Abstract
Trichogramma (Hymenoptera: Trichogrammatidae) are species used worldwide for the biological control of Lepidopteran pests, notably through inundative releases on millions of hectares. The optimal use of Trichogramma parasitoids in crop protection requires an accurate knowledge of their biology. More specifically, the importance of age factor in parasitoids during the time they forage in crops for host eggs (after initial release) and how the aging of host eggs could impact parasitoid biological traits may be important for overall efficiency in terms of crop protection. In this context, the importance of parasitoid female and host egg ages on parasitism rate and the development of offspring was studied in laboratory conditions on Trichogramma cacoeciae Marchal (Hymenoptera: Trichogrammatidae) and the eggs of the pest Lobesia botrana Denis and Schiffermüller (Lepidoptera: Tortricidae). Host eggs tested were 1–2- and 3–4-day-old, while the ages of T. cacoeciae adult females varied from 1-day-old to 4-day-old post-emergence. When L. botrana eggs were 3–4-day-old, they were less parasitized by T. cacoeciae than 1–2-day-old eggs, and this was not linked to the age of T. cacoeciae females. The age of parasitoid females has an effect on parasitism, as 1-day-old females produced fewer parasitized eggs than 2, 3, and 4-day-old females. For the total number of L. botrana eggs killed by T. cacoeciae, the two factors did not show significant effects. When L. botrana eggs were 1–2-day-old, parasitoid emergence increased according to the age of parasitoid females with the highest success observed for 3-day-old females. The lowest emergence rates were obtained with T. cacoeciae females 1-day-old. The development time was also longer with the young 1-day-old parasitoid females. This study demonstrated that both the aging of parasitoids and host eggs play a role in the subsequent development of parasitoid offspring. The importance of these results in the context of biological control programs involving Trichogramma parasitoids is discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Trichogramma (Hymenoptera: Trichogrammatidae) species are used extensively on millions of hectares for biological control throughout the world against Lepidoptera (Li-Ying 1994; Smith 1996; Pintureau 2009; Agamy 2010; Desneux et al. 2010; Andrade et al. 2011). In Western Europe, the use of Trichogramma is relatively recent, particularly in France where research on the European corn borer dates from 1970 (Voegelé et al. 1975; Pintureau 2009), and from 1980 many studies have focused on the grapevine moth Lobesia botrana Denis and Schiffermüller (Lepidoptera: Tortricidae).
French vineyards cover nearly one million hectares and losses related to the attacks of many pests and pathogens can reach four hundred million euros per year (Mezière and Gary 2009). Among the vine pests, Tortricid moths are certainly the most harmful, notably, the European grapevine moth L. botrana, the grape berry moth Eupoecilia ambiguella Hübner, and Sparganothis pilleriana Denis and Schiffermüller (Thiéry et al. 2008). The first two species are polyvoltin pests and cause damage by feeding directly on berries and there is also indirect damage giving rise to the development of various mold diseases in damaged berries (e.g., Botrytis cinerea and Aspergilus carbonarius). Direct and indirect damage may result in up to 40 % losses at harvest (Boudon-Padieu et al. 2000; Thiéry et al. 2008).
Management of these pests relies on the use of insecticides, i.e., 6–8 chemical treatments per season for table grapes and 1–2 for wine grapes (ACTA 2010). These treatments ultimately result in insecticide residues on grapes and in wines (Cabras et al. 1995), which can delay harvesting date. In addition, potential problems have arisen with extensive pesticide use, i.e., resistant strains could appear in pests (Roush and Mckenzie 1987) as well as well-known negative impacts on human health (Weisenburger 1993) and non-target organisms (Desneux et al. 2006; Han et al. 2010; Biondi et al. 2012 and see Desneux et al. 2007 for a thorough review). Aiming at reducing the impact of chemical insecticides in vine crops, the development of biological control programs relying on egg parasitoids and the use of mating disruption methods may be promising for sustainable and environmentally sound pest management (Thiéry 2011; Xuéreb and Thiéry 2006).
In the case of management of L. botrana in France, inundative releases of Trichogramma cacoeciae Marchal (Hym. Trichogrammatidae), found naturally on eggs of L. botrana (Richard 1979; Dugast 1982; Babi 1990; Schubert and Stengel 1992; Barnay 1999), were made during a decade in the Alsace region. The results have been irregular (i.e., ranging from <10 to 100 % parasitism) and reduction in damage below economic thresholds (Barnay 1999; Hommay et al. 2002; Pizzol 2004). Effective parasitism by Trichogramma parasitoids can be influenced by environmental factors such as humidity, photoperiod and temperature (Pizzol et al. 2010) as well as by biotic factors such as egg size (Berrigan 1991; Martel et al. 2011). Recent studies have also shown that the quality of larval food and the grape cultivar are important for the reproductive performance of L. botrana (Thiéry and Moreau 2005; Moreau et al. 2006a, b).
The age of Trichogramma parasitoid females and/or of host eggs can influence parasitism of various pests (Pak et al. 1986; Garcia et al. 2001; Reznik and Vaghina 2007). These two factors should receive more attention to insure overall efficiency of parasitism by Trichogramma parasitoids when developing biological control programs involving the use of inondative releases of such natural enemies. In the case of T. cacoeciae searching for suitable L. botrana or E. ambiguella eggs, age factors may be critical. Eggs are mainly located on or inside bunches and are almost always scattered (Gabel and Thiéry 1996); this may lead to increased age of parasitoid females when they encounter host eggs. Moreover, the age of host eggs encountered by the parasitoid females varies, due to varying dates of diapause emergence, the egg laying dynamics by L. botrana in the first generation may last over 1 month (Thiéry et al. 2008).
In this context, tests were conducted in the laboratory on the effects of the host egg age (1–2-day-old and 3–4-day-old) and of the age of T. cacoeciae (1, 2, 3 and 4-day-old) in order to assess the influence of these factors on parasitism of L. botrana by T. cacoeciae and on various biological traits in the parasitoid.
Materials and methods
The host: Lobesia botrana
Mated females of L. botrana were grouped by ten in laying arenas (paper bags) (25 °C, 80 % R.H. and L16:D8) as described in Thiéry and Moreau (2005). In these conditions, egg incubation lasts 6–7 days; after 5 days on an average, eggs evolve to the so-called black head stage. Every day, females were transferred to new arenas and freshly laid eggs were collected by cutting off the bags around the eggs, then pooled according to age and stored in petri dishes in a climatic chamber (22 °C, 90 % R.H. and L16:D8). This insured that eggs were kept in good condition till they reach the age needed for the experiments. Two ages of host eggs were used: 1–2-day-old and 3–4-day-old. We used these two classes because (i) preliminary studies showed that older eggs (i.e., >4-day-old) were too close to the hatching stage for effective parasitism of L. botrana eggs by Trichogramma parasitoids (Pizzol J, unpublished data), (ii) a previous study reported that survivorship of T. evanescens was higher in younger L. botrana eggs than in older ones (Moreau et al. 2009), and (iii) of practical reasons.
The parasitoid: Trichogramma cacoeciae
The T. cacoeciae colony was established in the laboratory from L. botrana eggs collected in French vineyards (in Alsace region) (Pizzol and Pintureau 2008). After initial identification of parasitoids emerging from collected eggs, T. cacoeciae were selected and reared on UV-irradiated eggs of the alternative host Ephestia kuehniella Zeller (Lepidoptera: Pyralidae) (25 °C, 70 ± 5 % R.H. and L16:D8) (Daumal et al. 1975; Mansour 2010). Females were collected when they emerged and were kept to reach the various ages needed for the experiments (see below). To this end, they were placed in climatic chambers in plastic boxes (length: 36 cm, width: 24 cm, height: 14 cm) (22 °C, 75 % R.H. and L16:D8). All adult parasitoids were fed at emergence with a drop of honey.
Exposure of host eggs to the parasitoid
We studied the effect of age of L. botrana eggs and age of T. cacoeciae females on parasitism. To do so, we used a 2 × 4 factorial design. The two-level treatment corresponds to the two ages of L. botrana eggs exposed to parasitism (1–2-day-old and 3–4-day-old). The four-level treatment corresponds to four ages of T. cacoeciae females (1, 2, 3, and 4-day-old). This resulted in 8 combinations, and 60 replicates were performed per combination. Each T. cacoeciae female was isolated in a glass tube (length: 7.5 cm, diameter: 1 cm), fed with a drop of honey, and supplied with 25 eggs of L. botrana placed on a card (6 × 0.9 cm) (25 °C, 70 ± 5 % R.H. and L16:D8). Wasp females stayed with these host eggs for 24 h and were then withdrawn from the tubes. The tubes were then stored in climatic chambers (25 °C, 70 ± 5 % R.H. and L16:D8). The date of emergence of T. cacoeciae adults was recorded to estimate the duration of pre-imaginal development. In addition, 6 days after the first emergence, we counted the parasitized eggs (i.e., black eggs, emerged, or not emerged) and the L. botrana aborted eggs (i.e., L. botrana eggs that did not hatch owing to parasitoid stinging activity). These values were used to estimate (i) the emergence rate of T. cacoeciae on L. botrana eggs, and (ii) the total mortality of L. botrana eggs (i.e., parasitism + aborted eggs) when exposed to T. cacoeciae. Only data from tubes containing live females of T. cacoeciae after the 24-h laying period were taken into account to analyze the data.
Statistical analysis
The number of parasitized eggs, the total number of L. botrana eggs killed (i.e., parasitized + aborted eggs) and the number of days before parasitoid emergence (duration of pre-imaginal development) were analyzed by a factorial ANOVA with the “host egg age” and “parasitoid age” as factors. The same analysis was carried out on arcsi-transformed data regarding the parasitoid emergence rate.
Results
Parasitism and mortality of L. botrana eggs
The statistical results are summarized in Table 1A, B. There was a significant impact of both host age and parasitoid age factors on the number of parasitized eggs (i.e., black eggs). However, the two factors did not show any significant interaction which meant that both factors acted independently on the number of parasitized L. botrana eggs by T. cacoeciae. When L. botrana eggs were 3–4-day-old, they were less parasitized by T. cacoeciae than 1–2-day-old eggs (a decrease ranging between 7 and 13 %), and this was not connected to the age of T. cacoeciae females (Fig. 1a). The age of parasitoid females did have an effect on parasitism as 1-day-old females produced fewer parasitized eggs than 2, 3, and 4-day-old females. However, females in these three age groups (2, 3, and 4) produced similar numbers of black eggs. When considering the total number of L. botrana eggs killed by T. cacoeciae, the two factors did not show significant effects or a significant interaction (Fig. 1b) (all P > 0.05).
Emergence of T. cacoeciae from L. botrana eggs
There was a significant effect of the “parasitoid age” factor on the emergence rate of T. cacoeciae (Table 1C; Fig. 2). By contrast, the age of L. botrana eggs did not significantly affect successful development of T. cacoeciae (“Host egg age” factor not significant, Table 1C). The parasitoid emergence rate increased in older parasitoid females when L. botrana eggs were 1–2-day-old. The highest value observed when the females were 3-day-old (97.6 %) and it dropped to 94.8 % when parasitoids were 4-day-old. The pattern was not exactly the same when L. botrana eggs were 3–4-day-old; the highest emergence rate (96.7 %) was observed for 2-day-old parasitoid. Despite different patterns between the two host egg ages tested, the two factors “Parasitoid age” and “Host egg age” did not interact significantly.
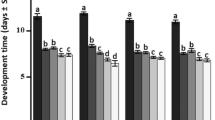
Parasitoid development time
Results are summarized in Table 1D. There was a significant impact of both host age and parasitoid age factors on the development time of parasitoid offspring in L. botrana eggs (Fig. 3). However, the two factors did not interact significantly, i.e., the two factors acted independently on the duration of T. cacoeciae offspring development. The duration of parasitoid development decreased with the increasing age of T. cacoeciae female. In addition, when parasitoid females parasitized older L. botrana eggs, the development time of offspring development was shorter.
Discussion
Several studies have documented the factors potentially increasing or decreasing the efficiency of T. cacoeciae, including the effect of rearing and oviposition temperature (Ozder and Kara 2010; Pizzol et al. 2010) and the effect of substitution host species used (Ozder and Kara 2010). Few studies have precisely documented the potential effects of host egg age (Pak et al. 1986; Moreau et al. 2009; Moreno et al. 2009) and of parasitoid age (Garcia et al. 2001; Pizzol 2004) on key parasitism traits such as parasitism rate, duration and successful offspring development. However, most studies have focused on either the effect of the age of host eggs or the effect of parasitoid age, but testing for potential interactions between these two factors has not been carried out. Our study demonstrates that these two parameters act independently on various biological traits in the Trichogramma parasitoid, T. cacoeciae.
Effect of parasitoid age
In our conditions, 1-day-old females showed the lowest parasitism rate independently of L. botrana egg age. However, we found no difference for total L. botrana egg mortality (i.e. parasitism + aborted eggs). Therefore, when parasitoid females were 1 day old or under, they most likely stung as many eggs as older females, but they actually did not parasitize as many eggs as older females. The youngest females may have stung L. botrana eggs for host feeding. On the other hand, (and in a non-exclusive way) females may have been more selective in the hosts they encountered (Klomp et al. 1980). Indeed, host choice behavior in parasitoids is known to be dependant on the physiological state of the parasitoid (Minkenberg et al. 1992). Low egg loads early in life often lead to higher choosiness when encountering hosts (Papaj 2000). It may have led T. cacoeciae females to sting host eggs (either to feed or to assess host quality, Vinson 1998) without actually effectively parasitizing them, i.e., no eggs laid. Such stinging is known to potentially induce host death (eggs in case of Trichogramma parasitoids) through venom/virus injection by the females (Asgari and Rivers 2011), e.g., the T. australicum Girault sting on Helicoverpa armigera Hübner egg always leads to immediate death of developing embryo because of parasitoid venom (Jarjees and Merritt 2004). When females were older, more stings actually led to parasitism (i.e., egg deposited into the host). Therefore, the choosiness behavior is weaker when the ovarian pressure is higher (i.e., more mature eggs in ovaries) due to increasing age of females (Klomp et al. 1980).
It is known that fecundity schedule in parasitoids usually shows a rise in the number of eggs laid per day until it reaches a maximum rate (Jervis and Kidd 1996). Then a gradual decrease occurs until reproduction ceases altogether at or shortly before the time of death. Although we did not test parasitoid females older than 4 days old in our study, we did not observe a great difference in parasitism rates among 2, 3, and 4-day-old females. Therefore, such periods may correspond to the maximum parasitism rate in our conditions and potential reduction of parasitism rate might have been observed if we had tested females older than 4 days. A lower parasitism rate (after a peak was reached) was reported in T. cordubensis that were 5–6-day-old (Garcia et al. 2001) and the authors reported that this species behaved accordingly to the “static optimization model” (Godfray 1994).
The emergence rate of T. cacoeciae offspring was also lower and development time higher when ovipositing females were younger (1-day-old). It is unclear why the progeny of these females developed slower than those of older females. However, it might be related to factors linked to the parasitism itself, e.g., venom which is injected into the host at the time of the stinging (to cope with host immune defense and to kill host embryo) might be less efficient in young females (if venom glands need several hours after parasitoid emergence to be fully active). It might delay parasitoid embryo development because host degeneration is known to rely exclusively on female venom rather than on factors derived from the parasitoid embryo or larva (Jarjees and Merritt 2004). It may be consistent with the lower emergence rate of the progeny of these young females (though it only occurred when host eggs were also young). In addition, eggs from young females might not be as good because they are not totally developed yet, i.e., most vitellogenesis and maturation of eggs in T. cacoeciae are known to occur on the second day of life (Volkoff and Daumal 1994).
Effect of host egg age
Host egg age is known to affect the parasitism rate of Trichogramma parasitoids (Pizzol 2004) and we observed that older host eggs, i.e., 3–4-day-old, were less parasitized than younger eggs in the case of L. botrana/T. cacoeciae. These results match those in previous studies (Brand et al. 1984; Calvin et al. 1997; Pak et al. 1986; Moreno et al. 2009). The effect of host egg age appeared totally unrelated to parasitoid age as we clearly observed the same trend for all parasitoid ages tested (Fig. 1a). By contrast, the age of host eggs did not affect on the whole the mortality of host eggs and parasitoid emergence rate (i.e., success in parasitoid development). The lack of consistency between the effects of parasitoid age and host egg age on parasitism rate and the absence of such effects on host egg mortality (Fig. 1a vs. b) could be attributed to two hypotheses. Firstly, parasitoid females could have laid eggs in a similar way independently of host egg age, but parasitoid larvae could have developed less efficiently in older eggs because of higher competition with developing host embryo (Benoit and Voegelé 1979). According to Benoit and Voegelé (1979) Trichogramma parasitoids do not oviposit on host eggs older than 5 days, but venom injected at the time of the stinging ultimately prevents the developing host embryo from reaching adulthood. This could explain lower parasitism success in older L. botrana eggs without actually allowing host embryo to successfully develop (i.e., resulting in host egg death). Second, parasitoid females may have stung the host eggs in a similar way regardless of their eggs’ age, but have oviposited only in hosts showing optimal physiological suitability for the subsequent development of the parasitoid larvae (Desneux et al. 2009b, 2012). Parasitoid females are known to assess hosts encountered and the behavior of females is based on cues perceived notably during ovipositor probing, i.e., at the time of stinging (Godfray 1994; Vinson 1998), though other mechanisms could be involved (Outreman et al. 2001; Desneux et al. 2009a). T. cacoeciae females may have rejected older eggs because of the potential competition with host embryo that had already developed. Such probing, despite resulting in the deposition of a parasitoid egg, probably induced death in all probed host eggs because of the venom injected at the time of the stinging (Asgari and Rivers 2011), as has already been reported in Trichogramma parasitoid species (Benoit and Voegelé 1979; Klomp et al. 1980). Further studies would have to be carried out to clarify this point.
Importance for biological control based on releasing Trichogramma parasitoids
Parasitism rates by Trichogramma parasitoids which are usually recorded in the field may often underestimate the total mortality induced in the host populations (Barnay 1999; Pizzol 2004; Tabone et al. 2010). Therefore, this should be considered to optimize the releases, as has been done when developing the use of T. brassicae against the European corn borer in France (it includes successive waves of parasitoid emergence and seasonal inundative releases, Hawlitzky 1992; Frandon et al. 2002, 2005). Data on the impact of host and parasitoid ages on the efficiency of Trichogramma parasitoids for biological control programs have been already used, notably when diapause was studied for optimizing production, storage, and release techniques (Pizzol and Voegelé 1987). This could also be important because the age of T. cacoeciae and the host eggs age significantly affect parasitism and it has consequences in the field, e.g., release points should be carefully distributed in fields/greenhouses. The variable parasitism rate owing to the quality of the host eggs (e.g. age) encountered/parasitized may partly explain the varying efficiency of Trichogramma parasitoid releases in vineyards during field trials though other factors can affect parasitism, such as the number of parasitoid adults released per hectare (Kast and Hassan 1986, Castaneda-Samayoa et al. 1993), the spatial distribution pattern of host eggs as a function of pest density, the vine cultivars (Moreau et al. 2009) or the choice of Trichogramma species (Kast and Hassan 1986; Castaneda-Samayoa et al. 1993).
References
ACTA (2010) Index phytosanitaire, 46e edn. ACTA, Paris
Agamy E (2010) Field evaluation of the egg parasitoid, Trichogramma evanescens West. against the olive moth Prays oleae (Bern.) in Egypt. J Pest Sci 83:53–58
Andrade GS, Pratissoli D, Dalvi LP, Desneux N, Gonçalves HJ (2011) Performance of four Trichogramma species (Hymenoptera: Trichogrammatidae) as biocontrol agents of Heliothis virescens (Lepidoptera: Noctuidae) under various temperature regimes. J Pest Sci 84:313–320
Asgari S, Rivers DB (2011) Venom proteins from endoparasitoid wasps and their role in host-parasite Interactions. Annu Rev Entomol 56:313–335
Babi A (1990) Bioécologie de Trichogramma cacoeciae Marchal et T. daumalae Dugast and Voegelé (Hym. Trichogrammatidae). Utilisation en lutte biologique contre Lobesia botrana Den. and Schiff. (Lep. Tortricidae). Thèse Doctorat d’Etat, Université de Marseille
Barnay O (1999) Dynamique des populations et relation hôte-parasitoïde chez le couple Lobesia botrana Den. and Schiff.—Trichogramma cacoeciae Marchal, dans le cadre de la lutte biologique en vignoble. Thèse Doct, Univ. Paris VI
Benoit M, Voegelé J (1979) Choix de l’hôte et comportement trophique de Trichogramma evanescens Westw. (Hym., Trichogrammatidae) en fonction du développement embryonnaire d’Ephestia kuehniella Zell. et d’Ostrinia nubilalis Hubner (Lep., Pyralidae). Entomophaga 24:199–207
Berrigan D (1991) The allometry of egg size and number in insects. Oikos 60:313–321
Biondi A, Desneux N, Siscaro G, Zappalà L (2012) Using organic-certified rather than synthetic pesticides may not be safer for biological control agents: selectivity and side effects of 14 pesticides on the predator Orius laevigatus. Chemosphere 87:803–812
Boudon-Padieu E, Esmenjaud D, Kreiter S, Roehrich R, Sforza R, Stockel J, van Helden M (2000) Ravageurs de la vigne. Editions Féret, Bordeaux
Brand AM, Van Dijken MJ, Kole M, Van Lenteren JC (1984) Host-age and host-species selection of three strains of Trichogramma evanescens Westwood, an egg parasite of several lepidopteran species. Meded Fac Landbouww Rijksuniv Gent 49 (3a):839–847
Cabras P, Garau VL, Pirisi FM, Cubrddu M, Cabitza F (1995) Fate of some insecticides from vine to wine. J Agric Food Chem 43(10):2613–2615
Calvin DD, Losey JE, Knapp MC, Poston FL (1997) Oviposition and development of Trichogramma pretiosum (Hym., Trichogrammatidae) in three age classes of southwestern corn borer eggs. Environ Entomol 26(2):385–390
Castaneda-Samayoa OR, Holst H, Ohnesorge B (1993) Evaluation of some Trichogramma species with respect to biological control of Eupoecilia ambiguella and Lobesia botrana Schiff. (Lep., Tortricidae). Z Pflanzenkr Pflanzenschutz 100(6):599–610
Daumal J, Voegelé J, Brun P (1975) Les Trichogrammes II. Unité de production massive et quotidienne d’un hôte de substitution Ephestia kuehniella Zeller. Ann Zool Ecol Anim 7:45–59
Desneux N, Ramirez-Romero R, Kaiser L (2006) Multi-step bioassay to predict recolonization potential of emerging parasitoids after a pesticide treatment. Environ Toxicol Chem 25:2675–2682
Desneux N, Decourtye A, Delpuech JM (2007) The sublethal effects of pesticides on beneficial arthropods. Annu Rev Entomol 52:81–106
Desneux N, Barta RJ, Hoelmer KA, Hopper KR, Heimpel GE (2009a) Multifaceted determinants of host specificity in an aphid parasitoid. Oecologia 160:387–398
Desneux N, Barta RJ, Delebecque CJ, Heimpel GE (2009b) Transient host paralysis as a means of reducing self-superparasitism in koinobiont endoparasitoids. J Insect Physiol 55:321–327
Desneux N, Wajnberg E, Wyckhuys KAG, Burgio G, Arpaia S, Narvaez-Vasquez CA, Gonzalez-Carbrera J, Catalan Ruescas D, Tabone E, Frandon J, Pizzol J, Poncet C, Cabello T, Urbanrja A (2010) Biological invasion of European tomato crops by Tuta absoluta: ecology, history of invasion and prospects for biological control. J Pest Sci 83:197–215
Desneux N, Blahnik R, Delebecque CJ, Heimpel GE (2012) Host phylogeny and host specialization in parasitoids. Ecol Lett 15:453–460
Dugast JF (1982) Les trichogrammes parasites des « vers de la grappe ». Influence de divers hôtes de substitution sur leur biologie. D.D.A., E.N.S.A, Montpellier
Frandon J, Kabiri F, Pizzol J (2002) La lutte biologique contre la pyrale du maïs avec les Trichogrammes. Bilan des derniers développements. In: 2ème Conférence Internationale sur les Moyens Alternatifs de Lutte Contre les Organismes Nuisibles des Végétaux, Lille, pp 33–40
Frandon J, Kabiri F, Pizzol J (2005) Amélioration du conditionnement des trichogrammes pour la lutte biologique contre la pyrale du maïs: vers plus de simplicité et d’efficacité. In: AFPP, 7e Conférence Internationale sur les ravageurs en Agriculture. AFPP, Montpellier, 26 et 27 Octobre 2005, [CD-ROM]
Gabel B, Thiéry D (1996) Oviposition response of Lobesia botrana females to long chain free fatty acids and esters from its eggs. J Chem Ecol 22:161–171
Garcia P, Wajnberg E, Oliveira L, Tavares J (2001) Is the parasitization capacity of Trichogramma cordubensis influenced by the age females? Entomol Exp Appl 98:219–224
Godfray HCJ (1994) Parasitoids: behavioural and evolutionary ecology. Princeton University Press, Chichester
Han P, Niu CY, Lei CL, Cui JJ, Desneux N (2010) Use of an innovative T-tube maze assay and the proboscis extension response assay to assess sublethal effects of GM products and pesticides on learning capacity of the honey bee Apis mellifera L. Ecotoxicology 19:1612–1619
Hawlitzky N (1992) La lutte biologique à l’aide de Trichogrammes. Courrier de la Cell. Environnement de l’INRA 16:9–23
Hommay G, Gertz C, Kienlen JC, Pizzol J, Chavigny P (2002) Comparison between the control efficacy of Trichogramma evanescens Westwood and of two Trichogramma cacoeciae Marchal strains against vine moth (Lobesia botrana Den. and Schiff.) depending on their release density. Biocontrol Sci Tech 12:569–581
Jarjees EA, Merritt DJ (2004) The effect of parasitization by Trichogramma australicum on Helicoverpa armigera host eggs and embryos. J Invertebr Pathol 85:1–8
Jervis MA, Kidd NAC (1996) Parasitoid adult feeding ecology and biocontrol. A review. Biocontrol News Info 16:11–26
Kast WK, Hassan SA (1986) Massenproduction und Anwendung von Trichogramma: 9. Wirksame Bekämpfung des Einbindigen Traubenwicklers Eupoecilia ambiguella Hbn. Wein-Wiss 41(4):278–286
Klomp H, Teerink B, Wei CM (1980) Discrimination between parasitized and unparasitized hosts in the egg parasite Trichogramma embryophagum (Hym.: Trichogrammatidae): a matter of learning and forgetting. Neth J Zool 30(2):254–277
Li-Ying L (1994) Worldwide use of Trichogramma for biological control on different crops: a survey. In: Wajnberg E, Hassan SA (eds) Biological control with eggs parasitoids. CAB International, Wallingford, pp 37–51
Mansour M (2010) Effects of gamma radiation on the Mediterranean flour moth, Ephestia kuehniella, eggs and acceptability of irradiated eggs by Trichogramma cacoeciae females. J Pest Sci 83:243–249
Martel V, Darrouzet E, Boivin G (2011) Phenotypic plasticity in the reproductive traits of a parasitoid. J Insect Physiol 57:682–687
Mezière D, Gary C (2009) Ecophyto R&D, vers des systemes de culture economones en produits phytosanitaires, Tome 3, volet 1. Institut National de la Recherche Agronomique, Paris
Minkenberg OPJM, Tatar M, Rosenheim JA (1992) Egg load as a major source of variability in insect foraging and oviposition behavior. Oikos 65:134–142
Moreau J, Benrey B, Thiery D (2006a) Assessing larval food quality for phytophagous insects: are facts as simple as it appears? Funct Ecol 20:592–600
Moreau J, Benrey B, Thiery D (2006b) Grape variety affects larval performance and also female reproductive performance of the European grapevine moth (Lobesia botrana). Bull Entomol Res 96:205–212
Moreau J, Richard A, Benrey B, Thiéry D (2009) The influence of plant cultivar of the grapevine moth Lobesia botrana on the life history traits of an egg parasitoid. Biol Control 50:117–122
Moreno F, Perez-Moreno I, Marco V (2009) Effect of Lobesia botrana (Lepidoptera: Tortricidae) egg age, density, and UV treatment on parasitism end development of T. cacoeciae (Hymenoptera: Trichogrammatidae). Environ Entomol 38:1513–1520
Outreman Y, Le Ralec A, Plantegenest M, Chaubet B, Pierre JS (2001) Superparasitism limitation in an aphid parasitoid: cornicle secretion avoidance and host discrimination ability. J Insect Physiol 47:339–348
Ozder N, Kara G (2010) Comparative biology and life tables of Trichogramma cacoeciae, T. brassicae and T. evanescens (Hymenoptera: Trichogrammatidae) with Ephestia kuehniella and Cadra cautella (Lepidoptera: Pyralidae) as hosts at three constant temperatures. Biocontrol Sci Tech 20(3):245–255
Pak GA, Buis HCEM, Heck ICC, Hermans MLG (1986) Behavioural variations among strains of Trichogramma spp.: host-age selection. Entomol Exp Appl 40:247–258
Papaj DR (2000) Ovarian dynamics and host use. Annu Rev Entomol 45:423–448
Pintureau B (2009) La lutte biologique et les Trichogrammes. Application au contrôle de la pyrale du maïs. Ed. Le Manuscript, Paris
Pizzol J (2004) Etudes bioécologiques de Trichogramma cacoeciae Marchal, parasitoïde oophage de l’eudémis de la vigne, en vue de son utilisation en lutte biologique. Diplôme d’Ingénieur Diplômé par l’Etat, option Agriculture ENSAM, Montpellier
Pizzol J, Pintureau B (2008) Effect of photoperiod experienced by parents on diapause induction in Trichogramma cacoeciae. Entomol Exp Appl 127:72–77
Pizzol J, Voegelé J (1987) La diapause de Trichogramma maïdis Pintureau and Voegelé en relation avec certaines caractéristiques de son hôte de substitution Ephestia kuehniella Zell, vol 48. European Workshop Lyon, September 7–10. Les Colloques de l’INRA, pp 93–94
Pizzol J, Pintureau B, Khoualdia O, Desneux N (2010) Temperature-dependent differences in biological traits between two strains of Trichogramma cacoeciae (Hym., Trichogrammatidae). J Pest Sci 83:447–452
Richard JP (1979) Les trichogrammes: essais d’efficacité en lutte biologique contre les vers de la grappe Lobesia botrana Den. and Schiff. et Eupoecilia ambiguella Hb. (Lep., Tortricidae). Mémoire Ingénieur, INRA, Antibes
Roush RT, Mckenzie JA (1987) Ecological genetics of insecticide and acaricide resistance. Annu Rev Entomol 32:361–380
Schubert G, Stengel M (1992) Des hyménoptères parasites efficaces de tordeuses. Viti 2:44–45
Smith SM (1996) Biological control with Trichogramma: advances, successes, and potential of their use. Annu Rev Entomol 41:375–406
Tabone T, Bardon C, Desneux N, Wajnberg E (2010) Comparative assessment of parasitism of different Trichogramma spp. on Plutella xylostella L. on greenhouse cauliflower. J Pest Sci 83:251–256
Thiéry D (2011) Gaps in knowledge for modern integrated protection in viticulture: lessons from controlling grape berry moths. IOBC/WPRS Bull 67:305–311
Thiéry D, Moreau J (2005) Relative performance of European grapevine moth (Lobesia botrana) on grapes and other hosts. Oecologia 143:548–557
Thiéry D, Esmenjaud D, Kreiter S, Martinez M, Sforza R, Van Helden M, Yvon M (2008) Les insectes de la vigne: les tordeuses nuisibles à la vigne. In: Kreiter S (ed) Ravageurs de la vigne. Féret, Paris, pp 214–246
Vinson SB (1998) The general host selection behavior of parasitoid hymenoptera and a comparison of initial strategies utilized by larvaphagous and oophagous species. Biol Control 11:79–96
Voegelé J, Stengel M, Schubert G, Daumal J, Pizzol J (1975) Les Trichogrammes. V.a. Premiers résultats sur l’introduction en Alsace sous forme de lâchers saisonniers de l’écotype Moldave de Trichogramma evanescens Westw. contre la Pyrale du maïs Ostrinia nubilalis Hubn. Ann Zool Ecol Anim 7(4):535–551
Volkoff AN, Daumal J (1994) Ovarian cycle in immature and adults stage of Trichogramma cacoeciae and T. brassicae (Hym., Trichogrammatidae). Entomophaga 39:303–312
Weisenburger DD (1993) Human health-effects of agrichemicals use. Human Pathol 24:571–576
Xuéreb A, Thiéry D (2006) Does natural larval parasitism of Lobesia botrana vary between years, generation, density of the host and vine cultivar? Bull Entomol Res 96:105–110
Ya Reznik S, Vaghina NP (2007) Effect of experience on response of Trichogramma buesi Voeg. and T. principium Sug. et Sor. (Hymenoptera, Trichogrammatidae) females to different ages of host eggs. Entomol Rev 8:3–14
Acknowledgments
We thank Jean-Michel Rabasse for his comments on an earlier version of the manuscript, Arlette Marconi and Julien Pfister for their technical assistance, Caroline Fouchet, and Marie-Jeanne Arguel for their participation in this work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by M. Traugott.
Rights and permissions
About this article
Cite this article
Pizzol, J., Desneux, N., Wajnberg, E. et al. Parasitoid and host egg ages have independent impact on various biological traits in a Trichogramma species. J Pest Sci 85, 489–496 (2012). https://doi.org/10.1007/s10340-012-0434-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10340-012-0434-1