Abstract
Egg parasitoids of the genus Trichogramma Westwood play an important role in the control of the velvetbean caterpillar Anticarisa gemmatalis Hübner in soybean crops in Southern Brazil. The effectiveness of Trichogramma species as biocontrol agents is dependent of several factors, but their adaptation to the climatic conditions of the region where they will be released is one of the most important. In this context, this study evaluated the effects of five constant temperatures ranging from 14 to 30°C on parasitism, age-specific survival, progeny production and longevity of Trichogramma pretiosum Riley, Trichogramma atopovirilia Oatman & Platner, Trichogramma acacioi Brun, Moraes & Soares, Trichogramma lasallei Pinto, and Trichogramma rojasi Nagaraja & Nagarkatti. We demonstrate that temperature differently affected the species of Trichogramma investigated when using eggs of the velvetbean caterpillar. We also demonstrate that T. pretiosum and T. atopovirilia are better adapted to a wide range of temperatures than the other species evaluated, and are therefore better suited as biocontrol agents for applied biological control programs of A. gemmatalis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The velvetbean caterpillar Anticarsia gemmatalis Hübner (Lepidoptera: Noctuidae) is one of the most important pests attacking soybean crops in New World agroecosystems. The caterpillar causes significant losses in yield in all areas where soybean is cultivated (Sosa-Gómez 2004, Silva et al 2012). Nowadays, control of A. gemmatalis is almost exclusively dependent on chemical insecticides. However, in the last years a large number of parasitoids and entomopathogens were found associated with the velvetbean caterpillar (Polaszek & Foerster 1997, Foerster & Avanci 1999, Moscardi 1999), making the implementation of management programs involving the preservation and augmentation of these natural enemies feasible.
Field surveys conducted in southern Brazil recorded seven species of microhymenoptera parasitizing eggs of A. gemmatalis, of which five were of the genus Trichogramma Westwood (Hymenoptera: Trichogrammatidae): Trichogramma pretiosum Riley, Trichogramma atopovirilia Oatman & Platner, Trichogramma acacioi Brun, Moraes & Soares, Trichogramma lasallei Pinto, and Trichogramma rojasi Nagaraja & Nagarkatti (Polaszek & Foerster 1997, Foerster & Avanci 1999). In southern Brazil, these egg parasitoids are part of the local ecosystem and play an important role in the natural control of the velvetbean caterpillar, reaching levels of parasitism of up to 29% (Avanci et al 2005).
The effectiveness of parasitoids in the control of insect pests in biological control programs is dependent of several factors, but among the most important is their adaptation to the climatic conditions of the region where they will be released (DeBach 1965). Temperature is one of the most important environmental factors influencing various aspects of insect physiology, biology, and behavior (Ratte 1985); and the growth, survival, parasitism rate, and longevity of Trichogramma are strongly influenced by temperature (Ratte 1985, Reznik et al 2009).
In a previous study, Foerster & Foerster (2009) evaluated the effects of temperature on the development and emergence of five Trichogramma species collected in eggs of A. gemmatalis on soybean crops in southern Brazil. They estimated the parasitoids’ thermal requirements and found that the lower temperature threshold of subtropical populations were lower than those estimated from tropical populations, indicating the adaptation of subtropical species/lineages to cold conditions. These data suggest that other biological parameters may also vary in response to lower temperatures experienced in the subtropics as compared to those recorded in tropical regions.
Apart from the study of Foerster & Foerster (2009), little is known regarding the biology of the complex of Trichogramma species associated to the velvetbean caterpillar in southern Brazil. Except for T. pretiosum, none of the species had their fitness assessed on eggs of A. gemmatalis, and the influence of temperature on parasitism rate, age-specific survival, and longevity remains unclear, especially in the case of the poorly studied species T. lasallei and T. rojasi. Understanding the effects of temperature on these important life-history traits will facilitate the development of mass rearing procedures for inundative programs of biological control using Trichogramma (Prasad et al 2002, Maceda et al 2003), and contribute to the knowledge on the role of temperature on parasitoid population dynamics. We compared the effects of constant temperatures on the rate of parasitism, age-specific survival, progeny production, and longevity of the five species of Trichogramma associated with the velvetbean caterpillar in Brazil.
Material and Methods
Parasitoids origin and maintenance
Stock colonies of T. pretiosum, T. atopovirilia, T. acacioi, T. lasallei, and T. rojasi were established with parasitized eggs of A. gemmatalis collected on soybean crops in the counties of Lapa (25°46′11″S, 49°42′57″W) and Fazenda Rio Grande (25°39′27″S, 49°18′29″W), south-eastern of the state Paraná, Southern Brazil. Parasitoid-rearing procedure followed Foerster & Foerster (2009).
Temperature effects on parasitism of Trichogramma spp
The experiment was conducted under controlled conditions in climatic chambers regulated at 14, 18, 21, 26, and 30 ± 0.5°C; 70 ± 10% RH; and 12L:12D photoperiod. Adult parasitoids used in the experiment were reared since larval eclosion at the evaluated temperatures. For each temperature, ten pairs of each parasitoid species were individually kept in glass tubes (0.5 × 6 cm) immediately after emergence. Thirty eggs of A. gemmatalis were glued onto blue cardboard cards (0.5 × 4 cm) and offered for parasitization to each pair for 24 h. This procedure was repeated during three consecutive days, which is the period when most of the eggs are laid by the female wasps (Hansen & Jensen 2002). To compensate for the lower parasitism rate at 14 and 18°C, eggs were offered to parasitism every other day at these temperatures. Adult parasitoids were fed with droplets of pure honey deposited on the side of the glass tubes throughout the experiment.
The number of parasitized eggs and parasitoids emerged per host, age-specific survival, progeny production, and longevity were used to assess the effect of temperature on the five Trichogramma species selected. The number of parasitoids per host was calculated by dividing the total number of emerged parasitoids in each glass tube by the total number of eggs parasitized. The longevity of each adult parasitoid was recorded, but all data from one replicate was grouped and we used the mean longevity of each replicate (glass tube) when performing the statistical analysis. Therefore, a total of ten replicates were used for longevity and the other parameters evaluated.
Statistical analysis
Differences in life-history parameters were compared using a factorial analysis of variance (ANOVA), considering temperature and parasitoid species as factors. Parasitoid sex was considered as a third factor when longevity in each temperature was compared. When differences were detected by ANOVA, Tukey’s HSD test (p < 0.05) was used for average comparisons. Before proceeding with the analysis, we tested the ANOVA assumptions of normality and homogeneity of variance by using the Shapiro-Wilk’s and Levene’s tests, respectively. Age-specific survival curves were constructed and statistically compared according to Kaplan & Meier (1958). All statistical procedures were performed using the software Statistica v. 8 (Statsoft Inc 2008).
Results
Total number of parasitized eggs
All five Trichogramma species were able to parasitize eggs in a range from 14 to 30°C (Table 1), but the number of parasitized eggs was significantly affected by temperature (F (4,214) = 47.63; p < 0.001), parasitoid species (F (4,214) = 57.66; p < 0.001) and the interaction between these two factors (F (16,214) = 11.83; p < 0.001). The number of parasitized eggs was lower at 14°C, but no significant differences were recorded among parasitoid species at this temperature. By contrast, at 18, 21, 26, and 30°C, the number of parasitized eggs varied significantly according to the parasitoid species. In general, T. pretiosum and T. atopovirillia showed significantly higher rates of parasitism at temperatures above 18°C as compared to the other species. At 21 and 26°C, T. atopovirilia showed the highest number of parasitized eggs, whereas at 30°C T. pretiosum showed a significant higher number of parasitized eggs compared to T. lasallei, T. acacioi, and T. rojasi (Table 1).
Progeny production
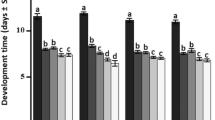
The number of descendents was significantly affected by temperature (F (4,214) = 30.55; p < 0.001), parasitoid species (F (4,214) = 10.83; p < 0.001) and the interaction between these two factors (F (16,214) = 4.02; p < 0.001). In general, progeny production tended to be reduced at 14 and 30°C for all egg parasitoid species. Trichogramma atopovirilia produced the highest number of descendents in all temperatures, except at 14°C (Fig 1).
Mean number of adult parasitoids produced after 3 days of parasitism (±SEM) by five Trichogramma species reared at different temperature regimes (70 ± 10% RH; 12L:12D photoperiod). The statistic shows differences in progeny production between species within each temperature. Means followed by the same letter in bars are not significantly different from each other according to ANOVA, Tukey’s HSD test (p ≥ 0.05).
Age-specific survival
According to the Kaplan–Meier’s analysis, differences in age-specific survival among Trichogramma species were recorded at 14 (χ 2 (4) = 35.17; p < 0.001), 18 (χ 2 (4) = 15.00; p = 0.004), 21 (χ 2 (4) = 14.15; p < 0.006), 26 (χ 2 (4) = 14.15; p < 0.007), and 30°C (χ 2 (4) = 35.82; p < 0.001). In all temperatures evaluated, T. pretiosum was the longest living species compared to the other parasitoids, while T. lasallei and T. rojasi presented the lowest survival rate (Fig 2).
Longevity
Longevity of the five Trichogramma species were affected by temperature (F (4,448) = 244.35; p < 0.001), parasitoid species (F (4,448) = 91.77; p < 0.001), parasitoid sex (F (1,448) = 37.95; p < 0.001), and by the interactions among these three factors (F (16,448) = 2.50; p = 0.001). For all parasitoid species, longevity decreased as temperature increased. At 14 and 18°C significant differences among species were recorded, with T. pretiosum living longer than the other species (Table 2).
Parasitoids emerged per host
The mean number of parasitoids emerged per host was also affected by temperature (F (4,214) = 73.49; p < 0.001), parasitoid species (F (4,214) = 81.50; p < 0.001), and the interaction between these factors (F (16,214) = 14.71; p < 0.001; Table 3).
Discussion
Temperature significantly affected parasitism, age-specific survival, progeny production, and longevity of all five Trichogramma species studied. Although all parasitoids survived and were able to parasitize eggs in the range between 14 and 30°C, the response to the extreme temperatures varied according to the species. For instance, T. acacioi showed a higher tolerance to low temperatures compared to T. pretiosum and T. atopovirilia. A similar adaptation of T. acacioi to low temperatures was recorded by Pratissoli et al (2009) using factitious hosts.
Our data is in accordance with previous data reported for different species of Trichogramma from various regions of the world, demonstrating a large variation in the rate of parasitism at temperatures below 15°C and above 30°C (Pak & van Heiningen 1985, Pizzol et al 2010). In general, egg parasitoids of the genus Trichogramma tend to reduce their rates of parasitism at temperatures below 25°C. One possible explanation is the loss of turgidity of the host egg at higher temperatures, making difficult the insertion of ovipositor through the host egg chorion (Pereira et al 2007). Another possibility is based on the fact that parasitoids need more energy to maintain their activity at high temperatures, and thus feed more frequently causing higher mortality through multiple feeding and stinging instead of parasitism (Shipp & Wang 1998).
Survival rate is also a good indicator of the ability of a species to tolerate a given temperature. Among the parasitoids evaluated in our study, T. pretiosum was the one with the highest survival rate at all temperatures, indicating its ability to tolerate a broader range of temperatures compared to the other species evaluated. On the other hand, both T. lasallei and T. rojasi showed comparatively low survival rates, suggesting they may be effective biocontrol agents of A. gemmatalis only under a restricted range of temperatures.
It is expected that the significant differences found in survival and parasitism rates differently affect the parasitoid fitness. These differences in fitness can partially explain the variation recorded in the abundance of Trichogramma species in the field. Surveys carried out during summer in soybean crops in south-eastern Brazil, when the average temperature is ca. 23°C, demonstrated that T. pretiosum was the dominant parasitoid, being in some seasons responsible for 70% of the parasitized eggs of A. gemmatalis (Avanci et al 2005). These findings are in accordance with the results obtained in our laboratory study regarding the high rates of parasitism and survival of T. pretiosum between 21 and 30°C. Also, T. lasallei and T. rojasi presented the lowest fecundity and survival rate and were the least abundant parasitoids in the field (Avanci et al 2005).
However, data obtained under laboratory conditions should be carefully extrapolated to field conditions due to the occurrence of other factors that might affect the dispersal and ability of parasitoids to locate their hosts, such as plant and habitat-related traits (Romeis et al 2005), interespecific competition (Pak & Oatman 1982), and the number of hosts that can be located and parasitized (Godfray 1994, West & Rivero 2000). In our study, T. atopovirillia also showed high parasitism rate and progeny production from 21 to 26°C, but was not as abundant in the field as T. pretiosum (Avanci et al 2005). Similarly, T. acacioi, which was the second most abundant parasitoid in the field, showed low survival rates and progeny production if compared to T. atopovirilia and T. pretiosum. Parasitism rate alone may not be a good predictor of field success, as already demonstrated for T. carverae Oatman & Pinto (Thomsom & Hoffmann 2002).
As observed in other studies with several species of Trichogramma, the mean longevity decreased as temperature increased (Hansen & Jensen 2002, Maceda et al 2003, Bueno et al 2010, 2012). This is possibly associated with a decrease in parasitoid activity and, consequently, in their metabolism at low temperatures (Bleicher & Parra 1990). An interesting result, however, is the fact that the longevity we recorded for T. pretiosum females was markedly longer than those reported for tropical strains of this species (Alencar et al 2000, Bueno et al 2010). Differences in the longevity of populations of a species collected in distinct regions suggest their acclimatization to the specific climatic conditions of their area of origin. The acclimatization explains the fact that the subtropical strains we used lived longer than those from the tropics. However, comparisons between studies should be conducted carefully, because differences in life-history parameters can also be attributed to factors related to experimental conditions, such as host quality (Corrigan & Laing 1994), photoperiod (Rounbehler & Ellington 1973). and parasitoid adult access to food sources (Hansen & Jensen 2002).
Other studies came to similar conclusions by comparing the lower temperature threshold (T 0) estimated for populations of Trichogramma collected in different regions (Poorjavad et al 2011, Pizzol et al 2010, Samara et al 2011). Foerster & Foerster (2009) compared the lower temperature threshold estimated for subtropical populations of Trichogramma with those available in the literature for tropical populations and concluded subtropical strains show a lower T 0. Similarly, Samara et al (2011) evaluated the role of temperature on the biology of different strains of T. aurosum Sugonjaev & Sorokina, and based on the estimated demographic parameters concluded that some strains showed good adaptability to high temperatures, while others were well adapted to low temperatures.
In conclusion, we demonstrated that temperature differently affect the species complex of the genus Trichogramma parasitizing eggs of the velvetbean caterpillar in southern Brazil. These results are useful to understand the differences in parasitoids abundance in the field. Furthermore, considering that success in biological control by Trichogramma species depends on the understanding of their ecological requirements (Parra et al 1987, van Lenteren et al 1997), the data we provided can also aid in the selection of species better adapted to the climatic condition experienced during the critical period of pest attack in soybean crops. Our data indicated T. pretiosum and T. atopovirilia as the best adapted to a wide range of temperatures among the species we analyzed and the most suitable for use in applied biological control programs of A. gemmatalis.
References
Alencar JA, Haji FNP, Oliveira JC, Moreira AN (2000) Biologia de Trichogramma pretiosum Riley em ovos de Sitotroga cerealella (Oliver). Pesq Agropec Bras 35:1669–1674
Avanci MRF, Foerster LA, Cañete CL (2005) Natural parasitism in eggs of Anticarsia gemmatalis Hübner (Lepidoptera, Noctuidae) by Trichogramma spp. (Hymenoptera, Trichogrammatidae) in Brazil. Rev Bras Entomol 49:148–151
Bleicher E, Parra JRP (1990) Espécies de Trichogramma parasitoides de Alabama argillacea. I. Biologia de três populações. Pesq Agropec Bras 25:215–219
Bueno RCOF, Bueno AF, Parra JRP, Vieira SS, Oliveira LJ (2010) Biological characteristics and parasitism capacity of Trichogramma pretiosum Riley (Hymenoptera, Trichogrammatidae) on eggs of Spodoptera frugiperda (J.E. Smith) (Lepidoptera, Noctuidae). Rev Bras Entomol 54:322–327
Bueno RCOF, Parra JRP, Bueno AF (2012) Trichogramma pretiosum parasitism of Pseudoplusia includens and Anticarsia gemmatalis eggs at different temperatures. Biol Control 60:154–162
Corrigan JE, Laing JE (1994) Effects of the rearing host species and the host species attacked on performance by Trichogramma minutum Riley (Hymenoptera: Trichogrammatidae). Environ Entomol 23:755–760
DeBach P (1965) Weather and the success of parasites in population regulation. Can Entomol 97:848–863
Foerster LA, Avanci MRF (1999) Egg parasitoids of Anticarsia gemmatalis Hübner (Lepidoptera: Noctuidae) in soybeans. An Soc Entomol Bras 28:545–548
Foerster MR, Foerster LA (2009) Effects of temperature on the immature development and emergence of five species of Trichogramma. BioControl 54:445–450
Godfray HCJ (1994) Parasitoids. Behavioural and evolutionary ecology. Princeton University Press, Princeton, p 488
Hansen LS, Jensen KMV (2002) Effect of temperature on parasitism and host-feeding of Trichogramma turkestanica (Hymenoptera: Trichogrammatidae) on Ephestia kuehniella (Lepidoptera: Pyralidae). J Econ Entomol 95:50–56
Kaplan EL, Meier P (1958) Nonparametric estimation from incomplete observations. J Am Stat Assoc 53:457–481
Maceda A, Hohmann CL, Santos HR (2003) Temperature effects on Trichogramma pretiosum Riley and Trichogrammatoidea annulata De Santis. Braz Arch Biol Techn 46:27–32
Moscardi F (1999) Assessment of the application of baculoviruses for the control of Lepidoptera. Annu Rev Entomol 44:257–289
Pak GA, Oatman ER (1982) Comparative life table, behaviour and competition studies of Trichogramma brevicapillum and T. pretiosum. Entomol Exp Appl 32:68–79
Pak GA, Van Heiningen TG (1985) Behavioural variations among strains of Trichogramma spp.: adaptability to field-temperature conditions. Entomol Exp Appl 38:3–13
Parra JRP, Zucchi RA, Silveira-Neto S (1987) Biological control of pests through egg parasitoids of the genera Trichogramma and Trichogrammatoidea. Mem Inst Oswaldo Cruz 82:153–160
Pereira FF, Barros R, Pratissoli D, Pereira CLT, Vianna UR, Zanuncio JC (2007) Capacidade de parasitismo de Trichogramma exiguum Pinto & Platner, 1978 (Hymenoptera: Trichogrammatidae) em ovos de Plutella xylostella (L., 1758) (Lepidoptera: Plutellidae) em diferentes temperaturas. Cienc Rural 37:297–303
Pizzol J, Pintureau B, Khoualdia O, Desneux N (2010) Temperature-dependent differences in biological traits between two strains of Trichogramma cacoeciae (Hymenoptera: Trichogrammatidade). J Pest Sci 83:447–452
Polaszek A, Foerster LA (1997) Telenomus cyamophylax, n. sp. (Hymenoptera:Scelionidae) attacking eggs of the velvetbean caterpillar, Anticarsia gemmatalis Hübner (Lepidoptera:Noctuidae). An Soc Entomol Bras 26:177–181
Poorjavad N, Goldansaz SH, Hosseininaveh V, Nozari J, Dehghaniy H, Enkegaard A (2011) Fertility life table parameters of different strains of Trichogramma spp. collected from eggs of the carob moth Ectomyelois ceratoniae. Entomol Sci 14:245–253
Prasad RP, Roitberg BD, Henderson DE (2002) The effect of rearing temperature on parasitism by Trichogramma sibericum Sorkina at ambient temperatures. Biol Control 25:110–115
Pratissoli D, Bueno AF, Bueno RCOF, Zanúncio JC, Polanczyk RA (2009) Trichogramma acacioi (Hymenoptera, Trichogrammatidae) parasitism capacity at different temperatures and factitious hosts. Rev Bras Entomol 53:151–153
Ratte HT (1985) Temperature and insect development. In: Hoffmann KH (ed) Environmental physiology and biochemistry of insects. Springer, Berlin, pp 33–66
Reznik SY, Voinovich ND, Vaghina NP (2009) Effect of temperature on the reproduction and development of Trichogramma buesi (Hymenoptera: Trichogrammatidae). Eur J Entomol 106:535–544
Rounbehler MD, Ellington JJ (1973) Some biological effects of selected light regimes on Trichogramma semifumatum (Hym: Trichogrammatidae). Ann Entomol Soc Am 66:6–10
Romeis J, Babendreier D, Wäckers FL, Shanower TG (2005) Habitat and plant specificity of Trichogramma egg parasitoids—underlying mechanisms and implications. Basic Appl Ecol 6:215–236
Samara R, Monje JC, Zebitz CPW, Qubbaj T (2011) Comparative biology and life tables of Trichogramma aurosum on Cydia pomonella at constant temperatures. Phytoparasitica 39:109–119
Shipp JL, Wang K (1998) Evaluation of commercially produced Trichogramma spp. (Hymenoptera: Trichogrammatidae) for control of tomato pinworm, Keiferia lycopersicella (Lepidoptera: Gelechiidae), on greenhouse tomatoes. Can Entomol 130:721–731
Silva DM, Hoffmann-Campo CB, Bueno AF, Bueno RCOF, Oliveira MCN, Moscardi F (2012) Biological characteristics of Anticarsia gemmatalis (Lepidoptera: Noctuidae) for three consecutive generations under different temperatures: understanding the possible impact of global warming on a soybean pest. B Entomol Res 102:285–292
Sosa-Gómez DR (2004) Intraspecific variation and population structure of the velvetbean caterpillar, Anticarsia gemmatalis Hübner, 1818 (Insecta: Lepidoptera: Noctuidae). Genet Mol Biol 27:378–384
Statsoft Inc (2008) Statistica for windows. Statsoft Inc, Tulsa
Thomsom LJ, Hoffmann AA (2002) Laboratoy fecundity as predictor of field success in Trichogramma carverae (Hymenoptera: Trichogrammatidae). J Econ Entomol 95:912–917
van Lenteren JC, Roskam MM, Timmer T (1997) Commercial mass production and pricing of organisms for biological control of pests in Europe. Biol Control 10:143–149
West SA, Rivero A (2000) Using sex ratios to estimate what limits reproduction in parasitoids. Ecol Lett 3:294–299
Acknowledgments
The authors are grateful to Dr John Pinto (University of California, Riverside, USA), Dr Roberto A. Zucchi (Universidade de São Paulo—ESALQ/USP), Dr Ranyse BQ da Silva (Empresa Brasileira de Pesquisa Agropecuária—Embrapa) for the identification of the Trichogramma species. This research was performed with scholarships provided by the Brazilian National Research Council (CNPq) and Brazilian Federal Agency for Support and Evaluation of Graduate Education (CAPES).
Author information
Authors and Affiliations
Corresponding author
Additional information
Edited by Fernando L Cônsoli – ESALQ/USP
Rights and permissions
About this article
Cite this article
Foerster, M.R., Marchioro, C.A. & Foerster, L.A. Temperature-Dependent Parasitism, Survival, and Longevity of Five Species of Trichogramma Westwood (Hymenoptera: Trichogrammatidae) Associated with Anticarsia gemmatalis Hübner (Lepidoptera: Noctuidae). Neotrop Entomol 43, 176–182 (2014). https://doi.org/10.1007/s13744-013-0189-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13744-013-0189-2