Abstract
Portunus trituberculatus broodstock were stocked in plastic tanks to evaluate the effects of starvation and feeding on gonadal development, blood chemistry, fatty acid composition, and expression of vitellogenin (Vtg) and fatty acid-binding protein genes (FABP) in females. Two treatments (starved and fed) were randomly assigned to triplicate groups of 90 swimming crab broodstock (approximately 230 ± 45 g). In the starved treatment, crabs were starved for 30 days, whereas in the fed treatment crabs were fed once a day with clams. The gonadosomatic index decreased significantly in starved crabs (P < 0.05), as did the serum glucose and cholesterol concentrations; conversely, the total protein concentration in serum significantly increased (P < 0.05). In the ovary, there was a significant relative decline of 18:0, 16:1n-7 and 20:1n-9 fatty acids and relative increases of 20:4n-6, 22:6n-3, 18:1n-9 and 20:5n-3 in starved crabs compared to fed crabs (P < 0.05). Relative expression of Vtg in the ovary decreased significantly in starved crabs (P < 0.05), while there was no significant difference in hepatopancreas Vtg expression between starved and fed crabs (P > 0.05). Starvation suppressed gonadal development in female swimming crab broodstock.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The swimming crab Portunus trituberculatus is an important commercial mariculture species, which is widely distributed in the coastal waters of Japan, Korea and China [1–4]. The global production of this species was almost 429,959 t in 2012 [5]. In China, the swimming crab has become one of the most well-known aquaculture crustacean species for its high nutritional value and fast growth rate [3], and production exceeded 99,500 t in 2013 [6]. The swimming crab inhabits sandy or pebble-covered benthic habitats, but in its natural environment rarely experiences optimal conditions. It must cope with natural stressors for most of its life cycle, including starvation, extreme temperatures, oxygen depletion and pathogens [7, 8]. Recently, some studies have been undertaken on the natural stressors of the swimming crab, including salinity and pH [4], contaminants [9], temperature [10–12], and ammonia [13–15]. However, there are no studies on starvation stress in P. trituberculatus broodstock.
Farmed and wild aquatic animals usually face starvation stress caused by food deprivation due to many factors such as habitat destruction, seasonal change and the temporal and spatial fluctuations of their food resources [16–18]. Molting, reproduction, migration and changes in temperature can cause reduced food intake for many animals [19]. Aquatic animals employ various behavioral, physiological and biochemical responses to decrease maintenance metabolism under starvation conditions, and these responses are integrated at all levels of organization [17]. Crustaceans, like most poikilotherms, are able to withstand relatively long periods of food shortage [20–24]. However, the response to starvation stress differs from species to species. Blood-borne nutrients and metabolites have been proven to be direct and effective indicators for the estimation of the nutritional condition of animals under various feeding conditions [20, 25]. These nutrients or metabolites include serum total protein (TP), cholesterol, triglycerides, high-density lipoprotein (HDL), low-density lipoprotein (LDL) and glucose (GLC) [25, 26]. All of these indicators may be important tools for revealing the connection between the nutritional conditions and feeding activities of female swimming crabs. However, there is little information clearly showing how blood chemistry variables respond to starvation in crustacean species.
Metabolism in crustaceans is primarily centered on glycogen and lipids/fatty acids. The nature of fatty acid utilization for energy during starvation may differ among species [23]. For example, in the African catfish Clarias gariepinus, starvation resulted in an initial utilization of 14:0, 16: 1n-9 and 18: 1n-9 fatty acids and a relative conservation of 22:6n-3 and 20:5n-3 [27]. Similarly, De Silva et al. [28] reported that the relative amount of polyunsaturated fatty acids (PUFA), in particular docosahexaenoic acid (DHA), increased considerably and varied significantly with starvation in the hybrid red tilapia Oreochromis mossambicus × Oreochromis niloticus. On the other hand, Webster et al. [29] pointed out that little change occurred in the fatty acid profile of visceral fat when channel catfish had been starved for 80 days. In decapod broodstock, nutritional status, especially for fatty acids, can affect ovarian maturation, reproductive performance and offspring quality [30–33]. Till now, no studies have been carried out on the effect of starvation or feeding on the fatty acid composition of the tissues of the swimming crab. In addition, little attention has been paid to the relative expression of the fatty acid-binding protein gene (FABP) in relation to starvation or feeding.
In many species, reduced feeding and starvation can also restrain ovarian maturation and decrease fecundity [34, 35]. Vitellogenesis is a crucial reproductive process in oviparous animals. In mature females, vitellogenin (Vtg) is usually synthesized in response to endogenous estrogens, released into the blood and deposited in developing oocytes. In males, although present, Vtg is usually silent [36]. Hence, the expression of Vtg might be useful in revealing the process of ovarian maturation. Previous studies have reported the effects of starvation on larval/juvenile survival, growth and development [20–24]; however, little is known about the effects of starvation on crustacean broodstock. Also, there have been no investigations of the mRNA expression of Vtg under starvation conditions in crustacean species. This study investigated the effects of starvation and feeding on blood chemistry, fatty acid composition, and mRNA expression levels of Vtg and FABP in the female swimming crab P. trituberculatus broodstock.
Materials and methods
Experimental animals and design
The experiment was conducted at the Xing-Yi Nursery Farm (Ningbo, China). Wild, healthy adult female P. trituberculatus (body weight 230 ± 45 g) were caught in natural habitats in the East China Sea. Before the experiment, crabs were acclimated to culture conditions for 7 days and fed with fresh clam Ruditapes philippinarum. A total of 180 female crab broodstock were then randomly allocated to 180 individual plastic baskets (50 cm × 35 cm × 35 cm), which were divided into two experimental treatments (the starved group and the fed group); each treatment had three replicates (30 crabs per replicate). The plastic basket had two compartments, one section filled with sand to mimic the swimming crab’s habitat and the other section as the feeding area.
The plastic baskets were set down in a cement pool (70 m × 100 m × 0.6 m, length × width × depth) provided with continuous aeration through an air stone to maintain dissolved oxygen levels at, or near, saturation level. During the experimental period, the temperature in the pool was 17–23 °C, the salinity was 26–28 g l−1 and the pH was 7.5–8.4. The dissolved oxygen was not less than 6.0 mg l−1, and the ammonia–nitrogen concentration was lower than 0.05 mg l−1. Salinity, pH, dissolved oxygen and ammonia–nitrogen were measured using a YSI Pro Plus (YSI, Yellow Springs, OH). From the beginning of the experiment, the fed crabs were continuously fed fresh clams once a day at a rate of 3–5% of body weight, while the starved crabs did not receive any food. The experiment lasted for 30 days.
Sample collection techniques
At the termination of the feeding trial, the fed crabs were starved for 24 h before being sampled to reduce handling stress and clear the alimentary canal. Hemolymph samples from six crabs in each replicate were taken immediately using the method described by Li et al. [37]. The samples were stored at 4 °C for 24 h and then centrifuged at 4 °C, 1912 g for 10 min. Then, the supernatant was collected, packaged and stored at −80 °C until the analysis of blood chemistry. The hepatopancreas and the ovary from six crabs in each replicate were dissected and weighed to determine the hepatosomatic index (HSI) and the gonadosomatic index (GSI). The dissected hepatopancreas and ovary samples were used to analyze the fatty acid composition of the crabs. The hepatopancreas and the ovary samples for the analysis of relative gene expression of four crabs in each replicate were collected, homogenized with Trizol reagent (TaKaRa, Dalian, China), and immediately frozen in liquid nitrogen. All samples were stored at −80 °C until needed.
Hemolymph analysis
The TP, GLC, triacylglycerol (TG), total cholesterol (TC), HDL and LDL levels in the hemolymph were assayed using an automatic blood analyzer (Hitachi 7170A, Japan) at a clinical laboratory in Ningbo University Hospital.
Fatty acid analysis
The fatty acid profiles of the hepatopancreas and ovary samples were determined as described by [38] with few modifications. The freeze-dried samples (~80 mg hepatopancreas sample and ~100 mg ovary sample) were added to a 12-ml volumetric glass tube with lid (containing a Teflon gasket). Then 3 ml potassium hydroxide methanol (1 N) was added and the tubes heated in a water bath at 72 °C for 20 min. After cooling, 3 ml HCl–methanol (2 N) was added and the mixture was heated at 72 °C in a water bath for another 20 min. Previous tests were conducted to make sure that all fatty acids can be esterified following the procedures above. Finally, 1 ml hexane was added, the mixture shaken vigorously for 1 min, and then allowed to separate into two layers. Fatty acid methyl esters were separated and measured by a gas chromatograph mass spectrometer (GC–MS) (Agilent-GCMS 7890-5975C; Agilent Technologies, Santa Clara, CA) equipped with a capillary HP-5MS column (30 m × 0.250 mm; film thickness, 0.25 μm; Agilent Technologies) using helium as the carrier gas in a split mode (20:1). Mass spectra were scanned from m/z 50-800. Identification of fatty acids was confirmed by comparing the mass spectra with a commercially available standard library (the National Institute of Standards and Technology Mass Spectral Library 2011). Results are relative percentages of each fatty acid, calculated as the proportion of the area under the considered peak to the total area of all peaks.
RNA extraction and gene expression analysis
Total RNA from each crab sample was extracted from the hepatopancreas and the ovary using trizol reagent according to the manufacturer’s instructions (TransGen, Beijing, China). RNA quantity, purity and integrity were detected by a ND-2000 NanoDrop UV spectrophotometer (NanoDrop Technologies, USA) (A260/A280) and by electrophoresis on 1.2% agarose gels. Genomic DNA contamination was eliminated by RNase-free DNase (TransGen, Beijing, China), according to the manufacturer’s protocol. The RNA was reverse transcribed at 42 °C for 60 min using a reversed first strand cDNA synthesis kit (TransGen) and stored at −20 °C.
The levels of Vtg and FABP mRNA were detected by real-time quantitative polymerase chain reaction (PCR) (Light Cycler 96; Roche, Switzerland). The primers used for real-time quantitative PCR [Vtg F and Vtg R, FABP F and FABP R (Table 1)] were designed based on the Vtg cDNA sequence of P. trituberculatus (GenBank accession no. DQ000638) [39] and the FABP cDNA sequence of P. trituberculatus from the transcriptome [40]. The PCR was performed in a 20 μl reaction volume containing 10 μl of SYBR Green premix, 1 μl of cDNA template, 1 μl of each primer (10 μM) and 7 μl of diethyl pyrocarbonate-treated water. The PCR conditions were as follows: 95 °C for 10 min; 45 cycles of 95 °C for 15 s, 58 °C for 15 s and 72 °C for 20 s. RNA was extracted from each of the three crabs sampled from a triplicate. As the internal controls, β-actin F and β-actin R (Table 1) were used to amplify the β-actin gene for each RT reaction product [4, 41, 42]. Normalized gene expression of the ovary in the fed crabs was set to 1, and the expression of each target gene for the hepatopancreas in the fed crabs as well as the ovary and the hepatopancreas in the starved crabs were expressed relative to the ovary in the fed crabs. The data were optimized using the comparative Ct (2−ΔΔCt) value method as described by Livak and Schmittgen [43] and then subjected to statistical analysis.
Calculations of GSI and HSI
The GSI and HSI of the crabs were calculated using the following formulae:
GSI (%) = 100 × gonad wet weight/body wet weight;
HSI (%) = 100 × hepatopancreas wet weight/body wet weight.
Statistical analysis
The values are expressed as the mean ± SD. The results were compared by Student’s t-test. Differences among means were considered significant when P < 0.05. All statistical analyses were performed using the SPSS 17.0 software package (SPSS, IL).
Results
GSI and HSI data
There was no significant difference in final body weight between fed and starved crabs (P > 0.05) (Fig. 1). After 30 days without feeding, a significant decrease in the GSI was found in the starved crabs (P < 0.05) (Fig. 2). However, statistical analysis showed no significant effect of starvation and feeding on the HSI during the period of the experiment (P > 0.05) (Fig. 3).
Hematological characteristics
The effect of starvation and feeding on the hematological characteristics of the crabs are presented in Table 2. After 30 days of starvation, TG, HDL and LDL concentrations in the hemolymph did not differ significantly between the starved and fed crabs (P > 0.05). The GLC and TC levels in hemolymph were significantly lower in starved crabs than in crabs fed clams (P < 0.05); however, the TP concentration in the starved crabs was higher than in crabs fed clams (P < 0.05).
Fatty acid composition in ovary and hepatopancreas
The effects of starvation and feeding on ovarian fatty acid composition (% total fatty acid) are shown in Table 3. For saturated fatty acid (SFA), starved crabs contained a significantly higher relative percentage of 12:0, but significantly lower relative percentages of 18:0 fatty acid and ΣSFA than the fed crabs (P < 0.05). Among monounsaturated fatty acids (MUFA), starved crabs had significantly higher relative percentages of 18:1n-9 than fed crabs, but significantly lower relative percentages of 16:1n-7 and 20:1n-9 than fed crabs (P < 0.05); however, there were no significant differences in ΣMUFA between starved and fed crabs (P > 0.05). Regards the PUFA, starved crabs contained significantly higher relative percentages of 20:4n-6, 20:5n-3, and 22:6n-3 fatty acids, as well as ΣPUFA and ΣHUFA, than fed crabs.
The effects of starvation and feeding on hepatopancreas fatty acid composition in the crabs are presented in Table 4. The relative percentages of 18:1n-9, 22:1n-9, 20:2n-6, 20:4n-6, and 22:6n-3 fatty acids as well as ΣPUFA and ΣHUFA were significantly higher in starved crabs than in fed crabs, while starved crabs had significantly lower relative percentages of 14:0, 16:0, 16:1n-7, 22:2n-6 and 20:4n-3 fatty acids as well as ΣSFA (P < 0.05). The percentages of C18:0, C20:0, C22:0, C18:1n-7, C20:1n-9, C18:2n-6 and C20:5n-3 were not significantly different in the hepatopancreas between starved and fed crabs (P > 0.05).
Relative expression of Vtg and FABP
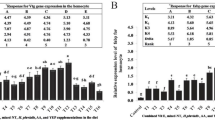
Starvation significantly influenced the relative expression of Vtg and FABP in the ovary and the hepatopancreas in female swimming crabs (P < 0.05) (Figs. 4, 5). The expression of ovarian Vtg in starved crabs was significantly lower than in fed crabs (P < 0.05), whereas the expression of hepatopancreas Vtg was not influenced by starvation stress (P > 0.05) (Fig. 4). The relative expression of FABP in the ovary and the hepatopancreas of starved crabs was significantly higher than in those of fed crabs (P < 0.05) (Fig. 5).
Discussion
In some aquatic animals, such as Atlantic salmon Salmo salar [44, 45], winter flounder Pleuronectes americanus [46, 47] and sea bass Dicentrarchus labrax [34], maturation was suppressed or reduced by starvation at specific periods. In the present study, starvation of female swimming crabs for 30 days caused a significant decrease in the GSI with a concomitant deterioration of nutritional status (Fig. 2), indicating that starvation affected gonadal development. The effect on gonadal development may be attributed to nutritional factors which play a critical role in the development of the ovary in female swimming crabs. There is abundant evidence for the influence of feeding ration and feeding levels on gonadal maturation and reproduction under culture conditions [48–50]. In the present study, starvation deprived the gonads of energy sources leading to reduced gonad size, and also affected gonad maturation. Thus good nutritional status of female swimming crabs is very important for gonadal development and maturation under culture conditions.
There were no significant differences in the HSI between starved and fed crabs (Fig. 3). Sureshkumar and Kurup considered [51] that the HSI of Macrobrachium rosenbergii could be expected to decline during fasting because the relative weight of the hepatopancreas reflects the provision of energy utilization for metabolism, i.e., this species compensates for the fasting condition with its own reserves. However, Uglow reported [52] that marine decapods utilize protein as the predominant metabolic source at a higher rate in ovaries than in the hepatopancreas [53]. In the present study, the starved crabs had no external energy supply to maintain their basic life activities and may have absorbed nutrients from the ovaries. Meanwhile, the HSI in swimming crab broodstock showed no significant difference during the over-wintering period [54]. These results could explain the lack of a significant difference in the HSI and decreased GSI in starved crabs in the present study.
Vitellogenesis is an important step in the oocyte maturation of decapods, involving the synthesis and accumulation of yolk proteins in the ovary. The site of synthesis of Vtg (precursor of vitellin) in crustaceans is controversial [55]. Several studies have confirmed that Vtg is synthesized in the ovaries of Callinectes sapdius [56] and Penaeus semisulcatus [57]. Other studies have shown that Vtg is synthesized exclusively in the hepatopancreas of Oziothelphusa senex senex [55], Pandalus hypsinotus [58], Macrobrachium rosenbergii [59] and Charybdis feriatus [60]. In crustaceans, the mRNA expression of Vtg has been used to determine the site of vitellogenesis [61]. In the present study (Fig. 4), Vtg gene expression in the hepatopancreas and the ovary indicated that the swimming crab could utilize these two tissues for vitellogenesis. Like some other decapod species, such as C. sapidus [62, 63], Homarus americanus [64], Eriocheir sinensis [65] and Scylla paramamosain [36], the swimming crab was shown to synthesize Vtg mainly in the hepatopancreas because the gene expression level of Vtg here was higher than in the ovary. The gene expression of ovarian Vtg in starved crabs was significantly lower than in fed crabs (Fig. 4), which could explain why starvation stress affects gonadal development in the female swimming crab. The stable Vtg mRNA expression in the hepatopancreas and the subsequent reduction in the level of Vtg mRNA in the ovary indicated that the crabs may have absorbed nutrients from the ovaries, which may have affected the maturation of the gonads, and caused the decreased relative gene expression of Vtg here.
The GLC concentration in serum is significantly affected by starvation or feeding. The reduction in serum GLC levels induced by starvation in female swimming crabs after 30 days of starvation (Table 2) is in agreement with most studies performed in other crustaceans and fish [25, 66–70]. The lower serum GLC levels in starved swimming crabs may be due to the utilization of GLC, a primary metabolic response to starvation. Previously, our studies have shown that serum lipid concentrations in the swimming crab, particularly those of triglycerides and cholesterol, are highly dependent on the crab’s nutritional or physiological state [26]. In the present study, a significant decrease in serum cholesterol levels was observed for the starved crabs (Table 2). Cholesterol is one of the most important sterol lipids. During starvation, stored carbohydrates are primarily utilized to yield energy, followed by lipid mobilization [71]. Decreases in the cholesterol content during starvation have been observed by various workers, such as Gatsko et al. [72], Prasad [71] and Shreni [73]. The plasma TP level is usually regarded as an indicator of protein mobilization during starvation [8, 74]. Several studies have shown that food deprivation in animals results in a reduction in plasma protein concentration [75–77]. Hu et al. [20] reported that no significant reduction were observed in the plasma TP in Tachypleus tridentatus and Carcinoscorpius rotundicauda. However, Pellegrino et al. [78] indicated that an increase in the activity of the gluconeogenic pathway occurred after 15 days of fasting in the crab Chasmagnathus granulatus previously fed a high protein (HP) diet. In our study, the levels of serum TP in starved crabs were significantly higher than in fed crabs (Table 2), which may have been due to the mobilization of protein from the ovary and/or muscle for gluconeogenesis in the starved condition [78, 79].
The energy metabolism of crustaceans under starvation primarily centers on glycogen and lipids. Halver [80] reported that the oxidation of fatty acids plays a very important role in the energy supply. In the present study, the relative percentages of ΣSFA in the ovary and hepatopancreas of the starved crabs were significantly lower than in the fed crabs (Tables 3, 4). Conversely, the relative percentages of ΣPUFA and ΣHUFA in the starved crabs were significantly higher than in fed crabs. Usually SFAs and MUFAs are utilized as energy sources. However, some of the PUFAs and HUFAs were thought to be conserved because HUFAs, such as 20:4n-6 (ARA), 20:5n-3 (EPA) and 22:6n-3 (DHA), are the major components of phospholipids, which may play a role in the structure of the cell membrane and appear to be preferentially conserved [81, 82]. Similarly, changes in SFA, PUFA and HUFA utilization during starvation have been reported in other species [23, 83]. According to the changing relative amounts of these fatty acids between starved and fed crabs, we inferred that the utilization of fatty acids in the ovary was 18:0 > 16:1n-7 > 20:1n-9 > 16:0, whereas the conservation was 20:4n-6 > 22:6n-3 > 18:1n-9 > 20:5n-3 > 18:2n-6 during the starvation period. During the 30-day starvation period, the utilization of fatty acids in the hepatopancreas was 14:0 > 22:2n-6 > 16:1n-7 > 16:0, whereas the conservation was 20:4n-6 > 20:2n-6 > 22:1n-9 > 22:6n-3 > 18:1n-9. Ravid et al. [84] reported that food consumed by the shrimp affects the abundance of specific fatty acids in the ovary, and that as the ovary matures, there is a gradual decrease in the relative abundance of PUFA. In the present study, the significant changes of relative fatty acid levels in ovary tissue during starvation might indicate that the ovary is an important organ for fatty acid metabolism in female swimming crab broodstock. In juvenile crustaceans, the hepatopancreas is the primary organ for fatty acid metabolism [23, 85]. There were different strategies for utilizing fatty acids between the ovary and hepatopancreas during starvation in female swimming crabs. For example, 20:4n-6 (ARA) was primarily conserved in starved crabs in the ovary and hepatopancreas, indicating that it may play a prominent role in ovarian development in starved crabs. Wu et al. reported [33] that the ARA level in eggs from swimming crabs had an important function in determining egg hatchability. Moreover, Silva et al. [28] reported that ARA was the precursor of the eicosanoids, thromboxanes, prostaglandins, and leukotrienes, which are biologically active molecules, and it was preserved in starved hybrid tilapia [28] and zebrafish [86] because of these roles; this is a possible explanation for increased ARA level in starved crabs. FABPs primarily bind fatty acids [87] and have a wide range of crucial biological roles, including intracellular targeting of fatty acids to specific organelles as well as the uptake and utilization of fatty acids [88, 89]. Moreover, studies with E. sinensis have shown that FABP expression levels increased with ovarian development, which reflected lipid nutritional requirements [90]. In the present study, the FABP expression levels in the ovary and the hepatopancreas in starved crabs were higher than in the fed crabs (Fig. 5), indicating that starved swimming crabs need more lipids for energy metabolism.
In conclusion, the results of the present study indicate that:
-
1.
Starvation suppressed or reduced gonadal development in the female swimming crab.
-
2.
The swimming crab modulated its biochemistry to cope with starvation, which significantly influenced blood GLC and lipid levels.
-
3.
The SFAs and MUFAs, 14:0, 16:0, 18:0; and 16:1n-7, are the main energy sources of swimming crabs during starvation. Thus, not only sufficient HUFAs but also sufficient SFAs and MUFAs must be supplied in the diets of female swimming crab broodstock.
References
Dai AY, Yang SL, Song YZ (1986) Marine crabs in China Sea. Marine, Beijing, pp 194–196
Dan S, Oshiro M, Ashidate M, Hamasaki K (2016) Starvation of Artemia in larval rearing water affects post-larval survival and morphology of the swimming crab, Portunus trituberculatus (Brachyura, Portunidae). Aquaculture 452:407–415
Jin M, Wang MQ, Huo YW, Huang WW, Mai KS, Zhou QC (2015) Dietary lysine requirement of juvenile swimming crab, Portunus trituberculatus. Aquaculture 448:1–7
Pan L, Hu D, Liu M, Hu Y, Liu S (2016) Molecular cloning and sequence analysis of two carbonic anhydrase in the swimming crab Portunus trituberculatus and its expression in response to salinity and pH stress. Gene 576(1):347–357
FAO 2014 (2015) FISHSTAT Plus: universal software for fishery statistical time series. Version 2.11.4. Fishery Information, Data and Statistics Unit, FAO Fisheries Department. http://www.fao.org/fishery/statistics/software/fishstat/en. Accessed on 14 March 2015
China Fishery Statistical Yearbook (2014) Compiled by the Fishery Bureau of the China Agriculture Department, pp 28–29
Holmstrup M, Bindesbøl AM, Oostingh GJ, Duschl A, Scheil V, Köhler HR, Gerhardt A (2010) Interactions between effects of environmental chemicals and natural stressors: a review. Sci Total Environ 408(18):3746–3762
Rossi A, Cazenave J, Bacchetta C, Campana M, Parma MJ (2015) Physiological and metabolic adjustments of Hoplosternum littorale (Teleostei, Callichthyidae) during starvation. Ecol Indic 56:161–170
Wen J, Pan L (2015) Short-term exposure to benzo[a]pyrene disrupts reproductive endocrine status in the swimming crab Portunus trituberculatus. Comp Biochem Phys C 174:13–20
Lu Y, Wang F, Dong S (2015) Energy response of swimming crab Portunus trituberculatus to thermal variation: implication for crab transport method. Aquaculture 441:64–71
Meng XL, Liu P, Li J, Gao BQ, Chen P (2014) Physiological responses of swimming crab Portunus trituberculatus under cold acclimation: antioxidant defense and heat shock proteins. Aquaculture 434:11–17
Zhao Q, Pan L, Ren Q, Wang L (2015) Identification of genes differentially expressed in swimming crab Portunus trituberculatus response to low temperature. Aquaculture 442:21–28
Liu S, Pan L, Liu M, Yang L (2014) Effects of ammonia exposure on nitrogen metabolism in gills and hemolymph of the swimming crab Portunus trituberculatus. Aquaculture 432:351–359
Ren Q, Pan L, Zhao Q, Si L (2015) Ammonia and urea excretion in the swimming crab Portunus trituberculatus exposed to elevated ambient ammonia-N. Comp Biochem Phys A 187:48–54
Yue F, Pan L, Xie P, Zheng D, Li J (2010) Immune responses and expression of immune-related genes in swimming crab Portunus trituberculatus exposed to elevated ambient ammonia-N stress. Comp Biochem Phys A 157(3):246–251
Sun Y, Wang F, Dong S (2015) A comparative study of the effect of starvation regimes on the foraging behavior of Portunus trituberculatus and Charybdis japonica. Physiol Behav 151:168–177
Wang T, Hung CC, Randall DJ (2006) The comparative physiology of food deprivation: from feast to famine. Annu Rev Physiol 68:223–251
Zeng LQ, Li FJ, Li XM, Cao ZD, Fu SJ, Zhang YG (2012) The effects of starvation on digestive tract function and structure in juvenile southern catfish (Silurus meridionalis Chen). Comp Biochem Phys A 162(3):200–211
Antonopoulou E, Kentepozidou E, Feidantsis K, Roufidou C, Despoti S, Chatzifotis S (2013) Starvation and re-feeding affect Hsp expression, MAPK activation and antioxidant enzymes activity of European sea bass (Dicentrarchus labrax). Comp Biochem Phys A 165(1):79–88
Hu M, Wang Y, Tsang ST, Cheung SG, Shin PK (2010) Effect of prolonged starvation on body weight and blood-chemistry in two horseshoe crab species: Tachypleus tridentatus and Carcinoscorpius rotundicauda (Chelicerata: Xiphosura). J Exp Mar Biol Ecol 395(1):112–119
Walker SJ, Neill WH, Lawrence AL, Gatlin DM (2011) Effects of temperature and starvation on ecophysiological performance of the Pacific white shrimp (Litopenaeus vannamei). Aquaculture 319(3):439–445
Watts AJ, McGill RA, Albalat A, Neil DM (2014) Biophysical and biochemical changes occur in Nephrops norvegicus during starvation. J Exp Mar Biol Ecol 457:81–89
Wen X, Chen L, Ku Y, Zhou K (2006) Effect of feeding and lack of food on the growth, gross biochemical and fatty acid composition of juvenile crab, Eriocheir sinensis. Aquaculture 252(2):598–607
Vinagre AS, Chung JS (2016) Effects of starvation on energy metabolism and crustacean hyperglycemic hormone (CHH) of the Atlantic ghost crab Ocypode quadrata (Fabricius, 1787). Mar Biol 163(1):1–11
Congleton JL, Wagner T (2006) Blood-chemistry indicators of nutritional status in juvenile salmonids. J Fish Biol 69(2):473–490
Huo YW, Jin M, Zhou PP, Li M, Mai KS, Zhou QC (2014) Effects of dietary protein and lipid levels on growth, feed utilization and body composition of juvenile swimming crab, Portunus trituberculatus. Aquaculture 434:151–158
Zamal H, Ollevier F (1995) Effect of feeding and lack of food on the growth, gross biochemical and fatty acid composition of juvenile catfish. J Fish Biol 46(3):404–414
De Silva SS, Gunasekera RM, Austin CM (1997) Changes in the fatty acid profiles of hybrid red tilapia, Oreochromis mossambicus × O. niloticus, subjected to short-term starvation, and a comparison with changes in seawater raised fish. Aquaculture 153(3):273–290
Webster CD, Tidwell JH, Goodgame LS, Yancey DH (1994) Effects of fasting on fatty acid composition of muscle, liver, and abdominal fat in channel catfish Ictalurus punctatus. J World Aquacult Soc 25(1):126–134
Millamena OM, Quinitio E (2000) The effects of diets on reproductive performance of eyestalk ablated and intact mud crab Scylla serrata. Aquaculture 181(1):81–90
Wouters R, Lavens P, Nieto J, Sorgeloos P (2001) Penaeid shrimp broodstock nutrition: an updated review on research and development. Aquaculture 202(1):1–21
Wu X, Chang G, Cheng Y, Zeng C, Southgate PC, Lu J (2010) Effects of dietary phospholipid and highly unsaturated fatty acid on the gonadal development, tissue proximate composition, lipid class and fatty acid composition of precocious Chinese mitten crab, Eriocheir sinensis. Aquacult Nutr 16(1):25–36
Wu X, Cheng Y, Zeng C, Wang C, Yang X (2010) Reproductive performance and offspring quality of wild-caught and pond-reared swimming crab Portunus trituberculatus broodstock. Aquaculture 301(1):78–84
Chatzifotis S, Papadaki M, Despoti S, Roufidou C, Antonopoulou E (2011) Effect of starvation and re-feeding on reproductive indices, body weight, plasma metabolites and oxidative enzymes of sea bass (Dicentrarchus labrax). Aquaculture 316(1):53–59
Terashima J, Takaki K, Sakurai S, Bownes M (2005) Nutritional status affects 20-hydroxyecdysone concentration and progression of oogenesis in Drosophila melanogaster. J Endocrinol 187(1):69–79
Jia X, Chen Y, Zou Z, Lin P, Wang Y, Zhang Z (2013) Characterization and expression profile of vitellogenin gene from Scylla paramamosain. Gene 520(2):119–130
Li C, Song S, Liu Y, Chen T (2013) Hematodinium infections in cultured Chinese swimming crab, Portunus trituberculatus, in Northern China. Aquaculture 396:59–65
Zuo RT, Ai QH, Mai KS, Xu W, Wang J, Xu HG (2012) Effects of dietary n-3 highly unsaturated fatty acids on growth, nonspecific immunity, expression of some immune related genes and disease resistance of large yellow croaker (Larmichthys crocea) following natural infestation of parasites (Cryptocaryon irritans). Fish Shellfish Immunol 32(2):249–258
Yang F, Xu HT, Dai ZM, Yang WJ (2005) Molecular characterization and expression analysis of vitellogenin in the marine crab Portunus trituberculatus. Comp Biochem Phys B 142(4):456–464
Wang W, Wu X, Liu Z, Zheng H, Cheng Y (2014) Insights into hepatopancreatic functions for nutrition metabolism and ovarian development in the crab Portunus trituberculatus: gene discovery in the comparative transcriptome of different hepatopancreas stages. PLoS One 9(1):e84921
Gao W, Tan B, Mai K, Chi S, Liu H, Dong X, Yang Q (2012) Profiling of differentially expressed genes in hepatopancreas of white shrimp (Litopenaeus vannamei) exposed to long-term low salinity stress. Aquaculture 364:186–191
Liu KF, Chiu CH, Shiu YL, Cheng W, Liu CH (2010) Effects of the probiotic, Bacillus subtilis E20, on the survival, development, stress tolerance, and immune status of white shrimp, Litopenaeus vannamei larvae. Fish Shellfish Immun 28(5):837–844
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25(4):402–408
Reimers E, Kjørrefjord AG, Stavøstrand SM (1993) Compensatory growth and reduced maturation in second sea winter farmed Atlantic salmon following starvation in February and March. J Fish Biol 43(5):805–810
Rowe DK, Thorpe JE (1990) Suppression of maturation in male Atlantic salmon (Salmo salar L.) Parr by reduction in feeding and growth during spring months. Aquaculture 86(2):291–313
Burton MPM (1991) Induction and reversal of the non-reproductive state in winter flounder, Pseudopleuronectes americanus Walbaum, by manipulating food availability. J Fish Biol 39(6):909–910
Burton MPM (1994) A critical period for nutritional control of early gametogenesis in female winter flounder, Pleuronectes americanus (Pisces: Teleostei). J Zool 233(3):405–415
Bromage N, Jones J, Randall C, Thrush M, Davies B, Springate J, Duston J, Barker G (1992) Broodstock management, fecundity, egg quality and the timing of egg production in the rainbow trout (Oncorhynchus mykiss). Aquaculture 100:141–166
Kjesbu OS, Klungsoyr J, Witthames PR, Walker MG (1991) Fecundity, atresia, and egg size of captive Atlantic cod (Gadus morhua) in relation to proximate body composition. Can J Fish Aquat Sci 48:2333–2343
Imsland AK, Jonassen TM (2005) The relation between age at first maturity and growth in Atlantic halibut (Hippoglossus hippoglossus) reared at four different light regimes. Aquac Res 36:1–7
Sureshkumar S, Kurup BM (1999) Variations in hepatosomatic index and biochemical profiles among the male morphotypes of Macrobrachium rosenbergii. Aquaculture 176(3):285–293
Uglow RF (1969) Hemolyph protein concentrations in portunid crabs. II. The effects of imposed fasting on Carcinus maenas. Comp Biochem Physiol 31A:959–967
He J, Xuan F, Shi H, Xie J, Wang W, Wang G (2017) Comparison of nutritional quality of three edible tissues of the wild-caught and pond-reared swimming crab (Portunus trituberculatus) females. Lwt Food Sci Technol 75:624–630
Jia L, Ma S (2007) The ovarian development of Portunus trituberculatus during overwintering and spawning periods. J Ocean Univ China (in Chinese) (s2):55–60
Girish BP, Swetha CH, Reddy PS (2014) Hepatopancreas but not ovary is the site of vitellogenin synthesis in female fresh water crab, Oziothelphusa senex senex. Biochem Biophys Res Commun 447(2):323–327
Lee CY, Watson RD (1995) In vitro study of vitellogenesis in the blue crab (Callinectes sapidus): site and control of vitellin synthesis. J Exp Zool 271(5):364–372
Browdy CL, Fainzilber M, Tom M, Loya Y, Lubzens E (1990) Vitellin synthesis in relation to oogenesis in in vitro-incubated ovaries of Penaeus semisulcatus (Crustacea, Decapoda, Penaeidae). J Exp Zool 255(2):205–215
Tsutsui N, Saido-Sakanaka H, Yang WJ, Jayasankar V, Jasmani S, Okuno A, Wilder MN (2004) Molecular characterization of a cDNA encoding vitellogenin in the coonstriped shrimp, Pandalus hypsinotus and site of vitellogenin mRNA expression. J Exp Zool Part A 301(10):802–814
Yang WJ, Ohira T, Tsutsui N, Subramoniam T, Huong DTT, Aida K, Wilder MN (2000) Determination of amino acid sequence and site of mRNA expression of four vitellins in the giant freshwater prawn, Macrobrachium rosenbergii. J Exp Zool 287(6):413–422
Mak ASC, Choi CL, Tiu SHK, Hui JHL, He JG, Tobe SS, Chan SM (2005) Vitellogenesis in the red crab Charybdis feriatus: hepatopancreas-specific expression and farnesoic acid stimulation of vitellogenin gene expression. Mol Reprod Dev 70(3):288–300
Subramoniam T (2011) Mechanisms and control of vitellogenesis in crustaceans. Fisheries Sci 77(1):1–21
Thongda W, Chung JS, Tsutsui N, Zmora N, Katenta A (2015) Seasonal variations in reproductive activity of the blue crab, Callinectes sapidus: vitellogenin expression and levels of vitellogenin in the hemolymph during ovarian development. Comp Biochem Phys A 179:35–43
Zmora N, Trant J, Chan SM, Chung JS (2007) Vitellogenin and its messenger RNA during ovarian development in the female blue crab, Callinectes sapidus: gene expression, synthesis, transport, and cleavage. Biol Reprod 77(1):138–146
Tiu SHK, Hui HL, Tsukimura B, Tobe SS, He JG, Chan SM (2009) Cloning and expression study of the lobster (Homarus americanus) vitellogenin: conservation in gene structure among decapods. Gen Comp Endocrinol 160(1):36–46
Li K, Chen L, Zhou Z, Li E, Zhao X, Guo H (2006) The site of vitellogenin synthesis in Chinese mitten-handed crab Eriocheir sinensis. Comp Biochem Phys B 143(4):453–458
De Pedro N, Delgado MJ, Gancedo B, Alonso-Bedate M (2003) Changes in glucose, glycogen, thyroid activity and hypothalamic catecholamines in tench by starvation and refeeding. J Comp Physiol B 173(6):475–481
Furné M, Morales AE, Trenzado CE, García-Gallego M, Hidalgo MC, Domezain A, Rus AS (2012) The metabolic effects of prolonged starvation and refeeding in sturgeon and rainbow trout. J Comp Physiol B 182(1):63–76
Kamiya M, Kamiya Y, Tanaka M, Shioya S (2008) Changes of plasma free amino acid concentrations and myofibrillar proteolysis index by starvation in non-pregnant dry cows. J Anim Sci 79(1):51–57
Soengas JL, Polakof S, Chen X, Sangiao-Alvarellos S, Moon TW (2006) Glucokinase and hexokinase expression and activities in rainbow trout tissues: changes with food deprivation and refeeding. Am J Physiol Reg I 291(3):R810–R821
Zauner C, Schneeweiss B, Kranz A, Madl C, Ratheiser K, Kramer L, Lenz K (2000) Resting energy expenditure in short-term starvation is increased as a result of an increase in serum norepinephrine. Am J Clin Nutr 71(6):1511–1515
Prasad NK (2015) Effects of prolonged starvation on cholesterol content of gonads in Clarias batrachus. Our Nature 13(1):26–30
Gatsko GG, Mazhul LM, Pozdnyakova EA (1982) Lipid peroxidation in tissues of normal and hungry rats of different ages. B Exp Biol Med 93(4):416–418
Shreni KD (1979) Influence of starvation on the brain and liver cholesterol levels of the cat-fish, Heteropneustes fossilis (Bloch). Proc Anim Sci 88(3):205–208
Almeida JS, Meletti PC, Martinez CB (2005) Acute effects of sediments taken from an urban stream on physiological and biochemical parameters of the Neotropical fish Prochilodus lineatus. Comp Biochem Phys C 140(3):356–363
Helland S, Nejstgaard JC, Fyhn HJ, Egge JK, Båmstedt U (2003) Effects of starvation, season, and diet on the free amino acid and protein content of Calanus finmarchicus females. Mar Biol 143(2):297–306
Matozzo V, Gallo C, Marin MG (2011) Can starvation influence cellular and biochemical parameters in the crab Carcinus aestuarii? Mar Environ Res 71(3):207–212
Sánchez-Paz A, García-Carreño F, Hernández-López J, Muhlia-Almazán A, Yepiz-Plascencia G (2007) Effect of short-term starvation on hepatopancreas and plasma energy reserves of the Pacific white shrimp (Litopenaeus vannamei). J Exp Mar Biol Ecol 340(2):184–193
Pellegrino R, Kucharski LC, Da Silva RSM (2008) Effect of fasting and refeeding on gluconeogenesis and glyconeogenesis in the muscle of the crab Chasmagnathus granulatus, previously fed a protein- or carbohydrate-rich diet. J Exp Mar Biol Ecol 358(2):144–150
Pellegrino R, Martins TL, Pinto CB, Schein V, Kucharski LC, Da Silva RSM (2012) Effect of starvation and refeeding on amino acid metabolism in muscle of crab Neohelice granulata previously fed protein- or carbohydrate-rich diets. Comp Biochem Phys A 164(1):29–35
Halver JE (1989) Fish nutrition, 2nd edn. Academic Press, New York, pp 239–345
Sargent JR, Bell JG, Bell MV, Henderson RJ, Tocher DR (1995) Requirement criteria for essential fatty acids. J Appl Ichthyol 11(3–4):183–198
Zabelinskii SA, Chebotareva MA, Kostkin VB, Krivchenko AI (1999) Phospholipids and their fatty acids in mitochondria, synaptosomes and myelin from the liver and brain of trout and rat: a new view on the role of fatty acids in membranes. Comp Biochem Phys B 124(2):187–193
Durazo E, Viana MT (2013) Fatty acid profile of cultured green abalone (Haliotis fulgens) exposed to lipid restriction and long-term starvation. Cienc Mar 39(4):363–370
Ravid T, Tietz A, Khayat M, Boehm E, Michelis R, Lubzens E (1999) Lipid accumulation in the ovaries of a marine shrimp Penaeus semisulcatus (De Haan). J Exp Biol 202(13):1819–1829
Wen X, Chen L, Ai C, Zhou Z, Jiang H (2001) Variation in lipid composition of Chinese mitten-handed crab, Eriocheir sinensis, during ovarian maturation. Comp Biochem Phys B 130(1):95–104
Ölmez A, Bayir M, Wang C, Bayir A (2015) Effects of long-term starvation and refeeding on fatty acid metabolism-related gene expressions in liver of zebrafish, Danio rerio. Turk J Vet Anim Sci 39:654–660
Esteves A, Ehrlich R (2006) Invertebrate intracellular fatty acid binding proteins. Comp Biochem Phys C 142(3):262–274
Corsico B, Liou HL, Storch J (2004) The alphahelical domain of liver fatty acid binding proteinis responsible for the diffusion-mediated transfer of fatty acids to phospholipid membranes. Biochemistry 43:3600–3607
Storch J, Veerkamp JH, Hsu KT (2002) Similar mechanisms of fatty acid transfer from human anal rodent fatty acid-binding proteins to membranes: liver, intestine, heart muscle, and adipose tissue FABPs. In: Cellular lipid binding proteins. Springer, New York, pp 25–33
Gong YN, Li WW, Sun JL, Ren F, He L, Jiang H, Wang Q (2010) Molecular cloning and tissue expression of the fatty acid-binding protein (Es-FABP) gene in female Chinese mitten crab (Eriocheir sinensis). BMC Mol Biol 11(1):71
Acknowledgements
This research was supported by the National Natural Science Foundation of China (41476125) and the Open Fund of Zhejiang Province (xkzsc1405). This research was also sponsored by the K. C. Wong Magna Fund and the K. C. Wong Education Foundation at Ningbo University. We would like to thank Y. W. Huo, M. Q. Wang, Z. M. Ren, T. L. Gao, S. K. Lu, Z. B. Yu, W. W. Huang, Y. Li and H. Qiu for their valuable help during the feeding trial and sample analysis.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ding, L., Fu, H., Hou, Y. et al. Effects of starvation and feeding on blood chemistry, fatty acid composition and expression of vitellogenin and fatty acid-binding protein genes in female swimming crab Portunus trituberculatus broodstock. Fish Sci 83, 455–464 (2017). https://doi.org/10.1007/s12562-017-1075-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12562-017-1075-3