Abstract
The thermal and pressure stability of tomato pectinmethylesterase (PME), polygalacturonase (PG), β-galactosidase (β-Gal), and α-arabinofuranosidase (α-Af) were investigated in situ. Enzyme inactivation by thermal and high-pressure processing (respectively 5 min at 25–95 °C at 0.1 MPa and 10 min at 0.1–800 MPa at 20 °C) was monitored by measuring the residual activity in crude enzyme extracts of treated tomato purée samples. PME was completely inactivated after a 5-min treatment at 75 °C. Only 30 % of the pressure stable PME was inactivated after a treatment at 800 MPa (20 °C, 10 min). A 5-min treatment at 95 °C and a treatment at 550 MPa (20 °C, 10 min) caused complete PG inactivation. β-Gal and α-Af activities were already reduced significantly by thermal treatments at 42.5–52.5 °C and 45–60 °C, respectively. These enzymes were, however, rather pressure resistant: treatments at respectively 700 and 600 MPa were necessary to reduce the activity below 10 % of the initial value. Assuming that first-order, fractional conversion or biphasic inactivation models could be applied to the respective enzyme inactivation data, inactivation rate constants and their temperature or pressure dependence for the different enzymes were determined. Based on differences in process stability of the enzymes, possibilities for the creation of specific “enzyme populations” in tomato purée by selective enzyme inactivation were identified. For industrially relevant process conditions, the enzyme inactivation data obtained for tomato purée were shown to be transferable to intact tomato tissue.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In fruits, vegetables, their preprocessed intermediates and processed end products, changes in functional properties (texture, rheology) are directly related to structural changes in the cell wall polysaccharides, particularly in pectic but also, to a lesser extent, in hemicellulosic and cellulosic material (Sila et al. 2009). Enzyme-catalyzed changes, related to a desired structural functionality of the end product, can be controlled to a certain extent by inactivating undesired structure-related enzymes and/or boosting the activity of the functionally important ones, for example by intelligent application of thermal or high-pressure processing (Van Buggenhout et al. 2009).
Tomato, one of the most popular and widely grown produce, is of great importance for the food industry (Nisha et al. 2011; Augusto et al. 2012). In the industrial production process of tomato-based products, commonly used thermal treatments affect structure-related enzymes. In case of a “cold-break” process, homogenized tomatoes are heated up to temperatures around 60 °C. The rather low processing temperature allows sufficient retention of color and taste of tomato products such as soups and juices while it does not completely inactivate pectin homogalacturonan-modifying enzymes such as pectinmethylesterase (PME; E.C. 3.1.1.11) and polygalacturonase (PG; E.C. 3.2.1.15). The incomplete inactivation of both enzymes is related to the rather low viscosity of cold-break-treated tomato products (Anthon et al. 2002). A “hot-break” treatment is applied for the production of tomato products with a high viscosity, such as, ketchup and pasta sauces (Anthon et al. 2002; Goodman et al. 2002). In this process, tomatoes are treated at temperatures around 85–90 °C at the beginning of the production process, causing inactivation of enzymes important for aroma development (e.g., lipoxygenase), as well as enzymes important for viscosity changes (i.e., PME and PG) (Goodman et al. 2002). High-pressure processing may create some opportunities concerning industrial processing of tomato-based products. Compared to thermal processing, high-pressure processing offers benefits related to the retention of nutritional and sensory food quality properties (Oey et al. 2008) and to selective enzyme inactivation. At particular pressure–temperature combinations (e.g., 500 MPa, 15 min, 25 °C), high-pressure processing enables selective knockout of tomato PG while PME activity is retained (Rodrigo et al. 2006).
Knowledge on the presence and processing stability of endogenous enzymes that act on cell wall polysaccharides other than PME and PG is also of interest in the context of tailoring the tomato polysaccharide structure via intelligent processing. Of particular interest are debranching enzymes as the importance of these enzymes has been shown in the context of fruit ripening. The loss of galactose and arabinose, sugars occurring in side chains of pectin and hemicellulose, during fruit ripening is well described (Brummell 2006). β-galactosidase (β-Gal; E.C. 3.2.1.23) and α-arabinofuranosidase (α-Af; E.C. 3.2.1.55) are enzymes reported to respectively hydrolyze terminal galactosyl and arabinosyl residues (Tateishi 2008). Although being exo-acting enzymes, the modifications of polysaccharide structures by β-Gal and α-Af could be outspoken. Release of terminal galactosyl and arabinosyl residues may lead to changes in the structure of sidechains and the interaction of neighboring polysaccharides and is believed to change the sensitivity to enzymatic degradation and the accessibility for other glycan hydrolases (Owino et al. 2005; Tateishi 2008). In the field of food rheology, α-Af activity has recently been shown to decrease the gel strength of carrot calcium–pectin gels by a reduction in sidechain entanglements (Ngouémazong et al. 2012). Even though the presence of both β-Gal and α-Af has been demonstrated in tomato fruit (Goulao and Oliveira 2008), studies concerning their processing stability are currently lacking.
This study aims at complementing the previously described processing stability data of the homogalacturonan-degrading enzymes PME and PG with the, so far unknown, thermal and pressure stability of the debranching enzymes β-Gal and α-Af in a tomato matrix. Tomato purée, chosen as an industrially relevant tomato matrix, was treated during a certain time period at different temperature and pressure levels, after which the residual enzyme activity was determined in crude tomato extracts. Based on differences in thermal and pressure stability between the enzymes, processing conditions for selective enzyme inactivation were selected. The transferability of enzyme inactivation data in tomato purée to intact tomato tissue was evaluated for industrially relevant process conditions.
Materials and Methods
A schematic overview of the experimental setup is displayed in Fig. 1, of which the details are described in the “Raw Material and Sample Preparation” section.
Raw Material and Sample Preparation
Processing tomatoes (Solanum lycopersicum cv. Patrona), originating from Spain, were acquired at the red-ripe stage.
Part of the fresh tomatoes, intended as a batch for the manufacture of tomato purée, was cut into quarters, frozen with liquid nitrogen and stored at −40 °C. For the production of tomato purée, non-pretreated tomato quarters were defrosted and subsequently homogenized (2 × 1 min) using a mixer (Büchi mixer B-400, Flawil, Switzerland), after which the homogenate was sieved (pore diameter = 1 mm) to remove seeds and peel remnants. The purées were thermally or high-pressure treated according to the conditions mentioned in the “Thermal Treatments” and “High-Pressure Treatments” section, after which residual enzyme activity in crude extracts was measured (cf. “Enzyme Extraction” and “Enzyme Assays” section).
Intact tomato tissue (slices/quarters), non-pretreated or treated at selected conditions (cf. “Thermal Treatments” and “High-Pressure Treatments” section), was frozen with liquid nitrogen and stored at −40 °C. Crude enzyme extracts, prepared from frozen samples, were assessed for residual enzyme activity (cf. “Enzyme Extraction” and “Enzyme Assays” section).
Thermal Treatments
For the thermal inactivation experiments of PME, PG, β-Gal, and α-Af in tomato purée, small amounts of purée (2.2 mL for PME, β-Gal and α-Af experiments; 10 mL for PG thermal stability study) were vacuum-packed in polyethylene bags followed by isothermal treatments in a temperature-controlled water bath at different temperature levels (25–95 °C) during a constant time period (5 min). Treatments were performed at least in duplicate (n = 2–4).
In the case of the thermal stability study of PME, PG, β-Gal, and α-Af in non-homogenized tomatoes, fresh tomatoes were cut into slices (±1 cm), and amounts of approximately 240 g were vacuum-packed in polyethylene bags. The tomato slices were treated in a temperature-controlled water bath using specific treatment conditions: 10 min, 65 °C, 0.1 MPa; 8 min, 95 °C, 0.1 MPa.
High-Pressure Treatments
For the high-pressure inactivation experiments of PME, PG, β-Gal, and α-Af in tomato purée, purée samples were treated at pressure levels between 0.1 and 800 MPa for 10 min at 20 °C. The high-pressure treatments of the purée samples were performed in duplicate (n = 2). The tomato purée was transferred to flexible microtubes (0.25 and 0.4 mL, Carl Roth, Karlsruhe, Germany) for samples intended to prepare PME, β-Gal, and α-Af crude extracts after treatment. Precaution to avoid air bubbles was taken. Treatments were performed using an eight-vessel laboratory scale high-pressure equipment (Resato, Roden, The Netherlands), equilibrated at 20 °C by using thermostatic mantles, surrounding the individual units and connected to a cryostat. A propylene glycol–water mixture (Resato) was used as pressure medium. Pressure buildup was performed slowly (100 MPa/min) in order to minimize temperature increase due to compression heating. Larger amounts (15 mL) of tomato purée, intended to prepare PG crude extracts after treatment, were vacuum-packed in double-film polyethylene bags. These samples were treated in a single-vessel laboratory scale high-pressure system (EPSI, Temse, Belgium) with a vessel volume of 500 mL, thermostated at 20 °C by using an external heat exchanger using 58 % ethylene glycol solution (Cryostat Haake N8-KT 50 W, Karlsruhe, Germany). A propylene–glycol mixture (60 % Dowcal, Dow Chemical Co., Horgen, Switzerland) was used as a pressure transmitting medium for this system.
In preparation of the high-pressure inactivation experiment of PME, PG, β-Gal, and α-Af in intact tomato tissue, fresh tomatoes were cut into quarters and amounts of approximately 180 g were vacuum-packed in double-film polyethylene bags. The treatment of the tomato quarters at the selected process conditions (10 min, 25 °C, 550 MPa) was performed using the setup of the single-vessel high-pressure system (EPSI, Temse, Belgium) already mentioned.
Enzyme Extraction
Crude enzyme extracts were prepared from treated tomato purées as well as from non-pretreated and processed intact tomato tissue. The processed tomato purées could be used as such for the enzyme extraction procedures. For the intact tomato samples, however, homogenates, including fruit flesh, peel and seeds, were prepared by homogenizing frozen tomato slices or quarters using a mixer (Grindomix GM 200 knife mill, Retsch, Haan, Germany). For the processed tomato purées, one enzyme extract was prepared for each individually treated sample. Extractions were at least duplicated in case of intact tomato tissue. The different enzymes were extracted from the tomato matrix using a specific procedure for each individual enzyme.
The extraction of PME was performed according to the method described by Verlent et al. (2004b). The tomato homogenate was centrifuged (10,000 × g, 30 min, 4 °C). Subsequently, the supernatant was discarded, and 0.2 M Tris–HCl extraction buffer pH 8.0 was added (volume extraction buffer (microliter) = 2 × the weight of the pellet (milligrams)). After homogenization for 1 min with a vortex (MELB 1719, Merck Eurolab, Germany), this mixture was centrifuged (10,000 × g, 60 min, 4 °C). The resulting supernatant represented the PME crude extract used for enzyme activity measurements.
PG extraction from the tomato homogenates was based on the procedure described by Pressey (1986). Cold distilled water was added to the tomato homogenate in a 1:1.5 (w/v) ratio, and the pH was subsequently adjusted to 3.0, followed by end-over-end mixing of the tomato mixture (15 min, 4 °C). Next, the suspension was centrifuged (14,400 × g, 30 min, 4 °C), the resulting pellet washed with distilled water (1:1 w/v), and the pH was adjusted to 3.0. This mixture was homogenized end-over-end (15 min, 4 °C) and centrifuged (15,300 × g, 20 min, 4 °C). The obtained pellet was dissolved (1:1 w/v) in a 1.2 M NaCl solution, and the pH of this mixture was adjusted to 6.0. Subsequently, the suspension was homogenized end-over-end at 4 °C for 3 h while keeping the pH constant by addition of NaOH. Afterwards, the mixture was centrifuged (18,000 × g, 30 min, 4 °C) and the resulting supernatant used for PG activity assays.
The extraction of β-Gal from the tomato homogenates was based on the method described by Tateishi et al. (2005). A 10 mM borate extraction buffer (pH 9.0) containing 1.0 M NaCl and 0.1 % (v/v) Triton X-100 was added to the tomato homogenate in a 1:1 (w/v) ratio. The mixture was homogenized by a vortexing device (MELB 1719, Merck Eurolab, Germany) for 1 h at 4 °C. The supernatant, obtained after centrifugation (12,000 × g, 30 min, 4 °C), was used as β-Gal crude enzyme extract.
The extraction procedure of α-Af was similar to the one described for β-Gal. The extraction buffer was, however, added in a different ratio (3:1 w/v) and was a 20 mM borate buffer (pH 9.0) containing 2.0 M NaCl, 0.2 % Triton X-100, 3 mM ZnCl2, and 2.0 % (w/v) sucrose (Sozzi et al. 2002). The subsequent steps in the extraction of α-Af are identical as described for the β-Gal extraction.
Enzyme Assays
Crude enzyme extracts of PME, PG, β-Gal, and α-Af from the tomato samples were analyzed for the respective enzyme activities.
PME activity was determined titrimetrically by measuring the enzyme-catalyzed formation of demethoxylated carboxyl groups on a pectin substrate (apple pectin; degree of esterification, 70–75 %; Fluka, Buchs, Switzerland) using an automatic pH stat titrator (Metrohm, Herisau, Switzerland) with 0.01 N NaOH. Assays were performed at pH 7.0 and 22 °C, using 30 mL of a 0.35 % (w/v) pectin solution containing 0.117 M NaCl (Jolie et al. 2009). One unit of PME activity is defined as the amount of enzyme catalyzing the hydrolysis of 1 μmol of ester bonds per minute at pH 7.0 and 22 °C.
PG activity was measured spectrophotometrically based on the formation of reducing groups from a polygalacturonic acid substrate (Fluka, Buchs, Switzerland) at 35 °C and pH 4.4 (Gross 1982). The reaction mixture consisted of 50 μL of PG crude extract and 350 μL of 0.3 % (w/v) polygalacturonic acid (washed previously with 80 % ethanol and dissolved in 40 mM Na acetate buffer pH 4.4). This mixture was incubated at 35 °C for 10 min after which addition of 2.0 mL cold borate buffer (pH 9.0, 0.1 M) stopped the reaction. Next, 0.4 mL of a 1 % 2-cyanoacetamide solution was added to the reaction mixture, after which the mixture was incubated at 100 °C for 10 min. After incubation, the reaction mixture was cooled immediately on ice water and subsequently equilibrated to room temperature. Finally, the absorbance was measured spectrophotometrically at 276 nm and 22 °C. Measurement of a blank sample was performed by replacing the enzyme solution with 40 mM Na acetate pH 4.4. A standard curve of monogalacturonic acid (Fluka, Buchs, Switzerland) (0 up to 1.4 mM solution) was used to calculate the amount of reducing groups formed, assuming that the concentration of monogalacturonic acid is proportional to the concentration of the reducing groups (Verlent et al. 2004a). One unit of PG activity represents the amount of enzyme producing 1 μmol of reducing groups from polygalacturonic acid per minute at pH 4.4 and 35 °C.
β-Gal activity was assessed by colorimetric measurement of the rate by which the enzyme hydrolyzes p-nitrophenyl-β-d-galactoside. The enzyme assay was based on the protocol described by Pressey (1983) with minor changes. The reaction mixture consisted of 0.5 mL of 0.1 M citrate pH 4.0, 0.1 mL of 0.4 % BSA, 0.4 mL of a 13 mM solution of p-nitrophenyl-β-d-galactoside (Sigma, Steinheim, Germany), and 0.4 mL of enzyme extract. After incubation for a fixed time at 37 °C, reactions were terminated by the addition of 2.0 mL of 0.2 M sodium carbonate, and the liberated p-nitrophenol was measured spectrophotometrically at 400 nm and 25 °C. One unit of β-Gal activity corresponds to the amount of enzyme that hydrolyzes 1 μmol of p-nitrophenyl-β-d-galactoside to p-nitrophenol and d-galactose per minute at pH 4.0 and 37 °C.
α-Af activity was determined by colorimetric measurement of p-nitrophenol that is liberated from p-nitrophenyl-α-l-arabinofuranoside (Tateishi et al. 1996). The enzyme extract (0.4 mL) was incubated for a fixed time at 37 °C with 0.5 mL of 0.1 M citrate pH 4.0, 0.1 mL of 0.4 % BSA and 0.4 mL of a 3.6 mM solution of p-nitrophenyl-α-l-arabinofuranoside (Sigma, Steinheim, Germany). Addition of 2.0 mL of 0.2 M sodium carbonate stopped the reactions, after which the liberated p-nitrophenol was measured at 400 nm and 25 °C. One unit of α-Af activity is defined as the amount of enzyme that hydrolyzes 1 μmol of p-nitrophenyl-α-l-arabinofuranoside to p-nitrophenol and l-arabinose per minute at pH 4.0 and 37 °C.
The enzyme activity data of treated tomato purée samples are expressed relatively to the enzyme activity of a control sample. Control samples for the thermal and high-pressure-treated purées consisted of tomato purée samples respectively treated for 5 min at 25 °C and 0.1 MPa, and for 10 min at 20 °C and 0.1 MPa. Each of the residual enzyme activity values of the processed purées is presented as the mean value of repeated treatments.
The enzyme activity data for the intact tomato tissue samples represent the mean value of the activities measured in multiple crude enzyme extracts. Residual enzyme activity values of the processed intact tomato tissue samples are expressed relatively to the activity of the enzymes in non-pretreated tomatoes.
Protein Content
The protein content of the different crude enzyme extracts was determined using the bicinchoninic acid kit according to Sigma procedure TPRO-562 (Sigma). This method is based on the reduction of Cu2+ to Cu+ by proteins in an alkaline environment. Bicinchoninic acid forms a colored complex with Cu+, quantified spectrophotometrically at 562 nm and 25 °C. The protein concentration (milligrams per milliliter) was calculated by comparison with a standard curve of bovine serum albumin. The protein content determination was performed in triplicate for each sample.
Kinetic Data Analysis
Thermal and high-pressure inactivation of enzymes is often described by a first-order kinetic model (Van Loey et al. 2003):
where A 0 is the initial activity, A is the residual activity after treatment time t (minutes) and k is the inactivation rate constant (per minute).
When several enzyme fractions with different processing stability (i.e., a labile and a stable fraction) are present, both inactivating according to a first-order model, a biphasic kinetic model can be used:
where subscripts L and S refer to the labile and the stable fraction, respectively.
When only the labile fraction inactivates, whereas the stable fraction is not affected under the processing conditions applied (i.e., if k S = 0), a fractional conversion kinetic model can be applied:
with A ∞ the non-zero residual activity after prolonged thermal or high-pressure treatment.
The temperature dependence (at constant pressure) of the inactivation rate constants, expressed in terms of activation energy (E a), is described by the Arrhenius equation:
where k ref is the inactivation rate constant at reference temperature T ref (K), E a is the activation energy (joules per mole) and R T is the universal gas constant.
The pressure dependence (at constant temperature) of the enzyme inactivation, expressed in terms of activation volume (V a), is described by the Eyring relationship:
where k ref is the inactivation rate constant at reference pressure p ref (megapascal), V a the activation volume (cubic centimeter per mole) and R p the universal gas constant.
In this study, the thermal or high-pressure inactivation data of each of the targeted enzymes were modeled using a one-step regression approach, in which the Arrhenius (Eq. 4) or Eyring equation (Eq. 5) were incorporated in an appropriate kinetic inactivation model (Eqs. 1, 2, 3). By plotting measured residual enzyme activity values against the corresponding treatment conditions (i.e., T or p levels), kinetic parameters ((k ref, E a) or (k ref, V a)) of the inactivation models were estimated by nonlinear regression analysis (SAS Statistical Software version 9.3, Cary, NC), for which T ref and p ref corresponded to T or p levels for which approximately 50 % residual enzyme activity was measured after treatment.
In order to express the quality of model fitting for nonlinear regression analysis, the corrected R 2 (Corr R 2) was considered:
where m is the number of observations, j is the number of model parameters and SSQ is the sum of squares (Fachin et al. 2003).
Because the present study did not investigate the course of enzyme inactivation in time, an irrefutable choice of enzyme inactivation models for kinetic modeling could not be made based on the experimental data. Hence, in order to select appropriate inactivation models for kinetic modeling of the in situ thermal or high-pressure inactivation of PME, PG, β-Gal, and α-Af, kinetic models applied in literature for thermal or high-pressure inactivation of these enzymes in a tomato matrix were considered. When several inactivation models were applicable or no kinetic models for enzyme inactivation were mentioned in literature, the Corr R 2 values for the applied models and the errors on the simultaneously estimated kinetic parameters, which were k ref and E a in case of thermal inactivation, and k ref and V a in case of high-pressure inactivation, were taken into account for the selection of the most appropriate model.
Statistical Analysis
The enzyme activity data and protein content values are presented as mean ± standard error of multiple measurements, and the results were processed using Microsoft Excel. Modeling of the enzyme inactivation by nonlinear regression analysis (SAS Statistical Software version 9.3, Cary, NC) generated simultaneously estimated kinetic parameters ((k ref, E a) or (k ref, V a)) which are displayed with their corresponding standard error of regression. The quality of model fitting was illustrated by the Corr R 2.
Results and Discussion
Presence of Targeted Enzymes in Tomato Fruit
Enzyme activity of PME, PG, β-Gal, and α-Af could be measured in crude enzyme extracts obtained from non-pretreated intact tomato tissue (Table 1), indicating their presence in the selected tomato variety. This is in agreement with data available in literature (Goulao and Oliveira 2008; Ishimaru et al. 2009).
Thermal Enzyme Stability in Tomato Purée
Figure 2 displays the effect of thermal processing of tomato purée on the in situ stability of PME, PG, β-Gal, and α-Af.
Residual activity of PME (circle), PG (diamond), β-Gal (triangle) and α-Af (square) as a function of the temperature level of the thermal treatments (t = 5 min, p = 0.1 MPa) applied to tomato purée. The residual activity values are expressed relative to the activity of a control sample (t = 5 min, T = 25 °C, p = 0.1 MPa). Each data point represents the mean of repeated treatments (n = 2–4, ±standard error). The areas T I and T II indicated by dashed lines refer to possible processing conditions allowing the creation of particular enzyme populations. (A color version of this figure is available in the Web version of this article.)
The residual enzyme activities after 5-min thermal treatments, as a function of temperature, clearly show differences in thermal stability of the studied enzymes in tomato purée.
The inactivation of PME in tomato purée by 5-min thermal treatments followed a sigmoidal inactivation curve: a gradual inactivation of PME was observed by treatments at 60–75 °C. Similar patterns of tomato PME thermal inactivation by 5-min treatments were previously published (Laratta et al. 1995; Rodrigo et al. 2006; Plaza et al. 2007). A 5-min treatment at 75 °C was necessary to inactivate the enzyme completely, which is similar to the findings of several other authors (Laratta et al. 1995; Lopez et al. 1997; Crelier et al. 2001; Anthon et al. 2002; Rodrigo et al. 2006; Plaza et al. 2007).
PG seemed to inactivate to a higher extent than PME at temperatures up to 65 °C. However, for treatment temperatures from 70 °C, PG was more thermostable than PME. The enzyme was only completely inactivated by a 5-min thermal treatment of the tomato purée at 95 °C, corresponding to data available in literature (Lopez et al. 1997; Anthon et al. 2002; Fachin et al. 2002a; 2003; Peeters et al. 2004; Rodrigo et al. 2006). Two phases in the inactivation profile can be observed, one starting already at temperatures below 60 °C and the second one at 80 °C, which is comparable to published results concerning the heat stability of PG in a crude tomato PG extract, tomato juice or tomato pieces (Fachin et al. 2002a; 2003; Peeters et al. 2004). This shape of the inactivation profile indicates the presence of two subpopulations of PG isozymes with different thermal stability. The occurrence of PG isozymes with different thermal stability in tomato is well established (Pressey and Avants 1973; Rodrigo et al. 2006). The thermostable PG isozyme seemed to account only for approximately 8 % of the activity of the total PG population. The occurrence of the two PG isoforms has been shown to depend largely on tomato variety: reported values for the contribution of thermostable PG to the PG population range from 14 to 75 % (Anthon et al. 2002; Fachin et al. 2003; Rodrigo et al. 2006). The presence of two PG populations in terms of thermal stability might be related to the production process of the tomato purée in this study, and the two populations thus might be absent in vivo in ripe tomato fruit. This is what was hypothesized by Peeters et al. (2004) who observed the thermostable form of PG in considerable amounts in tomato juice, unlike in tomato pieces.
β-Gal’s inactivation profile was found to be confined in a rather small temperature range: clear inactivation was already observed for temperature conditions ranging from 42.5 to 52.5 °C. The enzyme was completely inactivated by a treatment at 60 °C for 5 min. Like tomato PME, the thermal inactivation data of β-Gal followed a sigmoidal inactivation curve.
α-Af appeared to be rather sensitive towards thermal processing: a treatment for 5 min at 45 °C reduced the initial enzyme activity to 50 %, and only 6 % of enzyme activity was left after a treatment of the tomato purée at 60 °C. However, complete inactivation of the enzyme was only achieved by a 5-min treatment at 80 °C. These observations concerning the thermal inactivation of α-Af in tomato purée indicate the presence of two inactivation phases, one corresponding to temperatures from 25 to 65 °C and the other one corresponding to the temperature domain from 65 to 80 °C, which can respectively be related to the occurrence of a thermolabile and a thermostable fraction of the α-Af enzyme population. It can be observed from α-Af’s inactivation profile that this thermostable α-Af fraction accounted only for approximately 7 % of the activity of the total α-Af population.
It can be concluded that PME, PG, β-Gal, and α-Af appeared to possess distinct differences in thermal stability in tomato purée. For the temperature range up to 65 °C, PME showed to be the most thermostable among the studied enzymes while for temperatures from 70 °C, PG displayed the highest in situ thermostability. The in situ populations of α-Af and β-Gal showed a lower thermostability than the homogalacturonan-degrading enzymes PME and PG.
Thermal Enzyme Inactivation Rate Constants and Activation Energy Values
In this study, data concerning the thermal inactivation of PME, PG, β-Gal, and α-Af in tomato purée consist of residual enzyme activities obtained after thermal treatment for a constant time (t = 5 min) at varying temperatures (T = 25 – 95 °C). From these inactivation data, k ref and E a were simultaneously estimated for each enzyme using a one-step regression approach, in which the Arrhenius equation (cf. Eq. 4) was incorporated in a selected enzyme inactivation model (cf. Eqs. 1, 2, 3).
Under the assumption that thermal inactivation of PME in tomato purée can be described by a first-order model and the temperature dependence of the inactivation rate constants can be described by the Arrhenius equation (Crelier et al. 2001; Anthon et al. 2002; Fachin et al. 2002b), in situ PME thermal inactivation data were modeled using one-step nonlinear regression. The selected first-order model showed a good fit with the inactivation data, according to a Corr R 2 value of 0.9895.
The thermal inactivation of PG in tomato juice has been described by first-order (Crelier et al. 2001; Anthon et al. 2002) or fractional conversion inactivation kinetics (Fachin et al. 2003). The choice of different kinetic models to describe PG inactivation is related to the temperature ranges that were selected and to the fact that individual PG fractions (PG1 and PG2) were studied or not. In the study of Anthon et al. (2002), tomato juice was first thermally treated, after which the residual enzyme activity was determined from individual PG1 and PG2 crude enzyme extracts. This research group determined separately the inactivation kinetics of the thermolabile PG2 fraction and the thermostable PG1 fraction by respectively selecting the temperature ranges 64.9–72.8 °C and 84.8–92.8 °C. Conversely, in the studies of Fachin et al. (2003) and Crelier et al. (2001), residual enzyme activity was measured in crude PG extracts obtained from thermally treated tomato juice, without making a distinction between PG1 and PG2. The selected temperature range from 55 to 70 °C in the study of Fachin et al. (2003) was, however, presumably related to the inactivation of thermolabile PG2, assuming that the 14 % of initial activity that remained after a long treatment time at these temperatures could be ascribed to the thermostable PG1 fraction. In the study of Crelier et al. (2001), the selected temperature range from 80 to 105 °C provided information about the thermal inactivation of both PG fractions (PG1 and PG2). The inactivation data of PG in tomato purée obtained in this study presents two inactivation phases ((60–80 °C) and (80–95 °C)). The first inactivation phase is most probably related to thermal inactivation of the thermolabile PG2 while the second inactivation phase is related to the inactivation of thermostable PG1. The PG inactivation data of the current study allowed to estimate the thermal enzyme inactivation rate constant and the activation energy for the thermolabile PG fraction (in the T-domain ≤80 °C), by use of a fractional conversion inactivation model.
In the case of tomato β-Gal, no published data concerning thermal enzyme inactivation are available. Since visual inspection of the inactivation profile did not reveal multiple inactivation phases and the residual enzyme activity was reduced to zero, a first-order inactivation model was selected for the modeling of the β-Gal thermal inactivation data. Kinetic studies, measuring the enzyme inactivation after different treatment times at different temperatures, are, however, required to irrefutably validate this assumption of a first-order model.
To date, no thermal inactivation data of tomato α-Af can be found in published literature. In this study, the obtained α-Af inactivation profile indicates two inactivation phases ((25–65 °C) and (65–80 °C)) which are respectively related to the inactivation of a thermolabile and a thermostable α-Af fraction. The α-Af inactivation data obtained in the present study allowed to estimate kinetic parameters for the thermal inactivation of the thermolabile α-Af fraction (in the T-domain ≤65 °C), using a fractional conversion model.
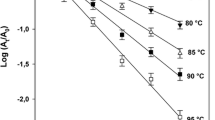
Figure 3 presents the observed thermal enzyme stability data versus the simulated residual enzyme activities obtained by applying one-step regression analysis (dashed lines). The selected inactivation models, estimated kinetic parameters k ref and E a, as well as Corr R 2 values as a measure for the quality of model fitting, are displayed in Table 2.
Modeling of the thermal stability data of PME, PG, β-Gal and α-Af in tomato purée by one-step nonlinear regression analysis. The modeled residual enzyme activities are displayed by a dashed line. The applied kinetic inactivation models and the obtained corresponding Corr R 2 values are presented in Table 2
Visual inspection of the model fitting (Fig. 3) and the obtained Corr R 2 values (Table 2) indicate that the thermal enzyme inactivation data could be accurately described by the selected inactivation models. The resulting k ref and E a values, displayed in Table 2, respectively deliver information concerning the rate of enzyme inactivation at reference temperature and the temperature dependence of k. A comparison of the E a values of the targeted enzymes indicates that the inactivation of PME in tomato purée is the most temperature dependent, followed successively by β-Gal, the thermolabile PG fraction and the thermolabile fraction of α-Af. This order is also reflected by the steepness of the inactivation profiles shown in Fig. 3.
From the estimated kinetic parameters for thermal PME inactivation, an inactivation rate constant at 70 °C of 0.495 min−1 can be derived. This inactivation rate constant and the observed activation energy of 336 kJ/mol are quite similar to the inactivation rate constant at 70 °C of 0.375 min−1 and activation energy value of 350 kJ/mol observed by Crelier et al. (2001) for thermal PME inactivation in tomato juice. Another thermal inactivation study of PME in tomato juice, performed by Anthon et al. (2002), delivered inactivation rate constants at 70 °C of 0.222 and 0.318 min−1 and activation energies of 477 and 440 kJ/mol, respectively, for the tomato cultivars CXD 199 and BOS 3155.
In case of thermal inactivation of the thermolabile PG fraction, a rate constant of 0.174 min−1 at 65 °C and an activation energy of 138 kJ/mol were estimated. Fachin et al. (2003) obtained an inactivation rate constant at 65 °C of 0.213 min−1 and an activation energy value of 228.35 kJ/mol for thermal inactivation of the thermolabile PG fraction in tomato juice, values which are higher than the kinetic inactivation parameters obtained in this study. From the thermal inactivation data displayed in Table 2, a rate constant of 0.358 min−1 at 70 °C can be derived for the inactivation of the PG2 fraction. Anthon et al. (2002) reported inactivation rate constants at 70 °C of 0.882 and 0.582 min−1, and activation energies of 208 and 215 kJ/mol, respectively, for thermal PG2 inactivation in tomato juice of the tomato cultivars CXD 199 and BOS 3155. These values are higher than the kinetic inactivation parameters determined in this study.
Pressure Stability of Enzymes in Tomato Purée
Figure 4 presents the effect of 10-min high-pressure treatments of tomato purée on the in situ stability of PME, PG, β-Gal, and α-Af.
Residual activity of PME (circle), PG (diamond), β-Gal (triangle) and α-Af (square) as a function of the pressure level of the high-pressure treatments (t = 10 min, T = 20 °C) applied on tomato purée. The residual activity values are expressed relative to the activity of a control sample (t = 10 min, T = 20 °C, p = 0.1 MPa). Each data point represents the mean of repeated treatments (n = 2, ±standard error). The areas p I and p II indicated by dashed lines refer to possible processing conditions allowing the creation of particular enzyme populations. (A color version of this figure is available in the Web version of this article.)
The inactivation data are displayed as residual enzyme activities as a function of the applied pressure level. It can be observed that the pressure stability of the four enzymes in the tomato purée shows clear differences.
PME appeared to be very pressure stable: approximately 70 % of the enzyme population was not inactivated after treatment for 10 min at 800 MPa and 20 °C. The high pressure stability of tomato PME has been mentioned before in literature (Tangwongchai et al. 2000; Shook et al. 2001; Rodrigo et al. 2006).
PG showed to be more pressure labile than PME. This enzyme was gradually inactivated in a rather narrow pressure range: a gradual reduction of PG activity to zero was observed after 10-min treatments of the tomato purée at 20 °C and pressure levels ranging from 300 to 550 MPa. Similar pressure stability curves of PG were published for 15-min high-pressure treatments of tomato juice or pieces at 25 °C (Fachin et al. 2003; Peeters et al. 2004; Rodrigo et al. 2006).
β-Gal showed an intermediate pressure stability compared to the pressure-stable PME and the pressure-labile PG. Its activity in tomato purée was gradually reduced to almost zero after treatments at 300–800 MPa for 10 min.
α-Af displayed a lower pressure stability than β-Gal for high-pressure treatments during 10 min in the range from 200 to 600 MPa at 20 °C. Treatment of the purée at the highest applied pressure level (800 MPa) did, however, not allow a complete inactivation of α-Af, retaining a residual enzyme activity of approximately 6 %.
In conclusion, distinct differences in pressure stability of the structure-related enzymes in tomato purée were observed. PME was clearly the most pressure-stable enzyme and tomato PG the most pressure-labile one. The polysaccharide-debranching enzymes, β-Gal and α-Af, showed an intermediate pressure stability: a reduction of enzyme activity below 10 % of residual activity was observed for treatments at 700 and 600 MPa, respectively.
High-Pressure Enzyme Inactivation Rate Constants and Activation Volume Values
High-pressure inactivation data of PME, PG, β-Gal, and α-Af in tomato purée were collected by measuring residual enzyme activities after high-pressure treatment for a constant time (t = 10 min) at varying pressure levels (p = 0.1–800 MPa) and a temperature of 20 °C. Using a one-step regression approach, in which the Eyring equation (cf. Eq. 5) was incorporated in a selected enzyme inactivation model (cf. Eqs. 1, 2, 3), the kinetic parameters k ref and V a were simultaneously estimated.
Kinetic parameters for PME inactivation in tomato homogenates at elevated pressure have only been estimated for combined high-temperature/high-pressure processing conditions (60–75 °C; 0.1–800 MPa) (Crelier et al. 2001; Stoforos et al. 2002). Modeling of high-pressure inactivation of PME in tomato homogenates at conditions approximating room temperature, like in the present study (i.e., T = 20 °C) is, however, not reported, probably due to the high-pressure stability of this enzyme (Fig. 4). Nonetheless, an attempt was made to model the pressure stability data of PME in tomato purée using a first-order model.
In earlier studies, high-pressure inactivation kinetics of PG in tomato homogenates at temperatures close to room temperature have been modeled according to a first-order reaction (Crelier et al. 2001; Fachin et al. 2003). Hence, a first-order inactivation reaction was selected for modeling of the experimentally obtained thermal PG inactivation data.
In the case of tomato β-Gal and α-Af, no high-pressure inactivation data are available in published literature. The high-pressure stability data of β-Gal in tomato purée obtained in this study indicate two inactivation phases of the enzyme, depending on the applied pressure levels: one phase related to treatments from 0.1 to 400 MPa and the other one to treatments above 400 MPa. This observation motivated for the application of a biphasic inactivation model. For α-Af, a fractional conversion reaction was chosen for kinetic modeling because a small fraction of enzyme activity (approximately 6 %) was still not inactivated after a 10-min treatment at 800 MPa and 20 °C.
Figure 5 presents the high-pressure enzyme stability data versus the modeled inactivation profile obtained by applying one-step regression analysis (dashed lines). The selected inactivation models, estimated kinetic parameters k ref and V a, as well as the quality of model fitting (Corr R 2), are displayed in Table 3.
Modeling of the high-pressure stability data of PME, PG, β-Gal and α-Af in tomato purée by one-step nonlinear regression analysis. The modeled residual enzyme activities are displayed by a dashed line. The applied kinetic inactivation models and the obtained corresponding Corr R 2 values are presented in Table 3
Visual examination of the model fitting (Fig. 5) and the determined Corr R 2 values (Table 3) indicate that the high-pressure inactivation data are properly described by the selected inactivation models. The simultaneously estimated parameters k ref and V a are indicative for respectively the rate of enzyme inactivation at reference pressure and the pressure dependence of k. It can be observed that the activation volumes all exhibit a negative value, showing that the pressure-induced inactivation of the enzymes was related to a volume decrease, which indicates that enzyme inactivation was accelerated by application of high pressure (Jolie et al. 2012). Analysis of the estimated V a values of the studied enzymes shows that enzyme inactivation in tomato purée is the most pressure dependent for the pressure-labile fraction of β-Gal, followed consecutively by PG, the pressure-stable β-Gal fraction, α-Af and PME. This order in pressure dependency corresponds to the slopes of the inactivation profiles displayed in Fig. 5.
In this study, an activation volume of −63.6 cm3/mol and inactivation rate constants of 0.128 and 1.747 min−1, respectively for high-pressure inactivation of PG at 400 and 500 MPa were determined. Fachin et al. (2003) obtained at a temperature of 20 °C first-order inactivation rate constants of 0.019 and 0.197 min−1 at 400 and 500 MPa, respectively, for PG inactivation in tomato juice, values which are quite lower than the results obtained in the current study. The use of different tomato varieties is a possible reason to explain these differences. However, a V a value of −57.15 cm3/mol at 20 °C was obtained by these researchers, which is similar to the value obtained in the current study.
Creation of Selective Enzyme Populations in Intact Tomato Tissue
The differences in temperature or pressure sensitivity of the studied enzymes in tomato purée offer possibilities for selective enzyme inactivation, which is visualized by the areas T I, T II, p I and p II, depicted in Figs. 2 and 4. These four areas represent processing conditions that allow the creation of various enzyme populations, consisting of one or more of the targeted structure-related enzymes with varying degrees of enzyme activities. Such created enzyme populations could be of interest in the context of structure creation in tomato matrices. The transferability of the obtained enzyme inactivation data and thus the selective enzyme inactivation in tomato purée towards the case of intact tomato tissue was evaluated. Hereto, two industrially relevant thermal treatments (“cold-break”, 10 min, 65 °C, 0.1 MPa; “hot-break”, 8 min, 95 °C, 0.1 MPa) and one high-pressure treatment (10 min, 25 °C, 550 MPa), respectively related to areas T I, T II, and p I (Figs. 2 and 4), were selected. The residual activity data of PME, PG, β-Gal, and α-Af in intact tomato tissue are presented in Table 4.
In the case of the cold-break-treated tomatoes, the residual activities of PG (12 %), β-Gal (2 %) and α-Af (12 %) comply with which can be inferred from the data displayed in Fig. 2. After a prolonged treatment (10 min instead of 5 min) at a temperature situated in domain T I, where clear enzyme inactivation was noticeable in the case of the tomato purées, most of the enzymatic activity of PG, β-Gal, and α-Af in the intact tomato tissue disappeared. PME, on the contrary, showed a residual activity of 74 % after a thermal treatment of intact tomato tissue for 10 min at 65 °C while a 5-min treatment at 65 °C of tomato purée resulted in a residual activity of only 68 %. It is known that colloidal material (proteins, pectins, etc.) surrounding enzymes in a food matrix can exert a protective effect on the enzyme structure against denaturation/inactivation (Fachin et al. 2002b; Van Boekel 2009). The observed difference in PME thermal stability in intact tomato tissue and tomato purée could hence possibly be explained by matrix effects. The hot-break treatment resulted in complete inactivation of the four studied enzymes in intact tomato tissue. The selected high-pressure treatment resulted in a 50 % reduction of PME, considerable inactivation of the two debranching enzymes and complete inactivation of PG in intact tomato tissue. In contrast to the similarity of the residual activities of PG, β-Gal, and α-Af in tomato purée (Fig. 4; area p I) to the activities of the respective enzymes in intact tomato tissue after a 10-min treatment at 550 MPa, the higher pressure stability of PME in tomato purée towards treatment at 550 MPa (94 % residual activity) compared to the case of intact tomato tissue (50 % residual PME activity) is striking. This difference in PME pressure stability could possibly be explained by a high sensitivity of the enzyme to differences in dynamic heating conditions intrinsic to the different high-pressure equipment used for the pressure stability study of PME in tomato purée on the one hand and the high-pressure treatment of intact tomato tissue on the other hand.
In general, the in situ enzyme inactivation data obtained for intact tomato tissue are clearly comparable to the results obtained for tomato purée and show the usefulness of inactivation studies as performed in the current study in the context of process design of products in which selective enzyme inactivation is desired to obtain specific functional properties (such as textural quality).
Conclusions
The thermal and pressure stability of the structure-related enzymes PME, PG, β-Gal, and α-Af were determined in tomato purée and intact tomato tissue. For the homogalacturonan-degrading enzymes, it was observed that PME possesses a higher thermal stability than PG at lower treatment temperatures (up to 65 °C) while PG was clearly more thermostable than PME at higher temperatures. The low pressure stability of PG compared to the pressure-stable PME was established. For the polysaccharide-debranching enzymes β-Gal and α-Af, thermal and pressure stability were described for the first time: despite displaying a rather low thermostability, β-Gal and α-Af possess a considerable resistance against high-pressure processing. These results permitted the identification of selective processing conditions which allow the in situ creation of various specific populations of endogenous tomato enzymes. In future research, it will be worthwhile evaluating the potential of such enzyme populations created by selective processing to tailor the structural properties of tomato matrices.
References
Anthon, G. E., Sekine, Y., Watanabe, N., & Barrett, D. M. (2002). Thermal inactivation of pectin methylesterase, polygalacturonase, and peroxidase in tomato juice. Journal of Agricultural and Food Chemistry, 50(21), 6153–6159.
Augusto, P. E. D., Falguera, V., Cristianini, M., & Ibarz, A. (2012). Rheological behavior of tomato juice: steady-state shear and time-dependent modeling. Food and Bioprocess Technology, 5(5), 1715–1723.
Brummell, D. A. (2006). Cell wall disassembly in ripening fruit. Functional Plant Biology, 33(2), 103–119.
Crelier, S., Robert, M. C., Claude, J., & Juillerat, M. A. (2001). Tomato (Lycopersicon esculentum) pectin methylesterase and polygalacturonase behaviors regarding heat- and pressure-induced inactivation. Journal of Agricultural and Food Chemistry, 49(11), 5566–5575.
Fachin, D., Van Loey, A., Indrawati, Ludikhuyze, L., & Hendrickx, M. (2002a). Thermal and high-pressure inactivation of tomato polygalacturonase: a kinetic study. Journal of Food Science, 67(5), 1610–1615.
Fachin, D., Van Loey, A. M., Ly Nguyen, B., Verlent, I., Indrawati, & Hendrickx, M. E. (2002b). Comparative study of the inactivation kinetics of pectinmethylesterase in tomato juice and purified form. Biotechnology Progress, 18(4), 739–744.
Fachin, D., Van Loey, A. M., Ly Nguyen, B., Verlent, I., Indrawati, & Hendrickx, M. E. (2003). Inactivation kinetics of polygalacturonase in tomato juice. Innovative Food Science & Emerging Technologies, 4(2), 135–142.
Goodman, C. L., Fawcett, S., & Barringer, S. A. (2002). Flavor, viscosity, and color analyses of hot and cold break tomato juices. Journal of Food Science, 67(1), 404–408.
Goulao, L. F., & Oliveira, C. M. (2008). Cell wall modifications during fruit ripening: when a fruit is not the fruit. Trends in Food Science and Technology, 19, 4–25.
Gross, K. C. (1982). A rapid and sensitive spectrophotometric method for assaying polygalacturonase using 2-cyanoacetamide. Hortscience, 17(6), 933–934.
Ishimaru, M., Smith, D., Mort, A., & Gross, K. (2009). Enzymatic activity and substrate specificity of recombinant tomato β-galactosidases 4 and 5. Planta, 229(2), 447–456.
Jolie, R. P., Christiaens, S., De Roeck, A., Fraeye, I., Houben, K., Van Buggenhout, S., et al. (2012). Pectin conversions under high pressure: Implications for the structure-related quality characteristics of plant-based foods. Trends in Food Science and Technology, 24(2), 103–118.
Jolie, R. P., Duvetter, T., Houben, K., Clynen, E., Sila, D. N., Van Loey, A. M., et al. (2009). Carrot pectin methylesterase and its inhibitor from kiwi fruit: Study of activity, stability and inhibition. Innovative Food Science & Emerging Technologies, 10(4), 601–609.
Laratta, B., Fasanaro, G., De Sio, F., Castaldo, D., Palmieri, A., Giovane, A., et al. (1995). Thermal inactivation of pectin methylesterase in tomato puree - implications on cloud stability. Process Biochemistry, 30(3), 251–259.
Lopez, P., Sanchez, A. C., Vercet, A., & Burgos, J. (1997). Thermal resistance of tomato polygalacturonase and pectinmethylesterase at physiological pH. Zeitschrift fur Lebensmittel-Untersuchung und Forschung A, 204(2), 146–150.
Ngouémazong, D. E., Kabuye, G., Fraeye, I., Cardinaels, R., Van Loey, A., Moldenaers, P., et al. (2012). Effect of debranching on the rheological properties of Ca2+-pectin gels. Food Hydrocolloids, 26(1), 44–53.
Nisha, P., Singhal, R. S., & Pandit, A. B. (2011). Kinetic modelling of colour degradation in tomato puree (Lycopersicon esculentum L.). Food and Bioprocess Technology, 4(5), 781–787.
Oey, I., Lille, M., Van Loey, A., & Hendrickx, M. (2008). Effect of high-pressure processing on colour, texture and flavour of fruit- and vegetable-based food products: a review. Trends in Food Science and Technology, 19(6), 320–328.
Owino, W. O., Amubko, J. L., & Mathooko, F. M. (2005). Molecular basis of cell wall degradation during fruit ripening and senescence. Stewart Postharvest Review, 1(3), 1–10.
Peeters, L., Fachin, D., Smout, C., Van Loey, A., & Hendrickx, M. E. (2004). Influence of β-subunit on thermal and high-pressure process stability of tomato polygalacturonase. Biotechnology and Bioengineering, 86(5), 543–549.
Plaza, L., Duvetter, T., Monfort, S., Clynen, E., Schoofs, L., Van Loey, A. M., et al. (2007). Purification and thermal and high-pressure inactivation of pectinmethylesterase isoenzymes from tomatoes (Lycopersicon esculentum): a novel pressure labile isoenzyme. Journal of Agricultural and Food Chemistry, 55(22), 9259–9265.
Pressey, R. (1983). β-galactosidases in ripening tomatoes. Plant Physiology, 71(1), 132–135.
Pressey, R. (1986). Extraction and assay of tomato polygalacturonases. Hortscience, 21(3), 490–492.
Pressey, R., & Avants, J. K. (1973). Two forms of polygalacturonase in tomatoes. Biochimica et Biophysica Acta (BBA) - Enzymology, 309(2), 363–369.
Rodrigo, D., Cortes, C., Clynen, E., Schoofs, L., Van Loey, A., & Hendrickx, M. (2006). Thermal and high-pressure stability of purified polygalacturonase and pectinmethylesterase from four different tomato processing varieties. Food Research International, 39(4), 440–448.
Shook, C. M., Shellhammer, T. H., & Schwartz, S. J. (2001). Polygalacturonase, pectinesterase, and lipoxygenase activities in high-pressure-processed diced tomatoes. Journal of Agricultural and Food Chemistry, 49(2), 664–668.
Sila, D. N., Van Buggenhout, S., Duvetter, T., Fraeye, I., De Roeck, A., Van Loey, A., et al. (2009). Pectins in processed fruit and vegetables: Part II - Structure-function relationships. Comprehensive Reviews in Food Science and Food Safety, 8(2), 86–104.
Sozzi, G. O., Greve, L. C., Prody, G. A., & Labavitch, J. M. (2002). Gibberellic acid, synthetic auxins, and ethylene differentially modulate α-L-arabinofuranosidase activities in antisense 1-aminocyclopropane-1-carboxylic acid synthase tomato pericarp discs. Plant Physiology, 129(3), 1330–1340.
Stoforos, N. G., Crelier, S., Robert, M. C., & Taoukis, P. S. (2002). Kinetics of tomato pectin methylesterase inactivation by temperature and high pressure. Journal of Food Science, 67(3), 1026–1031.
Tangwongchai, R., Ledward, D. A., & Ames, J. M. (2000). Effect of high-pressure treatment on the texture of cherry tomato. Journal of Agricultural and Food Chemistry, 48(5), 1434–1441.
Tateishi, A. (2008). β-galactosidase and α-L-arabinofuranosidase in cell wall modification related with fruit development and softening. Journal of the Japanese Society for Horticultural Science, 77(4), 329–340.
Tateishi, A., Kanayama, Y., & Yamaki, S. (1996). α-L-arabinofuranosidase from cell walls of Japanese pear fruits. Phytochemistry, 42(2), 295–299.
Tateishi, A., Mori, H., Watari, J., Nagashima, K., Yamaki, S., & Inoue, H. (2005). Isolation, characterization, and cloning of α-L-arabinofuranosidase expressed during fruit ripening of Japanese pear. Plant Physiology, 138(3), 1653–1664.
Van Boekel, M. A. J. S. (2009). Kinetics of protein and enzyme denaturation. In Kinetic modeling of reactions in foods (pp. 10-1-10-39). Florida: CRC Press, Taylor & Francis Group.
Van Buggenhout, S., Sila, D. N., Duvetter, T., Van Loey, A., & Hendrickx, M. (2009). Pectins in processed fruits and vegetables: Part III - texture engineering. Comprehensive Reviews in Food Science and Food Safety, 8(2), 105–117.
Van Loey, A., Indrawati, Smout, C., & Hendrickx, M. (2003). Inactivation of enzymes. From experimental design to kinetic modeling. In J. R. Whitaker, A. G. J. Voragen, & D. W. S. Wong (Eds.), Handbook of Food Enzymology (pp. 49–58). New York: Marcel Dekker Inc.
Verlent, I., Van Loey, A., Smout, C., Duvetter, T., & Hendrickx, M. E. (2004a). Purified tomato polygalacturonase activity during thermal and high-pressure treatment. Biotechnology and Bioengineering, 86(1), 63–71.
Verlent, I., Van Loey, A., Smout, C., Duvetter, T., Ly Nguyen, B., & Hendrickx, M. E. (2004b). Changes in purified tomato pectinmethylesterase activity during thermal and high pressure treatment. Journal of the Science of Food and Agriculture, 84(14), 1839–1847.
Acknowledgments
This research has been carried out with financial support from the Research Fund KU Leuven (KP/08/004) and from the Agency for Innovation by Science and Technology in Flanders (IWT-Vlaanderen). S. Van Buggenhout is a postdoctoral researcher, funded by the Research Foundation Flanders (FWO).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Houben, K., Jamsazzadeh Kermani, Z., Van Buggenhout, S. et al. Thermal and High-Pressure Stability of Pectinmethylesterase, Polygalacturonase, β-Galactosidase and α-Arabinofuranosidase in a Tomato Matrix: Towards the Creation of Specific Endogenous Enzyme Populations Through Processing. Food Bioprocess Technol 6, 3368–3380 (2013). https://doi.org/10.1007/s11947-012-0984-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-012-0984-5