Abstract
Trichogramma parasitoids are effective biocontrol agents and a reliable component of integrated strategies against lepidopterous pests. The success of these parasitoids in pest management relies not only on their ability to parasitize their hosts but also on their adaptation to the climatic conditions of the release area, particularly temperature. The expression of life history traits of Trichogramma spp. can vary significantly with temperature, depending on the species or strains being tested. Trichogramma cacoeciae (Marchal), T. euproctidis (Girault), T. minutum (Riley), and T. brassicae (Bezdenko) (Hymenoptera: Trichogrammatidae) are currently used in biocontrol programs against important lepidopteran pests. We aimed to assess the temperature sensitivity of these parasitoids during oviposition and preadult development, and to identify the most tolerant species to high temperatures conditions commonly encountered in Mediterranean Basin countries during the growing seasons. The biological characteristics of the four species were determined at seven temperature regimes expressed as temperatures during oviposition and preadult development (25/25, 25/30, 25/35, 25/40, 30/30, 35/35 and 40/40 °C), using Ephestia kuehniella Zeller (Lepidoptera: Pyralidae) eggs. Trichogramma cacoeciae showed the highest level of parasitism at 30/30 °C, while the other species exhibited the highest levels at 25/25 °C and 25/30 °C. All Trichogramma species were able to develop and survive from 25 °C to 35 °C, but not at 40 °C. Temperature significantly affected the longevity and fecundity of female progeny, with both decreasing when the temperature increased from 25 °C to 35 °C. When exposed to 35/35 °C, T. cacoeciae demonstrated the most optimal performance in terms of parasitization efficiency, developmental capacity, progeny longevity, and fecundity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Mediterranean region covers approximately 877 million hectares of land, out of which around 28% is allocated to agriculture (Mrabet et al., 2020). This sector plays a crucial role in supporting the socio-economic progress of the area (Ramdani et al., 2009). The specific climatic conditions of the Mediterranean region, characterized by prolonged hot and arid spells during the growing seasons, necessitate highly specialized cropping systems focusing on crops with significant nutritional, commercial, and environmental value (Ramdani et al., 2009; Ukhurebor et al., 2022). Cereals, vegetables, fruits, grapes, olives, and dates are among the most notable crops cultivated in this region (Leff et al., 2004; Mrabet et al., 2020). However, these crops are highly susceptible to damage caused by several harmful insects, including Lepidopteran pests (Abazaid et al., 2021; Caselli & Petacchi, 2021; Dhouibi et al., 2016; Gugliuzzo et al., 2019; Rahouma, 2018). The impact of climate change on Lepidoptera populations in the Mediterranean region has become increasingly apparent in recent years, with some native species expanding their ranges and several non-native species successfully establishing themselves, resulting in significant ecological and economic repercussions (Kocsis & Hufnagel, 2011; Ponti et al., 2016; Uhl et al., 2022).
In order to limit infestations caused by Lepidopteran pests, a diverse range of chemical insecticides has been widely employed in various agro-ecosystems throughout Mediterranean countries (Giorgini et al., 2018; Herz et al., 2005; Pavan et al., 2014; Ugurlu et al., 2013). However, their extensive application has caused several undesirable effects including emergence of pest resistance and resurgence, environmental contamination, and threat to non-target organisms and natural enemies (Carvalho, 2017; Karuppuchamy & Venugopal, 2016). The pursuit of sustainable agriculture and the need to mitigate economic losses caused by pests have led to a growing emphasis on the adoption of integrated pest management (IPM) programs (Karuppuchamy & Venugopal, 2016; Matthews, 2022). Egg parasitoids of the genus Trichogramma (Hymenoptera: Trichogrammatidae) have been proven to be a reliable component of IPM strategies, providing effective control against several lepidopteran pests in numerous agricultural crops and forests (Mills, 2010; Querino et al., 2010; Smith, 1996). The widespread success of Trichogramma parasitoids can be attributed to several key features, including their ease of mass rearing on factitious hosts, short life cycle, broad host range, ease of application as inundative biocontrol agents in both field and greenhouse settings, and their ability to significantly reduce pests egg hatching and subsequent crop damage resulting from larval feeding (Mills, 2010; Smith, 1996; Wu et al., 2015). Each year, over 32 million hectares of land worldwide are treated with Trichogramma spp., leading to a reduced reliance on chemical insecticides (Kumar et al., 2013).
The effective selection and implementation of Trichogramma parasitoids within biocontrol programs hinge upon meticulously considering several critical factors. These factors encompass the inherent capacity of Trichogramma species or strain to thrive within the targeted host, as well as the interplay between various abiotic and biotic factors (Bueno et al., 2012; Oliveira et al., 2017; Pratissoli & Parra, 2000). Notably, temperature emerges as the most preeminent environmental parameter, wielding a profound impact on the biology, survivorship, and distribution of these poikilothermic arthropods (Foerster et al., 2014; Ksentini et al., 2011; Moezipour et al., 2008; Mohammad et al., 2015). In some countries of the Mediterranean region, temperatures can even exceed 45 °C for short periods during the summer days, potentially impacting the activity of Trichogramma parasitoids and, consequently, the efficiency of inundative releases (Ksentini et al., 2010; Melo, 2011). Gaining a thorough and in-depth understanding of the intricate interplay between temperature and the biological parameters of the selected Trichogramma species/strain holds pivotal importance in formulating precise and effective laboratory mass rearing procedures (Greenberg et al., 1996), while also recognizing its potential as a biocontrol agent tailored to address the challenges posed by a particular pest species within a specific geographic region (Pratissoli & Parra, 2000; Pino et al., 2020).
The parasitoids Trichogramma cacoeciae (Marchal), T. euproctidis (Girault), T. minutum (Riley), and T. brassicae (Bezdenko) (Hymenoptera: Trichogrammatidae) have gained commercial significance and have been intentionally introduced into various agricultural settings across continents for their effective use as inundative biocontrol agents against important lepidopteran pests (Atashi et al., 2021; Aubry, 2008; Hegazi et al., 2007; Pease et al., 2016; Renou et al., 1992; Sigsgaard et al., 2017; Zouba et al., 2013a; Zougari et al., 2020). Some of the life history characteristics of these Trichogramma species have been studied previously (Moezipour et al., 2008; Özder & Kara, 2010; Pizzol et al., 2010; Schöller & Hassan, 2001; Tabebordbar et al., 2022a; Yu et al., 1984). However, it has been demonstrated that different strains (ecotypes) of Trichogramma species that originate from different geographic regions may exhibit distinct biological traits that influence their overall success (Pavlik, 1993; Pizzol et al., 2010; Ram et al., 1995; Smith, 1996). To identify a suitable candidate biocontrol agent that can withstand the challenging environmental conditions of the growing season (from May to late September) in Mediterranean Basin countries, we assessed the impact of high temperatures on the biological parameters of the four aforementioned species (originating from different countries) during oviposition and preadult development. This study enables the prediction of the response of these parasitoids to climate changes, and facilitates the identification of the ideal timing for the implementation of inundative release programs during the growing season.
Materials and methods
Parasitoid’s origin, culture and maintenance
Trichogramma cacoeciae, a thelytokous species that reproduces independently of Wolbachia symbiotic bacteria (Pintureau et al., 1999), was established as a local population in 2009 by collecting parasitoid wasps from host baits (sentinel eggs of Ephestia kuehniella Zeller (Lepidoptera: Pyralidae)) in date palm orchards located in southwest Tunisia. Molecular characterization by sequencing of the ITS2 gene was subsequently employed to identify the species (Zouba et al., 2013b). In 2016, this strain of T. cacoeciae was introduced into the National Agronomic Institute of Tunisia insectarium for research and conservation purposes. Additionally, three arrhenotokous species, T. euproctidis (originated from Egypt), T. minutum (originated from Canada), and T. brassicae (originated from Iran) were first introduced to Tunisia in 2017 and reared at the National Agronomic Institute of Tunisia insectarium using eggs of the factitious host E. kuehniella. These species were sourced from the Earth and Life Institute, Biodiversity Research Center, Université catholique de Louvain, Belgium.
Factitious host eggs used in this study were obtained from a culture of E. kuehniella massively reared on a standard diet containing wheat flour, mixed according to the methodology defined by Cerutti et al. (1992). Prior to the experiments, and for at least 15 successive generations, the tested Trichogramma species were maintained on fresh ultraviolet-sterilized eggs of E. kuehniella under controlled conditions (25 ± 1 °C, 60–80% RH and 16:8 h L:D photoperiod), as described by Ayvaz et al. (2008). Newly emerged parasitoids (arrhenotokous species: 50 ♀ + 50 ♂/ thelytokous species: 50 ♀) were introduced to glass vials (10 cm height * 1 cm diameter) containing a cardboard card (1 × 5 cm) with sufficient number (≈1000) of E. kuehniella eggs. The upper end of vials was covered by a fine mesh net to facilitate air flow. Parasitoids were provided with a honey–water solution (1:1) as a food source, which was smeared onto the internal surfaces of the vials. Afterwards, the parasitized eggs were incubated under the same conditions described above until the emergence of the adult wasps.
Experimental procedure
Part 1: Test on the parental generation
Female wasps of each species were allowed to lay eggs under climatic conditions similar to those used for rearing (25 ± 1 °C, 60–80% RH and 16:8 h L:D photoperiod). One day later, the parasitized eggs were randomly divided into four groups and subjected to different constant temperatures of 25, 30, 35, and 40 ± 1 °C in temperature-controlled cabinets. In parallel, another group of females from each species was allowed to lay eggs under the same temperature conditions used for preadult development of the parasitoids. This experimental design resulted in a total of 7 treatments, representing the temperatures experienced during both oviposition and preadult development: 25/25, 25/30, 25/35, 25/40, 30/30, 35/35 and 40/40 °C. Other climatic conditions were kept similar, i.e. 60–80% RH and 16:8 h L:D photoperiod.
For each treatment, 30 newly emerged females (< 24 h old) of each species were individually isolated in glass vials (5 cm height * 1 cm diameter). Each female was fed with a honey-water solution (1:1) and supplied with fresh eggs of E. kuehniella (100 ± 10 one-day old eggs sterilized with UV light) glued on a cardboard card with distilled water (1 × 2.5 cm). Glass vials were covered by a fine mesh net. Each isolated female represented a replicate. After 24 h of exposure, all parasitoids were removed from their vials using a thin brush, and the parasitized eggs were incubated at the respective preadult temperatures. The total number of eggs parasitized by a single female was recorded; successful parasitization could be identified by the blackening of host eggs. The emergence rate was calculated according to the method recommended by Van Driesche (1983) using the following equation:
The egg-to-adult period was measured as the period of time from the day when the females were removed to adult emergence. The sex ratio of the newly emerged adults was determined based on their antennal characters (Bowen & Stern, 1966). The female’s percentage was estimated using the following equation:
Part 2: Test on the F1 generation
Females from the F1 generation (obtained from above experiment) were randomly selected at each of the tested preadult temperatures. Since no adult parasitoids emerged at 40 °C in the F1 generation, we focused our investigation on recording the longevity and fecundity of the progeny at 25, 30, and 35 °C, with a 60–80% RH and 16:8 h L:D photoperiod. The selected females were allowed to lay eggs under the same temperature conditions experienced during their preadult development. For each temperature, 20 females less than one day old (< 24 h) of each species were placed individually into glass vials covered with mesh net and a water–honey (1:1) drop for feed. Cardboard cards with 100 ± 10 one-day old sterilized E. kuehniella eggs were supplied daily until the natural death of the female parasitoids. The replaced egg cards were kept in cabinets at the corresponding temperatures mentioned above.
Longevity (number of days) until death of the females was recorded. Cumulative female fecundity is defined as the total number of successfully parasitized eggs by a female over the full life span. The number of parasitized host eggs and the number of adults emerging from them were counted for each sample. The following parameters were derived from these records:
-
lx: the propotion of females still alive at age x (age-specific survival rate) (Southwood, 1978).
-
mx: the number of live female progeny per female at the age x (age-specific fecundity rate) (Southwood, 1978).
Where x is the age of the individuals in days.
Statistical analysis
The number of parasitized eggs, preadult development time, progeny longevity, and fecundity data under different temperature regimes were transformed to log10(x + 1), while emergence rate and sex ratio data were transformed to arcsine (√ (x⁄100)). All transformed data were analyzed by ANOVA test applying the General Linear Models (PROC GLM) procedure and the average values were compared by Tukey’s test (p = 0.05) by means of the statistical software IBM SPSS statistics software (Version 20), IBM, USA.
Results
Number of parasitized eggs
The mean number of E. kuehniella eggs parasitized by the four Trichogramma species was significantly influenced by temperature (F3.76 = 126.4; df = 13; p < 0.001) (Table 1). Trichogramma cacoeciae exhibited optimal parasitism performance at 30/30 °C, while the other species showed their highest parasitism levels when females laid eggs at 25 °C and preadult development occurred at either 25 °C or 30 °C. All Trichogramma species showed no significant differences in the number of parasitized eggs between the temperature regimes 25/25 °C and 25/30 °C. Trichogramma cacoeciae was the only species that did not show a significant decrease in parasitism at 25/35 °C when compared to 25/25 °C and 25/30 °C. We recorded a sharp decline in parasitism for all species when the preadult temperature increased to 40 °C (25/40 °C), with T. minutum exhibiting the lowest parasitism performance (1.3 ± 0.06 eggs/female/24 h). At 35/35 °C, the number of parasitized eggs was found to be highest in T. cacoeciae (20.55 ± 0.68 eggs/female/24 h), while T. minutum, T. euproctidis and T. brassicae demonstrated significantly lower values of 4.4 ± 0.04, 8.25 ± 0.19 and 13.75 ± 0.27 eggs/female/24 h, respectively. Parasitism was further reduced at 40/40 °C, with no significant differences observed among species.
Preadult development time
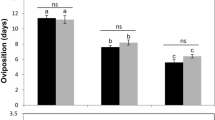
Analysis of variance revealed that temperature had a significant effect on the development time of immature parasitoids (F2.56 = 329.7; df = 14; p < 0.001) (Fig. 1). At 25 °C, all Trichogramma species experienced a significant delay in completing preadult development, taking on average 11–12 days. The shortest developmental times for all species were observed at 35 °C, ranging from 6 to 7 days.
Development time (days ± SE) of four Trichogramma species allowed to oviposit and developed in Ephestia kuehniella eggs under different temperature regimes expressed as temperatures during parasitism/preadult development. Development times (days) followed by the same letter do not differ significantly within the same species (Tukey test, P < 0.05)
Emergence rate
All four species of Trichogramma were able to complete their immature development and emerge successfully within the temperature range of 25–35 °C (Fig. 2). However, none of them succeeded in reaching adult stage at 40 °C. Temperature had a significant effect on the emergence rates (survival) of T. cacoeciae and T. euproctidis, with both species showing decreased survivorship as preadult temperature increased towards 35 °C. The emergence rates of T. brassicae and T. minutum remained statistically similar across all tested temperatures. With the exception of T. euproctidis, which only had a 35.2% emergence rate at 35/35 °C, all other Trichogramma species showed high survival rates, exceeding 75% at all temperature regimes.
Emergence rate (percentage ± SE) of four Trichogramma species allowed to oviposit and developed in Ephestia kuehniella eggs under different temperature regimes expressed as temperatures during parasitism/preadult development. Emergence rates (%) followed by the same letter do not differ significantly within the same species (Tukey test, P < 0.05)
Sex ratio (Female %)
Trichogramma cacoeciae did not produce males under any of the temperature regimes tested. Temperature had a significant effect on the sex ratio of T. brassicae and T. euproctidis. In both species, the percentage of females increased as the preadult temperature increased from 25 °C to 30 °C, where it peaked at 69.1% (25/30 °C) and 65.3% (30/30 °C) for T. brassicae and 63.2% (25/30 °C) and 62.9% at (30/30 °C) for T. euproctidis, and then decreased at 35 °C (Fig. 3). The female-biased sex ratio observed in T. minutum (female% > 57%) was not significantly affected by the tested temperatures.
Sex ratio (percentage of females ± SE) of three Trichogramma species allowed to oviposit and developed in Ephestia kuehniella eggs under different temperature regimes expressed as temperatures during parasitism/preadult development. Sex ratios (%) followed by the same letter do not differ significantly within the same species (Tukey test, P < 0.05)
Longevity and fecundity of female progeny (F1 generation)
The age-specific survivorship (lx) and the age-specific fecundity (mx) of the four Trichogramma species at different levels of temperature are illustrated in Figs. 4 and 5, respectively. They indicate that the F1 female parasitoids can successfully survive and reproduce on E. kuehniella eggs between 25 °C and 35 °C. For all Trichogramma species, lx and mx are inversely proportional to the increase of temperatures; as temperatures increased, survivorship and fecundity decreased. Temperature had a significant effect on the lifespan of F1 female parasitoids (Table 2). Across all studied species, the highest longevity of female progeny was observed at 25 °C. At 35 °C, the F1 female lifespan of T. cacoeciae (8.04 days) was significantly longer compared to the other species. As shown in Fig. 5, egg laying is more important during the two first days after female emergence. Temperature significantly affected the total number of eggs laid by F1 female parasitoids (Table 3). Trichogramma cacoeciae showed the highest progeny's fecundity at 30 °C, producing an average of 168.03 eggs per female. The total number of eggs parasitized by T. brassicae, T. minutum, and T. euproctidis was highest at 25 °C, with 136.53, 153.03, and 128.24 eggs per female, and lowest at 35 °C, with 25.72, 10.23, and 22.63 eggs per female, respectively. At 35 °C, T. cacoeciae showed a significantly higher progeny’s fecundity (39.41 eggs/ female) compared to the other species.
Discussion
Our experiments demonstrate a large effect of temperature on all the life history parameters of the investigated Trichogramma species. The study conducted by Yuan et al. (2012) underscores the importance of using Trichogramma species with broad temperature tolerance ranges to achieve effective pest control. According to Samara et al. (2011), temperature is recognized as the primary abiotic factor that directly influences various life history traits of Trichogramma spp., including development time, emergence rate, sex ratio, cumulative fertility and adult longevity. However, it is essential to note that the expression of these traits can differ significantly with temperature, depending on the species or strains tested (Foerster et al., 2014; Hassan, 1994; Pizzol et al., 2010; Ram et al., 1995; Tabone et al., 2010). In the context of a warming world (Funes et al., 2016), most insect species are exposed to thermal fluctuations that significantly influence their physiological processes (Feder et al., 1997) and ecological systems (Easterling et al., 2000). Nonetheless, the recurring exposure to extreme temperatures may lead to adaptive evolution in insects, facilitated by various mechanisms, including behavioral thermoregulation, phenotypic plasticity, and ontogenetic variations (Scharf et al., 2015). Besides temperature, numerous biological traits of Trichogramma spp. are subject to influence from various factors, encompassing host species, host egg size, host age, nutritional quality of the host egg, spatial density of host eggs, availability of food resources during the adult stage, humidity, and photoperiod (Altoé et al., 2012; Amalin et al., 2005; Atashi et al., 2021; Mashal et al., 2019; Pizzol et al., 2012; Pratissoli & Parra, 2001; Tabebordbar et al., 2022b).
Temperature had a significant effect on the mean number of E. kuehniella eggs parasitized by the four Trichogramma species. Trichogramma cacoeciae demonstrated the highest level of parasitism at 30/30 °C, while the other three species showed their optimal parasitism performance at 25/25 °C and 25/30 °C. Although the rate of parasitism peaked at 20/20 °C for some Trichogramma species, such as T. galloi Zucchi (Parra et al., 1991) and T. pretiosum Riley (Foerster et al., 2014), the majority of studies indicate that the optimal temperature regime for achieving the highest number of hosts parasitized within a specific time period is 25/25 °C (Ayvaz et al., 2008; Kalyebi et al., 2005; Ksentini et al., 2011; Reznik & Vaghina, 2006; Schöller & Hassan, 2001). However, some species, such as T. chilonis Ishii (Shirazi, 2006) and T. nubilale Ertle & Davis (Russo & Voegelé, 1982), achieve their best parasitism performance at 30/30 °C. Thus, our findings are consistent with the majority of earlier studies. At 35/35 °C, T. cacoeciae was able to successfully reproduce on E. kuehniella eggs, displaying a significantly higher level of parasitism in comparison to the other species.
The superior parasitism performance exhibited by T. cacoeciae at higher temperatures could be attributed to its prior exposure and adaptation to the challenging environmental conditions typically prevalent in southern Tunisia. In contrast, T. minutum showed a low parasitism performance at 35/35 °C (4.4 eggs/female), which may be attributed to its Canadian origin, where colder climatic conditions are predominant (Mols & Boers, 2001). According to Yu et al. (1984), T. minutum (originated from Canada) has evolved to optimize its reproductive success within a narrower temperature range of 20–25 °C. Our findings are consistent with earlier studies that have shown a correlation between the behavioral variability of Trichogramma species/strains and the climatic conditions of their respective habitats (Andrade et al., 2011; Pizzol et al., 2010; Schöller & Hassan, 2001). The level of parasitism recorded for all Trichogramma species at 40/40 °C was relatively low, with less than 1 egg per female. Reznik and Voinovich (2015) attribute the observed decrease in parasitism, in response to high preadult temperatures, to mortality among immature stages occurring before they reach the third larval stage, which is responsible for the characteristic black pigmentation. Pereira et al. (2007) showed that high temperatures can lead to an increase in turgidity of the host egg, which may impede the successful insertion of the parasitoid ovipositor through the chorion. Furthermore, Shipp and Wang (1998) reported that female parasitoids often show a preference for increased feeding and reduced parasitism at high temperatures to maintain their metabolic activity.
Our findings have shown that all four Trichogramma species were able to complete their egg-to-adult development successfully within a temperature range of 25 °C to 35 °C. Also, we observed an inverse relationship between temperature and preadult development time, with a significant decrease as the temperature increased from 25 °C to 35 °C. The current results are in line with the studies on other Trichogramma species (Altoé et al., 2012; Atashi et al., 2023; Bari et al., 2015; Melo et al., 2007; Negahban et al., 2021; Tabebordbar et al., 2022a). According to Zuim et al. (2017), the observed reduction in development time under high temperature conditions is likely due to a significant increase in metabolic activity during the immature stages, which speeds up the conversion process of reserved nutrients to required energy.
The survival rates of T. cacoeciae, T. brassicae and T. minutum were high (> 75%) at all temperature regimes. However, T. euproctidis showed a low emergence rate of 35.2% at 35/35 °C. This finding indicates that high temperatures can have a detrimental effect on the emergence of Trichogramma species, as previously noted by Bari et al. (2015) who reported reduced progeny production of T. zahiri Polaszek at 34 °C (6.73 offspring/female), and Nadeem et al. (2009) who observed a low emergence rate of approximately 33% for T. chilonis when incubated at 35 °C. Some species, such as T. brevicapillum (Pak & Oatman, 1982) and T. evanescens (Schöller & Hassan, 2001) did not emerge at all at 35 °C. Our study demonstrated that exposing parasitized eggs to 40 °C resulted in a complete failure of Trichogramma emergence. The eggs turned black and many collapsed, dried, and no viable progeny were observed, indicating a negative effect of the temperature exceeding the upper threshold. Nadeem et al. (2009) did not even observe blackened eggs when exposing a strain of T. chilonis from Pakistan to 40 °C. However, Hussain et al. (2013) reported a successful emergence rate of over 66% for the same species from Pakistan under this temperature regime. These contrasting results highlight the potential variation in temperature tolerance among strains of Trichogramma species, even within the same country.
Temperature is a crucial factor that strongly affects the sex ratio in Trichogramma spp. (Altoé et al., 2012; Atashi et al., 2023; Negahban et al., 2021; Pratissoli & Parra, 2000; Tabebordbar et al., 2022a). However, the impact of temperature on the sex ratio varies depending on the species/strain of Trichogramma (Bueno et al., 2012). In our study, T. cacoeciae did not produce any males across all temperature regimes. This finding corroborates earlier studies (Pizzol et al., 2010; Schöller & Hassan, 2001) that have also documented the stability of the sex ratio in this Trichogramma species. In general, Trichogramma wasps demonstrate haplodiploidy in their sex determination process, wherein unfertilized eggs develop into haploid males, while fertilized eggs give rise to diploid females (Moiroux et al., 2014). However, certain species of Trichogramma, such as T. cacoeciae, only produce females without fertilization, in a process known as thelytoky. According to Stouthamer (1993) and Zhou et al. (2020), thelytokous Trichogramma offers several benefits in biological control programs, such as the production of only female offspring, easier colonization without mating, higher efficacy at low host populations, and lower costs for mass rearing. Of the three arrhenotokous species studied, T. minutum stood out as the only one consistently exhibiting a female biased sex ratio across all temperature regimes. This finding aligns with previous research conducted on other Trichogramma species, such as T. evanescens (Schöller & Hassan, 2001), T. zahiri (Bari et al., 2015) and T. atopovirilia Oatman and Platner (Melo et al., 2007), which found that temperatures between 20 °C and 35 °C do not significantly affect the predominantly female-biased sex ratio. Our study has revealed a significant effect of temperature on the sex ratio of T. euproctidis and T. brassicae, with a female bias observed between 25 °C and 30 °C, while at 35 °C, the proportion of male offspring exceeded that of females. This finding is coherent with Harrison et al. (1985) and Cônsoli and Parra (1995), who noticed a bias towards male offspring production in the sex ratios of T. pretiosum and T. galloi, respectively, at 35 °C. According to Lauge (1985), extreme temperatures, limited food availability, and unfavorable rearing conditions may lead to a shift in the sex ratio towards male biased offspring.
Our experiments revealed that the lifespan of female Trichogramma progeny is highly dependent on temperature. Consistently with prior studies on other Trichogramma species (Ayvaz et al., 2008; Harrison et al., 1985; Schöller & Hassan, 2001), we observed that the mean adult longevity of female parasitoids decreased with increasing temperature, with the highest longevity value was recorded at 25 °C. However, our study uncovered differences in the lifespans of the species we tested, as compared to previous research. For instance, we observed a lifespan of 20 days for T. minutum at 25 °C, which is shorter than the lifespan of 28 days reported by Yu et al. (1984) on the same species at 25 °C. Likewise, we found a lifespan of 15 days for T. cacoeciae at 25 °C, while Schöller and Hassan (2001) reported a shorter lifespan of only four days for the same species at 26 °C. The above-mentioned dissimilarities underscore the crucial role played by both environmental factors and strains of Trichogramma spp. in determining the longevity of female progeny. Our findings have unveiled pronounced variations in the age-specific survivorship (lx) curves of the studied species across the different temperatures. Notably, at 35 °C, all species exhibited the highest mortality rate during the early stages of female life. However, at this temperature, only T. cacoeciae demonstrated the capacity to survive for up to eight days, while the other species had had significantly lower lifespans, ranging from 2.31 to 5.33 days. The high mortality rates of Trichogramma parasitoids in the field, caused by extreme temperature conditions, pose a significant obstacle to the success of inundative biological control programs (Zouba et al., 2022). However, a strategic approach involving the organization of successive emergence waves and an increase in the number of released wasps can effectively address this challenge (Wu et al., 2016). Furthermore, the availability of a reliable food source has been identified as a critical factor in promoting the longevity of Trichogramma adults, as it increases their chances of surviving for a longer period and encountering more hosts for egg-laying (McDougall & Mills, 1997). According to Gurr and Nicol (2000), providing food is the most practical and economically feasible approach to enhance the longevity of Trichogramma parasitoids in the field and increase their effectiveness as biocontrol agents.
Across all tested Trichogramma species, our experiments revealed that oviposition reached its peak within the first 24 h after female emergence. Subsequently, oviposition rates steadily declined until female death. This finding aligns with Ventura Garcia et al. (2002), who underscored the critical significance of the number of eggs laid by Trichogramma species during the first 24 h in determining their overall fecundity. Additionally, Bueno et al. (2012) reported that the reduction in the number of parasitized eggs over the female's lifespan is a characteristic commonly observed in pro-ovigenic species, such as the parasitoids of the genus Trichogramma. Our experiments demonstrated that the highest progeny fecundity in T. brassicae, T. euproctidis, and T. minutum was observed at 25 °C. However, at both 30 °C and 35 °C, T. cacoeciae parasitized significantly more eggs than the other species, with rates of 168.03 and 39.41 eggs per female, respectively. Notably, these values surpass those reported in previous studies for T. pretiosum (106.4 eggs at 30 °C) (Maceda et al., 2003), T. evanescens (87.62 eggs at 30 °C) (Özder & Kara, 2010), T. achaeae (0 egg at 35 °C) (Pino et al., 2020), T. evanescens (17.2 eggs at 35 °C) Schöller and Hassan (2001), and T. oleae (5.13 eggs at 35 °C) (Ksentini et al., 2011). Our data revealed a significant decrease in the progeny fecundity of Trichogramma species as the temperature increased from 25 °C to 35 °C. This finding is consistent with studies on other Trichogramma species (Ksentini et al., 2011; Pino et al., 2020; Samara et al., 2011). The adverse impact of high temperatures on fecundity may be attributed to a reduction in female longevity, as reported by Garcia et al. (2001). On the other hand, previous studies references have shown an important variation in fecundity, depending on the Trichogramma strain and rearing host. In our study, we observed that T. minutum females parasitized 153.03 eggs of E. kuehniella at 25 °C, which is lower than the value of 227.6 eggs reported by Yu et al. (1984) for the same Trichogramma species parasitizing Anagasta kuehniella Zeller. Similarly, our results showed that T. cacoeciae females parasitized 120.14 eggs at 25 °C, which is higher than the number of eggs parasitized by the same Trichogramma species in studies by Schöller and Hassan (2001) and Özder and Kara (2010) on Ephestia elutella Hubner (87.27eggs) and Cadra cautella Walker (80.64eggs), respectively.
The assessment of thermal sensitivity among the studied Trichogramma species not only highlights the variability in their susceptibility to high temperatures but also underscores the need for careful species and strain selection to ensure their proper adaptation to the environmental conditions of the release area. In the specific case of the Mediterranean Basin region, T. cacoeciae stands out as the preferential choice for mass-rearing and biological control of lepidopteran pests. This preference is attributed to its remarkable ability to survive and develop within the tested temperature range, particularly at high temperatures (30–35 °C). Our findings provide an explanation for the widespread presence and remarkable adaptation of T. cacoeciae to the prevailing extreme high temperature conditions in pomegranate and date palm orchards located in southern Tunisia (Ksentini et al., 2010; Zouba et al., 2013a). Furthermore, this species has demonstrated a remarkable ability to withstand the harsh climatic conditions prevalent in olive groves located within the Cairo desert, Egypt (Herz et al., 2007). Trichogramma cacoeciae exhibits an extensive worldwide distribution, occurring naturally in diverse regions including North Africa, Europe, Asia, and America (Herz et al., 2007; Pintureau, 2008). Pizzol et al. (2010) have highlighted the species' notable plasticity in terms of temperature tolerance, indicating the presence of multiple populations with finely-tuned biological characteristics adapted to the specific climatic conditions of their respective habitats.
Currently, T. cacoeciae is officially listed by the European Plant Protection Organization (EPPO) as a species approved for use in Euro-Mediterranean countries (EPPO, 2002). Trichogramma cacoeciae is commercially available and extensively employed as an integral component of integrated pest management strategies for managing the carob moth Ectomyelois ceratoniae Zeller (Lepidoptera, Pyralidae) in Tunisia, with studies proving its effectiveness in controlling this pest in pomegranate (Lebdi-Grissa & Ben Ayed 2005; Zougari et al., 2020), date palm (Khoualdia et al., 1996; Zouba et al., 2022), and citrus crops (Dhouibi et al., 2016). Furthermore, T. cacoeciae has been recognized as one of the highly efficient Trichogramma species suitable for combating the tomato leaf miner Tuta absoluta Meyrick (Lepidoptera, Gelechiidae) in protected and open field tomato crops in Mediterranean Basin countries (Zouba et al., 2013b; Cherif & Lebdi-Grissa, 2013; Cherif & Verheggen, 2019). Additionally, the remarkable potential of T. cacoeciae has been demonstrated through successful experimental inundative releases, effectively managing significant pests such as the European grapevine moth Lobesia botrana Denis & Schiffermüller (Lepidoptera, Tortricidae) (Hommay et al., 2002; Pizzol, 2004), the apple codling moth Cydia pomonella Linnaeus (Lepidoptera: Tortricidae) (Sigsgaard et al., 2017), and the olive moth Prays oleae Bernard (Lepidoptera, Plutellidae) (Herz et al., 2007), all of which pose serious threats in the Mediterranean basin region (Coscolla, 1997; Herz et al., 2007).
Conclusions
Through our study, we conducted experiments to investigate the influence of different oviposition and preadult temperatures that reflect the conditions typically experienced during the growing seasons in Mediterranean Basin countries on the biological parameters of four Trichogramma species originating from geographically diverse regions. Our findings have highlighted the notable impact of high temperatures, particularly those reaching 35 °C and 40 °C, on various biological traits exhibited by the studied species. Trichogramma cacoeciae showed the best performance in terms of parasitization efficiency, developmental capacity, sex ratio, progeny longevity, and fecundity at 35 °C. However, as temperatures increased from 35 °C to 40 °C, the strain of T. cacoeciae was unable to complete preadult development in E. kuehniella eggs. It is worth noting that temperatures in the field are subject to fluctuation throughout the day and night, and parasitoids are unlikely to encounter constant temperatures of 35–40 °C. Given these findings, T. cacoeciae appears to be a promising candidate for controlling thermophile lepidopteran pests across Mediterranean Basin countries via inundative releases. Nevertheless, to optimize the biological efficiency of T. cacoeciae and reduce the growing reliance on synthetic insecticides in diverse cropping systems, it is imperative to integrate this knowledge with other management strategies. Nonetheless, it is important to emphasize that additional efforts are necessary to validate these laboratory observations under natural field conditions.
Data availability
All data and materials are available on the reasonable request.
References
Abazaid, M. A. A., Shalaby, F. F., Hafez, A. A. & Ewaise, M. A. (2021). Survey and abundance of major insect pests on pomegranate fruits in Egypt. V International conference on biotechnology applications in agriculture (ICBAA), Bio- Pesticides and Biological Control (601–612). Benha University, 8 April 2021.
Altoé, T. S., Pratissoli, D., Carvalho, J. R., Gonçalves, H. J., Pereira, J. P., Oliveira, R. C., & Bueno, A. F. (2012). Trichogramma pretiosum (Hymenoptera: Trichogrammatidae) parasitism of Trichoplusiani (Lepidoptera: Noctuidae) eggs under different temperatures. Annals of the Entomological Society of America, 105(1), 82–89.
Amalin, D. M., Pena, J. E., & Duncan, R. (2005). Effects of host age, female parasitoid age, and host plant on parasitism of Ceratogramma etiennei (Hymenoptera: Trichogrammatidae). Florida Entomology, 88, 77–81.
Andrade, G. S., Pratissoli, D., Dalvi, L. P., Desneux, N., & Gonçalves, H. J. (2011). Performance of four Trichogramma species (Hymenoptera: Trichogrammatidae) as biocontrol agents of Heliothis virescens (Lepidoptera: Noctuidae) under various temperature regimes. Journal of Pest Science, 84(3), 313–320.
Atashi, N., Shishehbor, P., Seraj, A. A., Rasekh, A., Hemmati, S. A. & Riddick, E. W. (2021). Effects of Helicoverpa armigera egg age on development, reproduction, and life table parameters of Trichogramma euproctidis. Insectes, 12(7), 569. https://doi.org/10.3390/insects12070569
Atashi, N., Shishehbor, P., Seraj, A. A., Rasekh, A., Hemmati, S. A. & Ugine, T. A. (2023). The effect of temperature on the bionomics of Trichogramma euproctidis (Hym.: Trichogrammatidae) parasitizing the tomato fruitworm, Helicoverpa armigera (Lep.: Noctuidae). Plant Protection (Scientific Journal of Agriculture), 46(1), Spring. https://doi.org/10.22055/ppr.2023.42910.1677
Aubry, O. (2008). Lutte attracticide et lâchers inondatifs de trichogrammes contre le carpocapse de la pomme, Cydia pomonella (Lepidoptera : Tortricidae). Université du Québec à Montréal, 114 pp. https://archipel.uqam.ca/1052/. Accessed 1 Nov 2014
Ayvaz, A., Karasu, E., Karabörklü, S., & Aydln, ŞT. (2008). Effects of cold storage, rearing temperature, parasitoid age and irradiation on the performance of Trichogramma evanescens Westwood (Hymenoptera: Trichogrammatidae). Journal of Stored Products Research, 44, 232–244.
Bari, M. N., Jahan, M., & Islam, K. S. (2015). Effects of temperature on the life table parameters of Trichogramma zahiri (Hymenoptera: Trichogrammatidae), an egg parasitoid of Dicladispa armigera (Chrysomelidae: Coleoptera). Environmental Entomology, 44(2), 368–378.
Bowen, W. R., & Stern, V. M. (1966). Effect of temperature on the production of males and sexual mosaics in a uniparental race of Trichogramma semifumatum (Hymenoptera: Trichogrammatidae). Annals of the Entomological Society of America, 59, 823–834.
Bueno, R. C. O. F., Parra, J. R. P., & Bueno, A. F. (2012). Trichogramma pretiosum parasitism of Pseudoplusia includens and Anticarsia gemmatalis eggs at different temperatures. Biological Control, 60(2), 154–162. https://doi.org/10.1016/j.biocontrol.2011.11.005
Carvalho, F.P. (2017). Pesticides, environment, and food safety. Food and Energy Security, 6, 48–60. https://doi.org/10.1002/fes3.108
Caselli, A. & Petacchi, R. (2021). Climate change and major pests of mediterranean olive orchards: are we ready to face the global heating? Insects, 12, 802. https://doi.org/10.3390/insects12090802
Cerutti, F., Bigler, F., Eden, G., & Bosshart, S. (1992). Optimal larval density and quality control aspects in mass rearing of the Mediteranean flour moth, Ephestia kuehniella Zell. (Lep., phycitidae). Journal of Applied Entomology, 114, 353–361.
Cherif, A., & Lebdi-Grissa, K. (2013). Trichogramma cacoeciae as a biological control agent of the tomato pinworm Tuta absoluta in Northeastern Tunisia. Entomologia Hellenica, 22, 35–42.
Cherif, A., & Verheggen, F. (2019). A review of Tuta absoluta (Lepidoptera: Gelechiidae) host plants and their impact on management strategies. Biotechnology, Agronomy, Society and Environment, 23(4), 270–278.
Cônsoli, F. L., & Parra, J. R. P. (1995). Effects of constant and alternating temperatures on Trichogramma galloi Zucchi (Hym., Trichogrammatidae) biology. Journal of Applied Entomology, 119, 415–418.
Coscolla, R. (1997). La pololla dela racimo de la vid (Lobesia botrana Den. Y Schiff.). Sèrie tècnica. Generalitat Valenciana, Conselleria de agricultura, Pesca y Alimentación, Valencia, Spain, p 613.
Dhouibi, M. H., Hermi, H., Soudani, D., & Thlibi, H. (2016). Biocontrol of the carob moth Ectomyelois ceratoniae in pomegranate and citrus orchards in Tunisia. International Journal of Agriculture Innovations and Research, 4, 849–856.
Easterling, D. R., Meehl, G. A., Parmesan, C., Changnon, S. A., Karl, T. R., & Mearns, L. O. (2000). Climate extremes: Observations, modeling, and impacts. Science, 289, 2068–2074.
EPPO. (2002). PM 6/3(2). Safe use of biological control. List of biological control agents widely used in the EPPO region. Bulletin OEPP/EPPO Bulletin, 32, 447–461.
Feder, M. E., Blair, N. T., & Figueras, H. (1997). Natural thermal stress and heat-shock protein expression in Drosophila larvae and pupae. Functional Ecology, 11, 90–100.
Foerster, M. R., Marchioro, C. A., & Foerster, L. A. (2014). Temperature-dependent parasitism, survival, and longevity of five species of Trichogramma Westwood (Hymenoptera: Trichogrammatidae) associated with Anticarsia gemmatalis hübner (Lepidoptera: Noctuidae). Neotropical Entomology, 43, 176–182.
Funes, I., Aranda, X., Biel, C., Carbó, J., & Camps, F. (2016). Future climate change impacts on apple flowering date in a Mediterranean subbasin. Agricultural Water Management, 164, 19–27. https://doi.org/10.1016/j.agwat.2015.06.013
Garcia, P., Wajnberg, E., Oliveira, L., & Tavares, J. (2001). Is the parasitization capacity of Trichogramma cordubensis infuenced by the age females? Entomologia Experimentalis Et Applicata, 98, 219–224.
Giorgini, M., Guerrieri, E., Cascone, P., & Gontijo, L. (2018). Current strategies and future outlook for managing the Neotropical tomato pest Tuta absoluta (Meyrick) in the Mediterranean Basin. Neotropical Entomology, 48, 1–17.
Greenberg, S. M., Nordlund, D. A. & King, E. G. (1996). Mass production of Trichogramma spp.: Experience in the former Soviet Union, China, the United States and western Europe. Biocontrol News Information, 17(3), 51–60.
Gugliuzzo, A., Mazzeo, G., Mansour, R., & Giovanna, T. G. (2019). Carob pests in the Mediterranean region: Bio-ecology, natural enemies and management options. Phytoparasitica, 47, 605–628.
Gurr, G. M., & Nicol, H. I. (2000). Effect of food on longevity of adults of Trichogramma carverae Oatman and Pinto and Trichogramma brassicae bezdenko (Hymenoptera: Trichogrammatidae). Australian Journal of Entomology, 39, 185–187.
Harrison, W. W., King, E. G., & Ouzts, J. D. (1985). Development of Trichogramma exiguum and T. pretiosum at five temperature regimes. Environmental Entomology, 14, 118–121.
Hassan, S. A. (1994). Strategies to select Trichogramma species for use in biological control. In E. Wajnberg & S. A. Hassan (Eds.), Biological control with egg parasitoids (pp. 55–71). CAB International.
Hegazi, E. M., Herz, A., Hassan, S., Khafagi, W., Agamy, E., Zaitun, A., Abdel-Rahman, S. M., El-Said, S., & Khamis, N. (2007). Efficiency of endemic Trichgramma species of olive farms to control the olive (Prays oleae) and jasmine (Palpita unionalis) moths in Egypt. Journal of Insect Science, 7(16), 8–8.
Herz, A., Hassan, S. A., Hegazi, E., Khafagi ,W. E., Nasr, F. N., Youssef, A. A., Agamy, E., Jardak, T., Ksantini, M., Mazomenos, B. E., Konstantopoulou, M. A., Torres, L., Gonçalves, F., Bento, A. & Pereira, J. A. (2005). Towards sustainable control of lepidopterous pests in olive cultivation. Gesunde Pflanzen, 58, 117–128. https://doi.org/10.1007/s10343-005-0076-9
Herz, A., Hassan, S. A., Hegazi, E., Khafagi, W. E., Nasr, F. N., Youssef, A. A., Agamy, E., Blibech, I., Ksentini, I., Ksantini, M., Jardak, T., Bento, A., Pereira, J. A., Torres, L., Souliotis, C., Moschos, T., & Milonas, P. (2007). Egg parasitoids of the genus Trichogramma (Hymenoptera: Trichogrammatidae) in olive groves of the Mediterranean region. Biological Control, 40, 48–56.
Hommay, G., Gertz, C., Kienlen, J. C., Pizzol, J., & Chavigny, P. (2002). Comparison between the control efficacy of Trichogramma evanescens Westwood (Hymenoptera: Trichogrammatidae) and two Trichogramma cacoeciae. Biocontrol Science and Technology, 12(5), 569–581.
Hussain, A., Razaq, M., Saeed, R., Aslam, M., Rafiq, M., & Zaka, S. M. (2013). Effect of different temperatures on life history of Trichogramma chilonis (Ishii) in the laboratory conditions. Pakistan Entomology, 35(2), 123–127.
Kalyebi, A., Sithanantham, S., Overholt, W. A., Hassan, S. A., & Mueke, J. M. (2005). Parasitism, longevity and progeny production of six indigenous Kenyan trichogrammatid egg parasitoids (Hymenoptera: Trichogrammatidae) at different temperature and relative humidity regimes. Biocontrol Science and Technology, 15(3), 255–270.
Karuppuchamy, P. & Venugopal, S. (2016). Integrated pest management. Ecofriendly Pest Management for Food Security, 651–684. https://doi.org/10.1016/B978-0-12-803265-7.00021
Khoualdia, O., R’houma, A. & Marro, J. P. (1996).Utilisation de Trichogramma cacoeciae Marchal (Hymenoptera, Trichogrammatidae) contre la pyrale des dattes. Annales de l’INRAT, 69, 197-205.
Kocsis, M., & Hufnagel, L. (2011). Impacts of climate change on lepidoptera species and communities. Applied Ecology and Environmental Research, 9(1), 43–72.
Ksentini, I., Monje, J. C., Jardak, T., & Zeghal, N. (2010). Naturally occurring egg parasitoids of the genus Trichogramma (Hymenoptera: Trichogrammatidae) in a pomegranate orchard in Tunisia. Entomological Science, 13(1), 99–106.
Ksentini, I., Herz, A., Ksantini, M., Sabelis, M. W., & Hassan, S. A. (2011). Temperature and strain effects on reproduction and survival of Trichogramma oleae and Trichogramma cacoeciae (Hymenoptera: Trichogrammatidae). Biocontrol Science and Technology, 21(8), 903–916.
Kumar, P., Sekhar, J. C. & Kaur, J. (2013). Trichogrammatids: Integration with other methods of pest control. In Sithanantham, S., Ballal, C. R., Jalali, S. K. and Bakthavatsalam, N. (Eds.), Biological control of insect pests using egg parasitoids (pp. 191–208). Springer India.
Lauge, G. (1985). Sex determination: Genetic and epigenetic factors. In G. A. Kerkut & L. I. Gilbert (Eds.), Comprehensive insect physiology, biochemistry and pharmacology (Vol. I, pp. 295–318). Pergamon Press.
Lebdi-Grissa, K. & Ben Ayed, N. (2005). Lutte biologique contre Ectomyelois ceratoniae sur grenadier par des lâchers de Trichogramma cacoeciae. 7ème Conférence Internationale sur les Ravageurs en Agriculture, Montpellier, France, p 7.
Leff, B., Ramankutty, N. & Foley, J. A. (2004). Geographic distribution of major crops across the world. Global Biogeochemical Cycles, 18, GB1009. https://doi.org/10.1029/2003GB002108
Maceda, A., Hohmann, C. L., & Dos Santos, H. R. (2003). Temperature effects of Trichogramma pretiosum Riley and Trichogrammatoidea annulata De Santis. Brazilian Archives of Biology Andd Technology, 46(1), 27–32.
Mashal, S., Agamy, E., Abou-bakr, H., Abd El-Wahab, T. E., & El Behery, H. (2019). Effect of honeybee products, as food supplements, on the biological activities of three Trichogramma species (Hymenoptera: Trichogrammatidae). Egyptian Journal of Biological Pest Control, 29, 46. https://doi.org/10.1186/s41938-019-0149-1
Matthews, G. A. (2022). The need for Integrated Pest Management (IPM). Outlooks on Pest Management, 33(5), 174–176.
McDougall, S. J., & Mills, N. J. (1997). The influence of hosts, temperature and food sources on the longevity of Trichogramma platneri. Entomologia Experimentalis Et Applicata, 83, 195–203.
Melo, R. L., Pratissoli, D., Polanczyk, R. A., Melo, D. F., Barros, R. & Milanez, A. M. (2007). Biology and thermal requirements of Trichogramma atopovirilia Oatman & Platner (Hymenoptera: Trichogrammatidae) parasitizing eggs of Diaphania hyalinata L. (Lepidoptera: Pyralidae). Neotropical Entomology, 36(3), 431–435.
Melo, M. J. L. A. L. (2011). Dynamics study and temperature effect on biological traits of two Trichogramma species (Hymenoptera, Trichogrammatidae) of S. Miguel Island. Master’s Thesis, Universidade dos Açores, Açores, Portugal.
Mills, N. J. (2010). Egg parasitoids in biological control and integrated pest management. In F. L. Cônsoli, J. P. R. Parra, & R. A. Zucchi (Eds.), Egg parasitoids in agroecosystems with emphasis on Trichogramma (pp. 389–411). Springer.
Moezipour, M., Kafil, M., & Allahyari, H. (2008). Functional response of Trichogramma brassicae at different temperatures and relative humidities. Bulletin of Insectology, 62(2), 245–250.
Mohammad, J. K., Al-Jassany, R. F., & Ali, A. S. A. (2015). Influence of temperature on some biological characteristics of Trichogramma evanescens (Westwood) (Hymenoptera: Trichogrammatidae) on the egg of lesser date moth Batrachedra amydraula Meyrick. Journal of Biological Control, 29(3), 125–130.
Moiroux, J., Brodeur, J., & Boivin, G. (2014). Sex ratio variations with temperature in an egg parasitoid: Behavioural adjustment and physiological constraint. Animal Behaviour, 91, 61–66.
Mols, P. J. M. & Boers, J. M. (2001). Comparison of a Canadian and a Dutch strain of the parasitoid Aphelinus mali (Hald) (Hym., Aphelinidae) for control of woolly apple aphid Eriosoma lanigerum (Haussmann) (Hom., Aphididae) in the Netherlands: a simulation approach. Journal of Applied Entomology, 125(5), 255–262.
Mrabet, R., Savé, R., Toreti, A., Caiola, N., Chentouf, M., Llasat, M. C., Mohamed, A. A. A., Santeramo, F. G., Sanz-Cobena, A., Tsikliras, A. (2020). Food. In Cramer, W., Guiot, J., Marini, K. (Eds.), Climate and environmental change in the Mediterranean Basin – current situation and risks for the future. First Mediterranean Assessment Report (pp. 26). Union for the Mediterranean, Plan Bleu, UNEP/MAP.
Nadeem, N., Ashfaq, M., Hamed, M., Ahmed, S., & Nadeem, M. K. (2009). Comparative rearing of Trichogramma chilonis (Ishii) (Hymenoptera: Trichogrammatidae) at different temperature conditions. Pakistan Entomologist, 31(1), 33–36.
Negahban, M., Sedaratian-Jahromi, A., Ghane-Jahromi, M., Haghani, M. & Zalucki, M. P. (2021). Response of Trichogramma brassicae (Hym.: Trichogrammatidae) to temperature: Utilizing thermodynamic models to describe curvilinear development. Crop Protection, 143, 105562. https://doi.org/10.1016/j.cropro.2021.105562
Oliveira, C. M., Oliveira, J. V., Barbosa, D. R. S., Breda, M. O., França, S. M., & Duarte, B. L. R. (2017). Biological parameters and thermal requirements of Trichogramma pretiosum for the management of the tomato fruit borer (Lepidoptera: Crambidae) in tomatoes. Crop Protection, 99, 39–44. https://doi.org/10.1016/j.cropro.2017.04.005
Özder, N., & Kara, G. (2010). Comparative biology and life table of Trichogramma cacoeciae, T. brassicae and T. evanescens (Hymenoptera: Trichogrammatidae) with Ephestia kuehniella and Cadra cautella (Lepidoptera: Pyralidae) as hosts at three constant temperatures. Biocontrol Science and Technology, 20, 245–255.
Pak, G. A., & Oatman, E. R. (1982). Comparative life table, behavior and competition studies on Trichogramma brevicapillum and T. pretiosum. Entomologia Experimentalis Et Applicata, 32, 68–79.
Parra, J. R. P., Zucchi, R. A., Silveira Neto, S. & Haddad, M.L. (1991). Biology and thermal requirements of Trichogramma galloi Zucchi and T. distinctum Zucchi, on two factitious hosts. Colloques de l´INRA, 56, 81–84.
Pavan, F., Cargnus, E., Bigot, G., & Zandigiacomo, P. (2014). Residual activity of insecticides applied against Lobesia botrana and its influence on resistance management strategies. Bulletin of Insectology, 67(2), 273–280.
Pavlik, J. (1993). The size of the female and quality assessment of mass-reared Trichogramma spp. Entomologia Experimentalis Et Applicata, 66, 171–177.
Pease, C. E., López-Olguín, J. F., Pérez-Moreno, I., & Marco-Mancebón, V. (2016). Effects of kaolin on Lobesia botrana (Lepidoptera: Tortricidae) and its compatibility with the natural enemy, Trichogramma cacoeciae (Hymenoptera: Trichogrammatidae). Journal of Economic Entomology, 109(1), 1–6. https://doi.org/10.1093/jee/tov400
Pereira, F. F., Barros, R., Pratissoli, D., Pereira, C. L. T., Vianna, U. R. & Zanuncio, J. C. (2007). Capacidade de parasitismo de Trichogramma exiguum Pinto & Platner, 1978 (Hymenoptera: Trichogrammatidae) emovos de Plutella xylostella (L., 1758) (Lepidoptera: Plutellidae) em diferentes temperaturas. Ciência Rural, 37(2), 297–303.
Pino, M., Gallego, J. R., Hernández Suárez, E. & Cabello, T. (2020). Effect of temperature on life history and parasitization behavior of Trichogramma achaeae Nagaraja and Nagarkatti (Hym.: Trichogrammatidae). Insects, 11(8), 482. https://doi.org/10.3390/insects11080482
Pintureau, B., Petinon, S., & Nardon, C. (1999). Possible function of substances excreted by Trichogramma and darkening their host. Bulletin De La Société Zoologique De France, 124, 261–269.
Pintureau, B. (2008). Les espèces européennes de Trichogrammes. InLibroVeritas, Cergy-Pontoise (p. 96).
Pizzol, J. (2004). Etudes bioécologiques de Trichogramma cacoeciae Marchal, parasitoïde oophage de l’eudémis de la vigne, en vue de son utilisation en lutte biologique. Diplome d’Ingénieur Diplomé par l’Etat, Montpellier, option Agriculture ENSAM.
Pizzol, J., Pintureau, B., Khoualdia, O., & Desneux, N. (2010). Temperature-dependent differences in biological traits between two strains of Trichogramma cacoeciae (Hymenoptera: Trichogrammatidae). Journal of Pest Science, 83, 447–452.
Pizzol, J., Desneux, N., Wajnberg, E., & Thiéry, D. (2012). Parasitoid and host egg ages have independent impact on various biological traits in a Trichogramma species. Journal of Pest Science, 85, 489–496.
Ponti, L., Gutierrez, A. P., & Iannetta, M. (2016). Climate change and crop-pest dynamics in the Mediterranean Basin. ENEA Technical Report, 27(2016), p 18. https://doi.org/10.5281/zenodo.151161
Pratissoli, D., & Parra, J. R. P. (2000). Desenvolvimento e exigências térmicas de Trichogramma pretiosum Riley, criado em duas traças do tomateiro. Pesquisa Agropecuaria Brasileira, 35, 1281–1288.
Pratissoli, D., & Parra, J. R. P. (2001). Seleção de linhagens de Trichogramma pretiosum Riley (Hymenoptera, Trichogrammatidae) Para o controle das traças Tuta absoluta (Meyrick) e Phthorimaea operculella (Zeller) (Lep., Gelechiidae). Neotropical Entomology, 30, 277–282.
Querino, R. B., Zucchi, R. A. & Pinto, J. D. (2010). Systematics of the Trichogrammatidae (Hym.: Chalcidoidea) with a focus on the genera attacking Lepidoptera. In Cônsoli, F. L., Parra, J. R. P., Zucchi, R. A. (Eds.), Egg parasitoids in agroecosystems with emphasis on Trichogramma (pp: 191–219). Springer. https://doi.org/10.1007/978-1-4020-9110-0_7
Rahouma, A. K. (2018). The most economic lepidopterous pests attacking vegetable crops in Egypt. Journal of Plant Protection and Pathology, 7, 417–421.
Ram, P., Tshernyshev, W. B., Afonina, V. M., & Greenberg, S. M. (1995). Studies on the strains of Trichogramma evanescens weswood (Hym., Trichogrammatidae) collected from different hosts in northern Moldova. Journal of Applied Entomology, 119, 79–82.
Ramdani, M., Elkhiati, N., & Flower, R. J. (2009). Lakes of Africa: North of Sahara. In: G. E. Likens. (Ed.), pp. 544–554). Encyclopedia of inland waters. Academic Press.
Renou, M., Nagnan, P., Berthier, A., & Durier, C. (1992). Identification of compounds from the eggs of Ostrinia nubilalis and Mamestra brassicae having kairomone activity on Trichogramma brassicae. Entomologia Experimentalis Et Applicata, 63, 291–303.
Reznik, S. Y., & Vaghina, N. P. (2006). Temperature effects on induction of parasitization by females of Trichogramma principium (Hymenoptera, Trichogrammatidae). Entomology Review, 86(2), 133–138.
Reznik, S. Y. & Voinovich, N. D. (2015). The influence of temperature and photoperiod on the rate of development in Trichogramma principium Sug. et Sor. (Hymenoptera, Trichogrammatidae). Entomology Review, 95(3), 289–295.
Russo, J. & Voegelé, J. (1982). Influence de la temperature sur quatre espèces de trichogrammes (Hym.: Trichogrammatidae) parasites de la pyrale du mais, Ostrinia nubi1alis Hubn. (Lep: Pyralidae). Agronomie, 2(6), 509–516.
Samara, R., Monje, J. C., Zebitz, C. P. W., & Qubbaj, T. (2011). Comparative biology and life tables of Trichogramma aurosum on Cydia pomonella at constant temperatures. Phytoparasitica, 39, 109–119.
Schöller, M., & Hassan, S. A. (2001). Comparative biology and life tables of Trichogramma evanescens and T. cacoeciae with Ephestia elutella as host at four constant temperatures. Entomologia Experimentalis Et Applicata, 98, 35–40.
Scharf, I., Braf, H., Ifrach, N., Rosenstein, S., & Subach, A. (2015). The effects of temperature and diet during development, adulthood, and mating on reproduction in the red flour beetle. PLoS ONE, 10(9), e0136924. https://doi.org/10.1371/journal.pone.0136924
Shipp, J.L. & Wang, K. (1998). Evaluation of commercially produced Trichogramma spp. (Hymenoptera: Trichogrammatidae) for control of tomato pinworm, Keiferia lycopersicella (Lepidoptera: Gelechiidae), on greenhouse tomatoes. Canadian Entomologist, 130(5), 721–731.
Shirazi, J. (2006). Effect of temperature and photoperiod on the biological characters of Trichogramma chilonis Ishii (Hymenoptera: Trichogrammatidae). Pakistan Journal of Biological Sciences., 9(5), 820–824.
Sigsgaard, L., Herz, A., Korsgaard, M. & Wührer, B. (2017). Mass release of Trichogramma evanescens and T. cacoeciae can reduce damage by the apple codling moth Cydia pomonella in organic orchards under pheromone disruption. Insects, 8, 41.
Smith, S. M. (1996). Biological control with Trichogramma: Advances, successes, and potential for their use. Annual Review of Entomology, 41, 375–406.
Southwood, T. R. (1978). Ecological methods with particular reference to the study of insect population (2nd ed., pp. 1–6; 356–387). Chapman and Hall.
Stouthamer, R. (1993). The use of sexual wasps in biological control. Entomophaga, 38, 3–6.
Tabebordbar, F., Shishehbor, P., Ebrahimi, E., Polaszek, A., & Ugine, T. A. (2022a). Effect of different constant temperatures on life history and life table parameters of Trichogramma euproctidis (Hymenoptera: Trichogrammatidae). Journal of Economic Entomology, 115(2), 474–481. https://doi.org/10.1093/jee/toac007
Tabebordbar, F., Shishehbor, P., Ebrahimi, E., Polaszek, A., & Riddick, E. W. (2022b). Parasitoid age and host age interact to improve life history parameters and rearing of Trichogramma euproctidis. Biocontrol Science and Technology, 32(3), 267–280. https://doi.org/10.1080/09583157.2021.1990858
Tabone, E., Bardon, C., Desneux, N., & Wajnberg, E. (2010). Parasitism of different Trichogramma species and strains on Plutella xylostella L. on greenhouse cauliflower. Journal of Pest Science, 83, 251–256.
Uhl, B., Wölfing, M., & Bässler, C. (2022). Mediterranean moth diversity is sensitive to increasing temperatures and drought under climate change. Scientific Reports, 12(1), 14473. https://doi.org/10.1038/s41598-022-18770-z
Ugurlu, K. S., Konus, M., & Buyuk, M. (2013). Determination of susceptibility levels of Helicoverpa armigera (Hübner) (Noctuidae: Lepidoptera) strains collected from different regions to some insecticides in Turkey. Journal of Entomological Research Society, 15, 37–45.
Ukhurebor, K. E., Adetunji, C. O., Olugbemi, O.T., Nwankwo, W., Olayinka, A. S., Umezuruike, C., & Hefft, D. I. (2022). Precision agriculture: Weather forecasting for future farming. In AI, (Edge and IoT-based Smart Agriculture., pp. 101–121). Academic Press. https://doi.org/10.1016/b978-0-12-823694-9.00008-6
Van Driesche, R. G. (1983). Meaning of “percent parasitism” in studies of insect parasitoids. Environmental Entomology, 12, 1611–1622.
Ventura Garcia, P., Wajnberg, E., Pizzol, J., & Oliveira, M. L. M. (2002). Diapause in the egg parasitoid Trichogramma cordubensis: Role of temperature. Journal of Insect Physiology, 48, 349–355.
Wu, L. H., Hoffmann, A. A., & Thomson, L. J. (2015). Trichogramma parasitoids for control of Lepidopteran borers in Taiwan: Species, life-history traits and Wolbachia infections. Journal of Applied Entomology, 139(8), 609–618. https://doi.org/10.1111/jen.12202
Wu, L. H., Hoffmann, A. A., & Thomson, L. J. (2016). Potential impact of climate change on parasitism efficiency of egg parasitoids: A meta-analysis of Trichogramma under variable climate conditions. Agriculture Ecosystems and Environment, 231, 143–155.
Yu, D. S. K., Hagley, E. A. C., & Laing, J. E. (1984). Biology of Trichogramma minutum Riley collected from apples in southern Ontario. Environmental Entomology, 13, 1324–1329.
Yuan, X. H., Song, L. W., Zhang, J. J., Zang, L. S., Zhu, L., Ruan, C. C., & Sun, G. Z. (2012). Performance of four Chinese Trichogramma species as biocontrol agents of the rice striped stem borer, Chilo suppressalis, under various temperature and humidity regimes. Journal of Pest Science, 85, 497–504.
Zhou, J. C., Liu, Q. Q., Wang, Q. R., Ning, S. F., Che, W. N., & Don, H. (2020). Optimal clutch size for quality control of bisexual and wolbachia-infected thelytokous lines of Trichogramma dendrolimi Matsumura (Hymenoptera: Trichogrammatidae) mass reared on eggs of a substitutive host, Antheraea pernyi Guérin-méneville (Lepidoptera: Saturniidae). Pest Management Science, 76, 2635–2644. https://doi.org/10.1002/ps.5805
Zouba, A., Chermiti, B., Kadri, K., & Fattouch, S. (2013a). Molecular characterization of Trichogramma cacoeciae strains (Hymenoptera: Trichogrammatidae) from the South West of Tunisia. Biomirror, 4(03), 1–6.
Zouba, A., Chermiti, B., Chraiet, R., & Mahjoubi, K. (2013b). Effect of two indigenous Trichogramma species on the infestation level by tomato miner Tuta absoluta in tomato greenhouses in the south-west of Tunisia. Tunisian Journal of Plant Protection, 8, 87–106.
Zouba, A., Zougari, S., Attia, S., Abbes, K., Grissa-Lebdi, K., Chermiti, B., & Ben Hmida, F. (2022). Field performance of the egg parasitoid Trichogramma cacoeciae Marchal (hymenoptera: Trichogrammatidae) following releases against Ectomyelois ceratoniae Zeller (Lepidoptera: Pyralidae) in two types of oasis in Tunisia. Journal of Entomology and Zoology Studies, 10(2), 28–35.
Zougari, S., Attia, S., Zouba, A., & Lebdi-Grissa, K. (2020). Effectiveness of mass trapping and Trichogramma cacoeciae (Hymenoptera: Trichogrammatidae) releases against Ectomyelois ceratoniae (Lepidoptera: Pyralidae) in Tunisian oases. Biologia, 76(4), 1175. https://doi.org/10.2478/s11756-020-00628-2
Zuim, V., Rodrigues, H. S., Pratissoli, D., Torres, J. B., Fragoso, D. F. M., & Bueno, C. O. F. (2017). Age and density of eggs of Helicoverpa armigera influence on Trichogramma pretiosum parasitism. Acta Scientific, 39, 513–520.
Acknowledgements
Authors are very grateful to Mrs Sonia Jandoubi for her technical assistance in the rearings. This work was supported by the National Agronomic Institute of Tunisia (INAT).
Author information
Authors and Affiliations
Contributions
SZ conducted experiment. AZ and SZ contributed equally to the writing of the contents present under different sub-headings of the manuscript. MM revised the manuscript. All generated data were statistically analyzed by SZ and critically checked and verified by KGL. NK help in data entry evaluation. KGL and FBH supervise. All the authors read and approved the manuscript.
Corresponding author
Ethics declarations
Competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Anis Zouba and Sahar Zougari contributed equally to the work, and should be concidered as co-first authors.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zouba, A., Zougari, S., Mamay, M. et al. The effect of different oviposition and preadult development temperatures on the biological characteristics of four Trichogramma spp. parasitoids (Hymenoptera: Trichogrammatidae) species. Phytoparasitica 52, 19 (2024). https://doi.org/10.1007/s12600-024-01128-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12600-024-01128-8