Abstract
This study evaluated the NOx adsorption and desorption performance as well as the casual relationship underlying a Mn-incorporated catalyst (Pt/Ba/Ce/xMn/γ-Al2O3). NOx adsorption and desorption are regarded as a prominent index for the NOx removal performance of NOx storage and reduction; we utilized NOx storage experiments with various inlet NO and O2 concentrations and cycling adsorption/desorption experiments with a couple of adsorption time protocols for performance evaluation. In-suit DRIFT and NOx-TPD tests were implemented to reveal the instant stored species and their thermal stability. Eight percent of Mn catalyst at 350 °C was adopted in the described experiments for its desirable NOx adsorption characteristics. The optimal NOx storage performance was found under 10% O2, deteriorating when the concentration was further increased. Furthermore, elevating NO concentration impaired the NOx adsorption due to the low NO2/NOx ratio. It was also found that shorter adsorption time facilitated NOx removal via maintaining an unsaturated state for active storage components in terms of a fixed desorption time. The stored species existed as nitrites and nitrates with a good low-temperature thermal stability which however decayed at higher temperatures as exhibited in the DRIFT and NOx-TPD tests. These findings provided invaluable information for the application of Mn-incorporated catalyst for NOx removal in diesel exhaust purification to relieve the aerial pollution.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The global quest for safer environment as well as continuous agitation for the reduction of global warming has initiated more stringent emission regulatory demands especially towards the reduction of NOx which are considered as heavy air pollutants (Ting et al. 2018). It is therefore of great necessity to develop and apply novel and more advanced post-treatment technologies for the removal of NOx emissions especially for diesel engines which are known for higher fuel efficiency and lower greenhouse gas emission, but NOx reduction is more difficult to achieve due to excessive supply of oxygen during combustion (Sim et al. 2014).
In order to solve this drawback, the NOx storage and reduction (NSR) technology has been employed as one of the most promising and recognized methods (Constantinou et al. 2013; Ji et al. 2015). It has an excellent NOx removal performance under lean fuel condition and at low temperature (Constantinou et al. 2013; Ji et al. 2015). The NSR functions through the storage of NOx as nitrites and nitrates during regular lean conditions and is further reduced to N2 in the short rich periods (Du et al. 2018; Shakya et al. 2012). The NSR catalyst consists of a noble metal component, a storage component, and a support (Roy and Baiker 2008), among which Pt/Ba/γ-Al2O3 is currently known for its widespread use (Xu et al. 2016). Although γ-Al2O3 has been extensively utilized as a support component of the NSR catalyst, a number of disadvantages have accompanied its usage including sintering, phase changes at high temperatures, and inferior anti-sulfur capacity (Huang et al. 2013). Noble metal is a very fundamental component of the NSR due to its irreplaceable role in the NOx storage and reduction, despite its low level in the NSR catalyst (Gonzalez-Marcos et al. 2013).
Previous studies (Sim et al. 2014; Ryou et al. 2018) have shown that platinum (Pt) is easily deactivated due to its sintering effect at high temperatures albeit being the most effective noble metal when compared with palladium (Pd) and rhodium (Rh) with a relatively lower cost and better storage characteristics. Barium (Ba) is one of the alkaline-earth metals widely employed as storage component and it is widely utilized due to its superior storage capacity and NOx desorption in contrast to Sr, Ca, and K; however, due to its poor anti-sulfur ability, barium oxide (BaO) reacts with sulfur dioxide (SO2) and Pt within the lean and rich fuel conditions, generating BaSO4 and PtS, respectively, which is detrimental to catalytic activity (Nguyena et al. 2018; Yang et al. 2014; Corbos et al. 2008). Therefore, improvements on these drawbacks are urgently needed in order to perfect catalytic properties and efficiency.
The reactivity of the NSR catalyst is greatly influenced by the interaction between the three components of the NSR catalyst (noble metal components, storage components, and support). MnOx, CeOx, and CoOx acts on elevating the NOx storage capacity and anti-sulfur ability (Bai et al. 2017; Wang et al. 2017), while CeO2 enhances the removal of NOx by facilitating hydrocarbon reforming and water-gas shift (WGS) reactions (Wang et al. 2011). Some studies (Ji et al. 2018; Xiao et al. 2008; Tang et al. 2006) have demonstrated that Mn significantly improved the oxidation of NO to NO2, thus facilitating the storage and removal of NOx. Furthermore, it has been reported that Pd/Mn/Ba/Al catalytic system displayed a stronger NO oxidation and NOx removal capability than Pd/Ba/Al, suggesting the function of Mn in the reduction of NOx in NSR catalyst (Zhang et al. 2015). Notably, the inhibitory effect of H2O and CO2 on NOx adsorption and desorption can be alleviated by Mn as a result of the decomposition of MnCO3 at low temperature (Guo et al. 2009). Therefore, the application of a Pt/Ce/Ba/γ-Al2O3 catalyst which incorporates Mn would be a suitable and promising catalytic model for the removal of NOx.
To the best of our knowledge, the conversion of NO to NO2 is beneficial to NOx storage as NO2 is readily stored. Thus, NO conversion is significantly influenced by the dosages of NO and O2 as well as the adsorption time. An increase of NO and O2 has been suggested to facilitate the generation of more stable nitrates based on the fact that NO adsorption would proceed as a result of the weak bond between surface oxygen species and Mn (Liang et al., 2017; Guo et al. 2017). Besides, NOx storage/reduction performance has been reported to be closely related to temperature, since NO oxidation is retarded at low temperature and stored species would decompose at relatively high temperature (Andonova et al. 2017; Liu et al. 2017).
Despite the advantages of Mn as a component of NSR catalysts, the effect of internal/external factors on the NOx removal performance as well as the NOx adsorption and desorption mechanism under various conditions has not been fully understood and explored and thus requires further investigation. In this study, the NOx adsorption and desorption characteristics were investigated as a function of various NO and O2 concentrations, temperature, and storage time in order to investigate the NOx adsorption/desorption performance over a Mn-incorporated NSR catalyst (Pt/15Ba/15Ce/xMn/γ-Al2O3). These findings combined with in-suit DRIFT and NOx-TPD results carry a thorough and profound implication for the NOx adsorption and desorption performance over Mn-incorporated NSR catalysts and potential application in advanced post-treatment technology.
Experimental
Catalyst preparation
The NSR catalyst Pt/15Ba/15Ce/xMn/γ-Al2O3 was prepared as follows.
Ba, Ce, and Mn nanoparticles were deposited onto γ-Al2O3 by the sol-gel method. Briefly, a so-gel solution was prepared by dissolving stoichiometric ratio of barium acetate, cerium acetate, and manganese acetate aqueous solution into γ-Al2O3 turbid liquid, followed by the addition of citric acid and polyethylene glycol at 80 °C under continuous stirring. The reaction solution was dried at 110 °C for 24 h and calcined at 500 °C for 5 h in the muffle furnace. Pt was incorporated using a wetness impregnation method. The Ba/Ce/Mn/γ-Al2O3 sample was impregnated with an aqueous solution of chloroplatinic acid, dried at 110 °C for 24 h, and calcined at 500 °C for 5 h. The different Mn loadings (6, 8, and 10 wt.%) were loaded using the same method.
In-suit DRIFT and NOx-TPD tests
The in-suit DRIFT test was performed on a Nicolet 6700 (Thermo Fisher Scientific) at a wavelength range of 400–4000 cm−1 with 32 scans. Before the test, pretreatment was conducted by flushing the sample with nitrogen at 450 °C for 45 min. The spectrum was recorded during NO adsorption (500 ppm NO, 10% O2 and N2) consecutively for 30 min. The thermal stability of the adsorbed NOx species was measured using NOx temperature-programmed desorption (TPD) carried out on a two-zone furnace. The NOx adsorption was conducted at temperature from 100 to 450 °C and kept at 450 °C in 500 ppm NO, 10% O2, and balance N2 for some period of time. After cooling down the temperature of the sample to 100 °C, the catalysts was heated from 100 to 600 °C in a linear temperature ramp of 10 °C/min within N2.
Catalyst performance evaluation through a reactor system
The catalyst performance as the function of different parameters was carried out through a continuous flow quartz reactor as previously described in the literature (Ji et al. 2017). The catalyst powder was sustained with quartz wool in a reactor tube with an inner diameter of 7 mm. Four gas cylinders and a mass flow controller enabled the simulation of the composition and concentrations of various gas components for the supplied gas model. An electric furnace was used to control the reaction temperature. The NOx concentration was determined by a specific Fourier transform infrared spectroscope (FTIR) with the help of a combination of analysis software. All flow conditions were operated at a gas hourly space velocity (GHSV) of 52,000 h−1 and a total flow rate of 30 mL/min. The catalyst was pretreated at 450 °C for 1 h in a feed-stream composed of 1% H2 in N2 so as to ascertain the purity of the catalyst.
The lean feed consisting of 500 ppm NO, 10% O2, and N2 as balance gas was introduced into the reactor and adsorbed until saturated in NOx storage experiments. Alteration of the temperature and reaction atmosphere was allowed in order to evaluate its impacts on various parameters. The significance of NO and O2 during the NOx adsorption and desorption was investigated by NOx storage experiments at 350 °C with various inlet O2 (0, 5, 10, and 15%) and NO (500, 700, and 1000 ppm) concentrations. A fixed NO concentration of 500 ppm was employed for the evaluation of O2 effect, while 10% was used for NO effect. The cycling storage/reduction experiment was performed using different time protocols of 60 s/60 s, 120 s/60 s, and 180 s/60 s (lean/rich or adsorption/desorption) employing a rich feed composed of 1% H2 in N2 which was admitted after a time-restricted adsorption.
The NOx storage capacity and NOx storage efficiency are computed using the following formulae which act as an index of NOx adsorption/desorption performance:
where \( {\mathrm{F}}_{{\mathrm{NO}}_{\mathrm{x}}}^{\mathrm{inlet}} \) and \( {\mathrm{F}}_{{\mathrm{NO}}_{\mathrm{x}}}^{\mathrm{outlet}} \) represent the NOx concentration at the inlet (500 ppm) and outlet; tL denotes the storage time (s); m is the catalyst mass (g); and V is the molar volume of gas taken as 22.4 mol/L.
The average NO2/NOx ratio is defined with the formula below:
where \( {\mathrm{F}}_{{\mathrm{NO}}_{\mathrm{x}}}^{\mathrm{outlet}} \) and \( {\mathrm{F}}_{{\mathrm{NO}}_2}^{\mathrm{outlet}} \) represent the NOx and NO2 concentrations at the outlet; and tL denotes the storage time (s).
Results and discussion
Performance evaluation in temperature experiment
The effect of temperature was evaluated via NOx storage experiment. Figure 1a illustrates a lucid comparison of the storage efficiency for the Mn-incorporated catalyst with 6%, 8%, and 10% Mn loading. As clearly seen, 8% Mn-incorporated catalyst displayed a higher storage efficiency at 350 °C as compared with 6% and 10% Mn loadings. Therefore, the catalyst with 8% Mn loading was tested on its adsorption performance under various temperatures so as to obtain a deeper understanding of the mechanism involved in NOx storage and reduction.
As shown in Fig. 1b, the outlet NOx yield was almost not detected during the first few minutes indicating a desirable adsorption. Specifically, the breakthrough time and saturation time were defined as the time required for NOx to be detected and get saturated within the effluent, respectively; longer duration is representative of better NOx storage performance. It was observed that the breakthrough time increased with increase in temperature from 250 to 350 °C, while a downward trend was observed as the temperature was further increased to 450 °C. The saturation time was also observed to follow the same trend as the temperature was increased as shown in Table 1.
The NOx storage efficiency of 8% Mn catalyst described in Fig. 1a was also validated based on the results obtained from the foregoing breakthrough time and saturation time; the 8% Mn catalyst displayed the best adsorption characteristic at 350 °C. This may be probably caused by the inhibition of NO oxidation at low temperature like 250 °C and the thermal stability decay of stored species (such as nitrites and nitrates) surpassing the enhancement of catalytic reactivity at high temperature like 450 °C. As a result, 8% Mn catalyst was tested upon its adsorption and desorption performance at 350 °C in the experiments illustrated below.
Performance evaluation under various O2 concentrations
The effect of the concentration of O2 investigated in NOx storage experiments as well as its impacts on NOx adsorption is shown in Fig. 2. The results with 0% O2 in Fig. 2a were twofold. NO2 was not observed throughout the whole storage process and outlet NOx yield surged during the first 10 min; the former suggested the absence of NO oxidation while the latter implied inferior NOx storage performance. Table 2 provides a systematic analysis on the comparison of NOx adsorption performance in the presence of varying O2 concentrations. The NOx storage curves (observed in Figs. 1b and 2b) became those typical ones as analyzed in the “Performance evaluation in temperature experiment” section once O2 was admitted and ascribed to NO2 oxidization by NO in an atmosphere containing O2. As clearly observed, NO2/NOx ratio was low at 5% O2 but higher with increased O2 concentration. At 10% O2 concentration, an optimal adsorption capacity of 690 μmol/gcat was achieved as compared with 15% O2 where the adsorption capacity was only 450 μmol/gcat (Table 2). Figure 2c illustrates the storage efficiency of NOx as a function of time; NOx storage efficiency reduced with time and enhanced with increasing O2 concentration until 10% O2 was achieved. The storage efficiency under 10% O2 was sustained at a relatively high level (above 95%) over the first 20 min but decreased as the concentration of O2 was increased to 15%. Furthermore, the results indicated that O2 is essential for NOx storage for its function to increase the NO2/NOx ratio within a certain range facilitating NOx storage, since NO2 acts as the main force in the NOx adsorption as nitrites and nitrates, whereas the poor storage capacity at 15% O2 might majorly be caused by superfluous NO2, which could not be adsorbed by limited active sites in spite of the increase in average NO2/NOx ratio from 48 to 50% (Table 2). The adsorption mechanism could be explained with the help of the following reactions:
Similar experiments were carried out with 8% O2 (Zhang et al. 2015); a NOx storage capacity of 255.4 μmol/gcat was also found over 5Mn/10Ba/Al2O3 catalyst according to (Zhang et al. 2017), which is less than half of 690 μmol/gcat over Pt/15Ba/15Ce/10Mn/Al2O3 catalyst, as shown in Table 2. It indicated that the components Pt and Ce remarkably promoted the NOx storage capacity over NSR catalyst.
Performance evaluation under various NO concentrations
Figure 3 compares the NOx storage performance over Pt/Ba/Ce/8Mn/γ-Al2O3 at 10% O2 and at various NO concentrations. It was observed that the outlet NOx yield and the growth rate were both enhanced when inlet NO proportion was increased from 500 to 1000 ppm. This finding suggested an inhibition of the NOx storage process as supported by a decline in the breakthrough time from 8 to 2.5 min, saturation time from 64 to 36 min (Fig. 3), NOx storage capacity from 690 to 418 μmol/gcat, and NOx storage efficiency from 64 to 31% (Table 3). These may be as a result of the decrease in NO2/NOx ratio from 48 to 36% in average with NO concentration from 500 to 1000 ppm, as visibly illustrated in Table 3. There are two possible reasons for the decreased NO2/NOx ratio; the oxidizing capability of the active storage components, such as Mn and Ce, may not be able to sustain the excessive amount of NO, resulting in the exhaustion of NO at the effluents. In spite of the increase in NO2 oxidation from NO, the NO2 storage as nitrates was limited by a limited amount of active sites on the catalyst surface as stated in the “Performance evaluation under various O2 concentrations” section. Therefore, an increase in NO concentration would impair NOx storage and NOx removal by downregulating the NO2 proportion in NOx. As shown in Fig. 3d, for the NOx storage efficiency at 500 ppm, NO remained high after 40 min at about 80% and was much higher and steadier when compared with the NOx storage efficiency at 750 ppm and 1000 ppm. This results suggest that 500 ppm NO would produce an excellent NOx storage performance.
Effect of adsorption time on NOx adsorption/desorption
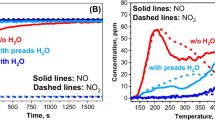
In order to investigate the effect imposed by adsorption time on catalytic performance, 10% O2 and 500 ppm NO were utilized in the cycling storage/reduction experiments with different adsorption time protocols as displayed in Fig. 4. A peak yield of NOx occurred when H2 was introduced into the reactor, which was attributed to the decomposition of stored species and their further aggravation as a result of the heat generated during the reduction. It was observed that the peak yield of NOx kept increasing until a steady state was achieved at the start of the cycles and the stable peak also increased when the adsorption time increased from 60 to 180 s, indicating excess NO spilling-off due to limited active storage sites (Table 4). The steady value of NOx concentration was also found to increase when the adsorption time was increased, which implied an unsaturated storage under an adsorption time of 60 s, 120 s, and probably 180 s. Notably at the beginning of storage, NO2 split off prior to NO, suggesting an excellent oxidation capability of Mn to convert NO into NO2.
Table 4 elaborated the NOx storage and removal performance by providing a quantitative analysis. As observed, the NOx storage and removal efficiency both exceeded 70%, implying favorable catalyst characteristics of Pt/15Ba/15Ce/8Mn/γ-Al2O3. However, the NOx removal performance decreased as the adsorption time increased. An optimal NOx desorption efficiency occurred at an adsorption time of 60 s; however, the NOx desorption efficiency was decreased from 82 to 75% when the adsorption time was increased from 60 to 180 s. This may possibly be due to the alleviation of adsorption burden as a result of low concentration/proportion of NO2 in the limited active sites which could be kept in a smooth working state without saturation under a shorter adsorption time. Interestingly, the desorption peak was increased from 153 to 226 ppm, which was probably due to the presence of a major part of NO in the desorption peak during the NOx storage and reduction. These results were quite the opposite under longer duration of adsorption time. A large amount of NOx was discharged directly without storage, resulting in higher NOx emission and lower NOx removal efficiency. Therefore, it is reasonable to suggest that a relatively shorter adsorption time (under a specific desorption time) is essential for the enhancement of NOx removal performance.
In-suit DRIFT and NOx-TPD results
An in-suit DRIFT test was performed at 250 °C to provide an insight into the intermediate species formed during the NOx adsorption. A spectra of absorbance against time was obtained as shown in Fig. 5. The spectra exhibited various absorption bands at 1122, 1250, 1332, 1530, 1624, and 1762 cm−1, and NO2− adsorption peaks were observed at 1380–1320 cm−1, 1250–1230 cm−1, and 840–800 cm−1, while NO3− was observed at 1380–1350 cm−1 and 840–815 cm−1 according to previous literature (Zheng et al. 2017; Say et al. 2016). Accordingly, the peaks in Fig. 5 correspond to a variety of nitrogen oxide species, including bidentate nitrate (1122 cm−1), nitrate adsorbed on Ba (1332 cm−1), and bridging nitro-nitrito (1624 cm−1). Notably, an adsorption peak of N2O4 was also observed at 1762 cm−1. From the transformation of stored species, it was observed that NOx was initially stored as nitrites (1122 cm−1) and then together with nitrate peaks as the intensity of nitrite peaks increased, thus suggesting that the stored species were mostly in the form of nitrites and partly as nitrates. Interestingly, the occurrence of nitrates at 250 °C conflicted with the findings in literatures (Castoldia et al. 2018). It was assumed that the incorporation of Mn and Ce into the NSR catalyst facilitated the oxidization of NOx species to stable nitrates at low temperature, promoting the NOx adsorption.
A desorption curve as a function of temperature in Fig. 6 was obtained via the NOx-TPD technology as a means of elucidating the thermal stability of the catalyst. It is clearly observed that the major NOx species desorped were NO and NO2, and the desorption peaks were found around 580 ppm at 450 °C and 60 ppm at 380 °C, respectively. Interestingly, the temperature of NO exceeded that of NO2 by 70 °C at the desorption peak, suggesting a better thermal stability for nitrites; and NO2 desorption was rather lower than NO, at least partly attributed to NO being favored compared with NO2 during the thermal dynamic equilibrium at high temperature. It is noteworthy to say that the catalyst oxidizing ability could get enhanced at higher temperature promoting NO2 generation and the conversion of Mn to Mn3+ and Mn4+ at high temperature also contributed to the oxidation of NO to NO2, thus facilitating NOx storage as nitrites and nitrates. It is assumed that the adsorption of NO2 was weakened and eventually terminated after 380 °C due to the increase in oxygen consumption stored on storage components Ba and Ce. Due to the thermometric impact, NOx (NO and NO2) desorption began at around 300 °C and declined due to temperature elevation after the desorption peak, suggesting fine and weaken thermal stability at low temperature and higher temperature, respectively. Presumably, the NO desorption peak at 450 °C was induced by nitrite and nitrate decomposition and NO2 adsorption and desorption were inhibited by the absence of stored oxygen to oxidize NO at high temperature. It could be deduced that NOx desorption arose from the weak bond between stored species and the active sites at low temperature and decomposition of stored nitrites and nitrates at high temperature, which is in line with the literature (Tamm et al. 2014). Evidently, a favorable thermal stability was achieved at low temperature, deteriorating at higher temperature which would assist in explaining the results in Fig. 1b. The desorption mechanism at high temperature can be illustrated by the following equations:
Conclusions
This study provides a deeper insight into the NOx adsorption and desorption performance as well as the underlying mechanism. The temperature experiments indicated that Pt/15Ba/15Ce/8Mn/γ-Al2O3 presented a satisfactory NOx adsorption performance at 350 °C. Under various NO and O2 concentrations in the NOx storage experiments, the NOx adsorption performance was found to be optimal under 10% O2 in this study and was decreased when O2 concentration was further increased owing to the surplus NO2 and limited active sites. In addition, elevation of NO concentration impaired the NOx adsorption probably due to the low NO2/NOx ratio. The results obtained from the cycling NOx storage/reduction experiment showed priority to the shorter adsorption time for NOx removal as it could help maintain an unsaturated state over active storage components. Notably, the stored species existed mainly as nitrites and partly nitrates at 250 °C in the DRIFT measurements implying that Mn facilitated the oxidation of NOx species to stable nitrates at a low temperature. NOx-TPD experiments demonstrated that the stored species displayed excellent low-temperature thermal stability promoted probably by Mn, albeit the degradation arising from decomposition of stored nitrites and nitrates at high temperature.
References
Andonova S, Marchionni V, Lietti L et al (2017) Micro-calorimetric studies of NO2 adsorption on Pt/BaO supported on γ-Al2O3 NOx storage and reduction (NSR) catalysts impact of CO2. Mol Catal 436:43–52
Bai Z, Zhang Z, Chen B, Zhao Q, Crocker M, Shi C (2017) Non-thermal plasma enhanced NSR performance over Pt/M/Ba/Al2O3 (M=Mn, Co, Cu) catalysts. Chem Eng J 314:688–699
Castoldia L, Matarresea R, Morandi S (2018) New insights on the adsorption, thermal decomposition and reduction of NOx over Pt- and Ba-based catalysts. Appl Catal B Environ 224:249–263
Constantinou C, Li W, Qi G, Epling WS (2013) NOx storage and reduction over a perovskite-based lean NOx trap catalyst. Appl Catal B Environ 134-135:66–74
Corbos EC, Courtois X, Bion N, Marecot P, Duprez D (2008) Impact of the support oxide and Ba loading on the sulfur resistance and regeneration of Pt/Ba/support catalysts. Appl Catal B Environ 80(1–2):62–71
Du SC, Wang SB, Guo YB et al (2018) Rational design, synthesis and evaluation of ZnO nanorod array supported Pt: La0.8Sr0.2MnO3 lean NOx traps. Appl Catal B Environ 236:348–358
Gonzalez-Marcos MP, Pereda B, Torre UDL, Gonzalez-Velasco JR (2013) On the effect of reduction and ageing on the TWC activity of Pt/Ce0.68Zr0.32O2 under simulated automotive exhausts. Top Catal 56:352–357
Guo H, Li X, Wang Z (2009) Preparation of manganese oxide with high density by decomposition of MnCO3 and its application to synthesis of LiMn2O4. J Power Sources 189:95–100
Guo LH, Guo L, Zhao DY, Gao ZN, Tian Y, Ding T, Zhang J, Zheng LR, Li XG (2017) Oxidizing, trapping and releasing NOx over model manganese oxides in alternative lean-burn/fuel-rich atmospheres at low temperatures. Catal Today 297:27–35
Huang B, Bartholomew CH, Smith SJ, Woodfield BF (2013) Facile solvent-deficient synthesis of mesoporous γ-alumina with controlled pore structures. Microporous Mesoporous Mater 165:70–78
Ji Y, Bai S, Crocker M et al (2015) Al2O3-based passive NOx adsorbers for low temperature applications. Appl Catal B Environ 170-171:283–292
Ji Y, Xu D, Bai S et al (2017) Pt- and Pd-promoted CeO2–ZrO2 for passive NOx adsorber applications. Ind Eng Chem Res 56:111–125
Ji Y, Xu D, Crocker M, Theis JR, Lambert C, Bueno-Lopez A, Harris D, Scapens D (2018) Mn-based mixed oxides for low temperature NOx adsorber applications. Appl Catal A Gen 567:90–101
Liang YL, Huang YF, Zhang HL et al (2017) Interactional effect of cerium and manganese on NO catalytic oxidation. Environ Sci Pollut Res 24:9314–9324
Liu Y, Guo L, Zhao D, Li X, Gao Z, Ding T, Tian Y, Jiang Z (2017) Enhanced activity of CuO/K2CO3/MgAl2O4 catalyst for lean NOx storage and reduction at high temperatures. RSC Adv 7:27405–27414
Nguyena HP, Valle SPD, Marie O et al (2018) NOx adsorption on K and Ba loaded on zirconia-titania NSR catalysts: a comparative study by in situ and operando IR spectroscopy. Appl Catal B Environ 231:391–399
Roy S, Baiker A (2008) NOx storage-reduction catalysis: from mechanism and materials properties to storage-reduction performance. Chem Rev 109:4054–4091
Ryou YS, Lee J, Lee H (2018) Low temperature NO adsorption over hydrothermally aged Pd/CeO2 for cold start application. Catal Today 307:93–101
Say Z, Tohumeken M, Ozensoy E (2016) Spectroscopic investigation of sulfur-resistant Pt/K2O/ZrO2/TiO2/Al2O3 NSR/LNT catalysts. Catal Today 267:167–176
Shakya BM, Harold MP, Balakotaiah V (2012) Crystallite-scale model for NOx storage and reduction on Pt/BaO/Al2O3: Pt dispersion effects on NOx conversion and ammonia selectivity. Catal Today 184:27–42
Sim JS, Gong MJ, Chang KS (2014) Preliminary performance studies of Al–Co–Mn mixed oxide and Ag-doping for the purpose of PGM-free DOC. Appl Catal A Gen 480:120–127
Tamm S, Andonova S, Olsson L (2014) The effect of hydrogen on the storage of NOx over silver, platinum and barium containing NSR catalysts. Catal Lett 144:1101–1112
Tang XF, Li YG, Huang XM et al (2006) MnOx–CeO2 mixed oxide catalysts for complete oxidation of formaldehyde: effect of preparation method and calcination temperature. Appl Catal B Environ 64:265–273
Ting AWL, Harold MP, Balakotaiah V (2018) Elucidating the mechanism of fast cycling NOx storage and reduction using C3H6 and H2 as reductants. Chem Eng Sci 189:413–421
Wang X, Yu Y, He H (2011) Effects of temperature and reductant type on the process of NOx storage reduction over Pt/Ba/CeO2 catalysts. Appl Catal B Environ 104(1–2):151–160
Wang P, Yi J, Gu W, Luo P, Lei LL (2017) The influence of xMnyCe/γ-Al2O3 on NOx catalysts on the properties of NOx storage and reduction over Pt-Ce-Ba/γ-Al2O3 catalysts. Chem Eng J 325:700–707
Xiao J, Li X, Deng S, Wang F, Wang L (2008) NOx storage-reduction over combined catalyst Mn/Ba/Al2O3–Pt/Ba/Al2O3. Catal Commun 9:563–567
Xu J, Ibrahim AR, Hu X, Hong Y, Su Y, Wang H, Li J (2016) Preparation of large pore volume γ-alumina and its performance as catalyst support in phenol hydroxylation. Microporous Mesoporous Mater 231:1–8
Yang L, Lin S, Yang X, Fang W, Zhou R (2014) Promoting effect of alkaline earth metal doping on catalytic activity of HC and NOx conversion over Pd-only three-way catalyst. J Hazard Mater 279:226–235
Zhang ZS, Chen BB, Wang XK, Xu L, Au C, Shi C, Crocker M (2015) NOx storage and reduction properties of model manganese-based lean NOx trap catalysts. Appl Catal B Environ 165:232–244
Zhang Z, Crocker M, Chen B et al (2017) Pt-free, non-thermal plasma-assisted NOx storage and reduction over M/Ba/Al2O3 (M = Mn, Fe, Co, Ni, Cu) catalysts. Catal Today 256:115–123
Zheng Y, Kovarik L, Engelhard MH, Wang Y, Wang Y, Gao F, Szanyi J (2017) Low-temperature Pd/zeolite passive NOx adsorbers: structure, performance and adsorption chemistry. J Phys Chem C 121:15793–15803
Acknowledgments
The authors acknowledge the contribution of Professor Guanjun Qiao for the technical supports.
Funding
This project was funded by the National Natural Science Foundation of China (No. 51676090), Natural Science Foundation of Jiangsu Province (No. BK20150513), and the Six Talent Peaks Project in Jiangsu Province.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Responsible editor: Philippe Garrigues
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wang, P., Yu, D., Wu, G. et al. NOx adsorption and desorption of a Mn-incorporated NSR catalyst Pt/Ba/Ce/xMn/γ-Al2O3. Environ Sci Pollut Res 26, 27888–27896 (2019). https://doi.org/10.1007/s11356-019-05847-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-05847-y