Abstract
Physical properties and stability are critical for delivering safe and healthy food to the consumers and thus is a theme that attracts food scientists for a long time. Recently, literature suggests that stability can be fully grasped only if food molecular dynamics and structure are taken into consideration, i.e. an appropriate understanding of the behaviour of food products requires knowledge of its composition, structure and molecular dynamics, through the three-dimensional arrangement of the various structural elements and their interactions. Food systems behaviour is strongly dependent on the water molecular dynamics. Understanding changes in location and mobility of water represents a significant step in food stability knowledge, since water “availability” profoundly influences the chemical, physical and microbiological quality of foods. Nuclear magnetic resonance has been presented as a powerful technique to investigate water dynamics and physical structures of foods through analysis of nuclear magnetisation relaxation times, because it provides information on molecular dynamics of different components in dense complex systems. The application of this technique may be very useful in predicting food systems physicochemical changes, namely texture, viscosity or water migration. This paper aims at reviewing some of the main aspects related to food physical properties and stability, and the role of water in these properties. More specifically, this paper intends to contribute to a deeper understanding of the relationship of molecular constituents–structure–function of food systems, contributing to the development of foods with improved functionality.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Overall Molecular Dynamics Concept
Molecular dynamics has been pointed as the actual most promising parameter for characterising multicomponent systems. Analysis of systems at a molecular scale has been demonstrated to be a useful methodology for investigating complex geometries and molecules, as well as studying structural and dynamic properties [71].
Molecular dynamics involves, at a microscopic level, the displacement of reactants, which promote chemical reactions. Macroscopically, molecular dynamics can be related to the viscosity of the material, which in turn controls the flow properties, structure collapse, mechanical properties and thus the product texture [57].
It is generally accepted that the knowledge of molecular dynamics is determinant for assessing physicochemical and microbiological stability of food systems [38, 57] and is quite dependent on composition and matrices microstructure.
Food stability is a critical parameter for different stakeholders. Concerning consumers, stability assures safety, nutritional and sensorial quality of food products and answers to the increasing demand for a diversity of ready-to-eat food with fresh appearance and health-promoting properties [46]. For industry, stability allows maximising shelf-life: minimising waste along the distribution chain, increasing profit and reducing the environmental impact [32, 53, 54, 67].
Food physical stability is assessed by shelf-life changes of mechanical, thermal or surface properties, which are often related to food product’s quality, processing behaviour or development of novel food products and processes [8, 36]. Physical state is directly affected and responsible for the molecular dynamics of a matrix [40, 51].
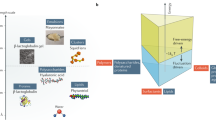
It is possible to observe in Fig. 1 a simplified scheme of how molecular dynamics covers several concepts related to food properties and stability, being a key and linking factor between all aspects involved in food systems assessment, including food structure/microstructure. The better understanding of these factors and relationship between them is essential for controlling degradation reactions rates and maintaining food integrity [53].
Food systems are complex mixtures of water, biopolymers (proteins and polysaccharides), low molecular weight ingredients (minerals, sugars, surfactants, etc.) and colloid particles (oil droplets or air bubbles). The molecular dynamics between these different components reflects on the stability of such systems, determining the physical state, microstructure and composition, which impacts on food characteristics [55]. Water, as one of the most important food constituents and its interactions with other food ingredients, controls both thermodynamic and dynamic properties of all aqueous elements [57]. These interactions affect mainly appearance and sensory attributes (texture/firmness) [47, 64, 72], nutrient quality [72] and the microbiological load [30]. The extensions of the reactions between food constituents, usually associated with metabolic processes, are responsible for the degradation of quality, safety and nutritional attributes.
Although molecular dynamics has been considered an useful methodology for investigating complex systems (geometries and molecules) [71] and the degradation reactions extension, a large number of studies have been focused on chemically pure or homogeneous materials, such as proteins or polysaccharides, instead of food systems. The data for “pure and simple” systems cannot be extrapolated when considering food systems, since it is necessary to take into account the heterogeneity of the systems, as well as their interactions with water [57]. Moreover, it is important to consider the system’s microstructure to understand the spatial and molecular distribution of water within its food matrix environment and determine whether water is already accessible for metabolic reactions.
Aspects of Water Molecular Dynamics
Water is the most important solvent, dispersion medium and plasticiser in biological and food systems [45]. It affects reactions, can be a substrate and a product of reactions and is involved in nutrient transport and dissolution of salts and other solutes. It establishes pH, acts as a polymer plasticiser and modulates viscosity, osmotic pressure, etc. [69]. Specifically, the state of water in food influences physical properties, such as rheological, electrical, optical, thermal or mass transfer [36].
For long, water has been recognised as one of the most important food components in impacting food physicochemical and microbiological attributes, shelf-life and deteriorative changes [24, 31, 32, 36, 44, 50, 54, 59, 61]. Therefore, determination of water content is one of the most frequent analyses in the food industry laboratories [44]. Water content of food systems normally ranges from 80 to 95 %, for high-moisture foods, to a percentage close to zero in semi-dry and dry foods [5, 50]. However, various foods with the same water content differ in stability [30], which demonstrates that the sole value of “water content” in a food does not inform about the nature of water [18, 30, 44]. In fact, in a food matrix, water molecules can be accessible or not to participate in degradation reactions [44].
The knowledge of each of these fractions is important, specifically because available water, its location and the interactions with the other food components (like proteins and polysaccharides) are responsible for the physicochemical and microbiological properties and stability of foods [45, 59].
As such, besides water content in a food material, it is important to understand the water state and dynamics for a proper comprehension of properties and stability of food products.
Water mobility/dynamics can thus be described as a manifestation how “freely” water molecules can participate in reactions or how easily water molecules diffuse to the reaction sites to participate in reactions [58]. Presence of molecules of different molecular weight and solubility in water can have a profound influence on water mobility/dynamics, as this is dependent on the physicochemical properties of other nonaqueous food constituents and their interactions with water and among themselves [58, 70].
Different parameters have been used in the literature to describe water dynamics in the food systems and its repercussion in stability: water activity, glass transition temperature or water relaxation time. These concepts are detailed in the next sub-sections.
Water Activity Concept and Shortcomings
Water activity (a w) concept was introduced in the mid-twentieth century as a critical parameter for estimating food stability [54] and has been one of the most widely used concepts to evaluate food’s water accessibility [30]. For a long time, a w was regarded as the most important parameter controlling the behaviour of food during processing and storage, with particular emphasis on its effects on reaction degradation rates [5, 31, 41, 59]. This parameter has been used thoroughly as the indicator for microbial growth and microbial stability of a food system [68]. Also, with respect to most of degradation reactions of a chemical, enzymatic, or physical nature, such as lipid oxidation, non-enzymatic and enzymatic activities, and the texture/mouthfeel of foods following production, water activity is currently used as an important parameter [41, 59, 61].
Despite the irrefutable significance of a w for food science and engineering, the limitations of this analysis are, actually, evident. Water activity is a thermodynamic measure of the chemical potential of water in the system, assuming that food is in equilibrium with the surrounding atmosphere [36]. However, it is well known that most foods are not in the state of equilibrium [24, 53]. Water activity measurements may not provide, for example, the relationship of the evolution of the structural changes of the food material with the changes of the water–macromolecules and water–water interactions that occur during food shelf-life [71], and studies have stressed that under many common circumstances, the thermodynamics activity of water is far less relevant to processing and storage than structure-related properties, which can restrict the mobility and diffusion of the reactants [5, 61].
Moreover, the water activity analysis does not consider microstructure or the possibility that there may be local regions differing in water content and presumably of water availability [24], fact that could be important for microbiological stability, since some authors [25, 27, 69] demonstrated that microorganisms are sensitive to the local properties of the system, i.e. local water activity, translational motions and microstructure, and not to the bulk water activity. Some authors also showed that microbial response in a solution is more dependent on the solute used to control a w values than on a w itself [12, 68], showing the importance of solute interaction.
Therefore, water activity, defined as a relative vapour pressure, reflects only the surface properties of a system and not necessarily the molecular dynamics that takes place in its interior [69].
Furthermore, it has been reported that solutions with the same water activity can present dramatic differences in the system’s “kinetics” (here assessed by viscosity) (see Fig. 2) [5, 41].
Viscosity versus water activity of model solutions produced with different solutes (adapted from [5])
Glass Transition Temperature
The glass transition temperature (T g) was often used, since the early 1980s, as a parameter that would be able to assess food stability and overcome the limitations of water activity. This concept has been extensively applied, giving way to a new important area of research and application: food material science [6, 53, 55, 62]. Essentially, this approach “simplifies” the foods as partially crystalline partially amorphous materials. The amorphous part is in a metastable state, which is very sensitive to changes in moisture content and temperature. Such amorphous matrix may exist either as a very viscous glass or as a more liquid-like “rubbery” amorphous structure. The characteristic temperature, T g, at which the glass–rubber transition occurs, is the physicochemical parameter that is a basis for product properties, stability and safety of foods [13, 56, 61] (Fig. 3).
Representation of T g effects on structural transformation and diffusion-controlled changes in biological food systems (adapted from [56])
The transition observed at T g resembles a second-order thermodynamical transition, at which the material undergoes a change of state but not a phase change [53], and the temperature at which it occurs is dependent on both composition and solid content of a material [19]. T g greatly influences food stability, as the water in the concentrated phase becomes less mobile and therefore less accessible to support or participate in reactions [53, 61]. Below T g, the food is expected to be physically stable, and above this temperature, the difference (T–T g) between T g and the storage temperature T is assumed to control the rate of physical, chemical and biological changes. As discussed already, these physical and chemical reactions, which are dependent on the diffusion of reactant molecules, would be quite slow in the supercooled liquid or rubber, in the vicinity of the T g, and kinetically controlled by mobility or viscosity [10].
T g is a very promising and innovative concept for food science and is considered as a future challenge when associated with other food mechanisms [53]. Despite this, experimental evidences demonstrate some fragility [12, 26, 38, 70]. Glass transition temperature considers mobility at a macromolecular level and, therefore, is a parameter descriptive of the physical state and overall mobility of macromolecules, which differs from the molecular mobility of smaller molecules such as water [38, 69].
Moreover, some experimental evidence does not support a clear correlation between T g and microbial activity [12, 70]. Similarly, many investigations demonstrate that glass transition alone cannot explain enzymatic and nonenzymatic activities below T g. In some cases, reactions occur slower in the rubbery state than in the glassy state (e.g. ascorbic acid oxidation, because the structural collapse in the rubbery state impeded O 2 diffusion through the system, which resulted in slower ascorbic acid degradation rates) [38].
Moreover, T g is not as easy to measure as, for example, water activity and may not be a representative parameter in multicomponent, multidomain complex foods [41].
Water Proton Relaxation Time and NMR as a Powerful Technique for Assessing Proton Relaxation Time
Biological systems, and particularly foods, consist largely of water and macromolecules, both rich in protons. Proton relaxation time is a characteristic of proton dynamics/mobility [10] and is a function of physical and chemical characteristics of individual chemical compounds as well as interactions among them [42, 58]. Water protons are one of the most important contributors to the proton relaxation in biological systems, and the interaction between water and macromolecules is the most important factor affecting proton relaxation process [58].
Nuclear magnetic resonance (NMR) spectroscopy is one of the most common investigative techniques used to evaluate systems molecular dynamics, by identifying molecular structures and evaluating the progress of chemical reactions [42]. This technique provides information on different food components that are considered as dense complex systems [16, 23, 58], both in solution and in solid state [15, 29, 74]. It also allows to study independently the dynamics of water and food solids [30].
Water dynamics/mobility can be analysed by NMR, through proton (1H), deuterium (2H) and oxygen-17 (17O) [70]. 1H NMR, as the most used NMR technique, has been used to investigate water dynamics and physical structures thought analysis of proton nuclear magnetisation relaxation times [20, 37]. Many researchers have found that the mobility of water, as measured by NMR, is related to the dynamics and “availability” of water in complex systems [23, 58], i.e. the higher the mobility of water, the higher the availability of water, very mobile water molecules take a long time to reach their equilibrium state, or relax very slowly, thus having a small relaxation rate or long relaxation time [58].
In these measurements, the samples are submitted to a static magnetic field and the protons are excited by means of a radiofrequency pulse. The analysis of the signal emitted while the samples return to equilibrium (FID) allows determining the spin–lattice (T 1 ) and spin–spin (T 2) relaxation. This later variable is related to the mobility of the protons in the sample matrix [20].
For example, in plant tissues different compartments can be discriminated, where water molecules or protons are in exchange. These exchange rates between compartments are controlled by the proton permeability of the membranes separating the compartments and/or by the diffusion process by which water molecules reach the membranes [63].
NMR can be applied in complex food systems [1, 7, 14] to do quantitative and conformational analysis (nutritional or functional aspects), quality control of packaging materials, process control [42] and also to evaluate food quality during storage period [11]. In the last case, the degradation changes that occur along the storage promote changes both in water and in solutes bounding and structure, which results in differences in NMR properties of the food [40, 58].
Literature reports diverse studies applying this technique to different foods and with different purposes. Some examples are discussed below (Practical application of NMR to assess molecular dynamics and structure).
Food Structure/Microstructure
Food “matrices” (systems) physical behaviour and stability depend strongly on its molecular mobility, but also on its microstructure. Food microstructure recognises that foods are highly structured and heterogeneous materials, composed of architectural elements. The types of such structural units and their interactions are decisive for the food physical behaviour and functional properties, such as texture or sensorial attributes, and also physical and chemical stability during storage. They influence the water/solute interactions and hence the water availability to participate in microbial growth and degradation reactions [2]. In fact, these intermolecular interactions in which the water molecules play a very important role can determine the structure of the food material at the beginning of a given process and during processing [71].
Also, the effective water diffusivity in foods, as well as free water content, highly depends on pore structure or particle size distribution [49, 50, 73].
In addition to water, other structural elements can be identified in foods, such as oil droplets, gas cells, fat crystals, strands, granules, micelles and interfaces [2]. These structural elements, composed of proteins, carbohydrates and lipids (in various combinations and proportions), can exist in different states (glassy/rubbery/crystalline/liquid and solubilised) even at uniform temperatures and water activity. This structural heterogeneity will necessarily affect the molecular dynamics in the system and consequently the macroscopic food quality attributes [40] and their behaviour along storage.
Designing the food structure during processing can also affect the behaviour during shelf-life. For example, physically separating the reactants in microstructural locations can control the biochemical activity by avoiding the reactants to be in contact, thus minimising the development of off-flavours and browning reactions [2].
Food microstructure can also be altered by controlling various intermolecular and inter-particle interactions among the different ingredients during processing and storage [35]. Engineering structures requires knowledge on the molecular organisation of the ingredients (short- and long-range molecule assemblies) and physical properties, such as charge density, hydrophobicity, molecular size and conformation under different environmental conditions [60]. The expression “structure–function”, nowadays widely used, describes basically the way in which physicochemical and functional properties of foods are related to their structure [3].
Practical Application of NMR to Assess Molecular Dynamics and Structure
As previously described, molecular mobility/dynamics has been identified as one of the actual most promising parameters for assessing physicochemical properties in multicomponent systems. This fact justifies the significant number and type of experimental works performed in food systems. This section briefly discusses examples of 1H NMR practical applications on food systems, considering matrices of different complexities.
Edible Films as Food Systems Models
Edible films have been studied for a long time for their potential to improve shelf-life and safety of food products [4, 17]. The literature is extensive in characterisation of such materials and particularly in reporting the thermomechanical behaviour and barrier properties of glassy biopolymers and polymers [9, 33]. These systems are partially crystalline/partially amorphous and easily reproducible materials. From a fundamental perspective, foods are mainly edible and digestible biopolymers that are partially crystalline/partially amorphous [71], and thus, edible films can be very interesting food model systems for mobility and microstructure studies. Also, in these films, water is one of the most important components, i.e. is used significantly as a plasticiser, creating hydrogen bonds with the polymeric chains present in the system and influencing its physical properties, e.g. relaxation [21].
However, the lack of systematic information about the relationship between the effect of films composition on the microstructure and molecular dynamics of polymeric systems behaviour is evident. A few published papers take advantage of these techniques.
1H NMR has been used to characterise starch–chitosan films with different levels of glycerol [39]. This technique proved to be useful in clarifying the interactions between films components. It was showed that the addition of glycerol promoted the interactions among chitosan, starch and glycerol through hydrogen bonding. The stronger glycerol/starch/chitosan interactions in samples containing higher glycerol concentration were confirmed by an observed decrease of glycerol mobility.
Another research work was developed with the purpose of investigating the effect of polysaccharide/plasticiser (chitosan/glycerol and water) concentration on the molecular dynamics and microstructure of the film [20]. In this case, results of molecular mobility contributed to the understanding of the films molecular rearrangement. NMR measurements showed two different populations with mobility in the matrix: water and glycerol. It was possible to conclude that, while glycerol is mainly bounded to the chitosan chain network, the water present in the system is predominantly free from the polymeric chain. However, it was observed that for lower glycerol concentrations, free chitosan binding sites can also be occupied by water molecules (Fig. 4a). Water content and water activity measurements also allowed concluding that not only the water content affects the water mobility, but also structural differences in the film may influence the water relaxation time. Water mobility relates to the water in the bulk and thus complements information on water activity of a system (Fig. 4b, c).
Films water proton transverse relaxation time (T 2) as function of films. a Polysaccharide/plasticiser ratio. b Water content. c Water activity (adapted from [20])
1H NMR experiments have also allowed understanding the differences on ascorbic acid stability observed in different films [34]. This study proved that the water dynamics influences the ascorbic acid stability and recognises which of the compounds added to film-forming solutions (e.g. calcium) interacted with this dynamics.
Real Food Matrices: Fruits
Fruits are high water content products, with a complex cellular structure where water can be present in both intra- and extra-cellular spaces. The general fruit constitution may be described as a watery solution of low molecular weight species, mainly sugars, salts and organic acids, and high molecular weight hydrocolloids, contained in a water-insoluble cellular matrix of macromolecules, mostly carbohydrates including insoluble pectic substances, hemicelluloses, proteins and, sometimes, lignins. Intracellular air spaces are present in parenchymous tissue, and these may be considered as true structural elements, having a very characteristic influence on the perceived texture. This complexity makes these systems of special interest for mobility studies.
Many studies have been performed on the application of 1H NMR techniques for evolution of quality in fruits. This technique allows using the changes in the distribution of water proton transverse relaxation times to monitor the subcellular compartmentation of water.
1H NMR has been a tool used for purposes as diverse as studying the effect of preservation processes [28, 48, 66], monitoring food quality changes during storage [75], analysing food quality characteristics [22, 43, 65] or just monitoring ripening [52].
The work of Hills and co-workers was an important milestone on the use of this technique. The group first identified the signals of water in the cellular wall, cytoplasm and vacuole [23, 24, 26] and applied the methodology for studying the effect of preservation processes in foods. An example is the study on changes in subcellular water compartmentation in parenchyma apple tissues during freezing/thawing [28]. Figure 5 shows the differences in water proton transverse relaxation time profile for fresh and freezing/thawing apple tissues. For the fresh apple, tissue behaviour presents a proton distribution following three peaks that can be assigned to water located in the vacuolar, cytoplasm and cell wall compartments. After thawing, the absence of the three peaks indicates membrane rupture and loss of turgor in the tissue, the cellular structure was broken and the vacuole, cytoplasm and cellular wall lost their integrity and become just one compartment.
Distribution of transverse water proton relaxation times in fresh and freeze-thawed apple tissues (adapted from [28]
As discussed, another example of the use of NMR is to understand the response of fruit quality parameters to different storage conditions, such as on pomegranate fruit [75]. In this case, NMR measurements allow analysing the microscopic structure changes during storage and confirm the water environment in each component. The authors found that water was redistributed between subcellular compartments of the pomegranate aril tissues during controlled atmosphere storage.
Another study has addressed the water proton relaxation times in different pear varieties with two different levels of internal damage (sound tissue and disordered tissue) and tried to find a relationship with the internal browning process and complement the observations with image techniques [22]. It was possible to conclude that, at least for one pear variety, internal browning (postharvest disorder) may be identified and correlated with the NMR parameters. Moreover, it was also possible to infer that the cell decompartmentation facilitates the accessibility of enzymes and subtracts (responsible for browning reactions). The analysis of firmness and soluble solids content was performed, and no correlation between internal browning was found, evidencing once again the relevance of NMR to support the internal inspection of the fruit.
One last example is a study aiming to understand the banana ripening phenomenon [52], showing the relationship between changes in water dynamics with variations in chemical composition. Results from NMR allow explaining the ripening process that happen for a period of 7 days, and where membrane-bound starch granules are almost converted to soluble sugars. Shortly, three components were determined, attributed to vacuole, cytoplasm and cell wall. T 2 values attributed to cytoplasmatic and vacuolar water show a gradual increase, correlated with the disappearance of starch that acts as a relaxation sink (Fig. 6). The disappearance of these granules during ripening increases the cytoplasm and vacuolar water fractions that can be influenced by the chemical diffusive exchange effect, increasing cytoplasm and vacuole T 2.
Banana proton transverse relaxation time, during 7 days of storage (adapted from [52])
Conclusions
This paper reviews some critical issues and highlights works in food systems molecular dynamics assessment. Molecular dynamics together with structure/microstructure is an important approach to study food systems properties and stability. Water is one of the most important food components and is a key factor in biological systems performance. Water activity, glass transition temperature and water proton relaxation time are three concepts that have been used to determine the water performance. Water proton relaxation time, assessed by NMR techniques, is one of the broadest methods to understand dynamics even in complex biological systems like foods. Dynamic properties play an important role in complementing the information provided by methods based on systems equilibrium and global kinetics. However, the lack of systematic information, even in straightforward model food matrices is evident.
Further work on relationships between water and solids mobility and glass transition or water activity in food systems is a fundamental and necessary approach to fully attain food physical properties and stability. The absence of studies on the relationship between degradation of quality factors and molecular mobility along shelf-life is also evident.
These studies may be extremely useful for food product and process design, safety and sensorial attributes and also for better understanding and predicting, for example, food storage stability conditions.
References
Agudelo-Laverde LM, Schebor C, Buera MP (2014) Proton mobility for the description of dynamic aspects of freeze-dried fruits. J Food Eng 125:44–50
Aguilera JM (2000) Food microstructure. Food engineering—encyclopedia of life support systems, vol 1. Aspen, Maryland
Aguilera JM, Stanley DW, Bakerc KW (2000) New dimensions in microstructure of food products. Trends Food Sci Technol 11:3–9
Aider M (2010) Chitosan application for active bio-based films production and potential in the food industry: review. LWT Food Sci Technol 43:837–842
Anese M, Shtylla I, Torreggiani D, Maltini E (1996) Water activity and viscosity: relations with glass transition temperatures in model food systems. Thermochim Acta 275:131–137
Angel CA (1996) The glass transition. Curr Opin Solid State Mater Sci 1:578–585
Assifaoui A, Champion D, Chiotelli E, Verel A (2006) Characterization of water mobility in biscuit dough using a low-field 1H NMR technique. Carbohydr Polym 64:197–204
Berk Z (2013) Physical properties of food materials food process engineering and technology, 2nd edn. Elsevier, London
Butler BL, Vergano PJ, Testin RF, Bunn JM, Wiles JL (1996) Mechanical and barrier properties of edible chitosan films as affected by composition and storage. J Food Sci 61(5):953–956
Champion D, Meste ML, Simatos D (2000) Towards an improved understanding of glass transition and relaxations in foods: molecular mobility in the glass transition range. Trends Food Sci Technol 11:41–55
Chen PL, Long Z, Ruan R, Labuza TP (1997) Nuclear magnetic resonance studies of water mobility in bread during storage. LWT Food Sci Technol 30(2):178–183
Chirife J, Buera MDP (1994) Water activity, glass transition and microbial stability in concentrated/semimoist food systems. J Food Sci 59(5):921–927
Chirife J, Buera MP (1995) A critical review of some non-equilibrium situations and glass transitions on water activity values of foods in the microbiological growth range. J Food Eng 25:531–552
Choi S-G, Kerr WL (2003) 1H NMR studies of molecular mobility in wheat starch. Food Res Int 36:341–348
Claridge TDW (2009) High-resolution NMR techniques in organic chemistry. In: Tetrahedron organic chemistry, vol 27, 2nd edn. Elsevier, Kidlington, Oxford
Domjan A, Bajdik J, Pintye-Hódi K (2009) Understanding of the plasticizing effects of glycerol and PEG 400 on chitosan films using solid-state NMR spectroscopy. Macromolecules 42:4667–4673
Epure V, Griffon M, Pollet E, Avérous L (2011) Structure and properties of glycerol-plasticized chitosan obtained by mechanical kneading. Carbohydr Polym 83:947–952
Fennema OR (1996) Water and ice. Food chemistry. Marcel Dekker Inc., New York
Ferry JD (1980) Viscoelastic properties of polymers, 3rd edn. Wiley, New York
Fundo JF, Fernandes R, Almeida PM, Carvalho A, Feio G, Silva CL, Quintas MAC (2014) Molecular mobility, composition and structure analysis in glycerol plasticised chitosan films. Food Chem 144:2–8
Hasegawa M, Isogai A, Onabe F, Usuda M, Atalla RH (1992) Characterization of cellulose–chitosan blend films. J Appl Polym Sci 45(11):1873–1879
Hernández-Sánchez N, Hills BP, Barreiro P, Marigheto N (2007) An NMR study on internal browning in pears. Postharvest Biol Technol 44:260–270
Hills BP, Cano C, Belton PS (1991) Proton NMR relaxation studies of aqueous polysaccharide systems. Macromolecules 24:2944–2950
Hills BP, Manning CE, Ridge Y (1996) New theory of water activity in heterogeneous systems. J Chem Soc 92(6):979–983
Hills BP, Manning CE, Ridge Y, Brocklehurst T (1996) NMR water relaxation, water activity and: bacterial survival in porous media. J Sci Food Agric 71:185–194
Hills BP, Manning CE, Ridge Y, Brocklehurst T (1996) NMR water relaxation, water activity and bacterial survival in porous media. J Sci Food Agric 71(2):185–194
Hills BP, Manning CE, Ridge Y, Brocklehurst T (1997) Water availability and the survival of Salmonella typhimurium in porous systems. Int J Food Microbiol 36:I187–I198
Hills BP, Remigereau B (1997) NMR studies of changes in subcellular water compartmentation in parenchyma apple tissue during drying and freezing. Int J Food Sci Technol 32(1):51–61
Keeler J (2002) Understanding NMR spectroscopy, 1st edn. Wiley, University of Cambridge, Department of Chemistry, Cambrige
Kou Y, Molitor PF, Schmidt SJ (1999) Mobility and stability characterization of model food systems using NMR, DSC, and Conidia germination techniques. J Food Sci 64(6):950–957
Labuza TP (1977) The properties of water in relationship to water binding in foods: a review. J Food Process Preserv 1(2):167–190
Labuza TP, Cassil S, Sinskey AJ (1972) Stability of intermediate moisture foods. 2. Microbiology. J Food Sci 37:160–162
Lazaridou A, Biliaderis CG (2002) Thermophysical properties of chitosan, chitosan–starch and chitosan–pullulan films near the glass transition. Carbohydr Polym 48:179–190
León PG, Lamanna ME, Gerschenson LN, Rojas AM (2008) Influence of composition of edible films based on gellan polymers on l-(+)-ascorbic acid stability. Food Res Int 41(6):667–675
Lesmes U, McClements DJ (2009) Structure function relationships to guide rational design and fabrication of particulate food delivery systems. Trends Food Sci Technol 20:448–457
Lewicki PP (2004) Water as the determinant of food engineering properties: a review. J Food Eng 61:483–495
Li R, Kerr WL, Toledo RT, Carpenter JA (2000) 1H NMR studies of water in chicken breast marinated with different phosphates. J Food Sci 65(4):575–580
Lin X, Ruan RR, Chen PL, Chung M, Ye X, Yang T, Doona C, Wagner T (2006) NMR state diagram concept. J Food Sci 71(9):136–143
Liu H, Adhikari R, Guo Q, Adhikari B (2013) Preparation and characterization of glycerol plasticized (high-amylose) starch–chitosan films. J Food Eng 116(2):588–597
Ludescher RD, Shah NK, McCaul CP, Simon KV (2001) Beyond Tg: optical luminescence measurements of molecular mobility in amorphous solid foods. Food Hydrocoll 15:331–339
Maltini E, Torreggiani D, Venir E, Bertolo G (2003) Water activity and the preservation of plant foods. Food Chem 82:79–86
Marcone MF, Wang S, Albabish W, Nie S, Somnarain D, Hill A (2013) Diverse food-based applications of nuclear magnetic resonance (NMR) technology. Food Res Int 51:729–747
Marigheto N, Venturi L, Hills B (2008) Two-dimensional NMR relaxation studies of apple quality. Postharvest Biol Technol 48:331–340
Mathlouthi M (2001) Water content, water activity, water structure and the stability of foodstuffs. Food Control 12(7):409–417
Matveeva YI, Grinberga VY, Tolstoguzov VB (2000) The plasticizing effect of water on proteins, polysaccharides and their mixtures. Glassy state of biopolymers, food and seeds. Food Hydrocoll 14:425–437
Olsen NV, Sijtsema SJ, Hall G (2010) Predicting consumers’ intention to consume ready-to-eat meals. The role of moral attitude. Appetite 55:534–539
Palzer S (2010) The relation between material properties and supra-molecular structure of water-soluble food solids. Trends Food Sci Technol 21:12–15
Panarese V, Laghi L, Pisi A, Tylewicz U, Rosa MD, Rocculi P (2012) Effect of osmotic dehydration on Actinidia deliciosa kiwifruit: a combined NMR and ultrastructural study. Food Chem 132:1076–1712
Peppas NA, Brannon-Peppas L (1994) Water diffusion and sorption in amorphous macromolecular systems and foods. J Food Eng 22(1–4):189–210
Pittia P, Sacchetti G (2008) Antiplasticization effect of water in amorphous foods: a review. Food Chem 106:1417–1427
Quintas MAC, Fundo JF, Silva CLM (2010) Sucrose in the concentrated solution or the supercooled “state”: a review of caramelisation reactions and physical behaviour. Food Eng Rev 2:204–215
Raffo A, Gianferri R, Barbieri R, Brosio E (2005) Ripening of banana fruit monitored by water relaxation and diffusion 1H-NMR measurements. Food Chem 89:149–158
Rahman MS (2006) State diagram of foods: its potential use in food processing and product stability. Trends Food Sci Technol 17:129–141
Rahman MS (2010) Food stability determination by macro–micro region concept in the state diagram and by defining a critical temperature. J Food Eng 99:402–416
Roos Y (1995) Characterization of food polymers using state diagrams. J Food Eng 24:339–360
Roos YH (1998) Phase transitions and structure of solid food matrices. Curr Opin Colloid Interface Sci 3:651–656
Roudaut G, Simatos D, Champion D, Contreras-Lopez E, Meste ML (2004) Molecular mobility around the glass transition temperature: a mini review. Innov Food Sci Emerg Technol 5:127–134
Ruan RR, Chen PL (1998) Water in foods and biological materials: a nuclear magnetic resonance approach. Technomic Publishing Company Inc, Lancaster
Sablani SS, Kasapis S, Rahman MS (2007) Evaluating water activity and glass transition concepts for food stability. J Food Eng 78:266–271
Scholten E, Moschakis T, Biliaderis CG (2014) Biopolymer composites for engineering food structures to control product functionality. Food Struct 1:39–54
Slade L, Levine H (1991) Beyond water activity: recent advances based on an alternative approach to the assessment of food quality and safety. Crit Rev Food Sci Nutr 30(2–3):115–360
Slade L, Levine H (1995) Water and the glass transition: dependence of the glass transition on composition and chemical structures: special implications for flour functionality in cookie baking. J Food Eng 24:431–509
Snaar JEM, Van As H (1992) Probing water compartments and membrane permeability in plant cells by 1H NMR relaxation measurements. J Biophys 63:1654–1658
Toivonen PMA, Brummel DA (2008) Biochemical bases of appearance and texture changes in fresh-cut fruit and vegetables. Postharvest Biol Technol 48:1–14
Tu SS, Choi YJ, McCarthy MJ, McCarthy KL (2007) Tomato quality evaluation by peak force and NMR spin–spin relaxation time. Postharvest Biol Technol 44(2):157–164
Tylewicz U, Panarese V, Laghi L, Rocculi P, Nowacka M, Placucci G, Rosa MD (2011) NMR and DSC water study during osmotic dehydration of Actinidia deliciosa and Actinidia chinensis kiwifruit. Food Biophys 6:327–333
Ubbink J, Kruger J (2006) Physical approaches for the delivery of active ingredients in foods. Trends Food Sci Technol 17(5):244–254
Vittadini E, Chinachoti P (2003) Effect of physico-chemical and molecular mobility parameters on Staphylococcus aureus growth. Int J Food Sci Technol 38:841–847
Vittadini E, Chinachoti P, Lavoie JP, Pham X (2005) Correlation of microbial response in model food systems with physico-chemical and mobility descriptors of the media. Innov Food Sci Emerg Technol 6:21–28
Vittadini E, Dickinson LC, Lavoie JP, Pham X, Chinachoti P (2003) Water mobility in multicomponent model media as studied by 2H and 17O NMR. J Agric Food Chem 51:1647–1652
Wang J-C, Liapis AI (2012) Water–water and water–macromolecules interactions in food dehydration and the effects of the pore structure of food on the energetics of the interactions. J Food Eng 110:514–524
Watada AE, Qi L (1999) Quality of fresh-cut produce. Postharvest Biol Technol 15(3):201–205
Xiong X, Narsimhan G, Okos MR (1992) Effect of composition and pore structure on binding energy and effective diffusivity of moisture in porous food. J Food Eng 15(3):187–208
Yan Z-Y, Mccarthy MJ, Klemann L, Otterburn MS, Finley J (1996) NMR applications in complex food systems. Magn Resson Image 14(7/8):979–981
Zhang L, McCarthy MJ (2013) Effect of controlled atmosphere storage on pomegranate quality investigated by two dimensional NMR correlation spectroscopy. LWT Food Sci Technol 54:302–306
Acknowledgments
Author Joana F. Fundo acknowledges Fundação para a Ciência e a Tecnologia (Grant SFRH/BD/62176/2009). This work was supported by National Funds from FCT through project PEst-OE/EQB/LA0016/2013.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fundo, J.F., Quintas, M.A.C. & Silva, C.L.M. Molecular Dynamics and Structure in Physical Properties and Stability of Food Systems. Food Eng Rev 7, 384–392 (2015). https://doi.org/10.1007/s12393-015-9109-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12393-015-9109-z