Abstract
High pressure can be applied for the inactivation of endogenous enzymes detrimental to fruit and vegetable products. One of the enzymes that affect the quality of fruits and vegetables is pectinmethylesterase (PME), responsible for cloud destabilization and consistency changes. Depending on the desired quality of the developed product, PME can be partly or fully inactivated. In this review paper, the cited results in the literature of the high pressure inactivation of PMEs in model systems after extraction and purification as well as in real food systems is comprehensively presented. It is discussed that the pressure stability of PMEs can vary significantly, especially when comparing the more pressure-sensitive types, like orange juice (Valencia cv.) PME, with the more barotolerant ones like purified banana PME (Cavendish cv.). This variation can be attributed to the type of enzyme, the coexistence with other enzymes, type of substrates, ionic strength, pH and nature of the medium in which the enzyme is dispersed. This review may support the systematic evaluation and optimal design of fruit and vegetable product high pressure (HP) processing aiming to control their shelf-life, especially when considering that milder conditions are necessary for the inactivation of microorganisms compared to endogenous enzymes. Using literature data, an exponential mathematical model was uniformly applied to enable a better comparative assessment of pressure effects, on all PMEs discussed in this manuscript, obtained from different plant sources.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Processing methods to control the growth of pathogenic or spoilage microorganisms as well as the activity of intrinsic enzymes play a key role in the food production industry. Failure to achieve specified requirements for enzyme and microbial inactivation can result in inadequate safety and quality of food products. One of the enzymes that affect the quality of fruit and vegetable products is pectinmethylesterase (PME); thus, control of its activity is a prerequisite in the food and, especially, beverage industries.
PMEs can play diverse roles in fruit and vegetable processing. They can aid processing, improve the product sensory characteristics and increase the efficiency of industrial operations. In the food industry, PMEs can be used for extraction and increase of juice yield (fruit and vegetable juice manufacturing) [1–3], fruit juice clarification [4], enzymatic peeling of fruits [2], rheological property characterization of purees and pastes (mainly for tomato products) [5], production of high-quality wines [6] and extraction of pigments and food colourings [7]. In most of these applications, PMEs are used for the complex degradation of pectin. On the other hand, they cause a serious quality defect in cloudy juices and concentrates (cloud loss), due to precipitation of pectin demethylated by PME with calcium ions [8, 9]. Hence, in most cases, one of the main concerns of the fruit industry is the inactivation of this enzyme, which deteriorates the quality of final juices.
During processing, PMEs can be denatured and partly or completely inactivated resulting in reduced pectin conversion. Under certain conditions, enzyme activity may increase resulting in a higher pectin conversion. Apart from enzymatic pectin conversions, under appropriate conditions of temperature and pH, chemical conversion reactions can be observed during processing of plant-based food products without the presence of PMEs (either β-elimination reaction or acid hydrolysis) [10]. Consequently, the processing-induced modifications on the structure and activity of the PMEs can have a positive or negative effect depending on the desired characteristics of the food product. To date, the food industry employs mainly conventional methods for the inactivation of PME in fruit products such as thermal methods (pasteurization). Thermal inactivation of PME has been the subject of many studies [11–13]. In the case of orange juice, PME is usually inactivated by pasteurization at 90 °C for 1 min [14]. Traditional thermal processing, however, can negatively affect heat-sensitive nutrients and food product quality factors such as flavour, colour and texture [15–18].
In recent years, novel technologies such as high pressure (HP) processing, pulsed electric fields (PEFs) and ultrasound or a combination of these technologies can also offer an alternative non-thermal processing method for (cold) pasteurization of food. These methods present some fundamental benefits related to the mild conditions involved, particularly because the process takes place at lower temperatures (usually ambient temperatures or temperatures even lower than 10 °C), than those used for thermal pasteurization. These technologies target microorganism and enzyme inactivation while maintaining the nutrient content and flavour of foods and, consequently, the quality of final products [19–22]. Among these technologies, HP is considered the most promising based on the results cited in the literature for the inactivation of microorganisms and enzymes and based on the potential applications and already established units in food industries. During the HP process, products, usually packed in flexible packaging, are introduced into the HP vessel and subjected to high hydrostatic pressure (mostly in the range of 100 to 650 MPa) transmitted by fluid (in most applications water). Products are practically considered to be pressurized instantaneously and uniformly to all directions independent of product size and geometry, although in cases of solid foods with constituents of highly different compressibility variations in the spatial distribution of pressure can occur. Adiabatic heating in the range of 2° to 5° per 100 MPa occurs during pressurization. The effect of HP treatment is a function of the process parameters, applied pressure (MPa), temperature (°C), holding time (min), pressure buildup time (min) and pressure release time (min). In addition, when applying high pressures, adiabatic heating should also be considered as an influence factor. Adiabatic heating is caused by compressive work against intermolecular forces resulting in temperature increase during pressurization. The temperature reached during pressurization can be readily derived assuming that there are no thermal losses [23].
In contrast to other technologies, HP may also be used for the selective enzyme inactivation where and when required, since different process parameters are required for different enzyme inactivation. Such a case is the selective inactivation of polygalacturonase (PG) in tomato products while simultaneously retaining most of PME activity, associated to alterations in the rheological properties of tomato purees and pastes, providing tomato products of superior quality [5, 24, 25]. In the conventional processing of tomatoes, when high consistency and viscosity is desired, a heat shock treatment (hot break) is used to inactivate both PME and PG. When high consistency is not a prerequisite, cold break is used resulting in lowering viscosity since PME and PG degrade pectin. Generally, PME activity control is of high significance in the food industry for fruit and vegetable process optimization, for high-quality final products.
In the literature, there is a significant number of papers describing the effect of high pressure (HP) and temperature on PME activity from different fruits and vegetables, such as citrus-based foods [15, 26–35], tomato-based foods [25, 36–39], peach [40], strawberry [27, 41], pepper [42, 43], carrot [41, 44–46], banana [47, 48], apple [49], persimmon [50] and sea buckthorn [51]. A number of the aforementioned studies have been performed on the purified forms in buffer solutions such as citrate and Tris–HCl buffer. In general, PME purification is mainly performed by a series of fractionations by which the enzyme is separated from other proteins present. A common first step to isolate PMEs is precipitation with ammonium sulphate (NH4)2SO4. This is performed by adding increasing amounts of ammonium sulphate and collecting the different fractions of precipitate protein. PMEs are subsequently purified using different chromatographic techniques considering differences in protein size, physico-chemical properties, binding affinity and biological activity among the protein molecules.
As evidenced by results cited in the literature, the degree of PME inactivation depends on the origin of the enzyme, since different behaviours have been observed.
A comparative assessment of the effect of high pressure processing parameters on the activity of PMEs from different sources in a quantitative manner is very important. Knowledge of PME stability dependence on pressure and temperature allows for the proper design of HP processes ensuring high quality of products. Mathematical models capable to describe the pressure inactivation kinetics of PME from different plant sources as a function of temperature and pressure serve as tools for such design and are critically presented in this review.
Mechanism of Action of PMEs
Pectinmethylesterase (PME, EC 3.1.1.11), an endogenous enzyme, can be found in the cell walls of fruits and plants and can be produced by several microorganisms [52, 53]. PMEs of higher plants such as citrus [26, 34, 54–58], banana [47, 48], apple [59], strawberry [60], apricot [61], persimmon [62], papaya [63, 64], cherry [65], peach [66], plum [67], pepper [42], carrot [41, 46, 68, 69] and tomato [70, 71] have been the focus of several studies that include purification, molecular investigations and kinetic research of enzyme activity and stability.
In general, the PMEs are moderate-sized enzymes with molecular weight ranging from 25 to 54 kDa [72]. They are mainly active as monomers. Most of the PMEs are glycoproteins, but lipoproteins have also been indentified mainly from bacteria such as Erwinia chrysanthemi [73]. With regard to the isoelectric point, values from 3.1 for fungal PME to 11 for a tomato PME have been reported [72]. It has been found that the stability of PMEs depends on several parameters such as matrix composition, model system in which the enzyme is dissolved, purification level of enzyme and pH [13, 33, 74]. According to the above statements, the stability of PME depends on the source of the enzyme.
PME is responsible for the de-esterification of pectin, releasing methanol, pectin with a low degree of esterification and hydrogen ion, as depicted in Picture 1.
Mechanism of action of PMEs on pectin substrate (Source: Jolie et al. [75])
Different mechanisms of PME action have been proposed. The mostly accepted hypothesis is that they could act either randomly or linearly along the chain of pectins [75–77]. When PMEs act randomly on homogalacturonans, demethylesterification releases protons that promote the action of endopolygalacturonases and contribute to cell wall loosening. When PMEs act linearly on homogalacturonans, they give rise to blocks of free carboxyl groups that could interact with Ca2+, creating a pectate gel. Because the action of endopolygalacturonases in such a gel is limited, this action pattern of PMEs contributes to cell wall stiffening. Three-dimensional crystallography of microbial (produced by E. chrysanthemi, PemA and Yersinia enterocolitica) [78] and plant PME obtained from carrot [79] and tomato [80] has been reported. Based on the 3D structure of PME and primary sequence alignments, Pelloux et al. [81] suggested a specific mechanism of action. In particular, two aspartates (Asp) and one arginine (Arg) are strictly conserved among PMEs. One of the Asp residues makes a hydrogen bond to Arg and is therefore most likely unprotonated. The other Asp is likely to be protonated. A water molecule adjacent to the unprotonated Asp may be activated by transferring its proton to the Asp. The hydroxyl generated can then attack the carbonyl carbon. Simultaneous protonation of one of the oxygens results in the formation of a tetrahedral intermediate, which collapses with the release of methanol and thus results in demethylation.
Effects of Combined High Pressure and Temperature on PME from Various Plant Sources
Changes in active site or enzyme denaturation (conformational alteration of protein molecule) can lead to a reversible or an irreversible loss of activity. HP can modify protein structure [82] and thus enzyme activity [83]. The principles of structure of proteins including optimum packing of the hydrophobic core, minimum hydrophobic surface area and ion pairs within and between subunits have to be taken into account when studying the effect of processing on the structural changes of the enzymes. The HP mechanism for enzyme denaturation is governed by the Le Chatelier principle, which predicts that application of pressure shifts an equilibrium to the state that occupies the smallest volume, so any reaction accompanied by volume decrease is accelerated by elevated pressures [27]. In view of the specificity of enzymatic reactions, enzymes may be affected by pressure in several ways [84]: (i) pressurization at ambient temperature may lead to reversible or irreversible, partial or complete enzyme inactivation resulting from conformational changes in the protein structure; (ii) enzymatic reactions may be accelerated or retarded by pressure, depending on the positive or negative reaction volume; (iii) a macromolecular substrate may become more sensitive to enzymatic depolymerization or modification once it has been pressurized and (iv) intracellular enzymes may be released in extracellular fluids or cell cytoplasm due to alteration of the membranes by pressure, thereby facilitating enzyme–substrate reactions.
For the controlled inactivation of many plant PMEs, due to their extreme pressure (needed pressures higher than 600 MPa at room temperature) and thermal (needed temperatures higher than 80 °C, at atmospheric pressure) stability, combined processes (HP in conjunction with mild or elevated thermal treatment) might be needed. Combined HP treatments may increase the efficiency of non-thermal processing and reduce the severity of non-thermal treatment needed to obtain a given level of enzyme inactivation. Thus, processing conditions required are generally less severe than those used for either treatment alone. The results found in most papers cited in the literature, as previously discussed, show that when processed at the same process conditions, the degree of PME inactivation from different sources varies. This variation can only be partly attributed to the type of enzyme, the presence of other enzymes, type of substrates, ionic strength, pH, nature of the medium in which the enzyme is dispersed, pressure, temperature and treatment time [33, 84, 85].
In general, more intense pressure and temperature process conditions enhance enzyme inactivation. In some cases, there is a synergistic effect of pressure and temperature (process combining pressure at a certain temperature results in faster inactivation when compared to enzyme inactivation by only thermal treatment at the same temperature), as expected (an alteration in the volume of hydration influences the denaturation of the enzyme under a high pressure environment that, in combination with thermal treatment, results in enzyme unfolding and higher inactivation due to synergistic effect) [86]. However, at high temperatures (close to temperatures resulting in thermal inactivation of enzymes at atmospheric pressure, i.e. >70 °C), an antagonistic effect of pressure and temperature could be observed. In these cases, the enzyme inactivation is slower when the enzyme is treated at a certain temperature combined with pressure, compared to the inactivation by only thermal processing. Such antagonistic effect of pressure on thermal inactivation can be explained by the fact that at atmospheric pressure, temperature increase affects both non-covalent and covalent bonds, resulting in aggregated or incorrectly folded enzymes. It entails that the active site becomes inaccessible (due to protein unfolding) and the enzyme loses its activity. On the contrary, when increasing pressure, some parts of the enzyme molecule (especially the active site) are ordered, resulting in partial or complete recovery of enzyme activity [74].
This trend has also been observed in several studies of plant PME inactivation, with pressure and temperature exerting counteracting effects on the low-pressure–high-temperature region. Fachin et al. [70] investigated the effects of HP on purified tomato PME and PME in tomato juice and found that PMEs were pressure-stable, with a distinct antagonistic effect of pressure and temperature. This is in line with the work of Stoforos et al. [25] who observed high inactivation rate of tomato PME during processing at 75 °C and ambient pressure and reduction of PME inactivation with increasing processing pressure (at pressures between 200 and 600 MPa) at the same temperature. Other researchers studied the HP inactivation of orange PMEs and found a synergistic effect of pressure and temperature on this enzyme under HP processing conditions, except in the high-temperature (>70 °C)–low-pressure (<300 MPa) region where an antagonistic effect was noted [32, 34]. Such a behaviour was also reported for various plant PMEs by other researchers, i.e. inactivation of carrot PME [45], banana PME [48], white grapefruit PME [30], green pepper PME [42] and peach PME [40]. A synergistic effect of pressure and temperature may be observed for some enzymes when treated up to a certain pressure or/and temperature, and an antagonistic effect may be observed for more intense process conditions and vice versa.
Structural changes in HP-treated PMEs may elucidate the mechanism underlying enzyme inactivation at the molecular level and may provide information for further assumptions. Taking into account the principles of structure of proteins, i.e. optimum packing of the hydrophobic core, minimum hydrophobic surface area and ion pairs within and between subunits, it is clear that HP has to be effective at the levels of both tertiary and quaternary structures and possibly secondary structure. Alterations in protein conformation may lead to changes in activity of those proteins.
Regarding PMEs, since structural changes are responsible for changes in their catalytic behaviour, HP-induced structural changes of enzymes are important aspects to be discussed. Alexandrakis et al. [26] investigated the HP-induced and the heat-induced structural changes upon the purified PME molecules from two different orange sources (Navel and Valencia cv.). Results showed that the pressure effects were negligible in the secondary structure of orange PMEs. This verifies that HP alone does not cause alterations on the molecule’s secondary structure. Instead, it supports the assumption that pressure bears a minimum effect upon the hydrogen bonds that are responsible for the secondary structure network maintenance [86, 87].
On the other hand, the near-UV CD spectra of PMEs, associated with the enzyme’s tertiary structure, reveal significantly altered patterns. The application of pressure led to extensive, irreversible changes of the enzyme. The magnitude of these structural changes was greater for higher pressure values and was otherwise independent of the duration of the treatment for temperatures below the PME’s thermal denaturation limits. Pressure is generally assumed to denature proteins by the destabilization of hydrophobic aggregates, thus allowing water molecules to be forced into the protein interior. This will, in turn, affect the molecule’s tertiary structure [88]. It is evidenced that exposure to HP may lead to a structurally molten globule-like state, where the PMEs maintain a secondary structure of untreated protein molecules, while a tertiary structure is substantially affected bearing subsequent impact on the substrate–enzyme binding interaction, leading to reduction of enzyme activity.
Effect of HP on PME Inactivation in Citrus Products
High pressure processing may affect the stabilization of citrus-related juices, resulting in an extension of their shelf-life. A number of studies have shown that PME in citrus-based products is not fully inactivated after certain pressure treatments [28, 31–34, 89, 90].
Basak and Ramaswamy [15] studied the effect of HP on PME activity in orange juice (freshly squeezed or reconstituted frozen concentrate with commercial citrus PME added) in the range of 100–400 MPa and investigated the effects of pH on pressure inactivation of PME. PME obtained from orange juice was found to be inactivated more rapidly at pH 3.2 than at pH 3.7. At the natural pH of 3.7, inactivation of PME was found to be relatively small (up to 25% at 400 MPa). However, the effect was clearly noticeable at pH 3.2 with inactivation increasing from about 25% at 100 MPa to as high as 90% at 400 MPa. They also reached a conclusion that total soluble solid content in orange juice affects the inactivation rate of PME. The baroprotective effect of orange juices containing large concentrations of soluble solids on PME activity was demonstrated.
Cano et al. [27], working in the pressure range of 50–400 MPa combined with heat treatment at 20–60 °C, reported that only combinations of low pressures and mild temperatures inactivated PME in freshly squeezed orange juice (Citrus aurantium, Salustiana, Spain), with a maximum 25% reduction of the initial activity of PME after treatment at 200 MPa and 30 °C. Goodner et al. [28] investigated the PME inactivation in orange juice with additional pulp (Valencia cv.) using high pressure processing in the range of 500–900 MPa. Results showed that the thermal-sensitive form of PME was effectively inactivated, while the thermal-tolerant form was slightly affected. Nienaber and Shellhammer [91] found that PME in non-concentrated frozen Florida oranges followed first-order kinetics in the range of 400–600 MPa and 25–50 °C with residual activity of the pressure-resistant enzyme. Other researchers used the response surface method in order to evaluate the combined effect of pressure cycle, pressure level and treatment duration on inactivation of PME in single strength and concentrated orange juice during high pressure processing [89]. Furthermore, kinetic studies on the inactivation of PME in model systems of commercial PME purified from orange peel (Valencia cv.) in the range of 50–900 MPa combined with temperatures from 15 to 67 °C were conducted by Van den Broeck et al. [34]. They found that high pressure inactivation of PME could be described by a first-order fractional conversion model, estimating the inactivation rate constant of the labile fraction and the remaining activity of the stable fraction.
The degree of PME inactivation depends on the environment of the enzyme-model solution or the particular food system; even on the variety and origin of the material used, other researchers examined the effect of intrinsic factors such as pH, food composition and purification level of enzyme on PME inactivation, combined with thermal and high pressure processing. Irwe and Olsson [85] investigated PME inactivation in different orange juices by applying pressures up to 600 MPa combined with moderate temperatures. They concluded that the degree of PME inactivation was dependent on the citrus variety used. This is in line with other works which indicated that PME from Valencia orange juice (inactivation rate constant, k = 2.99 min−1) was found to be more sensitive than Navel orange juice PME (inactivation rate constant, k = 0.35 min−1) at the same treatment conditions (400 MPa, 30 °C) [31, 32]. Similarly, PME from Navel orange juice was found to be more sensitive than purified PME from white grapefruit (Citrus paradisi) (k values were calculated as 1.76 and 0.113 min−1 respectively at 600 MPa and 50 °C) [30, 32]. Sampedro et al. [33] studied the inactivation kinetics of PME in an orange juice–milk-based beverage system as well as in different orange matrices under combined conditions of HP and heat. PME was found to be more thermostable in the orange juice–milk beverage than the other media, while it was more pressure-resistant in the purified enzyme in a buffer system (pH = 7.0). Residual enzyme activities of 17 and 6% were observed in the orange juice–milk system and orange juice after a treatment at 750 and 700 MPa respectively, while a remaining activity of 20% was observed after a treatment at 800 MPa in the case of the purified enzyme (pH 7.0). This fact could mean that pH, matrix composition and purity all contribute significant roles in the stability of PME against the different HP processing conditions.
Collectively, these studies demonstrated the potential of high pressure processing to inactivate PME to a level that preserves cloud. Bull et al. [90] made an effort to determine a commercially suitable HP that would allow production of a high-quality orange juice (Navel and Valencia cv.) with a refrigerated shelf-life sufficient to meet the requirements of the market. Other researchers pointed out that HP can be used for Valencia orange juice cold pasteurization, by inactivating the factors that cause quality deterioration, such as dominant spoilage microorganisms (lactic acid bacteria) and endogenous PME, while minimally affecting its nutritional and sensorial characteristics. They found that the optimal estimated process conditions for that type of orange juice were 360 MPa at 35 °C for 2 min [31].
Effect of HP on PME Inactivation in Carrot-Based Products
Many researchers support that high pressure processing would be satisfactorily implemented to carrot-based products in order to produce safe products of high quality. Ly-Nguyen et al. [41] found that the thermal and the pressure inactivation of purified carrot (“BELGIAN red carrot”) followed a fractional conversion model with a residual PME activity (3%), indicating the existence of both a pressure-labile and a pressure-tolerant isoenzyme in the pressure range of 600 to 700 MPa. In comparison to PME obtained from oranges, the carrot PME is more thermal-sensitive. Ly-Nguyen et al. [45] also reported that pressure and temperature act synergistically, except in the high-temperature (>50 °C) and low-pressure region (100–300 MPa) where an antagonistic effect was found. These authors also pointed out that a kinetic study of carrot PME in real carrot-related products is worth investigating. Hence, Balogh et al. [44] studied the carrot pieces and juice PMEs as well as purified PME of this vegetable using HP of 700 and 800 MPa, in the range of 10–40 °C, in order to evaluate this technology on these products. Results indicated that PME contained in carrot pieces (decimal reduction time, D-value of about 161 min at 700 MPa and 10 °C) appeared to be more sensitive than PME in carrot juice (188 min, respectively) and purified PME (172 min, respectively). They also examined the stability of carrot PME at pH 4.5, 5.5 and 6.0. Purified carrot PME seemed to be more thermostable and pressure stable at pH 6.0 (which is the pH of carrot juice) compared to its stability at pH 4.5 or pH 5.5.
Effect of HP on PME Inactivation in Tomato-Based Products
Several studies have dealt with the impact of HP on PME inactivation in tomato-based products [36, 37, 39, 71, 92–95]. Aiming at an optimal process design of this kind of product, the knowledge of the stability (inactivation) of the enzymes that affect the quality of the final product is necessary. Crelier et al. [93] compared heat inactivation and combined pressure–heat inactivation of PME and PG in tomato juice. Tomato PME and PG were inactivated by heat treatment at atmospheric pressure following first-order kinetics at temperatures of 60 to 75 and 80 to 105 °C, respectively. Tomato PG activity was inactivated completely by HP treatment at 600 MPa and 30 °C for 5 min. Stabilization of tomato PME was observed at temperatures of 60 to 75 °C under pressures ranging from 100 to 600 MPa. This is in line with the work of Shook et al. [95] which indicated that PME in tomato pieces is very stable in the range of 400–800 MPa and 25–45 °C. Van den Broeck et al. [38] studied the effect of HP and thermal treatment on activity of commercial tomato PME. Tomato PME was found to be heat-sensitive at atmospheric pressure, but it was very pressure-resistant [96, 97]. Fachin et al. [70] also examined the processing stability of purified tomato (Lycopersicon esculentum var. Flandria Prince) PME in buffer solution as well as in tomato juice. In both systems, PMEs were pressure-resistant and it was observed that pressure acts antagonistically to the temperature. Such a behaviour was also reported by Stoforos et al. [25] pointing out that the tomato processing should be described by considering two mechanisms of inactivation. One of the mechanisms can be related with pressure, the other one with temperature-induced changes in enzyme activity. Rodrigo et al. [37] reported that only high pressures (above 700 MPa) inactivated PME in different tomato varieties. This is in line with the findings observed by Houben et al. [98] mentioning that only 30% of pressure-stable PME in tomato puree was inactivated at 800 MPa (20 °C, 10 min).
A residual activity of 50% was observed in the tomato juice after a treatment at 850 MPa for 15 min. Nevertheless, Plaza et al. [36] reported the existence of a pressure-labile PME isoenzyme in tomato (L. esculentum, variety Perfect Peel).
Boulekou et al. [5] studied the HP inactivation of PME and PG in tomato variety (Red Sea). They concluded that high pressure processing can be used for the selective inactivation of PME and PG leading to products with improved quality characteristics such as viscosity, colour and consistency. According to the overall results of this work, high pressure processing could be used in order to replace the traditional industrial tomato processing methods leading to products with superior-quality characteristics.
Effect of HP on PME Inactivation in Other Products
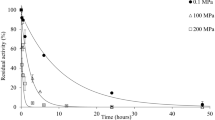
High pressure processing of several vegetables and fruits is advantageous from the point of nutrient content because non-significant detrimental impacts of this technology on nutrients have been reported. Ly-Nguyen et al. [47, 48] studied the effect of pressure (up to 900 MPa) combined with mild temperature on PME extracted and purified from banana (Cavendish cv.). High pressure inactivation of this enzyme can be described by a fractional conversion model. Residual activity of purified banana PME was estimated to be approximately 8% after HP treatment in the range of 600 to 700 MPa at 10 °C for prolonged times accounting for the presence of a pressure-stable fraction of PME in bananas. When pressure of 800 MPa was applied at temperatures higher than 70 °C, the inactivation of purified banana PME was decreased compared to equivalent heat treatments at atmospheric pressure indicating an antagonistic effect of pressure and heat. Banana PME was sensitive to pressure increases ranging from 700 to 800 MPa at 64 °C.
Ly-Nguyen et al. [60] also purified strawberry PME and subjected the PME to HP treatments at ambient temperature. Pressure stability of strawberry PME was characterized by a fractional conversion model suggesting the presence of both pressure-labile and pressure-tolerant forms with prolonged pressure treatment. Strawberry PME was extremely pressure-resistant with a smaller k value during pressure treatments at 1000 MPa (k = 0.0260 min−1). In addition, the effect of HP on PME in strawberry puree was investigated by Bodelon et al. [99] at 100–400 MPa/20 and 50 °C for a treatment duration of 15 min. Maximum inactivation of 13% was observed at 300 MPa/50 °C/15 min. On the other hand, Chakraborty [100] reported that the addition of sugar in strawberry puree enhanced PME inactivation during HP. A pressure–temperature synergy was observed with maximum inactivation (60%) achieved at 600 MPa/10 min/80 °C/30% added sugar. Nunes et al. [67] investigated the effect of high pressure on purified PME from greengage plums (Prunus domestica cv.) and found that its pressure inactivation could be described by a first-order kinetic model in a pressure range of 650–800 MPa at ambient temperature. PME from plums was more thermal- and pressure-tolerant for treatments below 600 MPa compared to PME from other fruits such as peach pulp [40] and orange juice [32]. Several studies dealt with the effect of HP on PME inactivation in pepper-based products. Castro et al. [42] investigated the HP inactivation of the labile fraction of purified pepper Capsicum annuum cv.) PME in a model system (pH = 5.6). It was found that pressure acts antagonistically to the temperature at lower pressures (P < 300 MPa) and high temperatures (>54 °C).
Baron et al. [101] working in the pressure range of 200–600 MPa combined with heat treatment at 15–65 °C observed partly inactivation of PME in apples (Golden Delicious). In particular, 61 and 68% inactivation of purified apple PME in citric–phosphate buffer (pH 4.0) was observed after application of HP at 100 and 650 Mpa, respectively, at 20 °C regardless of the treatment time, which appears to be due to the instantaneous pressure inactivation of the pressure-labile fraction. Boulekou et al. [40] studied the effect of HP (100–800 MPa) combined with temperature (30–60 °C) on PME in peach pulp (Everts cv.). They reported that pressure and temperature acted synergistically on PME inactivation, except at the high temperature of 70 °C and middle pressure range (100–600 MPa), where an antagonistic effect of pressure and temperature was observed. Pressure effects on PME in peach juice was investigated by Rao et al. [102] at 400–600 MPa and 25 °C for 5–25 min. Maximum 50% PME inactivation was reported at 600 MPa/25 min/25 °C, whereas no change was observed at 400 MPa. Bermúdez-Aguirre et al. [103] investigated the pressure stability of mango (Mangifera indica L. cv.) nectar PME at three different pressures (247, 345 and 415 MPa—17 °C). PME was found to be pressure-resistant, showing the highest decrease in enzymatic activity (45%) after 4 min at 345 MPa but with a significant activation at 414 MPa.
Katsaros et al. [50] found that the thermal and high pressure inactivation of persimmon PME was described by first-order kinetics both in thermal and in HP treatment. Persimmon PME appeared to be an enzyme of high pressure and temperature resistance. For a 90% enzyme inactivation, 5.5 min at 90 °C or 35 min at 800 MPa and 70 °C is required. Ortuno et al. [104] investigated the effect of HP on PME in feijoa (Acca sellowiana) puree. The residual PME activity of HP-treated samples at 600 MPa (25 °C, 5 min) was found to be equal to 65%. Also, they pointed out that using HP along with other techniques such as dense phase carbon dioxide (DPCD), lower HP pressures may be used for a given inactivation level.
HP inactivation of PME in watermelon juice was studied by several researchers [105, 106] where contradictory findings were noticed. According to Liu et al., inactivation of 77% was achieved at 600 MPa/25 °C/60 min, while Zhang et al. supported that more intense process conditions (900 MPa/60 °C/40 or 60 min) are required for a similar degree of inactivation.
Alexandrakis et al. [51] studied the effect of the conventional thermal pasteurization (60–80 °C) and high pressure (200–600 MPa) cold pasteurization (temperatures lower than 35 °C) on sea buckhorn juice. Based on the PME inactivation and antioxidant activity retention, they suggested that the optimal process conditions for commercial production of superior-quality sea buckthorn juice were 600 MPa, 35 °C and 5 min process time.
Factors Affecting Sensitivity of PMEs to Pressure Inactivation
As clearly demonstrated in the previous paragraphs, stability of PMEs against HP and thermal processes depends on the source of the enzyme. Among the PMEs investigated, tomato PME appears to be the most pressure-resistant with no inactivation at ambient condition even up to 800 MPa, while orange PME is the least pressure-resistant with the inactivation of the labile fraction to be achieved at ambient temperature and pressures close to 300 MPa. Purified strawberry and banana PMEs have also been reported to be pressure-stable since their inactivation requires pressures above 800 MPa in combination with mild heating. Reference could be made to Table 1 in which the most important findings of published works regarding the effect of HP parameters on the activity of plant PMEs in different matrices are summarized.
One possible explanation about the aforementioned enzyme’s behaviour against HP processing is that PMEs from different sources exist in several isoforms, which may be distinguished by their molecular weight, isoelectric point, biochemical activity and stability. PMEs from different plant sources or from the same source but different variety may vary towards pressure inactivation since different isoenzymes may be present or the same isoenzymes may be found in different proportions. Further to the above, the inactivation rate of PME is dependent on the nature of the medium in which the enzyme is dissolved or on food composition that the enzyme exists. In most cases, PME found to be more resistant in an intact tissue is protected by the presence of food components than in its purified form. In particular, Balogh et al. [44] investigated the inactivation of purified carrot PME in different buffer solutions (0.02 M Tris buffer at pH 6.5 and 7.0; 0.1 M citrate buffer at pH 4.5, 5.5, and 6.0) as well as in carrot juice and tissue. PME was found to be more heat- and pressure-resistant in carrot tissue than in carrot juice or its purified form. At 800 MPa and 40 °C, the k value of PME in carrot tissue was 0.03 min−1, whereas at 800 MPa and 10 °C, it was 0.06 and 0.08 min−1 for PME in carrot juice and buffer solution (pH 6.0), respectively. Nevertheless, the opposite has been reported in other cases that higher inactivation rate of PME was observed in matrix composition compared to purified enzymes in model systems. A study by Sampedro et al. [33] showed that purified orange PME in 20 mM Tris buffer (pH 7.5) was more pressure-resistant compared to PME in different orange matrices i.e. orange juice–milk beverage and orange juice. A remaining activity of 17 and 6% was observed in the orange juice–milk system and orange juice after a treatment at 750 and 700 MPa respectively. On the other hand, a remaining activity of 20% was observed after a treatment at 800 MPa for the purified enzyme. This could be explained by the differences in the amount of the pressure-labile PME fraction found in the different systems.
Another factor that affects significantly the stability of PMEs is the pH of the medium in which it dissolved the purified enzyme. Enzymes are typically more sensitive to acidic than to alkaline environments. As pH values increased, a significant increase in resistance to inactivation was observed. Basak and Ramaswamy [15] reported higher inactivation rate of PME in orange juice at pH 3.2 compared to the natural pH of the juice (pH 3.7). Similarly, Van den Broeck et al. [13] observed higher rate of pressure inactivation of the thermolabile orange PME in buffer of pH 3.7 compared to deionized water at a higher pH (4.5). However, the opposite was observed for PME obtained from tomato as the enzyme was found to be less stable at pH 4.2 compared to pH 7.0 [93]. Likewise, D values of 11.5, 33.5 and 64.8 were determined for the inactivation of purified heat-labile carrot PME at 750 MPa and 25 °C at pH 4.5, 5.5 and 6.0, respectively, indicating significantly higher-pressure stability at higher pH [44]. Apparently, the effect of pH on the stability of enzymes depends on both their structure and biochemical properties.
Modelling Inactivation of PME as a Function of Temperature and Pressure
Data cited in the literature on the inactivation of PME from different plant sources by HP processing were collected. Only studies on which processing and experimental conditions were well documented and suitable statistical methods were used in the subsequent interpretation of the data were considered. The studies used in the present review were also selected according to the adequacy of the experimental domain i.e. range and combinations of temperature and pressure conditions. The inactivation of these enzymes conducted by a significant number of researchers by high pressure has been investigated under various experimental conditions. In general, HP processing of plant PMEs was achieved in the wide range of pressures from 100 to 900 MPa combined with low to moderate temperatures (less than 80 °C). The selection of the process condition range was based on the pressure stability of enzymes. All pressure experiments were carried out in laboratory-scale HPP equipment, which allow pressurization even up to 1000 MPa in combination with temperatures ranging from −20 to 100 °C. In most of the cases, the pressure-transmitting fluid used was water, but polyglycol ISO viscosity class VG 15 has also been reported as pressure medium. In order to achieve the desired operating temperature during pressurization, the initial increase of temperature due to adiabatic heating during pressure buildup was taken into account. It has been reported that pressurization may increase the temperature of the foods by approximately 3 °C per 100 MPa, depending on the composition of the food. Moreover, one of the most important aspects frequently overlooked in the analysis of high pressure treatments is the pressure-induced pH change. Even though during depressurization pH might return to its initial value, the pressure-induced pH shift, while the food is under HP, may affect the inactivation rate of enzymes. The limited consideration in published works of the food pH changes induced by pressure could be justified by the lack of practical and widely available instruments. However, knowledge of the direction of pH shift and its magnitude for each product is necessary for the determination of optimal HP conditions.
A significant number of these studies have been carried out directly in fruit juices and pieces, others in model systems after extraction and purification of PME. Almost in all cases, PME in different systems (buffer, juice, tissue) followed first-order inactivation kinetics (Eq. 1). However, the existence of several isoenzymes of PMEs, which show different heat or/and pressure resistance, has also been observed (Tables 1 and 2) [107]. These data can be fitted in the biphasic model that could be explained based on the hypothesis of at least two PME isozymes, a pressure-resistant and a pressure-labile one. However, this hypothesis needs to be independently validated. Apart from the biphasic model, the series type model could also be used to describe these data (with different assumptions) [108] with similar fitting adequacy to the biphasic model. In the case of fractional conversion model (Eq. 2), first-order inactivation is applied taking into account a non-zero residual activity upon prolonged processing.
For comparison purposes, it is proposed that all the data cited in the literature be modelled using a uniform procedure and one-model equation, instead of different models. Specifically, the inactivation of all these enzymes can be described by a first-order kinetic model (Eq. 1.) [14]:
where A o and A t are the initial activity and the remaining activity at time t, respectively, and k is the inactivation rate constant (min−1).
In the case of the fractional conversion model for all pressure–temperature conditions, Eq. 2 could be used:
where A is the PME activity after processing for a treatment duration t, A f is the residual activity after processing, A o is the initial activity, t is the processing time (min) and k is the inactivation rate constant (min−1). The above kinetic model may describe adequately the loss of PME activity during processing, showing a first-order inactivation of the sensitive portion of the enzyme (labile isoenzyme) and the presence of a resistant enzyme fraction that is hardly inactivated by the pressure or temperature applied.
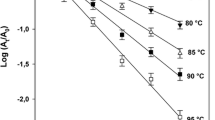
The temperature dependence of the inactivation rate constant, k, could be described adequately by Arrhenius equation and expressed in terms of activation energy, E a (kJ/mol):
where Tref is the reference temperature, k Tref is the inactivation rate (min−1) at Tref and R the universal gas constant (8.314 Jmol−1 K−1).
The effect of the pressure processing on the activation energy values could be expressed by an exponential equation.
With regards the pressure effect on the inactivation rate constant, k, the Eyring equation may be used (Eq. 5) and expressed through the activation volume, V a (ml/mol):
where Pref is the reference pressure, k Pref is the inactivation rate (min−1) at Pref and R is the universal gas constant (8.314 Jmol−1 K−1). The dependence of activation volume on temperature was expressed by a linear function:
Based on Eqs. (3) and (5) and taking also into consideration the effect of pressure on E a (Eq. 4) and the effect of temperature on V a (Eq. 6), Polydera et al. [32] developed a multi-parameter equation to mathematically predict the inactivation rate constant at any combination of pressure and temperature conditions.
Other researchers have used a higher-order polynomial model in order to describe the combined pressure and temperature dependence of the inactivation rate constant [48]. This equation results from the conversion of the thermodynamic model described by Hawley [109] into a kinetic model through the transition state theory of Eyring [110] and a subsequent small modification proposed by Smeller [111]. This type of kinetic model was successfully applied to model the combined pressure and temperature dependence of various enzymes [110, 112, 113].
Using literature data, the exponential mathematical model (Eq. 7) was uniformly applied to enable a better comparative assessment of pressure effects on all PMEs discussed in this manuscript, obtained from different plant sources.
All the data were fitted in Eq. 1 or 2, depending on the existence of resistant enzyme fraction. Applying Eqs. 3 and 5, the E a and V a values were estimated, while from Eqs. 4 and 6, the dependence of pressure on E a and temperature on V a was modelled. The estimated E a values for all the plant PMEs in different systems (buffer, juice, tissue) are presented in Tables 3 and 4. For Valencia orange and sea buckthorn juice PMEs, the E a values increased with increasing pressure indicating more temperature dependence of the enzyme inactivation rate at higher pressures, while for Navel orange, peach, persimmon, white grapefruit, carrot, pepper and banana PME, the opposite phenomenon was observed (PMEs are less temperature-dependent at elevated pressure).
The V a values for all the plant PMEs in different systems (buffer, juice, tissue) were also estimated (Tables 5 and 6). Negative activation volumes indicate that PME inactivation was favoured by pressure. In case that the increase of temperature results in reduced absolute values of activation volume, the inactivation rates became less pressure-dependent.
Estimated parameters for the model in Eq. 7 for various plant PMEs are summarized in Tables 7 and 8. Satisfactory agreement was found between fitting this model and PME inactivation data reported in the literature for various plant sources. For statistical assessment, the R 2 values (observed versus predicted values) were used to compare the experimental values with the predicted values obtained by Eq. 7. The higher the R 2 value, the better the adequacy of the model to describe the experimental data. For all fittings, R 2 values ranged from 0.663 to 0.994.
By inserting all model parameters of Tables 7 and 8 into Eq. 7, pressure–temperature combinations resulting in specific pre-set inactivation rate constants for PMEs can be simulated and can be depicted in 3D plots (Figs. 1 and 2). Under isothermal conditions, the pressure stability of PMEs was found to vary ranging from pressure-sensitive types like orange juice (Valencia cv.) PME (higher inactivation rates at the same process conditions compared to all other PME sources), to extremely barotolerant ones like purified banana PME (Cavendish cv.) (even 800 MPa pressure results in low inactivation rate constants equal to 0.05 min−1 at 50 °C). The half-life times, t 1/2 (min) (the time required by the enzyme to lose half of its initial activity), of several plant PMEs were estimated at 600 MPa and 50 °C and are presented in Fig. 3. The half-life time determination allows for easy and direct comparison of the inactivation of the studied enzymes. In general, banana PME was found to be the more difficult to inactivate (t 1/2 higher than 150 min), followed by persimmon PME (t 1/2 higher than 50 min), while for all the other sources the half-life times ranged from 1 (for tomato-labile enzyme and Valencia orange PMEs) to 25 min (for carrot PME). Excluding persimmon PME that appeared to be significantly resistant to pressure and temperature, one general comment comparing all data presented is that the purification affects the stability of the enzyme (purified enzymes were more resistant).
Predicted inactivation rate constants (k, min−1) at any combination of pressure and temperature predicted by the exponential mathematical model (Eq. 7) for PMEs from different fruits and vegetables: a banana (purified form), b carrot (purified form), c white grapefruit (purified form), d pepper (purified form), e tomato (purified form)
Predicted inactivation rate constants (k, min−1) at any combination of pressure and temperature predicted by the exponential mathematical model (Eq. 7) for PMEs from different fruits and vegetables: a Navel orange juice, b Valencia orange juice, c sea buckthorn juice, d peach pulp, e persimmon pulp
Estimated t 1/2 (min) values for plant pressure-tolerant PMEs at 600 MPa and 50 °C. Filled diamond, purified tomato PME (labile fraction); filled triangle, purified Valencia orange PME; filled circle, peach PME; open circle, white grapefruit PME, open diamond, purified pepper; open triangle, purified Navel orange PME; gray triangle, purified carrot PME; filled square, persimmon PME; asterisk, purified banana PME
The practical significance of the results presented in this manuscript can be shown by examples of how to use them for the optimization of processing (Fig. 3). The selection of the process conditions could be based on the sufficient process pressure–temperature and time for the total—or partial—inactivation of PMEs, by estimating the inactivation rate constant at any combination of pressure–temperature, thus calculating the necessary process time for PME inactivation (either partial or total). More specifically, the data obtained by Katsaros et al. [31] could be used for Valencia orange juice HP cold pasteurization, by inactivating the factors that cause quality deterioration, such as LAB and PME, while minimally affecting its nutritional and sensorial characteristics. They concluded and suggested that the optimal estimated process conditions for that type of orange juice are 360 MPa pressure, at 35 °C for 2 min and obtaining 90% inactivation of PME. Comparing these data with data cited in the literature [32] for the same fruit of different variety (Navel orange juice), more intense HP conditions (600 MPa, 40 °C, 4 min) are required for the pasteurization of Navel orange juice (Navel PME is more pressure-tolerant compared to Valencia PME).
Conclusions
This review has discussed the effects of a number of factors on the inactivation of PMEs treated with HP. It is clear from the in-depth analysis of the literature that the pressure inactivation of PME depends on numerous parameters including the type and the composition of the food, the purification level of enzyme, the pressure intensity and the treatment temperature. Data cited in the literature on PME inactivation by pressure were fitted in first-order and fractional conversion kinetic models under a range of conditions referred to the sources used. High efficiency in inactivating PMEs caused by HP, beneficial to preserving the food quality, is dependent on the parameters of the process (pressure, temperature and treatment duration). All data were uniformly modelled by an exponential mathematical model to allow quantitative assessment of the effect of the parameters of the process. The PME inactivation rate constant was expressed as a function of the temperature and pressure process conditions used. This function incorporates the observed exponential dependence of activation energy on the pressure conditions, as well as the linear dependence of activation volume on process temperature. Using one mathematical model as the one applied, the pressure–temperature combinations necessary to inactivate the PMEs can be estimated allowing for comparative studies and enabling a proper design of HP combined with mild temperature treatment in many fruit and vegetable products.
PME is usually known to be more heat- and pressure-resistant than the common spoilage microorganisms (i.e. in orange juice) [28]. For HP cold pasteurization, the necessary temperature or/and pressure process conditions sufficient for the inactivation of PME should be the selection criteria, since PME inactivation conditions are sufficient for the elimination of main juice spoilage factors [23].
Concluding, the inactivation of enzymes such as PME, at low or moderate temperatures without changing organoleptic and nutritional properties, shows that high pressure technology has the potential to be used in the development of a new generation of value-added foods. Some of the results that were discussed in this paper may be directly applied in the food industry. Puree, fruit preparations, juices (orange, apple, pomegranate, carrot, broccoli, beetroot, etc.) and smoothies are only some examples of a wide range of fruit and vegetable products found in the market processed by HP. The selection of the process conditions could be based on the sufficient process pressure–temperature and time for the total—or partial—inactivation of PMEs.
References
Alkorta I, Garbisu C, Llama MJ, Serra JL (1998) Industrial applications of pectic enzymes: a review. Process Biochem 33:21–28
Kashyap DR, Vohra PK, Chopra S, Tewari R (2001) Applications of pectinases in the commercial sector: a review. Bioresour Technol 77:215–227
West S (1996) Olive and other edible oils. In: Godfrey T, West S (eds) Industrial enzymology, 2nd edn. Stockholm Press, New York, pp. 293–300
Kilara A (1982) Enzymes and their uses in the processed apple industry: a review. Proc Biochem 23:35–41
Boulekou S, Mallidis C, Taoukis P, Stoforos N (2011) Quality evaluation of slightly concentrated tomato juice produced under high pressure conditions. Procedia–Food Sci 1:800–804
Pilnik W, Voragen AGJ (1993) Pectic enzymes in fruit juice and vegetable juice manufacture. In: Reeds G (ed) Food and science technology, enzymes in food processing. Academic Press, New York, pp. 363–399
Ceci NL, Lozano JE (2010) Use of enzymes for non-citrus fruit juice production. In: Bayindirli A (ed) Enzymes in fruit and vegetable processing: chemistry and engineering applications. CRC Press, Boca Raton
Baker RA, Cameron RG (1999) Clouds of citrus juices and juice drinks. Food Technol 53:64–69
Nienaber U, Shellhammer TH (2001a) High-pressure processing of orange juice: combination treatments and a shelf life study. J Food Sci 66:332–336
Moelants KRN, Cardinaels R, Van Buggenhout S, Van Loey A, Moldenaers P, Hendrickx M (2014) A review on the relationships between processing, food structure, and rheological properties of plant-tissue-based food suspensions. Compr Rev Food Sci Food Saf 13:241–260
Cameron RG, Grohmann K (1996) Purification and characterization of a thermally tolerant pectin methylesterase from a commercial Valencia fresh frozen orange juice. J Agric Food Chem 44:458–462
Lee JY, Lin YS, Chang HM, Chen W, Wu MC (2003) Temperature-time relationships of pectinesterases in orange juice. J Sci Food Agric 83:681–684
Van den Broeck I, Ludikhuyze LR, Van Loey AM, Weemaes CA, Hendrickx ME (1999) Thermal and combined pressure-temperature inactivation of orange pectinesterase: influence of pH and additives. J Agric Food Chem 47:2950–2958
Eagerman BA, Rouse AH (1976) Heat inactivation temperature-time relationships for pectin esterase inactivation in citrus juices. J Food Sci 41:1396–1397
Basak S, Ramaswamy HS (1996) Ultra high pressure treatment of orange juice: a kinetic study on inactivation of pectin methyl esterase. Food Res Int 29:601–607
Gervilla R, Ferragut V, Guamis B (2000) High pressure inactivation of microorganisms inoculated into ovine milk of different fat contents. J Dairy Sci 83:674–682
Gould G (2002) The evolution of high pressure processing of foods. In: Hendrickx M, Knorr D (eds) Ultra high pressure treatment of foods. Aspen food engineering series, Chapter 1. Kluwer Academic, New York
Krebbers B, Matser AM, Hoogerwerf SW, Moezelaar R, Tomassen M, Van den Berg RW (2003) Combined high-pressure and thermal treatments for processing of tomato puree: evaluation of microbial inactivation and quality parameters. Innovative Food Sci Emerg Technol 4:377–385
Arroyo G, Sanz PD, Prestamo G (1999) Response to high pressure, low-temperature treatment in vegetables: determination of survival rates of microbial populations using flow cytometry and detection of peroxidase activity using confocal microscopy. J Appl Microbiol 86(3):544–556
“Bala” Balasubramaniam VM, Farkas D, Turek E (2008) Preserving foods through high-pressure processing. Food Technol 62:32–38
Hayashi R (1995) Use of high pressure in bioscience and in biotechnology. High pressure bioscience and biotechnology 13:1–7
Parish ME (1998) Orange juice quality after treatment by thermal pasteurization or isostatic high pressure. Lebensmittel Wissenschaft und Tecnhnologie 31:439–442
Knoerzer K, Buckow R, Sanguansri P, Versteeg C (2010) Adiabatic compression heating coefficients for high-pressure processing of water, propylene-glycol and mixtures—a combined experimental and numerical approach. J Food Eng 96(2):229–238
Kalamaki MS, Stoforos NG, Taoukis PS (2006) Pectic enzymes in tomatoes. In: food biochemistry and food processing. In: Hui YH, Nip WK (eds) Blackwell publishing prof , Ames, Iowa, pp. 271–292h. 12
Stoforos NG, Crelier S, Robert MC, Taoukis PS (2002) Kinetics of tomato pectin methylesterase inactivation by temperature and high pressure. J Food Sci 67(3):1026–1031
Alexandrakis Z, Katsaros G, Stavros P, Katapodis P, Nounesis G, Taoukis P (2014a) Comparative structural changes and inactivation kinetics of pectin methylesterases from different Orange cultivars processed by high pressure. Food Bioprocess Technol 7:853–867
Cano MP, Hernandez A, De Ancos B (1997) High pressure and temperature effects on enzyme inactivation in strawberry and orange products. J Food Sci 62:85–88
Goodner JK, Braddock RJ, Parish ME (1998) Inactivation of pectinesterase in orange and grapefruit juices by high pressure. J Agric Food Chem 46:1997–2000
Goodner JK, Braddock RJ, Parish ME, Sims CA (1999) Cloud stabilization of orange juice by high pressure processing. J Food Sci 64:699–700
Guiavarch Y, Segovia O, Hendrickx M, Van Loey A (2005) Purification, characterization, thermal and high-pressure inactivation of a pectin methylesterase from white grapefruit (Citrus paradisi). Innovative Food Sci Emerg Technol 6:363–371
Katsaros G, Tsevdou M, Panagiotou T, Taoukis P (2010) Kinetic study of high pressure microbial and enzyme inactivation and selection of pasteurization conditions. Int J Food Sci Technol 45:1119–1129
Polydera A, Galanou E, Stoforos N, Taoukis P (2004) Inactivation kinetics of pectin methylesterase of greek Navel orange juice as a function of high hydrostatic pressure and temperature process conditions. J Food Eng 62:291–298
Sampedro F, Rodrigo D, Hendrickx M (2008) Inactivation kinetics of pectin methyl esterase under combined thermal–high pressure treatment in an orange juice–milk beverage. J Food Eng 86:133–139
Van den Broeck I, Ludikhuyze LR, Van Loey AM, Hendrickx ME (2000b) Inactivation of orange pectinesterase by combined high-pressure and -temperature treatments: a kinetic study. J Agric Food Chem 48:1960–1970
Versteeg C, Rombouts FM, Spaansen CH, Pilnik W (1980) Thermostability and orange juice cloud destabilizing properties of multiple pectinesterases from orange. J Food Sci 45:969–998
Plaza L, Duvetter T, Monfort S, Clynen E, Schoofs L, Van Loey A, Hendrickx M (2007) Purification and thermal and high-pressure inactivation of pectinmethylesterase isoenzymes from tomatoes (Lycopersicon esculentum): a novel pressure labile isoenzyme. J Agric Food Chem 55:9259–9265
Rodrigo D, Cortes C, Clynen E, Schoofs L, Van Loey A, Hendrickx M (2006) Thermal and high-pressure stability of purified polygalacturonase and pectinmethylesterase from four different tomato processing varieties. Food Res Int 39:440–448
Van den Broeck I, Ludikhuyze LR, Van Loey AM, Hendrickx M (2000a) Effect of temperature and/or pressure on tomato pectinesterase activity. J Agric Food Chem 48:551–558
Verlent I, Hendrickx M, Verbeyst L, Van Loey A (2007) Effect of temperature and pressure on the combined action of purified tomato pectinmethylesterase and polygalacturonase in presence of pectin. Enzym Microb Technol 40:1141–1146
Boulekou S, Katsaros G, Taoukis P (2010) Inactivation kinetics of peach pulp pectin methylesterase as a function of high hydrostatic pressure and temperature process conditions. Food Bioprocess Technol 3:699–706
Ly-Nguyen B, Van Loey A, Fachin D, Verlent I, Indrawati I, Hendrickx M (2002a) Partial purification, characterization, and thermal and high-pressure inactivation of pectin methylesterase from carrots. J Agric Food Chem 50:5437–5444
Castro S, Van Loey A, Saraiva J, Smout C, Hendrickx M (2006) Inactivation of pepper (Capsicum annuum) pectin methylesterase by combined high-pressure and temperature treatments. J Food Eng 75:50–58
Castro S, Saraiva J, Lopes-da-Silva J, Delgadillo I, Van Loey A, Smout C, Hendrickx M (2008) Effect of thermal blanching and of high pressure treatments on sweet green and red bell pepper fruits (Capsicum annuum L.). Food Chem 107:1436–1449
Balogh T, Smout C, Ly-Nguyen B, Van Loey A, Hendrickx M (2004) Thermal and high pressure inactivation kinetics of carrot pectinmethylesterase (PME): from model systems to real foods. Innovative Food Sci Emerg Technol 5:429–436
Ly-Nguyen B, Van Loey AM, Smout C, Ozcan SE, Fachin D, Verlent I, Vu Truong S, Duvetter T, Hendrickx M (2003a) Mild heat and high-pressure inactivation of carrot pectinmethylesterase: a kinetic study. J Food Sci 68:1377–1383
Sila D, Smout C, Satara Y, Truong V, Van Loey A, Hendrickx M (2007) Combined thermal and high pressure effect on carrot pectinmethylesterase stability and catalytic activity. J Food Eng 78:755–764
Ly-Nguyen B, Loey AV, Fachin D, Verlent I, Hendrickx IM (2002b) Purification, characterization, thermal, and high-pressure inactivation of pectin methylesterase from bananas (cv cavendish). Biotechnol Bioeng 78:683–691
Ly-Nguyen B, Loey AMV, Smout C, Verlent I, Duvetter T, Hendrickx ME (2003b) Effect of mild-heat and high-pressure processing on banana pectin methylesterase: a kinetic study. J Agric Food Chem 51:7974–7979
Riahi E, Ramaswamy HS (2003) High-pressure processing of apple juice: kinetics of pectin methylesterase inactivation. Biotechnol Prog 19:908–914
Katsaros GI, Apseridis I, Taoukis PS (2006) Modelling of high hydrostatic pressure inactivation of pectinmethylesterase from persimmon (Diospyros virginiana). IUFoST Edpsciences. doi:10.1051/IUFoST:20060753
Alexandrakis Z, Kyriakopoulou K, Katsaros G, Krokida M, Taoukis P (2014b) Process condition optimization of high pressure pasteurized sea buckthorn juice with long shelf-life and antioxidant activity. Food Bioprocess Technol 7:3226–3234
Ahlawat S, Dhiman S, Battan B, Mandhan P, Sharma J (2009) Pectinase production by Bacillus subtilis and its potential application in biopreparation of cotton and micropoly fabric. Process Biochem 44:521–526
El-Sheekh MM, Ismail AS, El-Ab MA, Hegazy EM, El-Diwany AI (2009) Effective technological pectinases by Aspergillus carneus NRC1 utilizing the Egyptian orange juice industry scraps. Int Biodeterior Biodegrad 63:12–18
Arias CR, Burns JK (2002) A pectinmethylesterase gene associated with a heat-stable extract from citrus. J Agric Food Chem 50:3465–3472
Cameron RG, Baker RA, Grohmann K (1998) Multiple forms of pectinmethylesterase from citrus peel and their effects on juice cloud stability. J Food Sci 63:253–256
Cameron RG, Savary BJ, Hotchkiss AT, Fishman ML (2005) Isolation, characterization, and pectin-modifying properties of a thermally tolerant pectin methylesterase from Citrus sinensis var. Valencia. J Agric Food Chem 53:2255–2260
Savary BJ, Hotchkiss AT, Cameron RG (2002) Characterization of a salt-independent pectin methylesterase purified from Valencia orange peel. J Agric Food Chem 50:3553–3558
Savary J, Vasu P, Nunez A, Cameron R (2010) Identification of thermolabile pectin methylesterases from sweet orange fruit by peptide mass fingerprinting. J Agric Food Chem 58:12462–12468
Denes JM, Baron A, Renard CM, Pean C, Drilleau JF (2000) Different action patterns for apple pectin methylesterase at pH 7.0 and 4.5. Carbohydr Res 4:385–393
Ly-Nguyen B, Van Loey AM, Fachin D, Verlent I, Duvetter T, CU ST, Smout C, Hendrickx ME (2002a) Strawberry pectin methylesterase (PME): purification, characterization, thermal and high-pressure inactivation. Biotechnol Prog 18:1447–1450
Ozler A, Karakus E, Pekyardimci S (2008) Purification and biochemical characteristics of pectinesterase from Malatya apricot (Prunus armeniaca. p. L. ). Prep Biochem Biotechnol 38:358–375
Alonso J, Howel LN, Canet W (1997) Purification and characterization of two pectinmethylesterases from persimmon (Diospyros kaki). J Sci Food Agric 75:352–358
Fayyaz A, Asbi BA, Ghazali HM, Man YBC, Jinap S (1995) Kinetics of papaya pectinesterase. Food Chem 53:129–135
Lim YM, Chung MC (1993) Isolation and characterization of pectin methylesterase from papaya. Arch Biochem Biophys 307(1):15420
Alonso J, Rodriguez MT, Canet W (1996) Purification and characterization of four pectinesterases from sweet cherry (Prunus avium L.). J Agric Food Chem 44:3416–3422
Javeri H, Wicker L (1991) Partial purification and characterization of peach pectinesterase. J Food Biochem 15:241–252
Nunes C, Castro S, Saraiva J, Coimbra M, Hendrickx M, Van Loey AM (2006) Thermal and high pressure stability of purified pectin methylesterase from plums (Prunus domestica). J Food Biochem 30:138–154
Alonso J, Canet W, Howell N, Alique R (2003) Purification and characterization of carrot (Daucus carota L.) pectinesterase. J Sci Food Agric 83:1600–1606
Markovič O, Cederlund E, Griffiths WJ, Jörnvall H (2002) Characterization of carrot pectin methylesterase. Cell Mol Life Sci 59 in press
Fachin D, Van Loey A, Ly-Nguyen B, Verlent I, Indrawat I, Hendrickx ME (2002) Comparative study of the inactivation kinetics of pectinmethylesterase in tomato juice and purified form. Biotechnol Prog 18:739–744
Verlent I, Van Loey A, Smout C, Duvetter T, Nguyen BL, Hendrickx ME (2004) Changes in purified tomato pectinmethylesterase activity during thermal and high pressure treatment. J Sci Food Agric 84:1839–1847
Benen JAE, Voragen AGJ, Visser J (2003) Pectic enzymes. In: Whitaker JR, Voragen AGJ, Wong DWS (eds) Handbook of food enzymology. Marcel Dekker, Inc, New York, Basel, pp. 169–188
Shevchik VE, Condemine G, Hugouvieux-Cotte- Pattat N, Robert-Baudouy J (1996) Characterization of pectin methylesterase B, an outer membrane lipoprotein of Erwinia chrysanthemi 3937. Mol Microbiol 19:455–466
Oey I (2010) Effect of novel food processing on fruit and vegetable enzymes. In: Enzymes in fruit and vegetable processing. Taylor and Francis Group, New York
Jolie R, Duvetter T, Van Loey AM, Hendrickx M (2010) Pectin methylesterase and its proteinaceous inhibitor: a review. Carbohydr Res 345:2583–2595
Limberg G, Korner R, Buchholt HC, Christensen T, Roepstorff P, Mikkelsen JD (2000) Analysis of different de-esterification mechanisms for pectin by enzymatic fingerprinting using endopectin lyase and endopolygalacturonase II from A. niger. Carbohydr Res 327:293–307
Ngouemazong DE, Jolie RP, Cardinaels R, Fraeye I, Van Loey A, Moldenaers P, Hendrickx M (2012) Stiffness of Ca2+-pectin gels: combined effects of degree and pattern of methylesterification for various Ca2+ concentrations. Carbohydr Res 348:69–76
Fries M, Ihrig J, Brocklehurst K, Shevchik VE, Pickersgill RW (2007) Molecular basis of the activity of the phytopathogen pectin methylesterase. EMBO J 26:3879–3887
Johansson K, El Ahmad M, Friemann R, Jornvall H, Markovic O, Eklund H (2002) Crystal structure of plant pectin methylesterase. FEBS Lett 514:243–249
Di Matteo A, Giovane A, Raiola A, Camardella L, Bonivento D, De Lorenzo G, Cervone F, Bellincampi D, Tsernoglou D (2005) Structural basis for the interaction between pectin methylesterase and a specific inhibitor protein. Plant Cell 17:849–858
Pelloux J, Rusterucci C, Mellerowicz EJ (2007) New insights into pectin methylesterase structure and function. Trends Plant Sci 12:267–277
Galazka VB, Dickinson E, Ledward DA (1996) Effect of high pressure on the emulsifying behaviour of b-lactoglobulin. Food Hydrocoll 10:213–219
Gomes MRA, Ledward DA (1996) Effect of high pressure treatment on the activity of some polyphenoloxidases. Food Chem 56:1–5
Cheftel JC (1991) Applications des hautes pressions en technologie alimentaire. Industries Alimentairs et Agricoles 108:141–153
Irwe S, Olsson I (1994) Reduction of pectinesterase activity in orange juice by high pressure treatment. In: Singh RP, Oliveira FAR (eds) Minimal processing of foods and process optimisation—an interface. CRC Press, Boca Raton, pp. 35–42
Mozhaev V, Heremans K, Frank J, Masson P, Balny C (1994) Exploiting the effects of high hydrostatic pressure in biotechnological applications. TIBTECH 12:493–501
Messens W, Camp JV, Huyghebaert A (1997) The use of high pressure to modify the functionality of food proteins. Trends Food Sci and Technol 8:107–112
Hummer G, Garde S, Garcia AE, Paulaitis ME, Pratt LR (1998) The pressure dependence of hydrophobic interactions is consistent with the observed pressure denaturation of proteins. Proc Natl Acad Sci U S A 95:1552–1555
Basak S, Ramaswamy HS, Simpson BK (2001) High pressure inactivation of pectin methyl esterase in orange juice using combination treatments. J Food Biochem 25:509–526
Bull M, Zerdin K, Howe E, Goicoechea D, Paramanandhan P, Stockman R, Sellahewa J, Szabo E, Johnson R, Stewart C (2004) The effect of high pressure processing on the microbial, physical and chemical properties of Valencia and Navel orange juice. Innovative Food Sci Emerg Technol 5:135–149
Nienaber U, Shellhammer TH (2001b) High-pressure processing of orange juice: kinetics of pectinmethylesterase inactivation. J Food Sci 66:328–331
Crelier S, Tache MC, Raemy A, Renken A, Raetz E (1995) High pressure for the inactivation of enzymes in food products: thermal and HHP treatment of tomato pectin methyl esterase. Poster presentation at the 9th World Congress on Food Science and Technology, Budapest, Hungary
Crelier S, Robert MC, Claude J, Juillerat MA (2001) Tomato (Lycopersicon esculentum) pectin methylesterase and polygalacturonase behaviors regarding heat- and pressure-induced inactivation. J Agric Food Chem 49:5566–5575
Hernandez A, Cano MP (1998) High pressure and temperature effects on enzyme inactivation in tomato puree. J Agric Food Chem 46:266–270
Shook CM, Shellhammer TH, Schwartz SJ (2001) Polygalacturonase, pectinesterase, and lipoxygenase activities in high-pressure-processed diced tomatoes. J Agric Food Chem 49:664–668
Hsu KC (2008) Evaluation of processing qualities of tomato juice induced by thermal and pressure processing. LWT—Food Sci Technol 41:450–459
Van Buggenhout S, Messagie I, Van der Plancken I, Hendrickx M (2006) Influence of high-pressure–low-temperature treatments on fruit and vegetable quality related enzymes. Eur Food Res Technol 223:475–485
Houben K, Kermani ZJ, Van Buggenhout S, Jolie RP, Van Loey AM, Hendrickx ME (2012) Thermal and high-pressure stability of pectinmethylesterase, polygalacturonase, β-galactosidase and α-arabinofuranosidase in a tomato matrix: towards the creation of specific endogenous enzyme populations through processing. Food Bioprocess Technol 6(12):3368–3380
Bodelon OG, Avizcuri JM, Fernandez-Zurbano P, Dizy M, Prestamo G (2013) Pressurization and cold storage of strawberry puree: color, anthocyanins, ascorbic acid and pectin methylesterase. LWT-Food Sci Technol 52(2):123–130
Chakraborty S (2012) Effect of added sugar on the inactivation of strawberry enzymes during combined high-pressure and temperature treatments. [MTech thesis]. Kharagpur, India: Indian Institute of Technology Kharagpur. 89 p. Available from: Agricultural and Food Engineering Department Library, IIT Kharagpur, India
Baron A, Dénes JM, Durier C (2006) High-pressure treatment of cloudy apple juice. LWT-Food Sci Technol 39:1005–1013
Rao L, Guo X, Pang X, Tan X, Liao X, Wu J (2013) Enzyme activity and nutritional quality of peach (Prunus persica) juice: effect of high hydrostatic pressure. Intl J Food Prop 17(6):1406–1417
Bermúdez-Aguirre D, Ángel Guerrero-Beltrán J, Barbosa-Cánovas GV, Welti-Chanes J (2011) Study of the inactivation of Escherichia coli and pectin methylesterase in mango nectar under selected high hydrostatic pressure treatments. Food Sci Technol Int 17:541
Ortuno C, Duong T, Balaban M, Benedito J (2013) Combined high hydrostatic pressure and carbon dioxide inactivation of pectin methylesterase, polyphenol oxidase and peroxidase in Feijoa puree. J Supercrit Fluids 82:56–62
Liu Y, Zhao X, Zou L, Hu X (2013) Effect of high hydrostatic pressure on overall quality parameters of watermelon juice. Food Sci Technol Intl 19(3):197–207
Zhang C, Trierweiler B, Li W, Butz P, Xu Y, Rufer CE, Ma Y, Zhao X (2011) Comparison of thermal, ultraviolet-C and high-pressure treatments on quality parameters of watermelon juice. Food Chem 126(1):254–260
Duvetter T, Sila DN, Van Buggenhout S, Jolie R, Van Loey A, Hendrickx M (2009) Pectins in processed fruit and vegetables: part I—stability and catalytic activity of pectinases, part II—structure–function relationships, part III—texture engineering. Compr Rev Food Sci Food Saf 8:75–117
Saraiva J, Oliveira J, Oliveira S, Hendrickx M (1996) Inactivation kinetics of horseradish peroxidase in organic solvents of different hydrophobicity at different water contents. Int J of Food Sc & Tec 31:233–240
Hawley SA (1971) Reversible pressure–temperature denaturation of chymotrypsinogen. Biochemist 10:2436–2442
Ludikhuyze L, Van den Broeck I, Weemaes C, Hendrickx M (1998) Effect of combined pressure and temperature on soybean lipoxygenase: II. Modeling inactivation kinetics under static and dynamic conditions. Agric Food Chem 46:4074–4080
Smeller L (2002) Pressure–temperature phase diagram of biomolecules. In: Balny C, Masson P, Heremans K (eds) Frontiers in high pressure biochemistry and biophysics. The Netherlands’ Elsevier, Amsterdam, pp. 11–29
Ly-Nguyen B (2004) Doctoral dissertation n-.630, Katholieke Universiteit Leuven, Faculty of Applied Bioscience and Engineering, Laboratory of Food Technology, Leuven, Belgium
Weemaes C, Ludikhuyze L, Van den Broek I, Hendrickx M (1998) Effect of pH on pressure and thermal inactivation of avocado polyphenoloxydase: a kinetic study. J Agric Food Chem 46:2785–2792
Acknowledgements
This work has been cofinanced by the European Union (European Social Fund—ESF) and Greek national funds through the Operational Program “Education and Lifelong Learning” of the National Strategic Reference Framework (NSRF)—Research Funding Program: Heracleitus II. Investing in knowledge society through the European Social Fund.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Katsaros, G.J., Alexandrakis, Z.S. & Taoukis, P.S. Kinetic Assessment of High Pressure Inactivation of Different Plant Origin Pectinmethylesterase Enzymes. Food Eng Rev 9, 170–189 (2017). https://doi.org/10.1007/s12393-016-9153-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12393-016-9153-3