Abstract
Two lean NOx trap (LNT) catalysts, Pt/BaO/CeO2 + Al2O3 and Pt/BaO/CeO2 − Al2O3, were prepared and compared for low-temperature (< 250 °C) NOx storage and reduction performance. The influence of the form of ceria on low-temperature NOx storage and reduction performance of LNT catalysts was investigated with the focus on NOx storage capacity, NOx reduction efficiency during lean/rich cycling, product selectivity and thermal stability. Inductively coupled plasma-atomic emission spectrometry (ICP-AES), Brunner–Emmet–Teller (BET), H2-pulse chemisorption and X-ray diffraction (XRD) were conducted to characterize the physical properties of LNT catalysts. NOx storage capacity and NOx conversion efficiency were measured to evaluate NOx storage and reduction performance of LNT catalysts. Pt/BaO/CeO2 − Al2O3 catalyst exhibits higher NOx storage capacity than Pt/BaO/CeO2 + Al2O3 catalyst in the temperature range of 150–250 °C. Meanwhile, Pt/BaO/CeO2 − Al2O3 catalyst shows better NOx conversion efficiency and N2 selectivity. XRD results indicate that the thermal stability of CeO2 − Al2O3 complex oxide is superior to that of pure CeO2. H2-pulse chemisorption results show that Pt/BaO/CeO2 − Al2O3 catalyst has higher Pt dispersion than Pt/BaO/CeO2 + Al2O3 catalyst over fresh and aged samples. The improved physical properties of Pt/BaO/CeO2 − Al2O3 catalyst are attributed to enhance the NOx storage and reduction performance over Pt/BaO/CeO2 + Al2O3 catalyst.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
To meet the stringent China Stage-VI light-duty diesel vehicles’ NOx emission regulations, a promising approach has been explored by a combination of lean NOx trap (LNT) and selective catalytic reduction (SCR) catalysts [1,2,3], which can enhance NOx reduction while avoiding the need for urea injection to the SCR catalyst [4]. However, additional improvements are required in LNT system, especially for low-temperature NOx conversion [5, 6].
LNT catalysts operate under cyclic lean/rich conditions [7, 8]. During lean conditions, NOx is stored on the catalyst; while during rich conditions, stored NOx is released and reduced into N2 along with possible byproducts, such as NH3 and N2O [9,10,11]. A typical LNT catalyst contains basic components (alkali metal or alkaline earth metal compounds) for NOx storage, noble metals (Pt, Pd, Rh), and support oxides [7]. In addition, ceria-based materials have been shown to be beneficial for LNT catalysts at low temperatures [12,13,14]. CeO2 is a common rare earth metal oxide with special structure and properties [15,16,17], and many current commercial LNT catalysts have already incorporated ceria [5, 18].
In the present work, the goal was to study the effects of the form of ceria on low-temperature NOx storage and reduction performance through surface/bulk analysis and activity tests. The first catalyst of Pt/BaO/CeO2 + Al2O3 used ceria as a support material as well as alumina, and the second catalyst of Pt/BaO/CeO2 − Al2O3 used complex ceria–alumina as a new support material. The low-temperature NOx storage capacity (NSC), NOx reduction efficiency, NH3 production and N2O emissions were measured. Thermal stability of LNT catalysts was also investigated after aging for 20 h at 800 °C.
2 Experimental
2.1 Catalyst preparation
Catalysts were prepared by incipient wetness impregnation technique, using aqueous solutions of Ba(CH3COO)2 and Pt(NO3)2. In the preparation of Pt/BaO/CeO2 + Al2O3 (1 wt% Pt and 9 wt% BaO), CeO2 (specific surface area 152 m2·g−1) and γ-Al2O3 (150 m2·g−1) were mechanically mixed at the weight ratio of 3:7. The impregnation was carried out in a sequential manner: The well-mixed CeO2 + Al2O3 support was firstly impregnated with the Ba acetate solution followed by Pt nitrate solution. On the other hand, Pt/BaO/CeO2 − Al2O3 (1 wt% Pt and 9 wt% BaO) was prepared similarly, but using a CeO2 − Al2O3 complex oxide (30 wt% CeO2 and 70 wt% Al2O3, 154 m2·g−1) as the support. After each impregnation step, the catalyst was dried at 100 °C in air for overnight and then calcined in air at 500 °C for 5 h. The obtained fresh catalysts were denoted as F-1 and F-2, respectively. In order to compare their thermal stability, the catalysts were further calcined in air at 800 °C for 20 h. The aged catalysts were denoted as A-1 and A-2.
2.2 Catalyst characterization
The actual elemental compositions of catalysts were analyzed by inductively coupled plasma-atomic emission spectrometry (ICP-AES, Optima 2100 DV, PerkinElmer). The Brunner–Emmet–Teller (BET) specific surface areas were measured by N2 adsorption–desorption at − 196 °C on a Micromeritics ASAP 2000 analyzer. Prior to measurements, the samples were pre-treated at 150 °C under vacuum for 3 h to eliminate the adsorbed species. The Pt dispersions were determined by H2-pulse chemisorption at − 80 °C by a Micromeritics AutoChem II Analyzer. The crystalline phases of the catalysts were characterized by X-ray diffractometer (XRD, XRD-600, Shimadzu) with a Cu Kα radiation (λ = 0.154056 nm) at 36 kV with a graphite monochromator.
2.3 Activity measurements
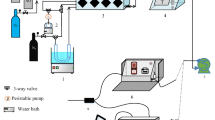
In NOx storage capacity (NSC) and NOx conversion efficiency measurements, 0.25 g Pt/BaO/Al2O3 catalyst was mixed with 0.75 g quartz sand. The total flow rate is 1 L·min−1, corresponding to a space velocity of 60,000 h−1. Before each experiment, the sample was oxidized in 10% O2/N2 balance at 350 °C for 30 min, and then reduced in 5% H2/N2 balance at 450 °C for 20 min. The reactor was then cooled in N2 to the target test temperatures at 150, 200 and 250 °C. The outlet gas of the reactor was maintained at 140 °C to avoid condensation and NH3 hold-up. A MKS MultiGas 2030 FT-IR analyzer was used to monitor NO, NO2, N2O, NH3, CO, C3H6, CO2 and H2O concentrations of the outlet gas.
NOx storage capacity (NSC) tests were conducted by exposing the catalyst to the flowing gas containing 250 × 10−6 NO, 8% O2, 5% H2O, 5% CO2 at 150, 200 and 250 °C. The storage time was 10 min at each temperature during NSC measurement. NOx conversion efficiency tests were also measured at 150, 200 and 250 °C, and LNT performance was evaluated in a lean/rich (100 s/17 s) cycle under the gas composition detailed in Table 1. The average value of the last 5 cycles was calculated after 15 lean/rich cycles.
3 Results and discussion
3.1 Catalyst characterization
The contents of Pt, Ba, Ce and Al in catalysts are summarized in Table 2. F-1 and F-2 samples have similar Pt and Ba contents. Ce content in F-1 is slightly lower than that in F-2 (26.24 wt% vs. 27.54 wt%).
BET specific surface areas and Pt dispersions of catalysts are listed in Table 3. The specific surface areas of F-1 and F-2 samples are 117 and 122 m2·g−1, respectively. Pt dispersion of F-1 is 60%, slightly lower than 62% of F-2. After thermal aging at 800 °C for 20 h, their surface areas decrease to 99 and 103 m2·g−1, respectively. The Pt dispersions reach significantly low values, only 12% for A-1 and 19% for A-2, indicating that the sintering of Pt particles occurs during aging due to the mobility of Pt crystallites and eventually merged to form larger particles [19].
Figure 1 shows XRD patterns of fresh and aged catalysts. The results show that Pt-related phases in all four samples were not detected because of either the low loading ratio or its small size. The main crystallite phases detected are CeO2, BaCO3 and γ-Al2O3 in all samples. The BaAl2O4 peak only appears after aging [20, 21]. Applying Scherrer equation, the average CeO2 crystal size was calculated based on CeO2 diffraction peak at 2θ = 28.5°. The CeO2 crystal size in F-1 is estimated to be 10 nm, while 6 nm in F-2, 16 nm in A-1, and 7 nm in A-2, respectively. These XRD results suggest that the thermal stability of CeO2 − Al2O3 complex oxide is superior to that of pure CeO2 + Al2O3 with regard to CeO2 crystal size.
3.2 NOx storage capacity (NSC)
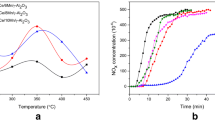
The results of NOx storage capacity tests are reported in Table 4. All samples’ NSCs increase substantially as the test temperature increases from 150 to 250 °C due to enhancing NO oxidation activity to produce NO2 which is known to be more effective than NO to be adsorbed on LNT catalysts [22]. F-2 sample displays higher NSC compared to F-1 sample at 150, 200 and 250 °C. NSCs of aged samples are lower than those of the fresh samples. A-2 sample still has higher NSC than A-1 sample. The NSC results indicate that CeO2 − Al2O3 complex oxide as the support loaded with Ba and Pt can trap NOx amount to more extent in comparison with CeO2 + Al2O3.
3.3 NOx conversion efficiency
NOx concentration profiles during the last 5 lean/rich cycles are shown in Fig. 2. Average NOx conversions during the last 5 cycles are presented in Table 5. For F-1 sample, NOx breakthrough is immediately observed after the feed gas switched into lean condition at 150 °C, and the outlet NOx concentration decreases with time and gradually approaches the inlet NOx concentration. When the feed gas is subsequently switched to the rich condition, a sharp and intense NOx release peak appears and then intensity quickly decreases. The similar profile was also observed in a previous study [23]. The average NOx conversion at 150 °C is 13.8%. At 200 °C, the outlet NOx concentration reaches only about 90 × 10−6 at the end of the lean duration. The amount of NOx release during switching to rich condition becomes significantly lower compared to that at 150 °C. Therefore, the average NOx conversion increases to 72.0%. At 250 °C, NOx concentration profile during lean duration is almost coincided with that at 200 °C. However, there is no NOx release detected during switching to rich condition. Average NOx conversion reaches 75.2%. Raising reaction temperature increases NOx storage capacity, and on the other hand, high temperature is beneficial to promoting NOx reduction ability. As a result, the average NOx conversion increases with reaction temperature increasing from 150 to 250 °C.
In the case of F-2 sample, NOx evolutions at three temperatures are similar to those of F-1 sample, but F-2 sample has slightly higher average NOx conversions, 14.6% at 150 °C, 73.4% at 200 °C, and 76.1% at 250 °C, in accordance with the results of NSC measurements.
After thermal aging, the amount of NOx trapped on the aged catalyst at lean phase at 150 °C becomes smaller in comparison with fresh catalysts and the outlet NOx concentrations gradually reach a constant level (around 240 × 10−6) for both aged catalysts. Higher NOx slip is observed during rich phase, and average NOx conversions of A-1 and A-2 samples are only 6.6% and 9.0%, respectively. At 200 °C, NOx-spill-out over A-1 is relatively larger in comparison with that over A-2 during switching to the rich condition, leading to a lower NOx conversion for A-1 (49.0% vs. 58.9% for A-2). NOx release still appears over aged samples at 250 °C, resulting in that NOx conversion decreases to some extent. Average NOx conversion sharply declines at 150 and 200 °C. Pt is known to have impacts on NO oxidation under lean conditions, NOx storage under rich/lean conditions and NOx reduction under rich conditions [7]. The difference in NOx conversion may be correlated with different Pt dispersions of catalysts. Clayton et al. [24] also found that the differences in storage and reduction activity were the largest among three catalysts which have different Pt dispersions at low temperatures (< 200 °C).
From the perspective of study on the law and production of NH3 and N2O, their concentrations evolution at 200 °C during lean/rich cycles is taken as an example and shown in Fig. 3. For fresh and aged samples, NH3 and N2O are both formed in lean and rich phases. During lean phase, NH3 and N2O are subsequently formed, NH3 intensity sharply decreases to below 10 × 10−6 at the initial lean phase, while a small N2O peak appears, and then the N2O intensity gradually becomes stabilized. A sharp N2O peak is immediately observed at the beginning of rich phase, and NH3 peak delays about 2 s. In comparison with the results obtained over fresh samples, the amount of NH3 and N2O formation decreases over aged samples. In the lean and rich phase, NH3 is mainly formed via the reduction in NOx by surface hydrogen, although NH3 is also a product of isocyanate hydrolysis reaction [25, 26], Dasari et al. [25] summarized the following reaction mechanism:
N2O formation in lean phase is related to the reactions between surface-deposited reductants/intermediates (CO, HC, NH3, isocyanate) and gaseous NO/O2, and N2O release peak during rich phase may be attributed to that NOx partially reduced over platinum group metal (PGM) sites [26,27,28].
Concerning the product selectivity, N2O is the main product at 150 °C. At 200 °C, selectivity of NH3, N2O and N2 is similar for fresh samples, but NH3 and N2O become the main products for aged samples. By increasing the temperature to 250 °C, N2 selectivity of all samples shows a substantial enhancement. Moreover, N2O selectivity decreases as the temperature increases from 150 to 250 °C. N2 selectivity decreases in the following order: F-2 > F-1 > A-2 > A-1. Thermal aging results are in an increase in NH3 and N2O selectivity, except NH3 selectivity at 150 °C. Compared to fresh samples, the higher NH3 selectivity over aged LNT catalysts above 200 °C was also found by Chatterjee et al. [29] and Easterling et al. [30]. Chatterjee et al. [29] suggested that the lower noble metal and oxygen storage activities correspond to the shifted light-off of the ammonia oxidation reactions to higher temperatures, which supports the faster NH3 breakthrough.
4 Conclusion
The influence of the form of ceria on low-temperature NOx storage and reduction performance of LNT catalysts was investigated, using CeO2 − Al2O33 complex oxide or CeO2 + Al2O3 mixed oxide as the support for BaO and Pt. CeO2 − Al2O3 support exhibits the improvements on NOx storage capacity, NOx conversion efficiency (especially for aged samples at 200 °C) and N2 selectivity in comparison with CeO2 + Al2O3 support in the temperature range of 150–250 °C. After aging for 20 h at 800 °C, Pt/BaO/CeO2 − Al2O3 catalyst exhibits better thermal stability and chemical distribution than Pt/BaO/CeO2 + Al2O3 catalyst. Overall, CeO2 − Al2O3-based catalysts show superior NOx storage and regeneration performance over CeO2 + Al2O3-based catalyst at low temperatures.
Concerning NH3 and N2O selectivity of NOx reduction, N2O is the main product at 150 °C, and its selectivity decreases as the temperature increases from 150 to 250 °C. Thermal aging results are in an increase in the NH3 and N2O selectivity at 200 and 250 °C.
References
Wang J, Ji YY, He ZW, Crocker M, Dearth M, McCabe RW. A non-NH3 pathway for NOx conversion in coupled LNT-SCR systems. Appl Catal B Environ. 2012;111–112:562.
Liu Y, Harold MP, Luss D. Coupled NOx storage and reduction and selective catalytic reduction using dual-layer monolithic catalysts. Appl Catal B Environ. 2012;121–122:239.
Wang J, Ji YY, Jacobs G, Jones S, Kim DJ, Crocker M. Effect of aging on NOx reduction in coupled LNT–SCR systems. Appl Catal B Environ. 2014;148–149:51.
Johnson T, Joshi A. Review of vehicle engine efficiency and emissions. SAE Technical Paper 2017-01-0907. 2017, https://doi.org/10.4271/2017-01-0907.
Zheng Y, Liu Y, Harold MP, Luss D. LNT–SCR dual-layer catalysts optimized for lean NOx reduction by H2 and CO. Appl Catal B Environ. 2014;148–149:311.
Neely GD, Sarlashkar JV, Mehta D. Diesel cold-start emission control research for 2015-2025 LEV III emissions. SAE Int J Engines. 2013;6(2):1009–20.
Roy S, Baiker A. NOx storage–reduction catalysis: from mechanism and materials properties to storage–reduction performance. Chem Rev. 2009;109(9):4054.
Epling WS, Campbell LE, Yezerets A, Currier NW, Parks JE. Overview of the fundamental reactions and degradation mechanisms of NOx storage/reduction catalysts. Catal Rev. 2004;46(2):163.
Lindholm A, Currier NW, Dawody J, Hidayat A, Li JH, Yezerets A, Olsson L. The influence of the preparation procedure on the storage and regeneration behavior of Pt and Ba based NOx storage and reduction catalysts. Appl Catal B Environ. 2009;88(1):240.
Mráček D, Kočí P, Marek M, Choi J-S, Pihl JA, Partridge WP. Dynamics of N2 and N2O peaks during and after the regeneration of lean NOx trap. Appl Catal B Environ. 2015;166–167:509.
Masdrag L, Courtois X, Can F, Duprez D. Effect of reducing agent (C3H6, CO, H2) on the NOx conversion and selectivity during representative lean/rich cycles over monometallic platinum-based NSR catalysts. Influence of the support formulation. Appl Catal B Environ. 2014;146:12.
Ocampo F, Ohtake N, Southward BW. Recent developments for CeBa hybrid materials with enhanced properties for BS-6 NOx storage applications. SAE Technical Paper 2017-26-123. 2017, https://doi.org/10.4271/2017-26-0123.
Ji Y, Toops TJ, Crocker M. Effect of ceria on the storage and regeneration behavior of a model lean NOx trap catalyst. Catal Lett. 2007;119(3):257.
Ocampo F, Harle V, Ohtake N, Rohe R, Southward BW. Innovative hybrid rare earth and barium materials with enhanced properties for NOx storage applications. SAE Int J Engines. 2015;8(3):1136.
He L, Sun DF, Wang TY, Xu YH, Li RX. Synthesis of nano-CeO2 hollow spheres with high adsorption activity via template-free solvothermal method. Chin J Rare Met. 2016;40(5):429.
Jin H, Wang YY, Wang YT, Yang HB. Synthesis and properties of electrodeposited Ni–CeO2 nano-composite coatings. Rare Met. 2018;37(2):148.
Yang Q, Hu H, Wang SS. Preparation and desulfurization activity of nano-CeO2/γ-Al2O3 catalysts. Rare Met. 2018;37(7):554.
DiGiulio CD, Pihl JA, Choi J-S, Parks JE, Lance MJ, Toops TJ, Amiridis MD. NH3 formation over a lean NOx trap (LNT) system: effects of lean/rich cycle timing and temperature. Appl Catal B Environ. 2014;147:698.
Auvray X, Pingel T, Olsson E, Olsson L. The effect gas composition during thermal aging on the dispersion and NO oxidation activity over Pt/Al2O3 catalysts. Appl Catal B Environ. 2013;129:517.
Yang M, Li YP, Wang J, Shen MQ. Effects of CO2 and steam on Ba/Ce-based NOx storage reduction catalysts during lean aging. J Catal. 2010;271(2):228.
Le Phuc N, Courtois X, Can F, Royer S, Marecot P, Duprez D. NOx removal efficiency and ammonia selectivity during the NOx storage-reduction process over Pt/BaO(Fe, Mn, Ce)/Al2O3 model catalysts. Part I: influence of Fe and Mn addition. Appl Catal B Environ. 2011;102(3):353.
AL-Harbi M, Epling WS. Investigating the effect of NO versus NO2 on the performance of a model NOx storage/reduction catalyst. Catal Lett. 2009;130(1):121.
Wang XY, Yu YB, He H. Effects of temperature and reductant type on the process of NOx storage reduction over Pt/Ba/CeO2 catalysts. Appl Catal B Environ. 2011;104(1–2):151.
Clayton RD, Harold MP, Balakotaiah V, Wan CZ. Pt dispersion effects during NOx storage and reduction on Pt/BaO/Al2O3 catalysts. Appl Catal B Environ. 2009;90(3):662.
Dasari PR, Muncrief R, Harold M. Elucidating NH3 formation during NOx reduction by CO on Pt–BaO/Al2O3 in excess water. Catal Today. 2012;184(1):43.
Wang J, Wang XT, Zhu JX, Wang JQ, Shen MQ. Elucidating N2O formation during the cyclic NOx storage and reduction process using CO as a reductant. Environ Sci Technol. 2015;49(13):7965.
Zhu JX, Wang J, Wang JQ, Lv LF, Wang XT, Shen MQ. New Insights into the N2O formation mechanism over Pt-BaO/Al2O3 model catalysts using H2 as a reductant. Environ Sci Technol. 2015;49(1):504.
Bártová Š, Kočí P, Mráček D, Marek M, Pihl JA, Choi J-S, Toops TJ, Partridge WP. New insights on N2O formation pathways during lean/rich cycling of a commercial lean NOx trap catalyst. Catal Today. 2014;231:145.
Chatterjee D, Kočí P, Schmeißer V, Marek M, Weibel M, Krutzsch B. Modelling of a combined NOx storage and NH3-SCR catalytic system for diesel exhaust gas aftertreatment. Catal Today. 2010;151(3):395.
Easterling V, Ji YY, Crocker M, Dearth M, McCabe RW. Application of spaciMS to the study of ammonia formation in lean NOx trap catalysts. Appl Catal B Environ. 2012;123–124:339.
Acknowledgements
This study was financially supported by the National Key R&D Program of China (No. 2017YFC0211100).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, T., Jia, LW., Wang, XT. et al. Enhancing low-temperature NOx storage and reduction performance of a Pt-based lean NOx trap catalyst. Rare Met. 38, 81–86 (2019). https://doi.org/10.1007/s12598-018-1184-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12598-018-1184-x