vandamme T, Yolou FS, Marchioni E (2011) In vitro effects of pH, bile salts and enzymes on the release and viability of encapsulated Lactobacillus plantarum strains in a gastrointestinal tract model. Int Dairy J 21:97–102. https://doi.org/10.1016/j.idairyj.2010.09.006
Parthasarathi S, Bhushani JA, Anandharamakrishnan C (2018) Engineered small intestinal system as an alternative to in-situ intestinal permeability model. J Food Eng 222:110–114. https://doi.org/10.1016/j.jfoodeng.2017.11.019
Jayan H, Maria Leena M, Sivakama Sundari SK et al (2019) Improvement of bioavailability for resveratrol through encapsulation in zein using electrospraying technique. J Funct Foods 57:417–424. https://doi.org/10.1016/j.jff.2019.04.007
Sharma A, Arora M, Goyal AK, Rath G (2017) Spray dried formulation of 5-fluorouracil embedded with probiotic biomass: in vitro and in vivo studies. Probiotics Antimicrob Proteins 9:310–322. https://doi.org/10.1007/s12602-017-9258-x
Dinoto A, Suksomcheep A, Ishizuka S et al (2006) Modulation of rat cecal microbiota by administration of raffinose and encapsulated Bifidobacterium breve. Appl Environ Microbiol 72:784–792. https://doi.org/10.1128/AEM.72.1.784-792.2006
Coelho-Rocha ND, de Castro CP, de Jesus LCL et al (2018) Microencapsulation of lactic acid bacteria improves the gastrointestinal delivery and in situ expression of recombinant fluorescent protein. Front Microbiol 9:2398. https://doi.org/10.3389/fmicb.2018.02398
Mai V, Waugh S, Byrd D et al (2017) Novel encapsulation improves recovery of probiotic strains in fecal samples of human volunteers. Appl Microbiol Biotechnol 101:1419–1425. https://doi.org/10.1007/s00253-016-7915-8
Arioli S, Koirala R, Taverniti V et al (2018) Quantitative recovery of viable Lactobacillus paracasei CNCM I-1572 (L. casei DG®) after gastrointestinal passage in healthy adults. Front Microbiol 9:1720. https://doi.org/10.3389/fmicb.2018.01720
Nambiar RB, Sellamuthu PS, Perumal AB (2018) Development of milk chocolate supplemented with microencapsulated Lactobacillus plantarum HM47 and to determine the safety in a Swiss albino mice model. Food Control 94:300–306. https://doi.org/10.1016/j.foodcont.2018.07.024
Ayyanna R, Ankaiah D, Arul V (2018) Anti-inflammatory and antioxidant properties of probiotic bacterium Lactobacillus mucosae AN1 and Lactobacillus fermentum SNR1 in Wistar albino rats. Front Microbiol 9:3063. https://doi.org/10.3389/fmicb.2018.03063
Wang Y, Dong Z, Song D et al (2018) Effects of microencapsulated probiotics and prebiotics on growth performance, antioxidative abilities, immune functions, and caecal microflora in broiler chickens. Food Agric Immunol 29:859–869. https://doi.org/10.1080/09540105.2018.1463972
de Simone C (2019) The unregulated probiotic market. Clin Gastroenterol Hepatol 17:809–817. https://doi.org/10.1016/j.cgh.2018.01.018
Probiotics - Health Professional Fact Sheet. National Institutes of Health (NIH). Office of Dietary Supplements. https://ods.od.nih.gov/factsheets/Probiotics-HealthProfessional/. Accessed 30 Apr 2020
World Gastroenterology Organisation Global Guidelines - Probiotics and prebiotics. https://www.worldgastroenterology.org/UserFiles/file/guidelines/probiotics-and-prebiotics-english-2017.pdf. Accessed 30 Apr 2020
International Probiotics Association - Best Practices Guidelines for Probiotics. International Scientific Association for Probiotics and Prebiotics (ISAPP). https://isappscience.org/for-consumers/probiotic-product-labels/. Accessed 30 Apr 2020
Draft Guidance for Industry: Policy Regarding Quantitative Labeling of Dietary Supplements Containing Live Microbials. U.S. Food and Drug Administration (U.S. FDA). https://www.fda.gov/regulatory-information/search-fda-guidance-documents/draft-guidance-industry-policy-regarding-quantitative-labeling-dietary-supplements-containing-live. Accessed 30 Apr 2020
Nag A, Das S (2013) Improving ambient temperature stability of probiotics with stress adaptation and fluidized bed drying. J Funct Foods 5:170–177. https://doi.org/10.1016/j.jff.2012.10.001
Awaisheh SS (2012) Probiotic food products classes, types, and processing. In: Everlon Rigobelo (ed) Probiotics. IntechOpen. https://doi.org/10.5772/51267
Zhao M, Wang Y, Huang X et al (2018) Ambient storage of microencapsulated Lactobacillus plantarum ST-III by complex coacervation of type-A gelatin and gum arabic. Food Funct 9:1000–1008. https://doi.org/10.1039/c7fo01802a
Fenster K, Freeburg B, Hollard C et al (2019) The production and delivery of probiotics: a review of a practical approach. Microorganisms 7:83. https://doi.org/10.3390/microorganisms7030083
Zolnikova O, Komkova I, Potskherashvili N et al (2018) Application of probiotics for acute respiratory tract infections. Ital J Med 12:32–38. https://doi.org/10.4081/itjm.2018.931
Enaud R, Prevel R, Ciarlo E et al (2020) The gut-lung axis in health and respiratory diseases: a place for inter-organ and inter-kingdom crosstalks. Front Cell Infect Microbiol 10:9. https://doi.org/10.3389/fcimb.2020.00009
Kanauchi O, Andoh A, AbuBakar S, Yamamoto N (2018) Probiotics and paraprobiotics in viral infection: clinical application and effects on the innate and acquired immune systems. Curr Pharm Des 24:710–717. https://doi.org/10.2174/1381612824666180116163411
Chai W, Burwinkel M, Wang Z et al (2013) Antiviral effects of a probiotic Enterococcus faecium strain against transmissible gastroenteritis coronavirus. Arch Virol 158:799–807. https://doi.org/10.1007/s00705-012-1543-0
Gao QY, Chen YX, Fang JY (2020) 2019 Novel coronavirus infection and gastrointestinal tract. J Dig Dis 21:125–126. https://doi.org/10.1111/1751-2980.12851
Solanki HK, Pawar DD, Shah DA et al (2013) Development of microencapsulation delivery system for long-term preservation of probiotics as biotherapeutics agent. Biomed Res Int 2013:620719. https://doi.org/10.1155/2013/620719
Gul O, Atalar I (2019) Different stress tolerance of spray and freeze dried Lactobacillus casei Shirota microcapsules with different encapsulating agents. Food Sci Biotechnol 28:807–816. https://doi.org/10.1007/s10068-018-0507-x
Chávarri M, Marañón I, Villarán MC (2012) Encapsulation technology to protect probiotic bacteria. In: Everlon Rigobelo (ed) Probiotics. Intech Open. https://doi.org/10.5772/50046
Patarroyo JL, Florez-Rojas JS, Pradilla D et al (2020) Formulation and characterization of gelatin-based hydrogels for the encapsulation of Kluyveromyces lactis – applications in packed-bed reactors and probiotics delivery in humans. Polymers (Basel) 12:1287. https://doi.org/10.3390/polym12061287
Vishali DA, Monisha J, Sundari SSK et al (2019) Spray freeze drying: emerging applications in drug delivery. J Control release 300:93–101. https://doi.org/10.1016/j.jconrel.2019.02.044
Moses JA, Norton T, Alagusundaram K, Tiwari BK (2014) Novel drying techniques for the food industry. Food Eng Rev 6:43–55. https://doi.org/10.1007/s12393-014-9078-7
Bernaert N, Van Droogenbroeck B, Van Pamel E, De Ruyck H (2019) Innovative refractance window drying technology to keep nutrient value during processing. Trends Food Sci Technol 84:22–24. https://doi.org/10.1016/j.tifs.2018.07.029
Bhushani JA, Anandharamakrishnan C (2014) Electrospinning and electrospraying techniques: potential food based applications. Trends Food Sci Technol 38:21–33. https://doi.org/10.1016/j.tifs.2014.03.004
Jacobsen C, García-Moreno PJ, Mendes AC et al (2018) Use of electrohydrodynamic processing for encapsulation of sensitive bioactive compounds and applications in food. Annu Rev Food Sci Technol 9:525–549. https://doi.org/10.1146/annurev-food-030117-012348
Champagne CP, Kailasapathy K (2011) Some current food products with microencapsulated probiotics. Agro Food Ind Hi Tech 22:54–56
Yogactive® - The world’s first and only probiotic cereal. http://www.vandewaterraymond.com/en/news/15-the-worlds-first-and-only-probiotic-cereal.html. Accessed 1 May 2020
EnCaptimusTM - AnaBio Encapsulation for health performance. https://www.anabio.ie/products/encaptimus/. Accessed 1 May 2020
PERKii Products. https://www.perkii.com/our-products. Accessed 1 May 2020
PERKii - A Queensland drink that’s good for your gut from the inside out. Advance Queensland (Queensland Government). https://advance.qld.gov.au/whats-happening/stories-about-innovation/perkii-queensland-drink-thats-good-your-gut-inside-out. Accessed 1 May 2020
Velobiotics - 1000x More Probiotics to Your Gut! Trust your gut. https://velobiotics.com/. Accessed 1 May 2020
FLORASSIST® - Probiotic for Digestive Health - Life Extension. https://www.lifeextension.com/vitamins-supplements/prebiotics-probiotics. Accessed 1 May 2020
Flying Embers - FSI announces world’s first shelf stable probiotic technology. https://www.nuffoodsspectrum.in/news/30/6272/fsi-announces-worlds-first-shelf-stable-probiotic-technology.html. Accessed 1 May 2020
Culturelle®: The Better Alternative to Yogurt. https://www.culturelleprobiotic.ca/resources/culturelle-the-better-alternative-to-yogurt. Accessed 1 May 2020
PRO15 Probiotics by Cognoa – The Probiotic Authority - Probiotics in the Philippines. http://www.pro15probiotics.com/. Accessed 1 May 2020
ProbioFerm - Probiotics Contract Manufacturing Our Technology. https://www.probioferm.com/technology.html. Accessed 1 May 2020
ActiveProbiotics - Live bacteria encapsulation technology was introduced at Life Science Baltic forum – Probiosanus. https://probiosanus.com/en/new-innovative-probiotic-product-line-including-live-encapsulated-probiotic-bacteria/. Accessed 1 May 2020
Ayanda products - Softgel formulation and production. https://ayanda.com/products/soft-gels/. Accessed 1 May 2020
Sirio’s Ayanda launches oil-based probiotic softgel capsules - Express Pharma. https://www.expresspharma.in/latest-updates/sirios-ayanda-launches-oil-based-probiotic-softgel-capsules/. Accessed 1 May 2020
Catalent® - RP Scherer Softgel Technology . https://www.catalent.com/oral-dose/softgel-technologies/rp-scherer-softgel-technology/. Accessed 1 May 2020
Bifa-15 The Superlative Probiotic. https://www.edenfoods.com/galleries/images/L702-sellsheet-bifa.pdf?__cf_chl_captcha_tk__=5e3cf162b3471d5910fb6436c9f41c216499b50e-1588273300-0-AbRHev0beIFHsPCadoXXrX0h9nzIs9DwRplmpkqwVMfz1MBMJhj5XosB3K5TYkpltN-2dv93490j87js3Hwgb3Jf-JduCxm8B5fiAoS7O0NBd-V_. Accessed 1 May 2020
Acidophilus Vcaps® - Naturesplus. https://naturesplus.com/products/productdetail.php?productNumber=4480. Accessed 1 May 2020
NutriDyn - UltraBiotic Dophilus. https://nutridyn.com/ultrabiotic-dophilus. Accessed 1 May 2020
Probio-40 Probiotics - Nutracraft. https://www.nutracraft.com/products/probio-40-probiotics. Accessed 1 May 2020
AB-Biotics: Fundamental report with strategy. https://www.ab-biotics.com/wp-content/uploads/2019/06/180713-Investement-Research-GCV.pdf. Accessed 1 May 2020
AB-Biotics: Probiotic supplements for women, kids - immune health. https://www.ab-biotics.com/c/probiotic-supplements/. Accessed 1 May 2020
Yoha KS, Anukiruthika T, Wilson A, Moses JA, Anandharamakrishnan C (2021) 3D printing of encapsulated probiotics: Effect of different post-processing methods on the stability of Lactiplantibacillus plantarum (NCIM 2083) under static in vitro digestion conditions and during storage. LWT 111461. https://doi.org/10.1016/j.lwt.2021.111461
Acknowledgements
The first author acknowledges the Indian Council of Medical Research (ICMR), Department of Health Research, Ministry of Health & Family Welfare, Government of India, for the “ICMR-SRF Fellowship” [3/1/2/194/2020-(Nut)].
Author information
Authors and Affiliations
Contributions
Yoha K.S., Sundus Nida: data curation, writing—original draft; Sayantani Dutta: writing—review and editing; J.A. Moses: supervision, writing—review and editing; C. Anandharamakrishnan: conceptualization, writing—review and editing, project administration.
Corresponding author
Ethics declarations
Conflicts of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yoha, K.S., Nida, S., Dutta, S. et al. Targeted Delivery of Probiotics: Perspectives on Research and Commercialization. Probiotics & Antimicro. Prot. 14, 15–48 (2022). https://doi.org/10.1007/s12602-021-09791-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12602-021-09791-7