Abstract
A kinetic study of the inactivation of endogenous pectin methylesterase (PME) in Greek commercial peach pulp under high hydrostatic pressure (HHP; 100–800 MPa) combined with moderate temperature (30–70 °C) was conducted. Thermal inactivation of the enzyme at ambient pressure conditions was also studied. PME inactivation was modeled by first order kinetics at all conditions tested. High pressure and temperature acted synergistically on PME inactivation, except at the high temperature of 70 °C at the middle pressure range (100–600 MPa), where an antagonistic effect of pressure and temperature was observed. At this specific middle pressure range, an increase of pressure processing led to increased inactivation rate constants of peach PME. A multiparameter model was developed to express the PME inactivation rate constant as a function of temperature and pressure process conditions, taking into account the dependence of both activation energy and activation volume on pressure and temperature, respectively. A good correlation between experimental and predicted values of inactivation rate constants was established. This modeling approach enables the quantitative estimation of the HHP–temperature conditions needed to achieve a targeted PME inactivation in the peach pulp.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pectin methylesterase (PME; EC 3.1.1.11) is a pectic enzyme found in different fruits and vegetable sources. As a de-esterifying enzyme, PME catalyzes the demethylation of carboxylate groups on pectin substances to form pectic acid, a pectin with a lower degree of esterification and methanol. Demethylated pectin interacts with calcium ions causing precipitation of insoluble calcium pectate, as well as other cloud-forming compounds entrapped. In plants, PME is bound to the cell wall by electrostatic interaction (Van den Broeck et al. 2000; Ly Nguyen et al. 2002a, 2003).
In the fruit process industry, the action of PME causes cloud loss in fruit and vegetable juices and nectars. In order to avoid the undesirable result of PME action in the final fruit products, it is necessary to inactivate it. Conventional thermal treatment is used to inactivate fruit enzymes such as PME. A typical flow sheet for production of nonconcentrated peach pulp includes a stage of preheating at 90 ± 2 °C for 30 s, mainly for enzyme inactivation, followed by finishing, cooling, deaeration and aroma recovery, pasteurization (at 110 °C for 115 s), and aseptic filling (information provided by leading Greek processing plant). However, there is a negative effect of temperature on the nutritional and organoleptic characteristics of fruit juices and nectars (Basak and Ramaswamy 1998; Hendrickx and Knorr 2001).
High hydrostatic pressure (HHP) has been proposed as an alternative technology for the stabilization of fruit juices and nectars. In contrast to thermal treatment, HHP processing minimally deteriorates the foods, so its use can supply consumers with fresh-like, high-quality, and safe food products (Farr 1990; Knorr 1993; Norton and Sun 2008).
Enzymes may be affected by pressure in several ways. Pressurization may cause reversible or irreversible, partial or complete inactivation/activation depending on the type of the enzyme, the microenvironmental conditions, the nature of the substrate, the applied pressure, temperature, and processing time. Besides, enzymatic reactions may be enhanced or inhibited by pressure, as expressed by the activation volume value (positive or negative) associated with the reaction (Cheftel 1992).
A number of studies on the effect of HHP on the inactivation of PME obtained from different sources (citrus fruits, tomato, apple, strawberry, banana, and carrot in model systems or real foods) are cited in the literature, indicating that the origin of the enzyme is of high importance as far as it concerns the obtained different inactivation behavior (Basak and Ramaswamy 1998; Hernández and Cano 1998; Goodner et al. 1998; Van den Broeck et al. 2000; Crelier et al. 2001; Nienaber and Shellhammer 2001; Ly Nguyen et al. 2002a, b, c, 2003; Stoforos et al. 2002; Fachin et al. 2003; Riahi and Ramaswamy 2003; Balogh et al. 2004; Polydera et al. 2004). Balogh et al. (2004) studied the effect of high pressure (700–800 MPa at 10–40 °C) on the inactivation of purified carrot PME and PME in carrot juice and carrot pieces. PME in situ was more thermostable and pressure stable than PME in carrot juice and PME in purified form. The D values for inactivation of purified PME and PME in carrot juice at 700 MPa and 10 °C were equal to 188 and 172 min (k values 0.0123 and 0.0134 min−1), respectively, whereas in order to inactivate PME in carrot pieces at a similar rate as PME in carrot juice, processing at 750 MPa and 40 °C was needed (D value 161 min). PME in navel orange juice was more sensitive than PME from white grapefruit (Citrus paradisi) under the same processing (e.g., at 600 MPa and 50 °C inactivation rate constants, k, were 1.76 and 0.113 min−1, respectively; Polydera et al. 2004; Guiavarc’h et al. 2005). Ly Nguyen et al. (2002a) reported a first order inactivation of purified strawberry PME in the range of 850–1,000 MPa and 10 °C with a residual activity (10%) of the pressure resistant enzyme. Strawberry PME was far more stable than the navel orange and white grapefruit PME (at 1,000 MPa and 10 °C; k was equal to 0.026 min−1).
Greece is among the leading peach producing and processing countries. In 2004, Greece was leader in production of processing peaches (550,000 ton) followed by Spain, while the annual production of table peaches was 250,000 ton, second after Italy among European producers. Juice extracted from peach (Prunus bertolius) is of high commercial interest. The preference of consumers for more fresh tasting peach juice justifies the evaluation of HHP processing as an alternative process. For such an evaluation, it would be necessary to study the effect of HHP on the main deteriorative agent of peach juice, endogenous PME. Due to the aforementioned specificity of the effect of HHP on PMEs of different origins and in different media, it is necessary to obtain systematic data on the particular system.
The objective of this work was to investigate the inactivation kinetics of PME in fresh Greek commercial peach (Everts var.) pulp under HHP (100–800 MPa) combined with moderate temperature (30–70 °C). Thermal inactivation (45–70 °C) of the same enzyme at ambient pressure was also tested.
Materials and Methods
Peach Samples
Peach pulp (Everts var.) was frozen directly after the cutting and pulping line of a peach processing plant in Northern Greece. The pulp was transported to the laboratory on the same day and stored at −30 °С until used for HHP processing and testing.
Peach PME Extraction
Peach PME was bound to the cells of the fruit (measured activity approximately 0.7 U/ml), so there was a need for extraction of the enzyme from the cells in order to measure the full PME activity. The extraction of PME was performed according to a modification of the method described by Ly Nguyen et al. (2002b). Peach pulp (5 kg) was centrifuged at 10,000 rpm for 12 min (Hermle Z 380, Hermle Labortechnik GmbH, Wehingen, Germany). The supernatant (3.5 kg), with a measured activity of approximately 2 Uml−1, was stored at 5 °C. Deionized water was added to the precipitate (1:1 [w/v]), mixed well, and the mixture was centrifuged at 10,000 rpm for another 12 min. The supernatant of the second centrifugation was discarded, whereas the pellet was mixed with buffer solution pH 7 (1:2.5 [w/v]; 0.1 M Na2HPO4—NaH2PO4, containing 1 M NaCl). The mixture was stirred for 20 h in a room at 5 °C. After extraction, the suspension was centrifuged at 10,000 rpm for 12 min and the precipitate was discarded. The supernatant of a total volume of 1,635 ml was filtered, desalinated, and concentrated to a final volume of 85 ml via ultrafiltration (Millipore Co., Bedford, USA using a Pellicon XL filter, PLCGC 10, Millipore Co.), with a measured activity of approximately 42 U ml−1. The final “juice sample” for testing was prepared by mixing 0.1 ml of the final concentrate with 1.9 ml of the supernatant of the first centrifugation (enzyme activity was approximately 4.1 U ml−1).
PME Activity Assay
PME activity was measured using a modification of Rouse and Atkins (1955) method. Titration (Autotitrator Titralab TIM 854, Radiometer Analytical, France) with 0.02 N NaOH of the carboxylic groups generated by PME during the hydrolysis of a pectin solution at pH 7.5 and a temperature of 30 °C was conducted. Juice sample of 2 ml adjusted to pH 7.5 with NaOH 0.2 N was added to 50 ml 1% apple pectin (70–75% degree of esterification; Fluka Bio-Chemika, Buchs, Switzerland) solution containing 0.3 M NaCl which was also previously adjusted to pH 7.5 with 0.2 N NaOH at 30 °C. The consumption of 0.02 N NaOH to keep the pH stable was recorded during a period of about 20 min. The slope dVNaOH/dt was determined in the linear part of the titration curve. PME activity of juice sample, which is calculated by Eq. 1 and expressed as microequivalents per min and ml juice, is directly proportional to the slope:
where N NaOH is the normality of NaOH solution used for the titration (μeq ml−1). Dilution of peach juice sample during pH adjustment was taken into consideration for the determination of PME activity of each sample.
Experimental Design
Thermal inactivation experiments at atmospheric pressure were performed at 45 °С, 50 °С, 55 °С, 60 °С, 65 °С, and 70 °С. HHP inactivation experiments were conducted at various combinations of pressure (100–800 MPa) and temperature (30–70 °С) for various time periods. The duration of the treatments ranged from 1 to 60 min, while the process time intervals, chosen for each treatment, depended on the expected inactivation rates.
Thermal Inactivation Experiments
PME of peach high enzyme juice extract was thermally inactivated at temperatures in the range of 45–70 °C. The experiments were conducted in triplicate, using capillaries for quick temperature transfer. The capillaries, with 2 ml of the sample, were immersed into a waterbath (WB/OB 7–45, MEMMERT GmbH + Co. KG, Schwabach, Germany) set at the desired temperature (45–70 °C). Temperature was constantly monitored and recorded at 2 s intervals in a multichannel datalogger (CR10X, Campbell Scientific, Leicestershire, UK) by a type T thermocouple placed inside a capillary used as a temperature indicator. The time temperature reached the desired value (less than 25 s in all experiments) was defined as time zero of the process and three capillaries were removed from the water bath and immediately dipped into iced water until PME activity was measured. The rest of the capillaries were removed at predetermined time intervals (three capillaries for each sampling) according to the experimental design, following the same procedure.
High Pressure Processing
High enzyme juice extract was placed into 10-ml pouches (film: polypropylene–aluminum–polyethylene laminate) for HHP experiments. HHP inactivation experiments were conducted in triplicate at various combinations of pressure (100–800 MPa) and temperature (30–70 °C) for appropriate processing times. The high pressure unit (Food Pressure Unit FPU 1.01, Resato International BV, Roden, Holland) comprised a pressure intensifier and a multivessel system consisting of six vessels of 45 mL capacity each, with a maximum operating pressure and temperature of 1,000 MPa and 90 °C. The pressure transmitting fluid used was polyglycol ISO viscosity class VG 15 (Resato International BV, Roden, Holland). Process temperature in the vessels was achieved by liquid circulation in the outer jacket controlled by a heating-cooling system (Valka-K-10W5, Val Electronic). For all experiments, the samples were initially preheated to a few degrees below the targeted temperature. Due to the small volume and the dimensions of the sample pouches, samples were assumed to obtain the temperature recorded at the vessel-sample interface. The desired value of pressure was set and after pressure build up (at a rate of 20 MPa s−1), the pressure vessels were isolated. This point defined the time zero of this process. Pressure of each vessel was released after a preset time interval (according to the experimental design) by opening the pressure valve. The initial adiabatic temperature increase during pressure build up was taken into consideration in order to achieve the desired operating temperature during pressurization. Pressure and temperature were constantly monitored and recorded (in 1 s intervals) during the process. In Fig. 1 is representatively illustrated the recorded pressure–temperature evolution for the 700 MPa and 70 °C experiment and shows that the initial temperature during pressure build up was over 80 °C.
Data Analysis
Values of enzyme activity (average of three samples) were plotted against time for different process conditions. For statistical fit of the inactivation curves, least square regression analysis was conducted. Dependence of inactivation rates on process parameters was described with appropriate mathematical equations, statistically treated by linear or nonlinear regression using SYSTAT 8.0 (SPSS 1998) software.
Results and Discussion
PME Inactivation at Ambient Pressure
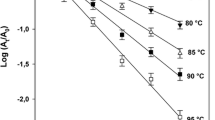
The reduction of PME activity as a function of processing time during thermal inactivation at 50 °С, 55 °С, 60 °С, 65 °С and 70 °С (ambient pressure) is representatively shown in Fig. 2. The inactivation of peach PME was adequately described by exponential curves, typical of first order kinetics for all pressure—temperature conditions tested:
where A is PME activity after processing for a time period t (μeq H+ min−1 ml−1), A o is the initial PME activity (at time zero of processing, in μeq H+ min−1 ml−1), t is the processing time (min), and k is the inactivation rate constant (min−1). Inactivation of PME from other sources has been reported as an apparent first order process (Polydera et al. 2004; Guiavarc’h et al. 2005). The values of k were calculated from the negative slope of the linear regression of ln(A/A o) vs. time. The R 2 coefficient was used to measure goodness of fit. Higher inactivation rates were observed as process temperature increased. The inactivation rate constants at all the tested temperatures of 45 °С, 50 °С, 55 °С, 60 °С, 65 °С, and 70 °С were determined as 0.0007, 0.0028, 0.0126, 0.0736, 0.4606, and 1.6450 min−1, respectively (see also Table 1; R 2 values ranged from 0.87 to 0.99).
Thermal inactivation of PME at 50 °C, 55 °C, 60 °C, 65 °C and 70 °C (ambient pressure). Lines represent the fit of Eq. 2 to experimental data
The temperature dependence of inactivation rate constant, k, was expressed by the value of the activation energy, E a, parameter of the Arrhenius equation:
where T ref is the reference temperature (323 K), k o is the inactivation rate constant (min−1) at T ref, and R is the universal gas constant (8.314 J mol−1 K−1). The parameters E a and k o were estimated by linear regression analysis using the linearized Arrhenius relationship. The activation energy value was found equal to 289 ± 39 kJ mol−1 (R 2 = 0.99). The inactivation rate constant, k o, at the reference temperature of 323 K (50 °С) was determined as 0.0031 min−1. Comparing the inactivation rate constants received from this work with other studies of the literature, it can be seen that peach PME is less temperature resistant than banana PME (at 60 °C, no inactivation of banana PME was observed, while for peach k = 0.0736 min−1) and more thermostable compared to PMEs obtained from strawberry, white grapefruit, and navel oranges (Polydera et al. 2004; Ly Nguyen et al. 2002a, 2003; Guiavarc’h et al. 2005).
Combined Thermal and High Pressure Inactivation of Peach PME
At all high pressures (i.e., excluding ambient pressure), PME inactivation increased with increasing processing pressure. Compared to the inactivation achieved thermally (ambient pressure), at the same temperature and high pressures, the inactivation that is observed up to a certain pressure value may be slower. In these cases, the conformational changes caused by the high pressure seem to act protectively (antagonistic effect) and enhance thermal stability. This was observed at the temperature range 50–60 °С only at the low range of pressures (100 MPa) where the inactivation rates were lower compared to the rates observed at the same temperatures and atmospheric pressure. At the higher tested temperature of 70 °С, the antagonistic effect was observed at pressures up to 600 MPa (Fig. 3a), above which the combined effect of temperature and pressure results in a faster inactivation than the thermal. A similar behavior was also observed for tomato, orange, and banana PME inactivation (Stoforos et al. 2002; Polydera et al. 2004; Ly Nguyen et al. 2003) with pressure and temperature acting antagonistically on PME inactivation in the low-pressure (P < 300–400 MPa)–high temperature (T > 60 °C) domain. The inactivation rate constant increased as temperature increased, at all high pressure processing conditions, as is representatively illustrated in Fig. 3b for the 700 MPa conditions. The effect of temperature (30–70 °С) and pressure (0.1–800 MPa) on peach PME inactivation is summarized in Table 1. The presented rate constants resulted from the fit of all the inactivation curves. Fit to Eq. 2 was satisfactory with R 2 values ranging from 0.89 to 0.99.
The high pressure effect on the inactivation rate constant, k, was expressed through the activation volume, V a, parameter of the Eyring equation:
where P ref is the reference pressure (600 MPa) and k o is the inactivation rate constant at P ref.
The parameters of the Eq. 4 were determined for each temperature by linear regression of the linearized Eyring equation (Table 1), using k values from Eq. 2, for processing under various pressures (R 2 values ranged from 0.92 to 0.99). High absolute value of the activation volume indicates high dependence of the inactivation rate constants on pressure, whereas the negative value denotes that PME inactivation is favored by pressure. Absolute values of activation volume decreased with increasing temperatures, meaning that inactivation rates became less pressure dependent (Table 1). The dependence of activation volume on temperature was expressed by a linear equation:
where T ref = 323 K (50 °C), \(V_{{\text{a}}_{\text{o}} } = - 20.6\,{\text{ml}}\,{\text{mol}}^{{\text{ - 1}}} \) (activation volume at T ref), and a = 0.69 ml mol−1K−1 (R 2 = 0.90). The slope a gives a measure of the change of activation volume as a function of temperature.
The activation energy values at each different pressure level were calculated through Eq. 3 (R 2 values ranged from 0.92 to 0.99). Activation energy decreased with increasing pressure (ranging from 183 kJ mol−1 at 100 MPa to 59 kJ mol−1 at 800 MPa; Table 1) indicating less temperature dependence of the enzyme inactivation rate at higher pressures. The dependence of activation energy on pressure was expressed by an exponential function:
where P ref = 600 MPa, \(E_{{\text{a}}_{\text{o}} } \) = 76.5 kJ mol−1 (activation energy at P ref), and b = 0.0018 MPa−1 (R 2 = 0.95).
PME Inactivation as a Function of Pressure and Temperature
Based on Eqs. 3 and 4 and taking into account the dependence of activation volume and activation energy on temperature and pressure, respectively (Eqs. 5 and 6), the inactivation rate constant at different temperature and pressure conditions was expressed by a single Eq. 7:
where k ref—the inactivation rate constant at P ref = 600 MPa and T ref = 323 K (50 °C), A—the parameter that expresses the effect of temperature on the activation volume value, and B—the parameter that expresses the effect of pressure on the activation energy value of PME inactivation.
Parameters of the above equation were determined through the SYSTAT nonlinear regression routine (Table 2) with satisfactory results (R 2 observed vs. predicted 0.98). A good correlation between experimental and predicted values of inactivation rate constants is demonstrated in Fig. 4. The proposed model can be used to predict PME inactivation at high pressure (100–800 MPa) and temperature range of 30–70 °C.
PME inactivation rate constant as a function of pressure and temperature. Lines represent values predicted from Eq. 7
Comparing the inactivation data measured and modeled in this work with relevant data reported in the literature, concerning inactivation of PME of different sources, peach PME appears to be more resistant to the same processing conditions compared to PME obtained from navel orange (k values were found equal to 0.1212 and 1.76 min−1, respectively, for processing at 600 MPa and 50 °C), but more sensitive, compared to banana PME, since for the latter one, lower inactivation rate values were reported for the same processing conditions (k values were found equal to 2.79 and 0.139 min−1 for peach and banana PME, respectively, for processing at 800 MPa and 70 °C) (Polydera et al. 2004; Ly Nguyen et al. 2003).
The inactivation of PME at different processing conditions can be calculated through Eq. 7. For example, processing at 600 MPa and 55 °C for 9 min will achieve a 90% enzyme inactivation.
Conclusions
Thermal and high-pressure inactivation of peach PME was modeled by first order kinetics. The inactivation rate constants of the treated enzyme were expressed as a function of temperature and pressure enabling a proper design of high pressure treatments. The effect of pressure on the activation volume V a was modeled by a linear function and the effect of temperature on the activation energy (E a) by an exponential function, respectively. A synergistic effect of pressure and temperature under HHP processing conditions was found, except in the middle pressure (100 to 600 MPa)–high temperature (70 °C) region where an antagonistic effect of pressure and temperature on PME inactivation was established. The developed kinetics allow the selection of the required HHP process conditions for achieving the desirable PME inactivation in the target peach products. The five-parameter mathematical model, which describes the PME inactivation rate as a function of pressure and temperature conditions, taking into account the dependence of both activation energy and activation volume on the applied pressure and temperature, respectively, allows a better quantitative comparison of different treatments in the quest for an optimal process design.
High pressure seems to offer only a marginal advantage over thermal treatment when seen from the point of view of processing but could potentially improve the organoleptic attributes of the product. The latter one could be the decisive criterion in considering the application of the HPP technology.
Further study concerning the effect of pressure and temperature on the inactivation of PME obtained from other plants and vegetables is of interest, as PME from different sources can exhibit markedly different inactivation kinetics. These evidenced differences in the high pressure changes connected to inactivation should also be investigated and explained by appropriate measurements of the pressure induced enzyme configuration changes using methods like Raman, Fourier transform infrared spectroscopy, and circular dichroism spectroscopies.
- A :
-
enzyme activity at time t (μeq H+ min−1 ml−1)
- A o :
-
initial enzyme activity (at time zero of processing; μeq H+ min−1 ml−1)
- a :
-
constant expressing the effect of temperature on V a (ml mol−1 K−1)
- b :
-
constant expressing the effect of pressure on E a (MPa−1)
- A :
-
constant expressing the effect of temperature on V a estimated by nonlinear regression analysis (in Eq. 7; ml mol−1 K−1)
- B :
-
constant expressing the effect of pressure on E a estimated by nonlinear regression analysis (in Eq. 7; MPa−1)
- E a :
-
activation energy (kJ mol−1)
- \(E_{{\text{a}}_o } \) :
-
activation energy at P ref (kJ mol−1)
- k :
-
first order inactivation rate constant (min−1)
- k o :
-
first order inactivation rate constant at a reference temperature or pressure (min−1)
- P :
-
pressure (MPa)
- P ref :
-
reference pressure (MPa)
- R :
-
universal gas constant (8.314 J mol−1 K−1)
- t :
-
time (min)
- T :
-
temperature (K)
- T ref :
-
reference temperature (K)
- V a :
-
activation volume (ml mol−1)
- \(V_{{\text{a}}_{\text{o}} } \) :
-
activation volume at T ref (ml mol−1)
References
Balogh, T., Smout, C., Ly Nguyen, B., Van Loey, A. M., & Hendrickx, M. E. (2004). Thermal and high-pressure inactivation kinetics of carrot pectinmethylesterase: From model system to real foods. Innovative Food in Science Emerging Technologies, 5(4), 429–436. doi:10.1016/j.ifset.2004.06.002.
Basak, S., & Ramaswamy, H. S. (1998). Effect of high pressure processing on texture of selected fruit and vegetables. Journal of Texture Studies, 29(5), 587–601. doi:10.1111/j.1745-4603.1998.tb00185.x.
Cheftel, J. C. (1992). Effects of high hydrostatic pressure on food constituents: An overview. In C. Balny, R. Hayashi, K. Heremans, & P. Masson (Eds.), High pressure and biotechnology, Colloque INSERM, Vol. 224 (pp. 195–209). Montrouge: John Libbey Eurotext.
Crelier, S., Robert, M. C., Claude, J., & Juillerat, M. A. (2001). Tomato (Lycopersicon esculentum) pectin methylesterase and polygalacturonase behaviors regarding heat- and pressure-induced inactivation. Journal of Agricultural and Food Chemistry, 49, 5566–5575. doi:10.1021/jf010202u.
Fachin, D., Van Loey, A. M., Ly Nguyen, B., Verlent, I., Indrawati, & Hendrickx, M. E. (2003). Inactivation kinetics of polygalacturonase in tomato juice. Innovative Food in Science Emerging Technologies, 4(2), 135–142. doi:10.1016/S1466-8564(02)00090-5.
Farr, D. (1990). High pressure technology in the food industry. Trends in Food Science & Technology, 1, 14–16. doi:10.1016/0924-2244(90)90004-I.
Goodner, J. K., Braddock, R. J., & Parish, M. E. (1998). Inactivation of pectinesterase in orange and grapefruit juices by high-pressure. Journal of Agricultural and Food Chemistry, 46(5), 1997–2000. doi:10.1021/jf9709111.
Guiavarc’h, Y., Segovia, O., Hendrickx, M., & Van Loey, A. (2005). Purification, characterization, thermal and high-pressure inactivation of a pectin methylesterase from white grapefruit (Citrus paradisi). Innovative Food in Science Emerging Technologies, 6(4), 363–371. doi:10.1016/j.ifset.2005.06.003.
Hendrickx, M. E., & Knorr, D. (2001). Ultra high pressure treatments of foods. New York: Kluwer Academic/Plenum.
Hernández, A., & Cano, M. P. (1998). High-pressure and temperature effects on enzyme inactivation in tomato puree. Journal of Agricultural and Food Chemistry, 46, 266–270. doi:10.1021/jf970455g.
Knorr, D. (1993). Effects of high-hydrostatic-pressure processes on food safety and quality. Food Technology, 47, 156–161.
Ly Nguyen, B., Van Loey, A. M., Fachin, D., Verlent, I., Duvetter, T., Vu, S. T., et al. (2002a). Strawberry pectin methylesterase (PME): Purification, characterization, thermal and high-pressure inactivation. Biotechnology Progress, 18(6), 1447–1450. doi:10.1021/bp0256622.
Ly Nguyen, B., Van Loey, A. M., Fachin, D., Verlent, I., & Hendrickx, M. E. (2002b). Purification, characterization, thermal and high-pressure inactivation of pectin methylesterase from bananas. Biotechnology and Bioengineering, 79, 363. doi:10.1002/bit.10401.
Ly Nguyen, B., Van Loey, A. M., Fachin, D., Verlent, I., Indrawati, Hendrickx, M. E. (2002c). Partial purification, characterization, and thermal and high-pressure inactivation of pectin methylesterase from carrots (Daucus carrota L.). Journal of Agricultural and Food Chemistry, 50(19), 5437–5444. doi:10.1021/jf011666v.
Ly Nguyen, B., Van Loey, A. M., Smout, C., Verlent, I., Duvetter, T., & Hendrickx, M. E. (2003). Effect of mild-heat and high-pressure processing on banana pectin methylesterase: A kinetic study. Journal of Agricultural and Food Chemistry, 51(27), 7974–7979. doi:10.1021/jf034658i.
Nienaber, U., & Shellhammer, T. H. (2001). High-pressure processing of orange juice: Kinetics of pectinmethylesterase inactivation. Journal of Food Science, 66(2), 328–331. doi:10.1111/j.1365-2621.2001.tb11341.x.
Norton, T., & Sun, D. W. (2008). Recent advances in the use of high pressure as an effective processing technique in the food industry. Food Bioprocess Technology, 1, 2–34. doi:10.1007/s11947-007-0007-0.
Polydera, A. C., Galanou, E., Stoforos, N. G., & Taoukis, P. S. (2004). Inactivation kinetics of pectin methylesterase of Greek navel orange juice as a function of high hydrostatic pressure and temperature process conditions. Journal of Food Engineering, 62(3), 291–298. doi:10.1016/S0260-8774(03)00242-5.
Riahi, E., & Ramaswamy, H. S. (2003). High-pressure processing of apple juice: Kinetics of pectin methylesterase inactivation. Biotechnology Progress, 19(3), 908–914. doi:10.1021/bp025667z.
Rouse, A. H., & Atkins, C. D. (1955). Pectinesterase and pectin in commercial orange juice as determined by methods used at the citrus experiment station. Bulletin of the University of Florida Agricultural Experiment Station, 570, 1–19 Lake Alfred, FL.
Stoforos, N. G., Crelier, S., Robert, M. C., & Taoukis, P. S. (2002). Kinetics of tomato pectin methylesterase inactivation by temperature and high pressure. Journal of Food Science, 67(3), 1026–1031. doi:10.1111/j.1365-2621.2002.tb09448.x.
SPSS (1998). SYSTAT 8.0 Statistics. Chicago, IL, USA: SPSS.
Van den Broeck, I., Ludikhuyze, L. R., Van Loey, A. M., & Hendrickx, M. E. (2000). Effect of temperature and/or pressure on tomato pectinesterase activity. Journal of Agricultural and Food Chemistry, 48(2), 551–558. doi:10.1021/jf990569n.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Boulekou, S.S., Katsaros, G.J. & Taoukis, P.S. Inactivation Kinetics of Peach Pulp Pectin Methylesterase as a Function of High Hydrostatic Pressure and Temperature Process Conditions. Food Bioprocess Technol 3, 699–706 (2010). https://doi.org/10.1007/s11947-008-0132-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-008-0132-4