Abstract
The potential health benefits of probiotics may not be cognized because of the substantial curtailment in their viability during food storage and passage through the gastrointestinal system. Intestinal flora composition, and resistance against pathogens are among the health benefits associated with probiotic consumption. In the gastric environment, pH 2.0, probiotics dramatically lose their viability during the transit through the gastrointestinal system. The challenge remains to maintain cell viability until it reaches the large intestine. In extreme conditions, such as a decrease in pH or an increase in temperature, encapsulation technology can enhance the viability of probiotics. Probiotic bacterial strains can be encapsulated in a variety of ways. The methods are broadly systematized into two categories, liquid and solid delivery systems. This review emphasizes the technology used in the research and commercial sectors to encapsulate probiotic cells while keeping them alive and the food matrix used to deliver these cells to consumers.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Probiotics are bacteria, moulds and yeasts, with lactic acid bacteria being the most common (Reid et al., 2019). It is typically believed that these microorganisms causes diseases and deteriorate health. The consumption of probiotics provides a number of health benefits, including improving immunity, reducing inflammation, improving digestion, making vitamins, and breaking down medications (Markowiak & Śliżewska, 2017). More than 50 genera of bacteria inhabit the human gut, particularly in the large intestine, some of which are harmful (toxins) and beneficial (synthesizing vitamins) (Zhang et al., 2015). The administration of probiotic bacteria stimulates the gut’s microbiome, potentially inhibiting harmful bacteria and augmenting the body’s natural defense mechanisms (Hemarajata & Versalovic, 2013). The total market for functional foods have been dominated by probiotic foods, with up to 70% of the market being probiotic foods. According to estimates, the global functional food market will reach $ 309 Billion in 2027 and may grow by 7.5% annually between 2020 and 2027 (Tripathi & Giri, 2014).
However, the viability of probiotics continues to be a technological and marketing challenge for industries. Reduction of the viability during processing and storage still continues to be one of the substantial challenges. Encapsulating probiotics has been proposed as an efficient way to perpetuate the viability and prevent metabolic activity from deteriorating in the gastrointestinal tract (Picot & Lacroix, 2004). Encapsulation has been successfully used to ameliorate cell viability during storage of Lacticaseibacillus paracasei NFBC 338 by spray-drying (Desmond et al., 2002), Lacticaseibacillus casei NCDC-298 by emulsification (Mandal et al., 2006) and more. This review analyses techniques used for encapsulation and factors that influence the viability of cells.
Bioengineering of probiotics
Many factors such as oxygen levels, redox potential, additives, antimicrobial compounds, and bacteriocins are found to affect the viability of cells present in probiotics during storage (Terpou et al., 2019). Biological factors include strain type, natural microflora product, enzymes produced, post acidification and various pathogenic or spoilage microorganism occurrences (Vieira da Silva et al., 2016). Physical factors include drying conditions, and temperature associated (Fenster et al., 2019). Strategies to enhance cell viability include selecting suitable strain, which serves a crucial role in improving viability. In order to prompt cell inactivation and to intensify cell stability, physical stress is applied (temperature stress, osmotic stress, oxygen stress) (Fenster et al., 2019). The selection of proper food packaging systems can influence viability. Including packaging methods like oxygen scavengers, and vacuum packaging can significantly improve viability (Souza et al., 2012).
Encapsulation technologies are expected to enhance stability, and ensure better handling and storage of probiotic cultures. As most probiotics are of intestinal origin, they are unsuitable for growth in dairy-based media and mostly get inactivated on exposure to high heat, and acid during processing (Zielińska & Kolożyn-Krajewska, 2018). Technological challenges associated with maintaining a high number of probiotic organisms in food, the capability of the culture to retain viability in the food matrix environment, and maintenance of its characteristics during consumption are also of concern. Spray-dried and freeze-dried cultures help introduce the culture of the human gastrointestinal system. But sometimes, these technological approaches used for the preparation of cultures may affect their viability and functionality. Sometimes cell injury can also happen during the application of these technologies (Iravani et al., 2015).
Probiotic encapsulation technology has rapidly emerged in the past decade. With the help of this technology, many microorganisms have been immobilized with semipermeable materials to facilitate their delivery (Hassan et al., 2019). Despite the benefit of increased viability and shelf life, it faces many challenges, including developing microencapsulation equipment, selecting non-toxic materials for encapsulation, developing beads or capsules from polymers, and determining the appropriate mechanism of probiotic release, carrying out these are assigning their costs. One of the most important challenges is of the cost, encapsulated end products can be very costly (Aragón-Rojas et al., 2019). This is because their development demands both time and financial resources. Using natural polymers will increase the cost further, milk proteins seem more costly than carbohydrates. These techniques also require certain raw materials, which include oil and emulsifiers, in order to stabilize the capsule (Aragón-Rojas et al., 2019).
Encapsulation of probiotics
Microencapsulation method describes the process of packing or encapsulating solid, liquid, or gaseous materials into tiny capsules that can protect active or functional materials from hazardous environment and release their contents under certain conditions at a controlled rate (Yao et al., 2020). Within the microcapsule core are probiotics that are present and survive, and surrounding the core is a very thin but strong membrane that allows contents to enter and exit the microcapsules. A microcapsule can be designed to release active ingredients gradually under the influence of heat, solvation, diffusion, and pressure. An engineered coating can also allow microcapsules to open at specific locations on the body (Bepeyeva et al., 2017). Having developed the science of microencapsulation and advanced biomaterials, biomaterial-based microencapsulation has become a popular method for encapsulating probiotics for enhanced stability (Dafe et al., 2017). Encapsulation of probiotics with carrier material, particle size, and its advantages are represented in Table 1.
Challenges with use of encapsulated probiotics
Microencapsulated probiotic stability when lyophilized or spray dried
Live microorganisms are those that are beneficial to the host and can be easily administered in adequate amounts. The study analyzed the effect of various encapsulating agents on the feasibility of freeze-dried Lacticaseibacillus casei capsules. The viable cells were determined using the spray drying technique. They were then subjected to heat treatment at temperatures of up to 90 °C (Reid et al., 2019). The use of different encapsulating agents significantly improved the survival rate of L. casei Shirota when exposed to acidic conditions. Microencapsulated cells exhibited the viability of being subjected to 1% bile salts in the presence of encapsulating agents. The cells were also stable during incubation (Gul, 2017). When subjected to 2% bile, the microcapsules exhibited a decrease in size of about 1.09 log. This effect was caused by the use of spray or dried freeze methods. L. casei Shirota was microencapsulated with various additives to improve its survival against the stresses of the environment. This included freeze-drying and spray formulations. The viability of microcapsules was maintained at 5 log CFU/g for up to 5 log CFU/g exposure (Gul & Atalar, 2019).
Solidness of microencapsulated microscopic organisms in gastrointestinal tract
The direction of the research was on the effects of different encapsulating agents upon growth and survival of freeze-dried L. casei capsules in the gastrointestinal tract. It is revealed that varying agents affected the bacteria’s viability. Encapsulated cells exhibited the ability to endure the presence of 1% bile salts. They also exhibited a stable state during incubation. When dehydrated, 2% bile microcapsules decreased in size by about 1.09 log (Castro-Cislaghi et al., 2012). The reason for the decrease in microcapsules size was due to the usage of spray or freeze methods. L. casei Shirota was microencapsulated with several compounds to improve its resistance to environmental stress. Freeze-drying and spray formulations were among them. Microcapsule viability was sustained at 5 log CFU/g exposure for up to 5 log CFU/g. Gul and Atala (2019) also stated that a great opportunity to improve the preservation of probiotics in severe acidic conditions in the stomach by using enzymes like rennet or TGases in the course of probiotic encapsulation in dairy-based matrices. Since then, other research has been successfully guided the improvement ofn feasible probiotics in regard to plant and probiotic materials (Andrade et al., 2019).
Biopolymer as stabilizer matrix
Biopolymers are naturally produced by cells and are made up of repeated chains of monomers that are covalently linked together (Brigham, 2018). The most commonly used biopolymers for probiotic encapsulation are carbohydrates (such as alginate, carrageenan, and gums), proteins (such as whey protein, casein protein, and soy protein) and lipids (such as waxes, and fats) (Gutierrez & Alvarez, 2017). The categorized materials employed for the preparation of encapsulation are represented in Fig. 1.
Carbohydrate-based system
Carbohydrates-based systems are proven to have thickening, stabilizing, and gelling properties. They are preferred because of their non-toxicity, biocompatibility, and bio-degradability. Some of the widely used carbohydrate systems are alginate, carrageenan, chitin, starch, and gum. (Martău et al., 2019).
Alginate, an potential encapsulation material does not dissolve in an acidic environment, thereby protecting the probiotic microbe (Oberoi et al., 2021). Emulsification and complex formation can be used to create alginate microspheres. Both methods can optimize the particle size as required. Coating the nanoparticles with chitosan to make the capsule less permeable to water-soluble molecules and for a smoother surface, thereby increasing the stability of the probiotic. Studies indicate that the viability increases with an increase in the capsule size but huge beads with a size > 1 mm does not significantly improve the viability (Ephrem et al., 2018) .
Carrageenan is a linear polymer formed by repeating units of β-d-glucopyranose and 3, 6-anhydro-α-d-glucopyranose (Ephrem et al., 2018). It is of three types, namely, κ-carrageenan, ι-carrageenan, and λ-carrageenan. An oxygen bridge present between the third and sixth carbon of d-galactose in the kappa and iota carrageenan, which makes gelation possible. Chitosan is a hydrophilic polymer with amazing muco-adhesive properties, which aids in the improvement of the release of bacteria in the gut and changes the gut microbiota through its antimicrobial activity. Alginate-chitosan system shows improved stability under stimulated GI conditions. It also increases the probiotics’ survival rate in simulated gastric and intestinal conditions and reduces viability losses during freeze-drying and freezing. The drawback of using this as the coating material is that chitosan does not dissolve in pH above 5.4 because the gut’s pH is greater than 5.4 (Călinoiu et al., 2019). However, studies have found that a combination of chitosan with other polymers can be utilized for a systme with better stability. Another study has shown that a mixture of chitosan and whey protein, chitosan and gelatin produce capsules with increased stability (Choińska-Pulit et al., 2015). When alginate beads are coated with chitosan, the surface becomes smoother and hence less permeable to water, thereby increasing the stability (Ephrem et al., 2018) .
Gellan gum and xantham gums are anionic bacterial linear tetrasaccharides (Milivojevic et al., 2019). L. casei was encapsulated into gellan gum and sodium caseinate. After subjecting both the immobilized and free cells to simulated gastric fluid for 120 min, the vitality of free cells dropped by 6.1 log CFU/g whilst the vitality of immobilized cells dropped by just 3.1 log CFU/g. It is clear from this that encapsulation increased the viability of the probiotic (Shori, 2017).
Protein-based systems
Proteins are polymers made up of peptides used as encapsulating materials due to their amphiphilic nature. Casein, whey protein, and gelatin are vastly used for encapsulating probiotics (Nguyen et al., 2015). Gelatin have been manufactured by partial hydrolysis which denatures collagen. It is preferred for encapsulation due to its thermo-reversible nature which makes it possible for gelatin to melt and become liquid when heated above its melting point and when cooled it hardens and forms gel (Fathi et al., 2018). Its benefits include non-toxicity, the excellent ability to form membranes, and biocompatibility (Agarwal et al., 2016). In order to improve its rigidity, cross-linking can be done (Skopinska-Wisniewska et al., 2021). Probiotics encapsulated with gelatin and coated with maltodextrin showed enhanced viability (Sohail et al., 2013). Recently, an experiment has been carried out in which the probiotic yeast Kluyveromyces lactis was encapsulated in gelatin hydrogels which were obtained by cross-linking the gelatin chemically. Hydrogels having the highest concentrations of gelatin (7.5%, w/v) which the concentrations of cross-linking agents were found to be 3.0% and 5.0% (w/w) were selected for encapsulation. The efficiency of encapsulation was found to be 10%, and there was 50% cell viability level when subjected to simulated gastric conditions (Albadran et al., 2020).
Casein, a milk protein, is a popular encapsulating material for probiotics (Shori, 2017). Casein has excellent gelation property, initiated by unfolding of protein by denaturation followed by aggregation (Wu et al., 2021). Non-covalent bonds aid in stabilizing the structure more than covalent bonds. It is water-insoluble in nature and protects the bacteria during gastric transit (Shori, 2017). Encapsulation of Bifidobacterium bb12 and Lactobacillus F19 in casein microstructures was done by enzymatic gelation, and their resistance against dehydration during cryodesiccation and storage was tested. It was found that Bifidobacterium showed an increase in viability during storage upto 90 days, whereas Lactobacillus didn’t show a substantial change in survivability in comparison to cells that were not encapsulated (Iravani et al., 2015).
Whey protein is frequently employed protein for probiotic encapsulation. It is preferred especially due to its amphoteric nature and its ability to be mixed easily with negatively charged polysaccharides. This happens when the net charge of the protein becomes positive as a result of the pH being set below the isoelectric point (Choińska-Pulit et al., 2015). Whey protein has been used to encapsulate Lacticaseibacillus rhamnosus strain by extrusion. 96% of bacterial cells had been incorporated into whey protein capsule successfully (Doherty et al., 2012).
Natural support
The non-digestible carbohydrates present in fruits acts as the foundation for encapsulating probiotics. In this context, using quince and apple pieces as support, L. casei cells were effectively immobilized. Immobilized biocatalysts were also used to synthesize lactic acid and probiotic additive fermented milk, whilst immobilized microbial cells regained activity after 129 days at 4 °C. Throughout the storage time, a fruity, unmistakable scent pervaded the fermented milk. Lacticaseibacillus casei, immobilised on fruits were also effectively employed in the manufacture of cheese containing probiotic (Aziz & Temiat, 2020).
Fruits and oats pieces were also considered to be used as support for transporting L. casei ATCC 393. Cell viability was examined throughout refrigerated conditions of the immobilized cells, which were utilised to make probiotic yoghurt. According to strain-specific multiplex PCR analyses, not only immobilized but free cells were also identified at sufficient quantities for conferring a probiotic effect for longer durations than needed by the dairy sector (30 days) while stored at 4 °C (Bosnea et al., 2017). By adopting a vacuum impregnation process, attempts were made to merge the health benefits of probiotics with the benefits of fruits and vegetables. Encapsulated L. salivarius is vacuum impregnated into apple discs which were later dried at 40 °C for 24 h, and after the gastrointestinal simulation for 30 days the viability of the cells were evaluated. It was found that the dried apple discs with the encapsulated cells that showed enhanced viability than the free cells (Ester et al., 2019).
Similar research revealed that alginate micro beads were used to encapsulate L. rhamnosus and Lactobacillus acidophilus at 25 °C and 4 °C their acidification carried out in orange juice was investigated for 9 days and around 30 days, respectively (Sohail et al., 2012). Orange juice has been considered as a great probiotic carrier since it has high antioxidant ascorbic acid that has the ability to protect the oxygen sensitive bacteria. Encapsulated L. rhamnosus showed enhanced viability compared to L. acidophilus, indicating that the increase in survival depends on the species. The findings also show that even though encapsulation does not protect the sensitive probiotic cells from death, it certainly reduces acidification in orange juice (Perricone et al., 2015).
Synthetic polymers
Synthetic polymers are not naturally produced by cells in nature. There are a lot of synthetic polymers used commercially for encapsulating probiotics today. Poly(lactide-co-glycolide) is a synthetic polymer used to encapsulate hydrophobic compounds that need time-dependent release. This is a desired encapsulating material due to its biocompatibility, biodegradability, and controlled delivery. The use of poly d, l-lactic-co-glycolic acid (PLGA) for encapsulating probiotics has its limitations, as the organic solvents used for polymer solubilizing can lead to cell damage (Asgari et al., 2020). Cellulose acetate phthalate is another synthetic polymer used as an encapsulating agent. Cellulose acetate phthalate has the property of being soluble at the pH above 6.0 but it is not soluble below the pH 5.0. This is a desired property since the biomaterials should dissolve in the gastrointestinal tract but not be soluble in the stomach (Amidon et al. 2015). Polyvinyl alcohol, a hydrophilic synthetic polymer that is widely used for encapsulating microbes. This is a desired biomaterial since it is inexpensive, non-toxic, durable and highly stable. It has been used in different eatables such as cereals, and yogurts. (Ephrem et al., 2018). Various encapsulation methods and their respective advantages and disadvantages are elaborated in Table 2.
Application of encapsulation technologies in probiotic food production
Although many microorganisms synthesize lactic acid, such as Enterococci, Streptococci, Lactococci, which are utilized as probiotics strains incorporated in food matrices, and the most frequently used ones belong to Bifidobacterium and Lactobacillus genera (Sarao & Arora, 2017). To enhance the host health benefits, it is commonly believed that the minimum concentration of probiotic cells that survive in the food matrix should be around 106–107 Colony forming unit (CFU) per gram or ml. Therefore, there is a technological barrier in preserving the viability of the microorganisms that are incorporated into the foods under the condition of processing, storage, distribution, and consumption. Various edibles have been utilized to deliver probiotics into the gut (Rodrigues et al., 2020).
Fermented foods are ideal vehicles used to deliver probiotic bacteria, especially fermented milk, as the number of proteins, lipids, and carbohydrates present in these matri can increase the bacteria’s viability. Encapsulation of probiotics is required since dairy foods are acidic and are not undersuitable atmosphere for the stabilization of the microbes. Therefore, immobilization methods can be employed to enhance the viability from the harsh environment (Alvarado-Reveles et al., 2019).
Proteins as well as essential micronutrients including zinc, iron, and vitamin B12 are abundant in meat products. It also contains a meagre quantity of monosaturated and polysaturated fatty acids (Ekmekcioglu et al., 2018). Probiotics can be incorporated into meat in order to increase the nutritional value of meat products, simultaneously reducing the negative effects. For this, thermos-resistant encapsulated probiotics are used to increase its viability (Chavarri et al., 2012).
Edible coatings as encapsulating matrices
Edible coatings are non-toxic polymers that can be utilized as encapsulating matrices for probiotics that provide a moisture and oxygen barrier (Quirós-Sauceda et al., 2014). The encapsulation provides a microenvironment that protects the core material from the outside environment. These help in extending the viability during storage and minimize the risk of contamination by pathogens and also protect the probiotic microbe from extreme environmental conditions. The method used to encapsulate depends upon the properties of the probiotic, edible coating material which are based on proteins, carbohydrates, and lipids (Pech-Canul et al., 2020). The applications of encapsulated probiotics are elaborated in Fig. 2.
Because of their outstanding mechanical and optical characteristics, proteins are regarded as excellent polymers for edible coatings and good barriers of CO2 and O2 (Pech-Canul et al., 2020). The lipids also make up effective edible coatings due to their low polarity, they largely obstruct moisture transfer. Usually, lipid-based films are in combination with polysaccharides for extra mechanical strength (Pech-Canul et al., 2020). The bacteria L. rhamnosus CECT8361 was coated with alginate enriched with inulin and oligofructose and used to coat blueberries. This edible coating increased the survival of the bacteria and enhanced the antimicrobial property of the end product, thus minimizing the quantity of Listeria inoculate (Bambace et al., 2019). These edible coverings have been used in a fresh-cut vegetables to increase shelf life. It has also been utilized to incorporate probiotics in meat and fish products.
Co-encapsulation of probiotics and bioactives
Synbiotics are now defined as “a mixture comprising live microorganisms and substrate(s) selectively utilized by host microorganisms that confers a health benefit on the host” by the ISAPP. The synbiotics are classified into complementary synbiotics and synergistic synbiotics. Complementary synbiotics constitute prebiotics and probiotics that are independent of each other. The substrate in synergistic synbiotics is intended to be exclusively used by the probiotic bacteria that is administered along with it (Swanson et al., 2020). Data suggesting the efficiency of synbiotics on the gut microbiota is compelling. These researches revealed that symbiotic is able to inhibit harmful microbes by direct antagonism and competitive exclusion and also expedite the recovery of the health of the intestinal microbiome.
The positive effects of these co-encapsulated synbiotics depend upon their specific combination, therefore the antimicrobial activity and strain specificity has to be taken into considerations. By controlling specific gut bacteria, synbiotics assist to balance the gut microbiota, paving the way for the creation of new forms of functional foods with a more precise effects than dietary supplements or other symbiotic-rich products. Moreover, it can also help to combat multi-drug resistant microbes. Co-encapsulation is used to promote synbiotic oral administration, durability, survivability, and also tailored discharge inside the gut. Therefore, selecting the right co-encapsulation technology is important since it needs to improve the survivability of the microbe against the extreme environment of the gastrointestinal tract. The structure and composition of the encapsulating material, as well as the selection of appropriate co-encapsulation technique, determine the encapsulation effectiveness and viability of co-encapsulated probiotics (Chen et al., 2017). A few examples of co-encapsulation are given below.
Gamma-aminobutyric acid (GABA) is a natural non-proteinogenic amino acid. It functions as a bioactive inhibitor of neurotransmission in the mammalian central nervous system. GABA is offered as supplements as it is found only in small quantities in its naturally occurring forms such as coffee, cereals, vegetables and fermented foods (Hepsomali et al., 2020). It also have anti-cancer, anti-anxiety, and anti-diabetic effects, and insufficient levels of GABA has been shown insomnia, anxiety and weaker immunity systems. Co-encapsulating GABA along with L. plantarum NCDC 414 can be used to improve its nutritional potential (Pandey & Mishra, 2021).
Omega-3 polyunsaturated fatty acids (PUFAs) are bioactive lipids that are naturally available in few plants, fish products, and a few vegetable oils. Omega-3 PUFAs incorporated into human diet by microencapsulation, thereby minimizing oxidative degradation, increasing stability and bioavailability (Chen et al., 2017). Furthermore, since probiotics enhance the action of PUFA and vice versa, co-encapsulating is a promising strategy.
Co-encapsulation of dietary fibres are advantageous to human health because they may change speciation diversity and size of the bacterial colonies and inhibit microbial adherence (Wu et al., 2020). Probiotics were effectively combined with several varieties of dietary fibers in microspheres, improving their shelf durability, processing resistance, and transit through the GI tract (Ying et al., 2016). Encapsulation of probiotics along with maize starch has shown improvement in viability compared to encapsulating just the probiotic alone (Etchepare et al., 2016).
Stability of encapsulated probiotics
Although encapsulation of the probiotics increases the stability considerably, there are a lot of factors that affect its stability. The major elements that determine the stability of immobilized probiotics are high temperature, UV light, solubility, controlled release, and anti-microbial effects (Mitropoulou et al., 2013). The glass transition temperature must be measured using differential scanning calorimetry in order to determine the stability of the encapsulated probiotics. At glass transition temperature, the permeability of the coating material is low and preventing oxygen entering into the capsule and thereby preserving the probiotic bacteria (Călinoiu et al., 2019). As the deterioration due to bacterial growth and other chemical reactions is very low when stored lower than the glass transition temperature, the shelf life of the encapsulated probiotics is extended. If it is stored at a temperature greater than the glass transition temperature, the entry of oxygen accelerates various chemical reactions (Liliana et al., 2014).
One of the important things that affect the efficiency of encapsulation and its ability to release probiotics in the desired region. The controlled release and the carrier material plays a vital role (Liao et al., 2020). Alginate is considered a great polymer for encapsulating probiotics as it is stable in an acidic environment but disintegrates when introduced into alkaline conditions, and thereby releasing the microbe. But due to the pores present in the alginate beads, it might decompose into hostile environment. This can be avoided by combining alginate with other materials, such as chitosan, gelatin, and pectin (Liao et al., 2020).
The bacteria Lacticaseibacillus rhamnosus, entrapped in alginate was coated with locust bean gum and xantham gum (Cheow et al., 2014). The presence of Locust gum makes it more resistant to acidic environment and not only protects it from the low pH and prevents the release of probiotic into the simulated gastric juice. This results in the dissolution of the capsule, thereby releasing the probiotic in the simulated intestinal juices. Overall the beads coated with locust bean gum showed a better release profile than the beads coated with xantham gum (Cheow et al., 2014).
The entrapped cells multiply when the microspheres are suspended in an appropriate medium. When the cells reach the critical concentration, which is the volume of the core’s volume, the capsules rupture thereby releasing all the cells (Călinoiu et al., 2019). This period is defined as the burst-release time. An experiment that was conducted using L. casei entrapped in an alginate matrix in which the burst-release method was practiced, showed that the encapsulation of bacteria can help to create a significant lag in time that protects the cells from harsh conditions of the external environment during production, storage and consumption (Basu et al., 2018). At the same time, the delay in releasing is not too long which ensures the release of the microbe in the target site.
The encapsulation of probiotics should not affect its antimicrobial activity negatively so that its efficiency is not affected. A study was conducted in which Pediocucus acidilactici, Limosilactobacillus reuteri, and L. salivarius was coated with inulin and alginate. The results showed a considerable reduction in the antimicrobial activity by the encapsulated cells than the microbial activity by the non-encapsulated cells (Atia et al., 2016).
To improve the survival of the probiotics, an antibacterial encapsulating material is needed. Poly glutamic acid films were developed with different concentration of polylysine in an attempt to produce antibacterial packaging material to protect a probiotic bacteria that produces GABA. When less PL was added, the antimicrobial activity was quite ineffective. But as the quantity of PL added to the PG increased, the antimicrobial activity of the material also increased significantly (Karimi et al., 2020).
Many bacteria are sensitive to high temperature and UV rays so encapsulating them would provide protection and increase their viability. In research, Lactiplantibacillus plantarum PCM 2675 was encapsulated in sodium alginate by electrospinning (Mahmoud et al., 2020). Both the free bacterial cells and the immobilized cells were subjected to harsh conditions such as UV radiation and high temperature. It was evident that both showed a reduction in metabolic activity after the exposure. But, it must be noted that the inhibition of growth of the immobilized cells was less compared to that of the free cells (Żur et al., 2016).
Synergistic interaction of bioactive compounds with probiotics
Over the years, probiotics have shown to be efficient, not only ameliorating the gut microbiome but also ameliorating the risks factors of various conditions like obesity, metabolic syndrome, and skin problems. (Li et al., 2021). The synergistic effect is defined as the cumulative effects of two active ingredients when they interact with each other leading to the cumulative effect of their activity. The synergistic effect of the probiotic strain along with bioactive agents has shown a positive impact on the gut by increasing the gut microbiota and reducing the risks of obesity, hypertension, and kidney disease. (Xavier-Santos et al., 2020). These have also proven to be efficient in weight loss in obese and overweight individuals. The interaction of the probiotic strains with bioactive agents has been studied over the years in humans, chickens, dogs, and mice (Wiciński et al., 2020; Xavier-Santos et al., 2020).
Antioxidants
When probiotics and antioxidants are administered in the appropriate quantities, their synergistic interaction results in reducing the risks of kidney disease. In dogs, the effects supplemented the parameters of blood along with the biochemical profile of the urinary system (Meineri et al., 2021). Probiotics, prebiotics, and antioxidants also showed low inflammation, and improved renal function due to antioxidant supplementation. Thus, the use of probiotics and antioxidants along with prebiotics maintains good nutritional status and improves blood and urinary parameters (Meineri et al., 2021). Few probiotic products also showed antioxidant properties. When fermented milk obtained from mammals like cows, goats, and camels was supplemented a with probiotic of the bacterial strain Pedicoccus pentosus, the antioxidative property was shown in Balakrishnan and Agrawal (2014). This also displayed increased radical scavenging activity (Meineri et al., 2021). It is observed that when hairless mice orally consumed live BBY, BBY prevented UV-induced trans epidermal water loss in the mice. The hydrogen peroxide level was suppressed and oxidation of proteins, lipids and xanthine was observed (Meineri et al., 2021).
The two strains of bacterial species Bacillus subtilis PB6 and Bacillus cereus were observed for their antioxidant activity and immune responses in chicken broiler (Abudabos et al., 2016). The use of probiotics showed positive modulation in oxidants and antioxidant levels. An increase in the antioxidant level, reduced glutathione concentration, and inhibited lipid peroxidation was observed (Abudabos et al., 2016). Reduced inflammation and obesity in Type 2 diabetic mice was observed in strains of S. boulardii. This particular effect of Saccharomyces boulardii resulted in dramatic differences in the gut microbial composition of the host organism. Human clinical trials also showed increased antioxidative activity and anti-atherogenic effects when treated with L. fermentum ME-3 from fermented milk. Thus, probiotics as antioxidants have exhibited reduced oxidative damage radicals, scavenging rats. The superoxide dismutase activity was also reduced significantly in the human body (Meineri et al., 2021).
Antimicrobials
Due to the increase in antibiotic resistance observed in pathogens, antimicrobial agents are introduced to combat the growing infections. The genus Bacillus is capable of producing antimicrobial agents which is an elementary alternative to combat antibiotic-resistant pathogen, it has proven to be an efficient alternative to the antibiotics used. Bacillus species possess properties such as antibacterial, antifungal, and antitumor. (Caulier et al., 2019). Polypeptides like bacitracin, along with antimicrobial agents, have successfully treated colitis and diarrhoea caused by Clostridium difficile. Cyclic and anionic AMP subtilisin exude antimicrobial activity against agents like bacterial vaginosis and related pathogens. Seaweed probiotics also synergistically improved the survival of shrimp. This seaweed probiotic has an antimicrobial effect against the pathogens hindering the survival of shrimp (Sumi et al., 2015).
Probiotics, with the help of antimicrobial activity, produce antimicrobial substances like bacteriocins, and organic acids, which modulate the host’s immune system (Igbafe et al., 2020). Bacteriocin-like compounds have proven to be responsible for the antimicrobial property exhibited by Bifidobacterium. This Bifidobacterium also inhibits pathogens like E. coli, and L. monocytogenes. Even Lactic acid bacteria (LAB) and certain yeasts have an antimicrobial effect against many pathogens (Igbafe et al., 2020). Bacterial species like Bifidobacterium longum, L. rhamnosus, and L. delbrueckii have the ability to self-aggregate and allow their antimicrobial activity to interact and aggregate with other microorganism like the wound pathogens Staphylococcus aureus and Candida albicans (Frassinetti et al., 2020).
Liquid delivery system or encapsulation through the liquid system
When delivered to the site of action, probiotics may be under several alterations which may weaken their overall effect due to changes in pH, oxygen, temperature, and storage condition. These probiotics show vulnerability towards several environmental factors such as temperature and pH, thus, the maintainance of the viability of probiotics in such stresses by developing successful probiotic delivery systems is important (Govender et al., 2014). Encapsulation protects the probiotic or the bacteria by forming a layer over it that can withstand extreme conditions upon its delivery to the site of action. The materials used in the process of encapsulation are sure that they are non-toxic to the particles to be encapsulated. Encapsulated materials can also be stored for long durations and increased shelf life (Govender et al., 2014). The liquid delivery systems include processes like sol–gel immobilization, extrusion, emulsification, coacervation, and emulsion gel.
Sol–gel immobilisation
This method encapsulates the bacteria in a three-dimensional framework by an aqueous polymerization or gelation reaction. In this method of encapsulation, bacteria or probiotic are covered with a layer of polymers that are stimuli-responsive soluble–insoluble polymers (Penhasi, 2015).The biomaterials used for encapsulating also include polysaccharides like alginate, chitosan, and agarose, and proteins like collagen and gelatin. Strains of Bifidobacteria are the most commonly used in experiments. Research have shown that encapsulation of these strains ensures protection and longevity (O’Callaghan & Van Sinderen, 2016). Under the survival test using Salt solution and PIF dispersion methods, the viability displayed by encapsulated cells was more than that of the free cells (non-encapsulated cells) (O’Callaghan & van Sinderen, 2016).
Ligilactobacillus salivarius, a probiotic strain, was coated using alginate and alginate–gelatin microgels and was subjected to various conditions to check viability. When both the encapsulated and free probiotics cells were introduced to artificial saliva, their viability was maintained. However, it was also noted that after incubation under simulated conditions of the GIT, the survival of encapsulated cells was greatly improved compared to that of the non-encapsulated cells (Kwiecień & Kwiecień, 2018). Further, these cells were stored for five weeks to verify the survival rate. Encapsulated L. salivarius cells showed improved viability than the free counterparts. Under thermal stability testing of the encapsulated cell, it was noted that the encapsulated cells displayed higher viability of cells after the heat treatment. This is to mimic the simulated thermal processing used in the food industry (Kwiecień & Kwiecień, 2018).
Extrusion
In this method, hydrocolloid is mixed with probiotics, which is then introduced in the extruder. This extruder is usually a syringe. The syringe, under pressure drops the contents present inside into a gelling mixture. The gelling mixture is subjected to constant stirring while the contents of the syringe drop into it (Muhardina et al., 2018). The drops formed in the gelling solution are called beads, and the size depends on the diameter of the syringe needle used, and it also depends on the gap between the syringe needle and the gelling solution. The core of the beads is a porous network. This way, the cells are trapped in a 3-dimensional network. L. bulgaricus probiotic in capsule was formed after extrusion encapsulation with alginate. These were tested under low temperatures, and it was observed that the encapsulated L. bulgaricus showed tolerance to lower temperatures. It can be concluded that this probiotic capsule is in a cold or frozen food product (Muhardina et al., 2018).
Emulsification
This technique is based on the homogenization of continuous and discontinuous phases. Probiotics are made into a slurry paste or lyophilized powder mixed with hydrocolloids like alginate, carrageenan, and pectin (discontinuous phase) (Tee et al., 2014). The stirring speed and the ratio of water in oil affect the size and diameter of the droplets formed. These droplets formed are called capsules which have a liquid core. This mixture is then stabilized using emulsifiers. The capsules are recovered by settling and recovered usually have unequal size and shape (Frakolaki et al., 2021).
Bifidobacterium longum was encapsulated the using emulsification method. The encapsulated and free cells were tested for survival ability under heat treatments. It was observed that at 65 °C, the cells that were encapsulated had higher viability when compared to the free cells. Similarly, when the survival of cells was checked under long-term storage, encapsulated cells showed resistance to a lower temperature (Ji et al., 2019). L. plantarum was encapsulated by the method of emulsification. The viability cells of the strain were noted when it was exposed to pH as low as pH 2.0 and exposed to bile salts. In pH 2.0, a comparison was drawn between the encapsulated and non-encapsulated cells of probiotic strain L. plantarum. It is observed that the encapsulated cells were higher in quantity when compared to free cells (Tee et al., 2014).
Coacervation
In the coacervation method, the core material is first scattered in the hydrophobic bifunctional phase. This core was emulsified in a continuous phase that has two or more different polymers (Bosnea et al., 2014). Bacterial strains Lactiplantibacillus paraplantarum, and L. paracasei were microencapsulated using coacervation. The viability of encapsulated L. paraplantarum, and L. paracasei, the non-encapsulated and encapsulated L. paraplantarum and L. paracasei were verified by subjecting it to low pH of 2.0. The coacervated L. paraplantarum and L. paracasei showed resistance to the low pH retaining their population with 89% of their initial viability (Bosnea et al., 2014). L. acidophilus was encapsulated by complex coacervation, after which was subjected to transglutaminase crosslinking, which yielded improved resistance of the encapsulated material and enhanced protection (Bosnea et al., 2014; Tee et al., 2014). The encapsulated cells showed increased viability when exposed to pepsin to mimic the gastro-intestinal environment. Under heat treatment, i.e., 63 °C for 1800 s and 72 °C for 15 s, the encapsulated cells showed improved resistance due to crosslinking with transglutaminase (Tee et al., 2014).
Emulsion gels
A 3-dimensional protein network is formed. This network entraps the oil droplets emulsified when gelling of the continuous phase occurs due to the treatment of heat, salt, and acid (Torres et al., 2016). The viability of B. breve cells that were encapsulated had notably increased when a comparison was drawn with the viability of non-encapsulated B. breve cells (Kwiecień & Kwiecień, 2018). Different strains of probiotic bacteria, like B. longum, and, L. plantarum were encapsulated using the emulsion method by materials like calcium alginate beads to verify the heat parameter. They were exposed to heat (65 °C). After an incubation period of 30 min, the bacteria that were encapsulated displayed increased survival potential than the non-encapsulated cells (Kwiecień & Kwiecień, 2018).
Solid delivery system or encapsulation through drying methodology
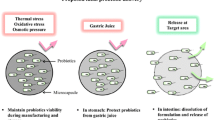
For the probiotics to be delivered under extreme stresses like heat, pH, and enzyme degradation these probiotics need to be encapsulated. This encapsulation will ensure the viability of the probiotics as well as protection from degradation by various factors (Govender et al., 2014). The various solid delivery systems include spray drying, electrospray drying, electrospinning, and spray chilling (Govender et al., 2014). Figure 3 shows the preparation of encapsulated probiotics and their types.
Spray drying
Spray drying is a conventional method to encapsulate heat-sensitive material like probiotics, and flavor. When L. reuteri was directly spray-dried using whey from the slurry fermentation, it was tested for survival rate under simulated intestinal conditions. It was observed that the encapsulated cell count of L. reuteri had increased upon introduction to the intestinal juice because the encapsulated bacteria were released while increasing the survival rate to 54% after 3 h (Jantzen et al., 2013). After incubation with digestive juices, free cells of L. reuteri showed loss of 86% in live cells, while the encapsulated bacteria showed a loss of only 54% under similar parameters. It was noted that the survivability of L. reuteri was increased when whey matrix was used for encapsulation by 32% against free/non-encapsulated ones (Jantzen et al., 2013).
Electrospray drying
The probiotics were first introduced into a capillary. This method relies on the application of electric fields, leading to deflection in the particles with variants. The electrospraying by deposition in solution method (Gomez-Mascaraque et al., 2016) has three variants of the electrospraying technique, electrospraying in solution technique, coaxial electrospraying technique, and electrospraying deposition technique (Tapia-Hernández et al., 2017). When L. plantarum was encapsulated using electrospray drying, it was proved efficient and reliable as 96% of L. plantarum culture notably retained upon the application of electric field and osmotic stresses (Tapia-Hernández et al., 2017).
Electrospinning
Electrospinning involves the continuous production of nanofibers for encapsulation of the probiotic. This method involves the liquid droplet getting electrified in order to generate a jet. An electrospinning setup comprises a spinneret, a high-voltage supply, a syringe pump, and a collector (Mojaveri et al., 2020). Utilizing chitosan/alginate of multi-layer fibre mats to encapsulate Bacillus coagulans, it was found that it protects these probiotic cells against gastrointestinal and improves its attachment, followed by growth in the intestine. Multiple layers of coating may ensure efficient protection of the incorporated probiotics (Anselmo et al., 2016). Electrospun bacteria have been in the form of polymers and have found applications in biomedical research. Staphylococcus epidermidis was introduced to carboxymethyl cellulose/polyethylene oxide fibres to treat diabetic foot ulcers. Bacterial strain Lactobacillus was loaded into PVA and polyvinyl pyrrolidone fibres to successfully treat bacterial vaginitis (Deng & Zhang, 2020). Electrospun probiotics show promising results as they display controlled delivery and active packaging. Encapsulated probiotics can replenish naturally occurring microorganisms that are destroyed when the body is invaded by diseases like psoriasis. These have also found use in tissue engineering. Even burns and scars have shown healing to some extent. Regeneration of skin tissues and protection from UV rays was also observed (Deng & Zhang, 2020). A summary of encapsulation techniques with their advantages and disadvantages are represented in Table 3.
In spite of the relative advantages of electrospinning blends for encapsulating probiotics, there are some limitations, like the lack of protection for active ingredients or controlled release mechanisms. Combining coaxial electrospinning with biomaterials has overcome these limitations in the delivery of probiotics. Plant cells contain cellulose, which is a polysaccharide that is good for strength and stiffness in nanofibers. Combining carboxymethyl cellulose with PEO has been shown to be effective for encapsulating S. epidermidis (Kurečič et al., 2018).
The addition of protective agents to nanofibers can greatly enhance the vitality of probiotics. In a study, (Škrlec et al., 2019), Lactobacillus plantarum was encapsulated in polymer materials with and without lyoprotectants. The protectants not just did not interfere with said electrospinning preparation procedure, but they significantly prevented bacterial survivability loss during the production and storage periods (Kurečič et al., 2018). They reported that probiotic quantities and lyoprotectant classes of the polymer were significantly linked to probiotic viability. Furthermore, Trehalose outperformed sucrose in regards to storage protection. In complement to lyoprotectants, prebiotics were useful as an additional ingredient in electrospinning. FOS is a saccharide that can help lactobacillus flourish. It may be utilized as a cladding for prophylactic encapsulation using electrospinning technology, which not only does not harm bacteria but also maintains vitality and improves thermal stability (Feng et al., 2018; Škrlec et al., 2019). Various parameters of all the encapsulation techniques related to its preparation were elaborated in Table 4.
Future perspective and summary
A wide array of food products containing probiotic strains are available in markets today, consumption of which in a prescribed amount is found to provide numerous health benefits. Various new technologies are used for the isolation and production of probiotics. Bioengineering of probiotics using new fermentation technologies using suspended and immobilized cell technologies are found to be effective. Several studies indicate favorable results when using different encapsulation procedures and encapsulation material. Although probiotic encapsulation technology has advanced significantly, further research is still needed to make this technology commercially more successful. There are still many challenges that needs to be overcome, such as selecting non-toxic encapsulation material, better encapsulation technology, more pH resistant polymers as coating materials and cost management. Further, we need to have a complete understanding of the physiochemical characteristics of the coating materials in order to predict or to have a control over the release of the probiotics under different environmental conditions such as pH, temperature, salinity. Cost of the product depends upon the polymer used for encapsulation, amount of product produced, and the encapsulation technique used. Moreover, the cost of the probiotic encapsulation technology has to be minimized.
References
Abudabos A, Alyemni A and Zakaria H. Effect of two strains of probiotics on the antioxidant capacity, oxidative stress, and immune responses of salmonella-challenged broilers. Revista Brasileira de Ciência Avícola. 18: 175-180 (2016). https://doi.org/10.1590/18069061-2015-0052
Agarwal P, Dubey S, Singh M and Singh RP. Aspergillus niger pa2 tyrosinase covalently immobilized on a novel eco-friendly bio-composite of chitosan-gelatin and its evaluation for l-dopa production. Frontiers in Microbiology. 7: (2016). https://doi.org/10.3389/fmicb.2016.02088
Al Aziz E and Temiat L. Preparation of probiotic fruits through encapsulation of bacteria. Medbiotech J. 4: 118–120 (2020). https://doi.org/10.22034/mbt.2020.120936
Albadran HA, Monteagudo-Mera A, Khutoryanskiy VV. and Charalampopoulos D. Development of chitosan-coated agar-gelatin particles for probiotic delivery and targeted release in the gastrointestinal tract. Applied Microbiology and Biotechnology. 104: 5749–5757 (2020). https://doi.org/10.1007/s00253-020-10632-w
Alonso S. Novel preservation techniques for microbial cultures. pp. 7–33. (2016). https://doi.org/10.1007/978-3-319-42457-6_2
Alvarado-Reveles O, Fernández-Michel S, Jiménez-Flores R, Cueto-Wong C, Vázquez-Moreno L and Montfort GR-C. Survival and goat milk acidifying activity of Lactobacillus rhamnosus gg encapsulated with agave fructans in a buttermilk protein matrix. probiotics and antimicrobial proteins. 11: 1340–1347 (2019). https://doi.org/10.1007/s12602-018-9475-y
Amidon S, Brown JE and Dave VS. Colon-targeted oral drug delivery systems: design trends and approaches. AAPS PharmSciTech. 16: 731–741 (2015). https://doi.org/10.1208/s12249-015-0350-9
Andrade DP, Ramos CL, Botrel DA, Borges SV, Schwan RF and Ribeiro Dias D. Stability of microencapsulated lactic acid bacteria under acidic and bile juice conditions. International Journal of Food Science & Technology. 54: 2355–2362 (2019). https://doi.org/10.1111/ijfs.14114
Anselmo AC, McHugh KJ, Webster J, Langer R and Jaklenec A. Layer-by-layer encapsulation of probiotics for delivery to the microbiome. Advanced Materials. 28: 9486–9490 (2016). https://doi.org/10.1002/adma.201603270
Aragón-Rojas S, Quintanilla-Carvajal MX, Hernández-Sánchez H, Hernández-Álvarez AJ and Moreno FL. Encapsulation of Lactobacillus fermentum K73 by Refractance Window drying. Scientific Reports. 9: 5625 (2019). https://doi.org/10.1038/s41598-019-42016-0
Asgari S, Pourjavadi A, Licht TR, Boisen A and Ajalloueian F. Polymeric carriers for enhanced delivery of probiotics. Advanced Drug Delivery Reviews. 161–162: 1–21 (2020). https://doi.org/10.1016/j.addr.2020.07.014
Atia A, Gomaa A, Fliss I, Beyssac E, Garrait G and Subirade M. A prebiotic matrix for encapsulation of probiotics: physicochemical and microbiological study. Journal of Microencapsulation. 33: 89–101 (2016). https://doi.org/10.3109/02652048.2015.1134688
Balakrishnan G and Agrawal R. Antioxidant activity and fatty acid profile of fermented milk prepared by Pediococcus pentosaceus. Journal of Food Science and Technology. 51: 4138–4142 (2014). https://doi.org/10.1007/s13197-012-0891-9
Bambace MF, Alvarez MV and Moreira M del R. Novel functional blueberries: Fructo-oligosaccharides and probiotic lactobacilli incorporated into alginate edible coatings. Food Research International. 122: 653–660 (2019). https://doi.org/10.1016/j.foodres.2019.01.040
Basu S, Banerjee D, Chowdhury R and Bhattacharya P. Controlled release of microencapsulated probiotics in food matrix. Journal of Food Engineering. 238: 61–69 (2018). https://doi.org/10.1016/j.jfoodeng.2018.06.005
Bepeyeva A, de Barros JMS, Albadran H, Kakimov AK, Kakimova ZKh, Charalampopoulos D and Khutoryanskiy VV. Encapsulation of lactobacillus casei into calcium pectinate-chitosan beads for enteric delivery. Journal of Food Science. 82: 2954–2959 (2017). https://doi.org/10.1111/1750-3841.13974
Blanco-Padilla A, Soto KM, Hernández Iturriaga M and Mendoza S. Food antimicrobials nanocarriers. The Scientific World Journal. 2014: 1–11 (2014). https://doi.org/10.1155/2014/837215
Bosnea LA, Kopsahelis N, Kokkali V, Terpou A and Kanellaki M. Production of a novel probiotic yogurt by incorporation of L. casei enriched fresh apple pieces, dried raisins and wheat grains. Food and Bioproducts Processing. 102: 62–71 (2017). https://doi.org/10.1016/j.fbp.2016.11.010
Bosnea LA, Moschakis T and Biliaderis CG. Complex coacervation as a novel microencapsulation technique to improve viability of probiotics under different stresses. Food and Bioprocess Technology. 7: 2767–2781 (2014). https://doi.org/10.1007/s11947-014-1317-7
Brigham C. Biopolymers. pp. 753–770. In: Green Chemistry. Elsevier (2018). https://doi.org/10.1016/B978-0-12-809270-5.00027-3
Călinoiu L-F, Ştefănescu B, Pop I, Muntean L and Vodnar D. Chitosan coating applications in probiotic microencapsulation. coatings. 9: 194 (2019). https://doi.org/10.3390/coatings9030194
Caulier S, Nannan C, Gillis A, Licciardi F, Bragard C and Mahillon J. Overview of the antimicrobial compounds produced by members of the bacillus subtilis group. Frontiers in Microbiology. 10: (2019). https://doi.org/10.3389/fmicb.2019.00302
Chavarri M, Maranon I and Carmen M. Encapsulation technology to protect probiotic bacteria. in: probiotics. InTech (2012). https://doi.org/10.5772/50046
Chen F, Fan GQ, Zhang Z, Zhang R, Deng ZY and McClements DJ. Encapsulation of omega-3 fatty acids in nanoemulsions and microgels: Impact of delivery system type and protein addition on gastrointestinal fate. Food Research International. 100: 387–395 (2017). https://doi.org/10.1016/j.foodres.2017.07.039
Cheow WS, Kiew TY and Hadinoto K. Controlled release of Lactobacillus rhamnosus biofilm probiotics from alginate-locust bean gum microcapsules. Carbohydrate Polymers. 103: 587–595 (2014) https://doi.org/10.1016/j.carbpol.2014.01.036
Choińska-Pulit A, Mituła P, Śliwka P, Łaba W and Skaradzińska A. Bacteriophage encapsulation: Trends and potential applications. Trends in Food Science & Technology. 45: 212–221 (2015). https://doi.org/10.1016/j.tifs.2015.07.001
Dafe A, Etemadi H, Dilmaghani A and Mahdavinia GR. Investigation of pectin/starch hydrogel as a carrier for oral delivery of probiotic bacteria. International Journal of Biological Macromolecules. 97: 536–543 (2017). https://doi.org/10.1016/j.ijbiomac.2017.01.060
de Araújo Etchepare M, Raddatz GC, de Moraes Flores ÉM, Zepka LQ, Jacob-Lopes E, Barin JS, Ferreira Grosso CR and de Menezes CR. Effect of resistant starch and chitosan on survival of Lactobacillus acidophilus microencapsulated with sodium alginate. LWT - Food Science and Technology. 65: 511–517 (2016). https://doi.org/10.1016/j.lwt.2015.08.039
de Castro-Cislaghi FP, Silva CDRE, Fritzen-Freire CB, Lorenz JG and Sant’Anna ES. Bifidobacterium Bb-12 microencapsulated by spray drying with whey: Survival under simulated gastrointestinal conditions, tolerance to NaCl, and viability during storage. Journal of Food Engineering. 113: 186–193 (2012). https://doi.org/10.1016/j.jfoodeng.2012.06.006
Deng L and Zhang H. Recent Advances in probiotics encapsulation by electrospinning. ES Food & Agroforestry: (2020). https://doi.org/10.30919/esfaf1120
Desmond C, Ross RP, O’Callaghan E, Fitzgerald G and Stanton C. Improved survival of Lactobacillus paracasei NFBC 338 in spray-dried powders containing gum acacia. Journal of Applied Microbiology. 93: 1003–1011 (2002). https://doi.org/10.1046/j.1365-2672.2002.01782.x
Doherty SB, Auty MA, Stanton C, Ross RP, Fitzgerald GF and Brodkorb A. Survival of entrapped Lactobacillus rhamnosus GG in whey protein micro-beads during simulated ex vivo gastro-intestinal transit. International Dairy Journal. 22: 31–43 (2012). https://doi.org/10.1016/j.idairyj.2011.06.009
Ekmekcioglu C, Wallner P, Kundi M, Weisz U, Haas W and Hutter HP. Red meat, diseases, and healthy alternatives: A critical review. Critical Reviews in Food Science and Nutrition. 58: 247–261 (2018). https://doi.org/10.1080/10408398.2016.1158148
Ephrem E, Najjar A, Charcosset C and Greige-Gerges H. Encapsulation of natural active compounds, enzymes, and probiotics for fruit juice fortification, preservation, and processing: An overview. Journal of Functional Foods. 48: 65–84 (2018). https://doi.org/10.1016/j.jff.2018.06.021
Ester B, Noelia B, Laura CJ, Francesca P, Cristina B, Rosalba L and Marco DR. Probiotic survival and in vitro digestion of L. salivarius spp. salivarius encapsulated by high homogenization pressures and incorporated into a fruit matrix. LWT. 111: 883–888 (2019). https://doi.org/10.1016/j.lwt.2019.05.088
Fathi M, Donsi F and McClements DJ. Protein-based delivery systems for the nanoencapsulation of food ingredients. Comprehensive Reviews in Food Science and Food Safety. 17: 920–936 (2018). https://doi.org/10.1111/1541-4337.12360
Feng K, Zhai MY, Zhang Y, Linhardt RJ, Zong MH, Li L and Wu H. Improved viability and thermal stability of the probiotics encapsulated in a novel electrospun fiber mat. Journal of Agricultural and Food Chemistry. 66: 10890–10897 (2018). https://doi.org/10.1021/acs.jafc.8b02644
Fenster K, Freeburg B, Hollard C, Wong C, Rønhave Laursen R and Ouwehand A. The production and delivery of probiotics: a review of a practical approach. microorganisms. 7: 83 (2019). https://doi.org/10.3390/microorganisms7030083
Frakolaki G, Giannou V, Kekos D and Tzia C. A review of the microencapsulation techniques for the incorporation of probiotic bacteria in functional foods. Critical Reviews in Food Science and Nutrition. 61: 1515–1536 (2021). https://doi.org/10.1080/10408398.2020.1761773
Frassinetti S, Gabriele M, Moccia E, Longo V and Di Gioia D. Antimicrobial and antibiofilm activity of Cannabis sativa L. seeds extract against Staphylococcus aureus and growth effects on probiotic Lactobacillus spp. LWT 124: 109149 (2020)
Gaur PK, Mishra S and Purohit S. Solid lipid nanoparticles of guggul lipid as drug carrier for transdermal drug delivery. BioMed Research International. 2013: 1–10 (2013). https://doi.org/10.1155/2013/750690
Gomez-Mascaraque LG, Morfin RC, Pérez-Masiá R, Sanchez G and Lopez-Rubio A. Optimization of electrospraying conditions for the microencapsulation of probiotics and evaluation of their resistance during storage and in-vitro digestion. LWT - Food Science and Technology. 69: 438–446 (2016). https://doi.org/10.1016/j.lwt.2016.01.071
Govender M, Choonara YE, Kumar P, du Toit LC, van Vuuren S and Pillay V. A review of the advancements in probiotic delivery: conventional vs. non-conventional formulations for intestinal flora supplementation. AAPS PharmSciTech. 15: 29–43 (2014). https://doi.org/10.1208/s12249-013-0027-1
Gul O. Microencapsulation of Lactobacillus casei Shirota by spray drying using different combinations of wall materials and application for probiotic dairy dessert. Journal of Food Processing and Preservation. 41: e13198 (2017). https://doi.org/10.1111/jfpp.13198
Gul O and Atalar I. Different stress tolerance of spray and freeze dried Lactobacillus casei Shirota microcapsules with different encapsulating agents. Food Science and Biotechnology. 28: 807–816 (2019). https://doi.org/10.1007/s10068-018-0507-x
Gutierrez TJ and Alvarez K (eds). Biopolymers as microencapsulation materials in the food industry. pp. 296–322. In: advances in physicochemical properties of biopolymers (Part 2). bentham science publishers (2017). https://doi.org/10.2174/9781681085449117010009
Hassan ME, Yang Q, Xiao Z, Liu L, Wang N, Cui X and Yang L. Impact of immobilization technology in industrial and pharmaceutical applications. 3 Biotech. 9: 440 (2019). https://doi.org/10.1007/s13205-019-1969-0
Hemarajata P and Versalovic J. Effects of probiotics on gut microbiota: mechanisms of intestinal immunomodulation and neuromodulation. Therapeutic Advances in Gastroenterology. 6: 39–51 (2013). https://doi.org/10.1177/1756283X12459294
Hepsomali P, Groeger JA, Nishihira J and Scholey A. Effects of oral gamma-aminobutyric acid (gaba) administration on stress and sleep in humans: a systematic review. Frontiers in Neuroscience. 14: (2020). https://doi.org/10.3389/fnins.2020.00923
Hong J, Yeo M, Yang GH and Kim G. Cell-electrospinning and its application for tissue engineering. International Journal of Molecular Sciences. 20: 6208 (2019). https://doi.org/10.3390/ijms20246208
Huang Y, Liu M, Gao C, Yang J, Zhang X, Zhang X and Liu Z. Ultra-small and innocuous cationic starch nanospheres: Preparation, characterization and drug delivery study. International Journal of Biological Macromolecules. 58: 231–239 (2013). https://doi.org/10.1016/j.ijbiomac.2013.04.006
Igbafe J, Kilonzo-Nthenge A, Nahashon SN, Mafiz AI and Nzomo M. Probiotics and antimicrobial effect of lactiplantibacillus plantarum, saccharomyces cerevisiae, and bifidobacterium longum against common foodborne pathogens in poultry. Agriculture. 10: 368 (2020). https://doi.org/10.3390/agriculture10090368
Iravani S, Korbekandi H and Mirmohammadi SV. Technology and potential applications of probiotic encapsulation in fermented milk products. Journal of Food Science and Technology. 52: 4679–4696 (2015). https://doi.org/10.1007/s13197-014-1516-2
Jantzen M, Göpel A and Beermann C. Direct spray drying and microencapsulation of probiotic Lactobacillus reuteri from slurry fermentation with whey. Journal of Applied Microbiology. 115: 1029–1036 (2013). https://doi.org/10.1111/jam.12293
Jayani T, Sanjeev B, Marimuthu S and Uthandi S. Bacterial Cellulose Nano Fiber (BCNF) as carrier support for the immobilization of probiotic, Lactobacillus acidophilus 016. Carbohydrate Polymers. 250: 116965 (2020). https://doi.org/10.1016/j.carbpol.2020.116965
Ji R, Wu J, Zhang J, Wang T, Zhang X, Shao L, Chen D and Wang J. Extending viability of bifidobacterium longum in chitosan-coated alginate microcapsules using emulsification and internal gelation encapsulation technology. Frontiers in Microbiology. 10: (2019). https://doi.org/10.3389/fmicb.2019.01389
Karimi M, Yazdi FT, Mortazavi SA, Shahabi-Ghahfarrokhi I and Chamani J. Development of active antimicrobial poly (l-glutamic) acid-poly (l-lysine) packaging material to protect probiotic bacterium. Polymer Testing. 83: 106338 (2020). https://doi.org/10.1016/j.polymertesting.2020.106338
Kurečič M, Rijavec T, Hribernik S, Lapanje A, Kleinschek KS and Maver U. Novel electrospun fibers with incorporated commensal bacteria for potential preventive treatment of the diabetic foot. Nanomedicine. 13: 1583–1594 (2018). https://doi.org/10.2217/nnm-2018-0014
Kwiecień I and Kwiecień M. Application of polysaccharide-based hydrogels as probiotic delivery systems. gels. 4: 47 (2018). https://doi.org/10.3390/gels4020047
Lancuški A, Abu Ammar A, Avrahami R, Vilensky R, Vasilyev G and Zussman E. Design of starch-formate compound fibers as encapsulation platform for biotherapeutics. Carbohydrate Polymers. 158: 68–76 (2017). https://doi.org/10.1016/j.carbpol.2016.12.003
Leng D, Thanki K, Foged C and Yang M. Formulating inhalable dry powders using two-fluid and three-fluid nozzle spray drying. Pharmaceutical Research. 35: 247 (2018). https://doi.org/10.1007/s11095-018-2509-z
Li HY, Zhou DD, Gan RY, Huang SY, Zhao CN, Shang A, Xu XY and Li HB. Effects and mechanisms of probiotics, prebiotics, synbiotics, and postbiotics on metabolic diseases targeting gut microbiota: a narrative review. Nutrients. 13: 3211 (2021). https://doi.org/10.3390/nu13093211
Liao N, Luo B, Gao J, Li X, Zhao Z, Zhang Y, Ni Y and Tian F. Oligosaccharides as co-encapsulating agents: effect on oral Lactobacillus fermentum survival in a simulated gastrointestinal tract. Biotechnology Letters. 41: 263–272 (2019). https://doi.org/10.1007/s10529-018-02634-6
Liao N, Pang B, Jin H, Xu X, Yan L, Li H, Shao D and Shi J. Potential of lactic acid bacteria derived polysaccharides for the delivery and controlled release of oral probiotics. Journal of Controlled Release. 323: 110–124 (2020). https://doi.org/10.1016/j.jconrel.2020.04.022
Liliana SC, Vladimir EVC and Estefana GG. Effects of wall materials and lyophilization on the viability of Weissella confusa. African Journal of Biotechnology. 13: 2661–2667 (2014). https://doi.org/10.5897/AJB2014.13860
Liu Y, Zhang Z and Hu L. High efficient freeze-drying technology in food industry. Critical Reviews in Food Science and Nutrition. 62: 3370–3388 (2022). https://doi.org/10.1080/10408398.2020.1865261
Mahmoud M, Abdallah NA, El-Shafei K, Tawfik NF and El-Sayed HS. Survivability of alginate-microencapsulated Lactobacillus plantarum during storage, simulated food processing and gastrointestinal conditions. Heliyon. 6: e03541 (2020). https://doi.org/10.1016/j.heliyon.2020.e03541
Mandal S, Puniya AK and Singh K. Effect of alginate concentrations on survival of microencapsulated Lactobacillus casei NCDC-298. International Dairy Journal. 16: 1190–1195 (2006). https://doi.org/10.1016/j.idairyj.2005.10.005
Markowiak P and Śliżewska K. Effects of probiotics, prebiotics, and synbiotics on human health. nutrients. 9: 1021 (2017). https://doi.org/10.3390/nu9091021
Martău GA, Mihai M and Vodnar DC. The use of chitosan, alginate, and pectin in the biomedical and food sector—biocompatibility, bioadhesiveness, and biodegradability. polymers. 11: 1837 (2019). https://doi.org/10.3390/polym11111837
Meineri G, Saettone V, Radice E, Bruni N, Martello E and Bergero D. The synergistic effect of prebiotics, probiotics and antioxidants on dogs with chronic kidney disease. Italian Journal of Animal Science. 20: 1079–1084 (2021). https://doi.org/10.1080/1828051X.2021.1940323
Milivojevic M, Pajic-Lijakovic I, Bugarski B, Nayak AK and Hasnain MS. Gellan gum in drug delivery applications. pp. 145–186. in: natural polysaccharides in drug delivery and biomedical applications. Elsevier (2019). https://doi.org/10.1016/B978-0-12-817055-7.00006-6
Mitropoulou G, Nedovic V, Goyal A and Kourkoutas Y. Immobilization technologies in probiotic food production. journal of nutrition and metabolism. 2013: 1–15 (2013). https://doi.org/10.1155/2013/716861
Mojaveri SJ, Hosseini SF and Gharsallaoui A. Viability improvement of Bifidobacterium animalis Bb12 by encapsulation in chitosan/poly(vinyl alcohol) hybrid electrospun fiber mats. Carbohydrate Polymers. 241: 116278 (2020). https://doi.org/10.1016/j.carbpol.2020.116278
Muhardina V, Aisyah Y, Anwar SH, Meutia Sari P, Haryani S and Mega FA. Extrusion encapsulation of Lactobacillus bulgaricus coated by carrageenan-alginate with additional tofu waste flour prebiotic. Article in International Journal of Engineering and Technology. 7: 5242–5244 (2018). https://doi.org/10.14419/ijet.v7i4.24255
Nguyen QV, Huynh DP, Park JH and Lee DS. Injectable polymeric hydrogels for the delivery of therapeutic agents: A review. European Polymer Journal. 72: 602–619 (2015). https://doi.org/10.1016/j.eurpolymj.2015.03.016
Oberoi K, Tolun A, Altintas Z and Sharma S. Effect of alginate-microencapsulated hydrogels on the survival of lactobacillus rhamnosus under simulated gastrointestinal conditions. Foods. 10: 1999 (2021). https://doi.org/10.3390/foods10091999
O’Callaghan A and van Sinderen D. Bifidobacteria and their role as members of the human gut microbiota. Frontiers in Microbiology. 7: (2016). https://doi.org/10.3389/fmicb.2016.00925
Okutan N, Terzi P and Altay F. Affecting parameters on electrospinning process and characterization of electrospun gelatin nanofibers. Food Hydrocolloids. 39: 19–26 (2014). https://doi.org/10.1016/j.foodhyd.2013.12.022
Pandey P and Mishra HN. Co-microencapsulation of γ-aminobutyric acid (GABA) and probiotic bacteria in thermostable and biocompatible exopolysaccharides matrix. LWT. 136: 110293 (2021). https://doi.org/10.1016/j.lwt.2020.110293
Pech-Canul A de la C, Ortega D, García-Triana A, González-Silva N and Solis-Oviedo RL. A Brief Review of Edible Coating Materials for the Microencapsulation of Probiotics. Coatings. 10: 197 (2020). https://doi.org/10.3390/coatings10030197
Penhasi A. Microencapsulation of probiotic bacteria using thermo-sensitive sol-gel polymers for powdered infant formula. Journal of Microencapsulation. 32: 372–380 (2015). https://doi.org/10.3109/02652048.2015.1028497
Perricone M, Bevilacqua A, Altieri C, Sinigaglia M and Corbo M. Challenges for the production of probiotic fruit juices. Beverages. 1: 95–103 (2015). https://doi.org/10.3390/beverages1020095
Picot A and Lacroix C. Encapsulation of bifidobacteria in whey protein-based microcapsules and survival in simulated gastrointestinal conditions and in yoghurt. International Dairy Journal. 14: 505–515 (2004). https://doi.org/10.1016/j.idairyj.2003.10.008
Quirós-Sauceda AE, Ayala-Zavala JF, Olivas GI and González-Aguilar GA. Edible coatings as encapsulating matrices for bioactive compounds: a review. Journal of Food Science and Technology. 51: 1674–1685 (2014). https://doi.org/10.1007/s13197-013-1246-x
Reid G, Gadir AA and Dhir R. Probiotics: Reiterating what they are and what they are not. Frontiers in Microbiology. 10: (2019). https://doi.org/10.3389/fmicb.2019.00424
Rodrigues FJ, Cedran MF, Bicas JL and Sato HH. Encapsulated probiotic cells: Relevant techniques, natural sources as encapsulating materials and food applications – A narrative review. Food Research International. 137: 109682 (2020). https://doi.org/10.1016/j.foodres.2020.109682
Sarao LK and Arora M. Probiotics, prebiotics, and microencapsulation: a review. Critical Reviews in Food Science and Nutrition. 57: 344–371 (2017). https://doi.org/10.1080/10408398.2014.887055
Shori AB. Microencapsulation improved probiotics survival during gastric transit. Hayathi Journal of Biosciences. 24: 1–5 (2017). https://doi.org/10.1016/j.hjb.2016.12.008
Sing CE. Development of the modern theory of polymeric complex coacervation. Advances in Colloid and Interface Science. 239: 2–16 (2017). https://doi.org/10.1016/j.cis.2016.04.004
Skopinska-Wisniewska J, Tuszynska M and Olewnik-Kruszkowska E. Comparative study of gelatin hydrogels modified by various cross-linking agents. Materials. 14: 396 (2021). https://doi.org/10.3390/ma14020396
Škrlec K, Zupančič Š, Prpar Mihevc S, Kocbek P, Kristl J and Berlec A. Development of electrospun nanofibers that enable high loading and long-term viability of probiotics. European Journal of Pharmaceutics and Biopharmaceutics. 136: 108–119 (2019). https://doi.org/10.1016/j.ejpb.2019.01.013
Sohail A, Turner MS, Coombes A and Bhandari B. The viability of lactobacillus rhamnosus gg and lactobacillus acidophilus ncfm following double encapsulation in alginate and maltodextrin. Food and Bioprocess Technology. 6: 2763–2769 (2013). https://doi.org/10.1007/s11947-012-0938-y
Sohail A, Turner MS, Prabawati EK, Coombes AGA and Bhandari B. Evaluation of Lactobacillus rhamnosus gg and Lactobacillus acidophilus NCFM encapsulated using a novel impinging aerosol method in fruit food products. International Journal of Food Microbiology. 157: 162–166 (2012). https://doi.org/10.1016/j.ijfoodmicro.2012.04.025
Souza R, Peruch G and dos Santos Pires AC. Oxygen Scavengers: An approach on food preservation. In: structure and function of food engineering. intech (2012). https://doi.org/10.5772/48453
Spyropoulos F, Lloyd DM, Hancocks RD and Pawlik AK. Advances in membrane emulsification. Part B: recent developments in modelling and scale-up approaches. Journal of the Science of Food and Agriculture. 94: 628–638 (2014). https://doi.org/10.1002/jsfa.6443
Sumi CD, Yang BW, Yeo I-C and Hahm YT. Antimicrobial peptides of the genus Bacillus : a new era for antibiotics. Canadian Journal of Microbiology. 61: 93–103 (2015). https://doi.org/10.1139/cjm-2014-0613
Suryabhan P, Lohith K and Anu-Appaiah KA. Sucrose and sorbitol supplementation on maltodextrin encapsulation enhance the potential probiotic yeast survival by spray drying. LWT. 107: 243–248 (2019). https://doi.org/10.1016/j.lwt.2019.03.002
Swanson KS, Gibson GR, Hutkins R, Reimer RA, Reid G, Verbeke K, Scott KP, Holscher HD, Azad MB, Delzenne NM and Sanders ME. The international scientific association for probiotics and prebiotics (isapp) consensus statement on the definition and scope of synbiotics. Nature Reviews Gastroenterology & Hepatology. 17: 687–701 (2020). https://doi.org/10.1038/s41575-020-0344-2
Tapia-Hernández JA, Rodríguez-Félix F and Katouzian I. Nanocapsule formation by electrospraying. pp. 320–345. In: Nanoencapsulation Technologies for the Food and Nutraceutical Industries. Elsevier (2017). https://doi.org/10.1016/B978-0-12-809436-5.00009-4
Tee W, Nazaruddin R, Tan Y and Ayob M. Effects of encapsulation on the viability of potential probiotic Lactobacillus plantarum exposed to high acidity condition and presence of bile salts. Food Science and Technology International. 20: 399–404 (2014). https://doi.org/10.1177/1082013213488775
Terpou A, Papadaki A, Lappa I, Kachrimanidou V, Bosnea L and Kopsahelis N. Probiotics in food systems: significance and emerging strategies towards improved viability and delivery of enhanced beneficial value. Nutrients. 11: 1591 (2019). https://doi.org/10.3390/nu11071591
Torres O, Murray B and Sarkar A. Emulsion microgel particles: Novel encapsulation strategy for lipophilic molecules. Trends in Food Science & Technology. 55: 98–108 (2016). https://doi.org/10.1016/j.tifs.2016.07.006
Tripathi MK and Giri SK. Probiotic functional foods: Survival of probiotics during processing and storage. Journal of Functional Foods. 9: 225–241 (2014). https://doi.org/10.1016/j.jff.2014.04.030
Vieira da Silva B, Barreira JCM and Oliveira MBPP. Natural phytochemicals and probiotics as bioactive ingredients for functional foods: Extraction, biochemistry and protected-delivery technologies. Trends in Food Science & Technology. 50: 144–158 (2016). https://doi.org/10.1016/j.tifs.2015.12.007
Wiciński M, Gębalski J, Gołębiewski J and Malinowski B. Probiotics for the treatment of overweight and obesity in humans—a review of clinical trials. Microorganisms. 8: 1148 (2020). https://doi.org/10.3390/microorganisms8081148
Wu Q, Chen T, El-Nezami H and Savidge TC. Food ingredients in human health: Ecological and metabolic perspectives implicating gut microbiota function. Trends in Food Science & Technology. 100: 103–117 (2020). https://doi.org/10.1111/1541-4337.12682
Wu C, Wang T, Ren C, Ma W, Wu D, Xu X, Wang L and Du M. Advancement of food‐derived mixed protein systems: Interactions, aggregations, and functional properties. Comprehensive Reviews in Food Science and Food Safety. 20: 627–651 (2021). https://doi.org/10.1016/j.tifs.2020.04.007
Xavier-Santos D, Bedani R, Lima ED and Saad SMI. Impact of probiotics and prebiotics targeting metabolic syndrome. Journal of Functional Foods. 64: 103666 (2020). https://doi.org/10.1016/j.jff.2019.103666
Yao M, Li B, Ye H, Huang W, Luo Q, Xiao H, McClements DJ and Li L. Enhanced viability of probiotics (Pediococcus pentosaceus Li05) by encapsulation in microgels doped with inorganic nanoparticles. Food Hydrocolloids. 83: 246–252 (2018). https://doi.org/10.1016/j.foodhyd.2018.05.024
Yao M, Xie J, Du H, McClements DJ, Xiao H and Li L. Progress in microencapsulation of probiotics: A review. Comprehensive Reviews in Food Science and Food Safety. 19: 857–874 (2020). https://doi.org/10.1111/1541-4337.12532
Yeung TW, Arroyo-Maya IJ, McClements DJ and Sela DA. Correction: Microencapsulation of probiotics in hydrogel particles: enhancing Lactococcus lactis subsp. cremoris LM0230 viability using calcium alginate beads. Food & Function. 7: 2909–2909 (2016). https://doi.org/10.1039/C6FO90021F
Yilmaz MT, Taylan O, Karakas CY and Dertli E. An alternative way to encapsulate probiotics within electrospun alginate nanofibers as monitored under simulated gastrointestinal conditions and in kefir. Carbohydrate Polymers. 244: 116447 (2020). https://doi.org/10.1016/j.carbpol.2020.116447
Ying D, Sanguansri L, Weerakkody R, Bull M, Singh TK and Augustin MA. Effect of encapsulant matrix on stability of microencapsulated probiotics. Journal of Functional Foods. 25: 447–458 (2016). https://doi.org/10.1016/j.jff.2016.06.020
Zhang YJ, Li S, Gan RY, Zhou T, Xu DP and Li HB. Impacts of gut bacteria on human health and diseases. International Journal of Molecular Sciences. 16: 7493–7519 (2015). https://doi.org/10.3390/ijms16047493
Zielińska D and Kolożyn-Krajewska D. Food-origin lactic acid bacteria may exhibit probiotic properties: review. BioMed Research International. 2018: 1–15 (2018). https://doi.org/10.1155/2018/5063185
Żur J, Wojcieszyńska D and Guzik U. Metabolic responses of bacterial cells to immobilization. molecules. 21: 958 (2016). https://doi.org/10.3390/molecules21070958
Acknowledgements
The authors would like to thank the financial support received from the fundamental research funds for SRMIST, Chennai, India.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declares that they have no conflict of interest to disclose.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pramanik, S., Venkatraman, S. & Vaidyanathan, V.K. Development of engineered probiotics with tailored functional properties and their application in food science. Food Sci Biotechnol 32, 453–470 (2023). https://doi.org/10.1007/s10068-023-01252-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10068-023-01252-x