Abstract
Over the past decades, the administration of probiotic bacteria as nutraceuticals has gained much attention. Probiotics are live microorganisms which confer a health benefit on the host when administered in an adequate amount. The health benefits of probiotics are dependent on the viability and sufficient number of probiotics in the target intestine. Due to probiotic’s vulnerability to several environmental factors such as temperature and pH, maintaining the viability of probiotics has long been a hurdle to develop successful probiotic delivery systems. In this review, we provide an overview of health benefits of probiotics, hurdles in probiotic delivery, commonly used encapsulating materials and recent probiotic delivery technologies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Probiotic is a term originated from Greek words meaning “for life” and the definition has been evolving since over time (Hill et al. 2014). More than a century ago, the concept of probiotic was introduced by Metchnikoff who stated that intake of Lactic acid bacteria would promote longevity. Since then, a term probiotic was often coined and used as an antonym of antibiotics (Lilly and Stillwell 1965). It was also suggested that feeding probiotics provide health benefit by modulating the microbial balance in the body (Fuller 1989). In the present, World Health Organization/Food and Agriculture Organization (WHO/FAO) defined that probiotics are live organisms that, when administrated in adequate amount, confers a health benefit to the host. Commonly used probiotic strains includes Lactobacillus rhamnosus, Lactobacillus acidophilus, Bifidobacterium breve and Bifidobacterium bifidum (Macfarlane and Cummings 1999).
Over the past decades, the market size of probiotics has greatly increased as modern consumer concern about health-promoting effect of nutraceuticals (Augustin and Sanguansri 2015). Since probiotic-containing products in general do not require for Food and Drug Administration approval, they are commonly available in the market in various food formats such as fermented milk, cheese, yogurt and juice (Sanders 2010). In recent years, probiotics have been extensively studied as a treatment option of various diseases such as obesity (Chen et al. 2014), diabetes (Lindsay 2015), cancer (Serban 2014), human immunodeficiency virus infection (Monachese et al. 2011), irritable bowel syndrome (Claes et al. 2010).
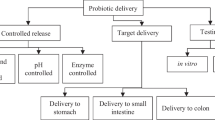
For probiotics to exert beneficial activities, a sufficient amount of probiotics should be alive and functionally active at the site of action as well as in a product (Cook et al. 2012). Probiotics are recommended to be present at a minimum level of 6 log colony forming unit (CFU)/g in a food product (Doleyres and Lacroix 2005) or 7 log CFU/g at the point of delivery (Lee and Salminen 1995). Due to the vulnerability of probiotics to harsh conditions during manufacturing, storage and passage through the gastrointestinal (GI) tract, however, it is difficult that viable probiotics successfully exert beneficial activities. During manufacturing and/or storage, the viability of probiotics can be negatively affected by several factors such as temperature, water activity and other food ingredients. Specifically, high temperature during manufacturing processes is a main reason for reduced viability because most probiotics have low thermo-resistance (Vesterlund et al. 2012). Maintaining viability in the stomach is another difficult task for probiotics to reach the target site because most of probiotics die or lose their functionality at acidic conditions. Next, survived probiotics should be released at the target site of action which is usually small or large intestine. Therefore, an ideal probiotic delivery system should protect probiotics from adverse conditions during fabrication and storage and in the acidic gastric environment so that the sufficient amount of probiotics is available in the site of action (Fig. 1).
In this review, we provide an overview of probiotic delivery, focusing on health benefits of probiotics, environmental factors affecting the probiotic viability and materials that are widely used for microencapsulation technology. Recently developed probiotic delivery technologies are also discussed.
Health benefits
The role of gut microbiota in human health has gained increasing attention. A number of studies found that human gut is colonized by diverse groups of bacteria species whose composition is strongly linked to GI health of each individual (DuPont and DuPont 2011). There are also growing evidences that administration of probiotics contributes to the microbial ecosystem which exerts a variety of health benefits including a prevention and/or treatment of diseases (Gareau et al. 2010). Recently the human microbiome project was launched to explore correlations of microbiomes with human health (Proctor and IHiR Network 2014). In this section, we briefly review the interaction of probiotics in a gut and their related health benefits. The potential mechanism of probiotics in gut is illustrated in Fig. 2.
Potential mechanisms of action of probiotics. 1 Probiotics inhibit pathogens by competing for nutrition and binding site, or by secreting anti-bacterial agents. 2 Probiotics enhance tight junction and promote secretion of mucins. 3 Probiotics contribute to intestinal homeostasis by immunomodulation effect
Probiotics are able to inhibit pathogens by competing for nutrition and binding site and by secreting antimicrobial factors. For the competition with pathogens, adhesion of probiotics to epithelial cells is crucial for antibacterial activity (Schluter et al. 2014). For example, the adhesion of Escherichia coli to Caco-2 cells reduced when it was co-cultured with Lactobacillus plantarum (Anderson et al. 2010). Similarly, Lactobacillus fermentum also inhibited adhesion of E. coli and G. Vaginalis to HeLa and HT-29 cell lines (Kaewnopparat et al. 2013). In another study, bifidogenic strains showed anti-Salmonella activity by inhibiting adhesion on HT29-MTX cell layers (Zihler et al. 2011). Saccharomyces boulardii, which is a probiotic yeast, protected mice from invasive property of Salmonella enterica (Martins et al. 2010). It was also found that five important pathogens, L. monocytogenes, Salmonella spp., C. jejuni, E. coli 0157:H7 and B. cereus were inhibited by antimicrobial property of Lactobacillus rhamnosus yoba (Mpofu et al. 2016).
An intestinal barrier plays an important role in keeping electrolytes and water not leaking into the intestinal lumen and in preventing permeation of harmful agents from an outer environment. Probiotics are known to strengthen epithelial barrier function by tightening the junctions between epithelial cells, modulating cell proliferation efficacy and promoting secretion of mucus (Saxelin et al. 2005). Lactobacillus rhamnosus GG also regulated intestinal epithelial homeostasis in a mouse colitis model by activation of the epidermal growth factor receptor and Akt pathway (Yoda et al. 2014). Escherichia coli Nissle 1917-derived protein increased the expression of tight junction proteins (Hering et al. 2014).
Probiotics are also related to immunomodulation. It was found that probiotics contribute to intestinal homeostasis by an interplay with innate or adaptive immune system (van Baarlen et al. 2013). Therapeutic effect of Lactobacillus lactis was assessed in a Crohn’s disease mouse model (del Carmen et al. 2011). This study found that Lactobacillus rhamnosus GG and Streptococcus thermophilus induced interleukin-10, an anti-inflammatory cytokine, and stimulated a cytokine signaling suppressor (Latvala et al. 2011). In another study, Lactobacillus plantarum attenuated the symptoms of colitis in a germ free interleukin-10 knock out mouse model (Schultz et al. 2002).
As discussed above, probiotics are generally considered advantageous in both healthy and diseased conditions. However, the probiotic-conferred effects varies depending on probiotic strains, environmental factors and each individual. Further studies are needed to elucidate the effect and mechanisms of probiotics in the body (Marco and Tachon 2013; van Baarlen et al. 2013).
Factors that affect viability of probiotics
Although the viability of probiotics is essential for functioning of probiotics, it is a difficult task to maintain the viability from fabrication/storage to the target site in the GI tract. For this reason, a majority of probiotic delivery studies focus on how to improve the probiotic viability. This section discusses factors that affect probiotic viability during manufacturing, storage and passage through the GI tract.
Thermal stress
The integrity of probiotics can be damaged by thermal stress during a long-term storage as well as commonly applied manufacturing processes such as drying and pasteurization (Burns et al. 2008). It is well known that probiotics, when exposed to a high temperature, are inactivated by denaturation of protein and subsequent cell damages (Perdana et al. 2012). Lactobacillus spp. were examined for heat tolerance at 60 °C for 5 min and the result showed that the viability decreased by 6 log cycle depending on their thermosensitivities (Paéz et al. 2012). In another study, more than 7 log cycle reduction of Lactobacillus rhamnosus was observed with incubation at 60 °C for 150 s (Ananta and Knorr 2009).
Oxidative stress
Since many of probiotic strains are anaerobes or microaerophiles, the viability of probiotics can be deteriorated by the existence of oxygen. Reactive oxygen species are generated under oxidative condition and they interact with probiotic components such as proteins, lipid or nucleic acid (Santivarangkna et al. 2008). A study showed that the growth rate of Bifidobacterium spp. were inhibited in the presence of oxygen (Simpson et al. 2005). In another study, oxygen concentration dependent toxicity was observed in Lactobacillus acidophilus and Bifidobacterium species (Talwalkar and Kailasapathy 2004).
Osmotic shock
Osmotic shock also impairs the viability of probiotics during a drying process. Dehydration that happens during a drying process leads to efflux of water from a probiotic cell, which causes the osmotic shock by increased intracellular molarity in probiotic cells, resulting in damaged cell functions (Poolman 2002). For example, decreased viability of Lactobacillus plantarum was enumerated due to air drying in a desiccator and a spray dryer (Perdana et al. 2012). The viability of Saccharomyces cerevisiae was decreased with increasing hyperosmotic shock (Beney et al. 2000).
Gastric juice
After intake of probiotics, the first and biggest barrier for maintaining the viability of probiotics is the harsh environment in the stomach, more specifically the gastric juice, which is extremely acidic. The pH of stomach is commonly ranged between 1 and 2.5 (Evans et al. 1988) and the gastric emptying time is around 2 h (Hellmig et al. 2006). Probiotics cannot survive under the acidic conditions for 2 h owing to disruption in metabolic and cytoplasmic activities (Hutkins and Nannen 1993). Since the passage through the stomach is inevitable for probiotic to reach the target site, acid resistance is considered an indispensable property of a effective probiotic delivery system. Acid resistance can be tested in vitro using a simulated gastric juice which possesses characteristics of human stomach fluid, such as buffer capacity, osmolality and surface tension (Charteris et al. 1998; Fredua-Agyeman and Gaisford 2015).
Materials for encapsulating probiotics
The probiotic-conferred benefits strongly depend on the ability of microorganisms to survive and multiply in the host. Microencapsulation is a technology to encapsulate probiotics into microparticles or beads and has long been utilized as a key strategy to maintain the viability of probiotics during storage and in an acidic condition of stomach in GI tract. Probiotics within encapsulating materials can be protected from adverse environments such as low pH and osmotic pressure. The objective of probiotic encapsulation, is not only to protect the cells against adverse environments, but also to liberate probiotics to the target intestine in a viable and functional state (Picot and Lacroix 2004). The viability of encapsulated probiotics depends on the physicochemical properties of the encapsulating material (Chen and Chen 2007). In this section, we describe commonly used materials for microencapsulation of probiotics.
Alginate
Alginate, a natural polysaccharide derived from brown algae or bacteria, has been widely used as an encapsulating material for probiotics due to biocompatibility and an easy gelling process by an ionic gelation with Ca2+ (Krasaekoopt et al. 2003). Two common methods to encapsulate probiotics in alginate are extrusion and emulsion (Cook et al. 2012). In the extrusion method, the mixture of aqueous alginate and concentrated probiotics is extruded through a syringe and dripped into a hardening solution containing divalent ion such as Ca2+ (Lee et al. 2015). The size of alginate beads is dependent on the diameter of the needle and free fall height to the surface of the alginate solution through the syringe needle. Extrusion is a relatively facile method to encapsulate probiotics with a low cell loss and small deviation; however, it is not an appropriate method to make the size of hundreds micrometer and is not easy to scale up (Anal and Singh 2007). On the other hand, emulsion can be used to make the hundreds micrometer size of alginate hydrogel particles and is easy to scale up. In the emulsion method, the mixture of alginate, probiotic cells and CaCO3 is added to an oil phase with agitation (Song et al. 2013). Due to the shearing force, the water phase containing alginate, probiotics and CaCO3 becomes a discrete phase. Organic acids such as acetic acid are subsequently added to liberate Ca2+ from CaCO3, resulting in the formation of alginate microcapsules. Then Ca2+ ion is liberated from CaCO3 as pH decreased. However, alginate also has some disadvantages such as an uncontrollable swelling behavior and susceptibility to the acid pH. To resolve this problem, additional coating materials such as chitosan or mixing with starch have be utilized (Krasaekoopt et al. 2003, Cook et al. 2011). Recent technologies that overcome the problems will be discussed in detail in the “Recent trends of probiotic delivery system” section.
Gums
Xanthan, an exopolysaccharide derived from Xanthomonas campestris, is the most commonly used gum and is composed of glucose, mannose and glucuronic acid (Garcia-Ochoa et al. 2000). Xanthan gum is known to possess resistance to a wide range of pH and thermal stress (Leela and Sharma 2000). Ding et al. evaluated effectiveness of xanthan gum based microencapsulation (Ding and Shah 2009). In the study, microcapsules were produced by an emulsion method in which the discrete water phase, containing xanthan gum was cross-linked with calcium chloride whilst suspended in oil. Gum acacia has also been used to protect probiotics (Desmond et al. 2002, Lian et al. 2003). The result demonstrated that microencapsulation of probiotics, such as Lactobacillus casei and Bifidobacterium spp., with gum acacia by a spray drying provide resistance to an acidic environment. Guar gum, locust bean gum, and carrageenan are other gums used as encapsulating matrices, all of which showed protective effect, to some extent, for the 10 strains of probiotic bacteria investigated (Ding and Shah 2009). Carrageenan, especially, used for microencapsulating Bifidobacterium bifidum BB-12 and Lactobacillus acidophilus LA-5 was effective in keeping the numbers of probiotic cells higher than the level of the therapeutic minimum (7 log CFU/g) while the counts of free cells declined approximately 3 log cycle during cheese manufacturing process (Özer et al. 2009).
Proteins
Proteins also can be used as a protective material for probiotics and become a popular choice in recent years. Probiotics are encapsulated into proteins by an enzymatic or chemical cross-linking or temperature-dependent gelation (Cook et al. 2012). Amphiphilic nature of the proteins provides unique property for the probiotic delivery system. Several proteins such as gelatin (Annan et al. 2008), whey protein (Doherty et al. 2011) and casein (Heidebach et al. 2009) have been used for microencapsulation of probiotics (Livney 2010, Poulin et al. 2011).
Gelatin is a protein composed of glycine proline and 4 hydroxyproline, which is derived from hydrolysis of collagen (Tabata and Ikada 1998). It can form a gel with a thermos-reversible property and an amphoteric nature (Burgain et al. 2011). Because of its low rigidity, gelatin-based microencapsulation needs cross-linking agent (Annan et al. 2007).
Milk proteins such as whey protein and casein has also been used for encapsulating probiotics (Cook et al. 2012). Whey is a protein derived from milk by extraction of cheese or yogurt. Whey-based microencapsulation is based on acid-induced gelation and heat-induced gelation. Doherty et al. demonstrated that microbeads made by whey protein increased the survival rate of Lactobacillus rhamnosus GG exposed to ex vivo porcine gastric contents (Doherty et al. 2011). Casein is able to form water insoluble matrix in acidic conditions (at below pH 6), indicating that casein-based microencapsulation could be used for protecting probiotics during the gastric transit. It was reported that Lactobacillus paracasei and Bifidobacterium lactis were successfully encapsulated into casein by transglutaminase-induced caseinate gelation and were protected from simulated gastric juice (Heidebach et al. 2009).
Synthetic polymer
Synthetic polymer such as poly (D,L-lactic-co-glycolic acid) (PLGA), polyvinyl alcohol (PVA) and polyacrylamide has been employed as an encapsulating material. PLGA is a FDA-approved biocompatible material which used for time-dependent release. Probiotics containing PLGA microparticles were produced using a water-in-oil-in-water double emulsion method with solvent evaporation (Della Porta 2012). However, use of synthetic polymers as an encapsulating material is still challenging due to involvement of organic solvents during fabrication, which causes cell damages. Preparation methods and solvents for the polymers should be carefully considered when developing synthetic polymer-based probiotic delivery systems.
Recent trends of probiotic delivery system
As described in the previous section, encapsulating probiotics into carrier materials had been a common strategy for probiotic delivery until recently. However, challenges still exist for effective protection of probiotics from tough conditions during a manufacturing process, a long-term storage and a transit in the GI tract in order to obtain a sufficient number of viable bacteria in the target site. This has propelled development of new strategies in probiotic delivery. In this section, we describe recent advancement in probiotic delivery systems. Some of them are summarized in Table 1.
Alginate has been the most extensively studied encapsulating material; however, a protective effect of bare alginate is not enough to obtain a sufficient number of viable probiotics in target sites due to a porous nature and an uncontrollable swelling behavior, which could allow H+ ion penetration and make the alginate system susceptible to acids. In addition, cell leakage by low mechanical durability in storage is a potential problem of alginate (Kim et al. 2014). Recent studies have employed various coating technologies to overcome the limitation by providing an additional protection to the surface of alginate microparticles or beads.
Chitosan coating on alginate beads has been used to provide probiotics for protection from acids by reducing pore size of alginate beads. Cook et al. evaluated the viability of probiotics from chitosan-coated alginate beads and compared to bare alginate beads (Cook et al. 2013b). After 60 min of incubation in an acidic pH, chitosan-coated alginate beads showed a higher viability by delayed H+ penetration as compared to bare alginate beads. In another work, chitosan-coated alginate beads were used for encapsulating Bifidobacterium breve, resulting in over 6 log CFU/ml of cells survived, while no viable cells were observed with non-coated in detectable range (Cook et al. 2011). As the number of chitosan-alginate coating increased, the protective effect for probiotics were also enhanced. That was verified with chitosan-alginate single and double-coated beads that encapsulate Lactobacillus plantarum (Nualkaekul et al. 2012). As compared to single-coated beads, the double-coated counterpart showed higher survivability, which was more than one log cycle after incubation with simulated gastric fluid. The protective effect of chitosan/alginate coating was also confirmed with multi-layer coated alginate beads encapsulating Lactobacillus plantarum (Cook et al. 2013a, b).
Polydopamine coating on alginate beads was used to encapsulate Saccharomyces cerevisiae (Kim et al. 2014). It was found that polydopamine coating enhanced mechanical durability of alginate beads. Unlikely to bare alginate beads, polydopamine coated alginate beads effectively prohibited cell leakages for up to 25 h in the presence of monovalent ions. Since monovalent ions can break alginate gel network which is formed by divalent calcium ions, disintegration of alginate beads was accelerated. In the study, polydopamine coating prevented bead swelling, enzymatic degradation and UV radiation, resulting in a good protection during storage and encapsulation process (Kim et al. 2014).
For enhanced target delivery of encapsulated probiotics to the target intestinal area, protamine was formulated with alginate. Inner alginate core containing Lactobacillus casei was surrounded by composite shell of alginate and protamine. Since protamine is digested by proteases in the GI tract, a rapid and selective release of probiotics was observed in the small intestine area (Mei et al. 2014).
Enteric-coating materials have been used for targeted delivery of probiotics. Eudragit L 100 55 was used with ethylcellulose to protect Bifidobacterium breve from gastric juice (de Barros et al. 2014). In the study, the results showed only less than 0.5 log reduction after 2 h of incubation in the simulated gastric fluid. When Eudragit L 100 and alginate was formulated to a tablet form to protect Lactobacillus fermentum, only 1 log cycle was decreased for 2 h of incubation at pH 1 (Villena et al. 2015a, b). Eudragit was also used as a coating material for Lactobacillus rhamnosus-containing microsphere (de Barros et al. 2015). The Eudragit-coated microparticles released more than 8 log log CFU/dose within 1 h incubation of the simulated intestinal fluid following 2 h exposure to simulated gastric juice.
To maximize probiotic-conferred health benefits, prebiotics such as galactooligosacchride and chicory have been added to probiotic delivery systems. Prebiotics is a non-dietary fiber that can selectively boost probiotic strains and confer synergistic effects (Kolida and Gibson 2011). When galactooligosaccharide-loaded PLGA particles were encapsulated in alginate beads with Bifidobacterium breve, the viability of Bifidobacterium breve increased up to 8 log log CFU/mL (Cook et al. 2014). Synergistic effect by prebiotics not only provides health beneficial effect, but also increases gastro resistance and thermos tolerance of probiotics. Alginate beads which encapsulate chicory and Staphylococcus succinus showed 95 % of survival while the viability of free cells decreased to 77 % after 35 day of storage (Sathyabama et al. 2014). In another paper, Bifidobacterium BB-12 was encapsulated in milk proteins blended with oligofructose-enriched inulin (Fritzen-Freire et al. 2012). In the study, more than 10.5 log CFU/g of cells were survived after 180 days at 4 °C storage (Fritzen-Freire et al. 2012).
Manufacturing processes can influence viability of probiotic bacteria (Grzeskowiak et al. 2011). Since many of probiotics can be exposed to high temperatures for pasteurization and spray-drying process and low temperatures for a freeze-drying process, maintaining viability during the manufacturing processes is also of importance (Tripathi and Giri 2014, Broeckx et al. 2016). Various technologies have been incorporated into formulations to improve the survival rate of probiotic bacteria during manufacturing processes and in storage. To enhance a survival rate of Lactobacillus reuteri in a heated condition, aluminum carboxymethyl cellulose-rice bran microcapsules were fabricated (Chitprasert et al. 2012). After 25 s of exposure to 85 °C, more than 8 log CFU/g of cells survived, while free cells survived less than 4.8 log CFU/g (Chitprasert et al. 2012). In another study, bacterial nanocellulose that encapsulate probiotics with pectin protected the probiotics well under a microwave drying and during a long-term storage at a variety of temperatures (Khorasani 2016). Nanocellulose was also used to decrease cell damage of Lactobacillus plantarum at freeze-drying processes by adhesion to the surface of probiotics (Nahr et al. 2015).
Recently, non-microencapsulation-based probiotic delivery systems have been attempted. For examples, tablet-based systems have been investigated as a probiotic delivery system. Govender et al. developed bi-layered mini-tablet-in-tablet system to deliver Lactobacillus acidophilus to both small intestine and colon (Govender et al. 2015). Ovalbumin which is known to possess gastro-resistant properties was used to prepare mini tablets where probiotics were incorporated. The ovalbumin mini tablets were then place inside each layer. Major excipient of two layers was lactose and Eudragit S100 which was chosen to deliver probiotics to intestinal and colon targeting, respectively. The tablets showed effective site-specific delivery of Lactobacillus acidophilus as intended. In another study, Eudragit L100–sodium alginate tablets were shown to improve the survival of Lactobacillus fermentum CECT 5716 when exposed to an acidic medium as compared to free cells, resulting in the survival of 9 log CFU/tablet after 2 h of incubation. The tablets also protected cells during storage at 4 °C for over 6 months (Villena et al. 2015a, b).
Cell surface engineering has also emerged as a non-microencapsulation-based technology to protect probiotics from gastric conditions. Cell permeability can be modulated by adhesion of polymer molecules on the cell surface (Fakhrullin et al. 2012). Pepsin is an enzyme with a large molecular weight which is present in gastric juice. Inhibition of pepsin penetration to a probiotic wall is of important for probiotic viability. By introducing chitosan and carboxymethyl cellulose onto bacterial surface, the penetration of large molecular weight enzyme was effectively inhibited while leaving a small molecular nutrition freely flow in and out (Priya et al. 2011). As a result, more than 8 log CFU/g of Lactobacillus acidophilus survived whereas the viability of non-coated cells decreased to less than 3.5 log CFU/g. The protective effect of chitosan and dextran sulfate coated Saccharomyces cerevisiae was also reported (Ben Thomas et al. 2014).
Conclusion
The field of probiotics has been growing due to well-documented health benefits. Since only viable probiotics can confer the health benefits, keeping the viability of probiotics up until reaching the site of action is of great importance in probiotic delivery. The viability can be affected by several environmental factors such as gastric pH, temperature and osmotic pressure. Microencapsulation has been widely used to improve the survival rate of probiotics. In general, alginate, gums or proteins have been used as an encapsulating material which provides sufficient protection to probiotics during storage and delivery to the target site. Recently, an array of novel technologies, such as coating systems, prebiotics and microencapsulation with newly developed materials, have been developed to enhance the viability. Another novel aspect of probiotic delivery is a controlled release of probiotics at the target site. Despite all the efforts, however, most delivery systems still suffer from loss of viable probiotics and a need for an ideal probiotic delivery system has yet to be met. Another issue that needs to be addressed in probiotic delivery is lack of tools for in vivo evaluation of probiotic viability and functioning. In conclusion, different aspects of this review may open new avenue for extensive research in the field of probiotic delivery.
References
Anal AK, Singh H (2007) Recent advances in microencapsulation of probiotics for industrial applications and targeted delivery. Trends Food Sci Technol 18(5):240–251
Ananta E, Knorr D (2009) Comparison of inactivation pathways of thermal or high pressure inactivated Lactobacillus rhamnosus ATCC 53103 by flow cytometry analysis. Food Microbiol 26:542–546
Anderson RC, Cookson AL, McNabb WC, Kelly WJ, Roy NC (2010) Lactobacillus plantarum DSM 2648 is a potential probiotic that enhances intestinal barrier function. FEMS Microbiol Lett 309(2):184–192
Annan N, Borza A, Moreau D, Allan-Wojtas P, Hansen LT (2007) Effect of process variables on particle size and viability of Bifidobacterium lactis Bb-12 in genipin-gelatin microspheres. J Microencapsul 24(2):152–162
Annan N, Borza A, Hansen LT (2008) Encapsulation in alginate-coated gelatin microspheres improves survival of the probiotic Bifidobacterium adolescentis 15703T during exposure to simulated gastro-intestinal conditions. Food Res Int 41(2):184–193
Augustin MA, Sanguansri L (2015) Challenges and solutions to incorporation of nutraceuticals in foods. Annu Rev Food Sci Technol 6:463–477
Ben Thomas M, Vaidyanathan M, Radhakrishnan K, Raichur AM (2014) Enhanced viability of probiotic Saccharomyces boulardii encapsulated by layer-by-layer approach in pH responsive chitosan–dextran sulfate polyelectrolytes. J Food Eng 136:1–8
Beney L, de Maranon IM, Marechal PA, Gervais P (2000) Influence of thermal and osmotic stresses on the viability of the yeast Saccharomyces cerevisiae. Int J Food Microbiol 55(1–3):275–279
Broeckx G, Vandenheuvel D, Claes IJ, Lebeer S, Kiekens F (2016) Drying techniques of probiotic bacteria as an important step towards the development of novel pharmabiotics. Int J Pharm 505(1–2):303–318
Burgain J, Gaiani C, Linder M, Scher J (2011) Encapsulation of probiotic living cells: from laboratory scale to industrial applications. J Food Eng 104(4):467–483
Burns P, Patrignani F, Serrazanetti D, Vinderola GC, Reinheimer JA, Lanciotti R, Guerzoni ME (2008) Probiotic Crescenza cheese containing Lactobacillus casei and Lactobacillus acidophilus manufactured with high-pressure homogenized milk. J Dairy Sci 91(2):500–512
Charteris WP, Kelly PM, Morelli L, Collins JK (1998) Development and application of an in vitro methodology to determine the transit tolerance of potentially probiotic Lactobacillus and Bifidobacterium species in the upper human gastrointestinal tract. J Appl Microbiol 84(5):759–768
Chen MJ, Chen KN (2007) Applications of probiotic encapsulation in dairy products. Encapsul Controll Release Technol Food Syst 23:83–112
Chen Z, Guo L, Zhang Y, Walzem RL, Pendergast JS, Printz RL, Morris LC, Matafonova E, Stien X, Kang L (2014) Incorporation of therapeutically modified bacteria into gut microbiota inhibits obesity. J Clin Investig 124(8):3391–3406
Chitprasert P, Sudsai P, Rodklongtan A (2012) Aluminum carboxymethyl cellulose–rice bran microcapsules: enhancing survival of Lactobacillus reuteri KUB-AC5. Carbohydr Polym 90:78–86
Claes I, Lebeer S, Shen C, Verhoeven T, Dilissen E, De Hertogh G, Bullens D, Ceuppens J, Van Assche G, Vermeire S (2010) Impact of lipoteichoic acid modification on the performance of the probiotic Lactobacillus rhamnosus GG in experimental colitis. Clin Exp Immunol 162(2):306–314
Cook MT, Tzortzis G, Charalampopoulos D, Khutoryanskiy VV (2011) Production and evaluation of dry alginate-chitosan microcapsules as an enteric delivery vehicle for probiotic bacteria. Biomacromolecules 12:2834–2840
Cook MT, Tzortzis G, Charalampopoulos D, Khutoryanskiy VV (2012) Microencapsulation of probiotics for gastrointestinal delivery. J Controll Release 162(1):56–67
Cook MT, Saratoon T, Tzortzis G, Edwards A, Charalampopoulos D, Khutoryanskiy VV (2013a) CLSM method for the dynamic observation of pH change within polymer matrices for oral delivery. Biomacromolecules 14:387–393
Cook MT, Tzortzis G, Khutoryanskiy VV, Charalampopoulos D (2013b) Layer-by-layer coating of alginate matrices with chitosan–alginate for the improved survival and targeted delivery of probiotic bacteria after oral administration. J Mater Chem B 1:52–60
Cook MT, Tzortzis G, Charalampopoulos D, Khutoryanskiy VV (2014) Microencapsulation of a synbiotic into PLGA/alginate multiparticulate gels. Int J Pharm 466:400–408
de Barros JMS, Scherer T, Charalampopoulos D, Khutoryanskiy VV, Edwards AD (2014) A laminated polymer film formulation for enteric delivery of live vaccine and probiotic bacteria. J Pharm Sci 103:2022–2032
de Barros JM, Lechner T, Charalampopoulos D, Khutoryanskiy VV, Edwards AD (2015) Enteric coated spheres produced by extrusion/spheronization provide effective gastric protection and efficient release of live therapeutic bacteria. Int J Pharm 493(1–2):483–494
del Carmen S, de Moreno A, de LeBlanc G, Bastos Perdigon V, Pereira A, Azevedo Miyoshi V, LeBlanc JG (2011) Evaluation of the anti-inflammatory effect of milk fermented by a strain of IL-10-producing Lactococcus lactis using a murine model of Crohn’s disease. J Mol Microbiol Biotechnol 21(3–4):138–146
Della Porta G, Castaldo F, Scognamiglio M, Paciello L, Parascandola P, Reverchon E (2012) Bacteria microencapsulation in PLGA microdevices by supercritical emulsion extraction. J Supercrit Fluids 63:1–7
Desmond C, Ross R, O’callaghan E, Fitzgerald G, Stanton C (2002) Improved survival of Lactobacillus paracasei NFBC 338 in spray-dried powders containing gum acacia. J Appl Microbiol 93(6):1003–1011
Ding W, Shah NP (2009) Effect of various encapsulating materials on the stability of probiotic bacteria. J Food Sci 74(2):M100–M107
Doherty SB, Gee VL, Ross RP, Stanton C, Fitzgerald GF, Brodkorb A (2011) Development and characterisation of whey protein micro-beads as potential matrices for probiotic protection. Food Hydrocoll 25(6):1604–1617
Doleyres Y, Lacroix C (2005) Technologies with free and immobilised cells for probiotic bifidobacteria production and protection. Int Dairy J 15(10):973–988
DuPont AW, DuPont HL (2011) The intestinal microbiota and chronic disorders of the gut. Nat Rev Gastroenterol Hepatol 8(9):523–531
Evans DF, Pye G, Bramley R, Clark AG, Dyson TJ, Hardcastle JD (1988) Measurement of gastrointestinal pH profiles in normal ambulant human-subjects. Gut 29(8):1035–1041
Fakhrullin RF, Zamaleeva AI, Minullina RT, Konnova SA, Paunov VN (2012) Cyborg cells: functionalisation of living cells with polymers and nanomaterials. Chem Soc Rev 41:4189–4206
Fredua-Agyeman M, Gaisford S (2015) Comparative survival of commercial probiotic formulations: tests in biorelevant gastric fluids and real-time measurements using microcalorimetry. Benef Microbes 6(1):141–151
Fritzen-Freire CB, Prudêncio ES, Amboni RDMC, Pinto SS, Negrão-Murakami AN, Murakami FS (2012) Microencapsulation of bifidobacteria by spray drying in the presence of prebiotics. Food Res Int 45:306–312
Fuller R (1989) Probiotics in man and animals. J Appl Bacteriol 66(5):365–378
Garcıa-Ochoa F, Santos V, Casas J, Gomez E (2000) Xanthan gum: production, recovery, and properties. Biotechnol Adv 18(7):549–579
Gareau MG, Sherman PM, Walker WA (2010) Probiotics and the gut microbiota in intestinal health and disease. Nat Rev Gastroenterol Hepatol 7(9):503–514
Govender M, Choonara YE, Vuuren S, Kumar P, Toit LC, Pillay V (2015) A gastro-resistant ovalbumin bi-layered mini-tablet-in-tablet system for the delivery of Lactobacillus acidophilus probiotic to simulated human intestinal and colon conditions. J Pharm Pharmacol 67(7):939–950
Grześkowiak Ł, Isolauri E, Salminen S, Gueimonde M (2011) Manufacturing process influences properties of probiotic bacteria. Br J Nutr 105(06):887–894
Heidebach T, Först P, Kulozik U (2009) Transglutaminase-induced caseinate gelation for the microencapsulation of probiotic cells. Int Dairy J 19(2):77–84
Hellmig S, Von Schoning F, Gadow C, Katsoulis S, Hedderich J, Folsch UR, Stuber E (2006) Gastric emptying time of fluids and solids in healthy subjects determined by C-13 breath tests: influence of age, sex and body mass index. J Gastroenterol Hepatol 21(12):1832–1838
Hering NA, Richter JF, Fromm A, Wieser A, Hartmann S, Gunzel D, Bucker R, Fromm M, Schulzke JD, Troeger H (2014) TcpC protein from E. coli Nissle improves epithelial barrier function involving PKCzeta and ERK1/2 signaling in HT-29/B6 cells. Mucosal Immunol 7(2):369–378
Hill C, Guarner F, Reid G, Gibson GR, Merenstein DJ, Pot B, Morelli L, Canani RB, Flint HJ, Salminen S (2014) Expert consensus document: the International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol 11(8):506–514
Hutkins RW, Nannen NL (1993) pH homeostasis in lactic-acid bacteria. J Dairy Sci 76(8):2354–2365
Kaewnopparat S, Dangmanee N, Kaewnopparat N, Srichana T, Chulasiri M, Settharaksa S (2013) In vitro probiotic properties of Lactobacillus fermentum SK5 isolated from vagina of a healthy woman. Anaerobe 22:6–13
Khorasani AC, Shojaosadati SA (2016) Bacterial nanocellulose-pectin bionanocomposites as prebiotics against drying and gastrointestinal condition. Int J Biol Macromol 83:9–18
Kim BJ, Park T, Moon HC, Park SY, Hong D, Ko EH, Kim JY, Hong JW, Han SW, Kim YG (2014) Cytoprotective alginate/polydopamine core/shell microcapsules in microbial encapsulation. Angew Chem Int Ed 53(52):14443–14446
Kolida S, Gibson GR (2011) Synbiotics in health and disease. Annu Rev Food Sci Technol 2:373–393
Krasaekoopt W, Bhandari B, Deeth H (2003) Evaluation of encapsulation techniques of probiotics for yoghurt. Int Dairy J 13(1):3–13
Latvala S, Miettinen M, Kekkonen RA, Korpela R, Julkunen I (2011) Lactobacillus rhamnosus GG and Streptococcus thermophilus induce suppressor of cytokine signalling 3 (SOCS3) gene expression directly and indirectly via interleukin-10 in human primary macrophages. Clin Exp Immunol 165(1):94–103
Lee Y-K, Salminen S (1995) The coming of age of probiotics. Trends Food Sci Technol 6(7):241–245
Lee BB, Ibrahim R, Chu SY, Zulkifli NA, Ravindra P (2015) Alginate liquid core capsule formation using the simple extrusion dripping method. J Polym Eng 35(4):311–318
Leela JK, Sharma G (2000) Studies on xanthan production from Xanthomonas campestris. Bioprocess Eng 23(6):687–689
Lian W-C, Hsiao H-C, Chou C-C (2003) Viability of microencapsulated bifidobacteria in simulated gastric juice and bile solution. Int J Food Microbiol 86(3):293–301
Lilly DM, Stillwell RH (1965) Probiotics: growth-promoting factors produced by microorganisms. Science 147(3659):747–748
Lindsay KL, Brennan L, Kennelly MA, Maguire OC, Smith T, Curran S, Coffey M, Foley ME, Hatunic M, Shanahan F (2015) Impact of probiotics in women with gestational diabetes mellitus on metabolic health: a randomized controlled trial. Am J Obstet Gynecol 212(4), pp 496, e491–496, e411
Livney YD (2010) Milk proteins as vehicles for bioactives. Curr Opin Colloid Interface Sci 15(1–2):73–83
Macfarlane GT, Cummings JH (1999) Probiotics and prebiotics: can regulating the activities of intestinal bacteria benefit health? West J Med 171(3):187
Marco ML, Tachon S (2013) Environmental factors influencing the efficacy of probiotic bacteria. Curr Opin Biotechnol 24:207–213
Martins FS, Dalmasso G, Arantes RM, Doye A, Lemichez E, Lagadec P, Imbert V, Peyron JF, Rampal P, Nicoli JR, Czerucka D (2010) Interaction of Saccharomyces boulardii with Salmonella enterica serovar Typhimurium protects mice and modifies T84 cell response to the infection. PLoS One 5(1):e8925
Mei L, He F, Zhou RQ, Wu CD, Liang R, Xie R, Ju XJ, Wang W, Chu LY (2014) Novel intestinal-targeted Ca-alginate-based carrier for pH-responsive protection and release of lactic acid bacteria. ACS Appl Mater Interfaces 6(8):5962–5970
Monachese M, Cunningham-Rundles S, Diaz MA, Guerrant R, Hummelen R, Kemperman R, Kerac M, Kort R, Merenstein D, Panigrahi P, Ramakrishna B, Safdar N, Shane A, Trois L, Reid G (2011) Probiotics and prebiotics to combat enteric infections and HIV in the developing world: a consensus report. Gut Microbes 2(3):198–207
Mpofu A, Linnemann AR, Nout MJ, Zwietering MH, Smid EJ, den Besten HM (2016) Inactivation of bacterial pathogens in yoba mutandabota, a dairy product fermented with the probiotic Lactobacillus rhamnosus yoba. Int J Food Microbiol 217:42–48
Nahr FK, Mokarram RR, Hejazi MA, Ghanbarzadeh B, Khiyabani MS, Benis KZ (2015) Optimization of the nanocellulose based cryoprotective medium to enhance the viability of freeze dried Lactobacillus plantarum using response surface methodology. LWT Food Sci Technol 64:326–332
Nualkaekul S, Lenton D, Cook MT, Khutoryanskiy VV, Charalampopoulos D (2012) Chitosan coated alginate beads for the survival of microencapsulated Lactobacillus plantarum in pomegranate juice. Carbohydr Polym 90:1281–1287
Özer B, Kirmaci HA, Şenel E, Atamer M, Hayaloğlu A (2009) Improving the viability of Bifidobacterium bifidum BB-12 and Lactobacillus acidophilus LA-5 in white-brined cheese by microencapsulation. Int Dairy J 19(1):22–29
Paéz R, Lavari L, Vinderola G, Audero G, Cuatrin A, Zaritzky N, Reinheimer J (2012) Effect of heat treatment and spray drying on lactobacilli viability and resistance to simulated gastrointestinal digestion. Food Res Int 48(2):748–754
Perdana J, Bereschenko L, Roghair M, Fox MB, Boom RM, Kleerebezem M, Schutyser MA (2012) Novel method for enumeration of viable Lactobacillus plantarum WCFS1 cells after single-droplet drying. Appl Environ Microbiol 78(22):8082–8088
Picot A, Lacroix C (2004) Encapsulation of bifidobacteria in whey protein-based microcapsules and survival in simulated gastrointestinal conditions and in yoghurt. Int Dairy J 14(6):505–515
Poolman B (2002) Transporters and their roles in LAB cell physiology. Antonie Van Leeuwenhoek Int J Gen Mol Microbiol 82(1–4):147–164
Poulin JF, Caillard R, Subirade M (2011) beta-Lactoglobulin tablets as a suitable vehicle for protection and intestinal delivery of probiotic bacteria. Int J Pharm 405(1–2):47–54
Priya AJ, Vijayalakshmi SP, Raichur AM (2011) Enhanced survival of probiotic Lactobacillus acidophilus by encapsulation with nanostructured polyelectrolyte layers through layer-by-layer approach. J Agric Food Chem 59:11838–11845
Proctor LM, IHiR Network (2014) The integrative human microbiome project: dynamic analysis of microbiome-host omics profiles during periods of human health and disease. Cell Host Microbe 16(3):276–289
Sanders ME, Marco ML (2010) Food formats for effective delivery of probiotics. Food Sci Technol 1:65–85
Santivarangkna C, Higl B, Foerst P (2008) Protection mechanisms of sugars during different stages of preparation process of dried lactic acid starter cultures. Food Microbiol 25(3):429–441
Sathyabama S, Kumar MR, Devi PB, Vijayabharathi R, Priyadharisini VB (2014) Co-encapsulation of probiotics with prebiotics on alginate matrix and its effect on viability in simulated gastric environment. LWT Food Sci Technol 57:419–425
Saxelin M, Tynkkynen S, Mattila-Sandholm T, de Vos WM (2005) Probiotic and other functional microbes: from markets to mechanisms. Curr Opin Biotechnol 16(2):204–211
Schluter J, Nadell CD, Bassler BL, Foster KR (2014) Adhesion as a weapon in microbial competition. Nat Publ Gr 9:139–149
Schultz M, Veltkamp C, Dieleman LA, Grenther WB, Wyrick PB, Tonkonogy SL, Sartor RB (2002) Lactobacillus plantarum 299 V in the treatment and prevention of spontaneous colitis in interleukin-10-deficient mice. Inflamm Bowel Dis 8(2):71–80
Serban DE (2014) Gastrointestinal cancers: influence of gut microbiota, probiotics and prebiotics. Cancer Lett 345(2):258–270
Simpson PJ, Stanton C, Fitzgerald GF, Ross RP (2005) Intrinsic tolerance of Bifidobacterium species to heat and oxygen and survival following spray drying and storage. J Appl Microbiol 99(3):493–501
Song H, Yu W, Gao M, Liu X, Ma X (2013) Microencapsulated probiotics using emulsification technique coupled with internal or external gelation process. Carbohydr Polym 96:181–189
Tabata Y, Ikada Y (1998) Protein release from gelatin matrices. Adv Drug Deliv Rev 31(3):287–301
Talwalkar A, Kailasapathy K (2004) The role of oxygen in the viability of probiotic bacteria with reference to L. acidophilus and Bifidobacterium spp. Curr Issues Intest Microbiol 5(1):1–8
Tripathi MK, Giri SK (2014) Probiotic functional foods: survival of probiotics during processing and storage. J Funct Foods 9:225–241
van Baarlen P, Wells JM, Kleerebezem M (2013) Regulation of intestinal homeostasis and immunity with probiotic lactobacilli. Trends Immunol 34:208–215
Vesterlund S, Salminen K, Salminen S (2012) Water activity in dry foods containing live probiotic bacteria should be carefully considered: a case study with Lactobacillus rhamnosus GG in flaxseed. Int J Food Microbiol 157(2):319–321
Villena MJM, Lara-Villoslada F, Martinez MAR, Hernandez MEM (2015a) Development of gastro-resistant tablets for the protection and intestinal delivery of Lactobacillus fermentum CECT 5716. Int J Pharm 487(1):314–319
Villena MJM, Lara-Villoslada F, Martínez MAR, Hernández MEM (2015b) Development of gastro-resistant tablets for the protection and intestinal delivery of Lactobacillus fermentum CECT 5716. Int J Pharm 487:314–319
Yoda K, Miyazawa K, Hosoda M, Hiramatsu M, Yan F, He F (2014) Lactobacillus GG-fermented milk prevents DSS-induced colitis and regulates intestinal epithelial homeostasis through activation of epidermal growth factor receptor. Eur J Nutr 53:105–115
Zihler A, Gagnon M, Chassard C, Lacroix C (2011) Protective effect of probiotics on Salmonella infectivity assessed with combined in vitro gut fermentation-cellular models. BMC Microbiol 11:264
Acknowledgments
This work was supported by a 2-Year Research Grant of Pusan National University. All authors (J. Kim, N. Muhammad, B.H. Jhun and J.-W. Yoo) declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Jihyun Kim and Naeem Muhammad contributed equally to this work.
Rights and permissions
About this article
Cite this article
Kim, J., Muhammad, N., Jhun, B.H. et al. Probiotic delivery systems: a brief overview. Journal of Pharmaceutical Investigation 46, 377–386 (2016). https://doi.org/10.1007/s40005-016-0259-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40005-016-0259-7