Abstract
This study investigated mass transfer and water state changes promoted by osmotic dehydration on two kiwifruit species, Actinidia deliciosa and Actinidia chinensis. Osmotic treatment was performed in a 61.5% w/v sucrose solution at three different temperatures (25, 35 and 45 °C), with treatment time from 0 to 300 min. Treatment time positively influenced kiwifruit water loss and solid gain while temperature significantly affected only water loss. Peleg’s model highlighted that the main response differences between the two species occurred during the initial phase of the osmotic treatment. Thermal properties and relaxation time measurements offered a complementary view concerning the effects of osmotic dehydration on kiwifruit. DSC parameters appeared to be sensitive to water and solid exchange between fruit and osmotic solution. LF-NMR proton T2 revealed the consequences of the water–solid exchange on the cell compartments, namely vacuole, cytoplasm plus extracellular space and cell wall. During the osmotic treatment, the initial freezing temperature and the freezable water content decrease was dependent on time and treatment temperature, showing a similar tendency for both the kiwifruit species. They evidenced the same treatment response also concerning the reduction of vacuole and the increase of cytoplasm plus extracellular space T2 values.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osmotic dehydration is a partial dewatering impregnation process usually carried out by immersion of cellular tissue in hypertonic solution.
The cells of plant tissue are characterized by semi-permeable membranes and the mass transfer phenomenon occurs because of the difference in the chemical potential of water between the food and the osmotic medium.1,2 The diffusion of water from the plant tissue to the solution is usually accompanied by the simultaneous counter-diffusion of solutes from the concentrated solution into the tissue.3,4 When the membrane responsible for osmotic transport is not perfectly selective, natural solutes present in the cells (vitamins, organic acids, minerals, pigments, etc.) can also leach into the osmotic solution.5,6
From the industrial point of view, the osmotic dehydration is mainly used as a pre-treatment for fruit and vegetables intended for further processing, like freezing and/or freeze drying.7,8 Moreover, when mild processing conditions are applied, this treatment could be useful to increase the shelf-life of minimally processed products with relatively high moisture content.9 In fact, the reduction of freezable water content and water activity at low temperature permits both to slow down degradative reactions and to guarantee a higher microbiological stability.10
Recently, the approach of combining different analytical techniques has been adopted to better investigate the status of water in several systems.11–13 Differential scanning calorimetry (DSC), and nuclear magnetic resonance (NMR) can offer a different but complementary point of view in studying the water mobility.11,13,14
The amount of unfreezable water within a sample after being cooled below zero is determined by DSC.15 As explained by Wolfe16, the amount of unfreezable water depends in general on three effects: (1) the presence of small solutes, for example ions, (2) the presence of macromolecules and membranes and (3) the viscosity of the solution.
The analysis of transverse relaxation time (T 2) curves obtained through LF-NMR yields an additional degree of details for the description of the embedded water. In fact, the T2 of foodstuff reveals a multicomponent behaviour that reflects the existence of different proton pools within the sample.
The main objectives of this work were (1) to investigate the mass transfer phenomena and (2) to analyze the water status provided by DSC and NMR measurements, during osmotic dehydration of two different kiwifruit species.
Materials and Methods
Raw Materials
Two species of kiwifruit (Actinidia deliciosa var. Hayward and Actinidia chinensis var. Hort 16A) were bought on the local market and stored at 4 ± 1 °C until they were processed. The osmotic dehydration treatment was applied on fruit hand peeled and cut into 10-mm thick slices.17 The fruit had homogeneous size (major axis of 60 ± 5 mm and minor axis of 45 ± 5 mm) and refractometric index of 12.0 ± 0.4 °Brix.
Osmotic Dehydration Treatment
The osmotic dehydration was carried out by dipping the samples in 61.5% (w/v) sucrose solution equilibrated at three temperatures (25, 35 and 45 °C) for pre-established contact period of 0, 15, 30, 60 and 300 min, as reported by Tylewicz.18 The product/solution ratio was about 1:4 (w/w), to avoid changes in the solution concentration during the treatment. The temperature of the solution was maintained constant by a thermo-controlled water bath. Three slices from the central part of each kiwifruit (about 180 g) were placed in mesh baskets and immersed in osmotic solution. The baskets were continuously stirred with a propeller. The rotational speed was experimentally determined to assure negligible resistance to mass transfer. After that, the slices were taken from the osmotic solution and each slice face was rinsed with distilled water for 3 s and placed on blotting paper for 2 s.
Analytical Determinations
Kiwifruit slices were weighted before and after osmotic dehydration process by means of technical balance (precision 0.01 g).
The moisture content of kiwifruit samples was determined gravimetrically by vacuum drying (pressure ≤ 100 mmHg) at 70 °C until a constant weight was achieved (AOAC 920.15, 2002).19
Soluble solids content was determined at 20 °C by measuring the refractive index with a digital refractometer (PR1, Atago, Japan).
Mass Transfer Parameters
Osmotic dehydration kinetics of kiwifruit were evaluated by calculating net change (∆) of kiwifruit slices total mass (M°), water mass (M w) and solids mass (M ST) adopting the following equations:20
where:
- m 0 :
-
Initial weight before osmotic treatment (kg)
- m t :
-
Weight after a time t (kg)
- x w :
-
Water mass fraction (kg kg−1)
- x ST :
-
Total solids mass fraction (kg kg−1)
Kinetic Model
Mass transfer data were modelled according to the equation proposed by Palou21 and Sacchetti22, using the Peleg23’s model:
In this work, the same equation rewritten as:
was also used in order to model total mass change kinetics.
As reported by Sacchetti22 at the equilibrium condition (t → ∞) the value for mass transfer parameters could be calculated as:
where P J could be respectively: M°; M w; M ST.
Similarly, the initial rate (t = 0) of mass transfer parameters is:
This kinetic model offers the advantage that by calculating the inverse of the constant (k 1 and k 2) it is possible to obtain the initial rate value of mass transfer parameters and the one at the equilibrium condition.22
DSC Measurements
DSC analysis was carried out on a Pyris 6 DSC (Perkin-Elmer Corporation, Wellesley, USA). The DSC was equipped with a low-temperature cooling unit Intacooler II (Perkin-Elmer Corporation, Wellesley, USA). Temperature and melting enthalpy calibrations were performed with ion exchanged distilled water (mp 0.0 °C), indium (mp 156.60 °C), and zinc (mp 419.47 °C); heat flow was calibrated using the heat of fusion of indium (ΔH = 28.71 J g−1). For the calibration, the same heating rate, as used for sample measurements, was applied under a dry nitrogen gas flux of 20 mL min−1. Samples of about 20–30 mg were encapsulated in 50 μl hermetic aluminium pans prior to measurements. An empty pan was used as a reference. DSC curves were obtained by cooling samples to −60 °C and then heating at 5 °C min−1 to 110 °C after an isothermal hold for 5 min at −60 °C.
According to Quinn24 the amount of freezable water (g g −1fw ) was determined as following reported:
where ∆H (g g −1fw ) is the measured latent heat of melting of water for gram of sample obtained by the integration of the melting endothermic peak; ∆H ice (334 J g−1) is the latent heat of melting of pure water at 0 °C.
NMR Measurements
Samples of about 400 mg of kiwifruit were placed inside 10 mm outer diameter NMR tubes so that they did not exceed the active region of the radio frequency coil, and they were analyzed at 24 °C with the Carr−Purcell−Meiboom−Gill (CPMG) pulse sequence using a Bruker Minispec PC/20 spectrometer operating at 20 MHz. Each measurement comprised 30,000 echoes, with a 2τ interpulse spacing of 80 μs and a recycle delay of 3.5 s. The number of scans was varied depending on moisture content, to obtain a S/N ratio in the range 900–1,400. The CPMG decays were normalized to the sample weight, and analyzed with the UPEN program, which inverts the CPMG signal using a continuous distribution of exponential curves, according to Eq. 9:
where 2τ is the CPMG interpulse spacing, n is the index of a CPMG echo, and I 0(T 2,i) provides a distribution of signal intensities for each T 2 component extrapolated at τ = 0 (the relaxogram), sampled logarithmically in the interval T 2, min − T 2, max set by the user. Default values for all UPEN parameters were used throughout this work.
Statistical Analysis
The osmotic dehydration process was performed once for each time–temperature treatment condition. Analytical determinations were performed in triplicates. Mass transfer, DSC, and NMR samples were collected from three kiwifruit slices for each treatment condition. Significance of the osmotic dehydration effects was evaluated by means of one- and two-way analyses of variance (ANOVA, 95% significance level) using the software STATISTICA 6.0 (Statsoft Inc., Tulsa, UK). In order to estimate the kinetic model constants, non-linear regression was carried out by means of the quasi-Newton calculus algorithm using STATISTICA 6.0.
Result and Discussion
Water Loss, Weight Reduction and Solid Gain Kinetic Model
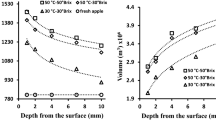
The kinetic model was used to fit mass transfer parameter data over processing time (0.83 < R 2 < 0.99). The predictive capability of the model can be observed in Figure 1, where, by way of example, the Eqs. 4, 5 and 6 were used to model the mass transfer parameters of A. deliciosa treated at 35 °C.
For both A. chinensis and A. deliciosa kiwifruit species, the highest water loss rates occurred during the first treatment hour as shown in the example in Figure 1. During that time water loss in A. chinensis was 18%, 20% and 35% of the initial fresh weight and in A. deliciosa 15%, 21% and 29% respectively, for 25, 35 and 45 °C. After 300 min of osmotic treatment, the percentage of water loss varied on average close to 38%, 45% and 62% in A. chinensis and 27%, 50% and 58% in A. deliciosa at 25, 35 and 45 °C. This agrees with the literature; in fact, Kowalska and Lenart4 showed noteworthy water content decreasing during the first 30 min of the osmotic process (30 °C, 61.5% sugar solution). After this period, the dehydration of carrots, apples and pumpkins appears slower. Besides kiwifruit, dehydration rate presented by Vial25 seems to decrease dramatically between 30 and 90 min of processing. The water loss rate was the highest at the beginning of the process because the dehydration driving force was the greatest. For both A. deliciosa and A. chinensis, the osmotic solution temperature seemed to positively influence the initial water loss rate as suggested by the behaviour of \( 1/k_1^{\rm{W}} \) reported in Figure 2a. This displays the initial water loss rate and water loss rate at equilibrium of both the kiwifruit varieties as a function of temperature. Higher process temperatures seem to promote several phenomena: faster water loss through swelling and plasticizing of cell membranes; faster water diffusion within the product; lower viscosity of the osmotic medium facilitating the water transfer on the surface.26 Kiwifruit has a porous structure; therefore, high temperature would release trapped air from the tissue, resulting in more effective removal of water by osmotic pressure.27
Effect of the temperature on mass changes kinetic parameters: 1/k 1 (open square–solid line) and 1/k 2 (open square–dashed line) of A. chinensis and 1/k 1 (filled circle–solid line) and 1/k 2 (filled circle–dashed line) of A. deliciosa. a Water mass change; b total mass change; c soluble solids mass change
The initial weight reduction rate of A. chinensis was higher than the one of A. deliciosa at 25, 35 and 45 °C (Figure 2b); water and weight loss initial rates of both kiwifruit species reached the maximum at 45 °C (Figure 2a,b).
In agreement with Vial’s findings25, A. deliciosa solid gain was minimally influenced by the increase of osmo-dehydration treatment temperature and time (Figure 2c). In fact during the first hour of osmotic treatment the solid gain in A. chinensis was about 2.5%, 2.5% and 3.6% and in A. deliciosa 3.6%, 3.2% and 6.1% for 25, 35 and 45 °C, respectively. After 300 min of osmotic treatment, the percentage of solid gain reached 5.6%, 6.5% and 6.3% in A. chinensis and 5.1, 8.8 and 9.6% in A. deliciosa for 25, 35 and 45 °C. These results disagree with those found by Bchir28 and Cao.27 Bchir28 found a significant temperature effect during the first 20 min of osmotic treatment on pomegranate seeds (55 °Brix solution; 30, 40 and 50 °C). Cao27 observed in kiwifruit a remarkable solid gain rising by varying the osmotic dehydration temperature (15–75 °C) and time (60–420 min) at different solution concentration (20–80% w sucrose/w solution). During the osmotic treatment, A. chinensis solid gain was modest and it seemed to be positively influenced by the temperature less than A. deliciosa, as shown in Figure 2c. These results are consistent with the higher weight reduction rate of A. chinensis and with Tylewicz18 results.
During the second phase of the treatment, described by the parameter 1/k 2, all the considered mass transfer rates (1/k W2 , 1/k ST2 , 1/k 2°) for both the kiwifruit species, underwent a significant and very similar increase by varying the temperature from 25 to 35 °C.
DSC Measurements
DSC measurements permitted to evaluate the changes of initial point of ice melting (T f,onset) and freezable water content (x FW ), that are related to product stability. The freezable water is water having enough mobility to freeze, as weakly bound to the macromolecular matrix.
During the osmotic treatment, the kiwifruit slices thermo-physical properties (T f,onset and x FW ) progressively changed as shown in Table 1. In agreement with Cornillon12 the depletion of the initial ice melting temperature (T f,onset) progressively increased along with the proceeding of the osmotic treatment and with the increase of the treatment temperature, following the trend of water loss and solids gain results. For T f,onset two-way ANOVA analysis evidenced a strong effect of both time (p < 0.001) and temperature (p < 0.01). With the proceeding of the osmotic treatment, all the samples showed a tendentious decrease of x FW (g g −1fw ); this behaviour was particularly evident for samples treated at high temperatures (35, 45 °C). Even for x FW two-way ANOVA analysis evidenced significant effects of both time (p < 0.001) and temperature (p < 0.01).
According to Quinn24, total non-freezable water corresponds to the maximum water content for which no enthalpic peak is detected. In previous dehydration studies13,24, this value has been considered as the moisture content when ∆H = 0 and thus calculated through the linear regression of ∆H (J g −1dw ) vs. water content (WC; g H2O 100 g −1dw ). This method applied to our results provided not trustable negative values of total non-freezable water content, but it is important to consider that the previous mentioned researches were focused on dehydration studies. During osmotic dehydration, in addition to water removal, a contemporaneous qualitative modification of the dry matter occurs, that in our experiments was promoted by sucrose soaking. In order to consider the latter phenomenon, ∆H values were normalized against soluble solids content (°Brix). Linear regressions of normalized ∆H (J g −1dw °Brix−1) data vs. WC are reported in Figure 3. Obtained fitting equations (∆H = 0.199 × WC − 25.453, R 2 = 0.914 and ∆H = 0.200 × WC − 23.279, R 2 = 0.967, respectively, for A. deliciosa and A. chinensis samples) showed that in our experimental conditions, the not freezable water content of A. deliciosa and A. chinensis was, respectively, 127.9 and 116.4 (gH2O 100 g −1dw ) or 56.12 and 53.79 (gH2O 100 g −1fw ). These last results are in agreement with those of Tocci29 that found disappearance of freezable water between 58.1 and 50.6 (gH2O 100 g −1fw ) for A. deliciosa kiwifruit treated at 20 °C in sucrose solution (60% w/w). However, this result needs to be confirmed by further experiments enabling to reach lower water content values.
NMR Measurements
The CPMG decays registered during the present investigation, when inverted to a continuous distribution of exponential curves, allowed the identification of three proton pools in both kiwifruit varieties, with T 2 for raw fruits around 40, 300 and 950 ms, respectively. Through a comparison with the results obtained on apples and carrots by Hills30,31, such pools were ascribed to cell wall, cytoplasm plus extracellular space and vacuole, respectively. Figure 4 shows the typical effect of osmotic dehydration on the intensity and T2 of the named pools, considered separately by fitting the raw T2 decays to the sum of three exponentials. Table 2 summarizes the proton pool values obtained on raw and 300 min osmo-dehydrated fruits at 25, 35, and 45 °C.
Intensities and T 2 of A. chinensis kiwifruit proton pools during the osmotic treatment at 25 °C: vacuole (open circle), cytoplasm and extracellular spaces (filled circle), cell walls (open upright triangle). To help in visualizing the value trends, the points are fitted to monoexponential curves presented as dashed lines
In raw kiwifruits, the protons located inside the vacuole represented the 61% of the total protons in A. deliciosa, and an even greater portion in A. chinensis (70%). During the osmotic treatment, the T 2 value and amount of vacuole protons of A. deliciosa decreased proportionally to the temperature. The reduction could be observed also in A. chinensis, resulting higher than A. deliciosa at 45 °C and lower at 25 and 35 °C. This behaviour can be explained considering that, at 20 MHz radiofrequency, T 2 value (ms) shorter than the one of pure water (≈1,600 ms) mainly reflects the proton exchange between water and solutes.30 Thus, the T 2 of the water protons pertaining to a certain compartment can decrease when the (solutes + biopolymers)/water ratio increases. The decrease of signal values from vacuole protons suggests that this compartment can undergo shrinkage during the dehydration due to water leakage32; consequent solutes concentration caused a shortening of the T 2. As far as the cytoplasm/extracellular space proton pool is concerned, with the proceeding of the treatment time, the proton pool intensity increased together with a T 2 reduction, because the extracellular space was filled with the osmotic solution.33
Conclusions
The present work shows the effect of osmotic dehydration on two kiwifruit species, A. deliciosa and A. chinensis, in terms of mass transfer and water state behaviours.
Treatment time positively influenced water loss and solid gain of both the studied species, while temperature significantly affected only water loss. Peleg’s model highlighted that the main response differences between the two species occurred during the initial phase of osmotic treatment.
Thermal properties and relaxation time measurements offered a complementary view concerning the effects of osmotic dehydration on kiwifruit. DSC parameters appeared to be sensitive to water and solid exchange between fruits and osmotic solution. LF-NMR proton T2 revealed the consequences of the water–solid exchange on the cell compartments, namely vacuole, cytoplasm plus extracellular space and cell wall. During the osmotic treatment, the initial freezing temperature and the freezable water content decrease was dependent on treatment time and temperature, showing a similar tendency for both the kiwifruit species. The two species evidenced the same treatment response also concerning the reduction of vacuole and the increase of cytoplasm plus extracellular space T 2 values.
This study confirms that DSC analysis could give key information about macroscopic water changes of vegetable tissue consequent to a technological process, whereas NMR analysis enables to assess the microscopic modifications of cell compartmentation.
To obtain a better understanding of the tissue changes promoted by osmotic treatment, microscopic studies and metabolic consequences determination are in progress in our laboratories.
References
C. Ratti, A.S. Mujumadar, Processing Fruit Science and Technology eds. by D.M. Barrett, L.P. Somogyi, H.S. Ramaswamy (CRC, 2005)
M.M. Khin, W. Zhou, C.O. Perera, J. Food Eng. 77, 84–95 (2006)
F. Kaymak-Ertekin, M. Sultanoğlu, J. Food Eng. 46, 243–250 (2000)
H. Kowalska, A. Lenart, J. Food Eng. 49, 137–140 (2001)
G.M. Dixon, J.J. Jen, J. Food Sci. 42, 1136–1140 (1977)
C.L. Lerici, G. Pinnavaia, M. Dalla Rosa, L. Bartolucci, J. Food Sci. 50, 1217–1219 (1985)
M. Robbers, R.P. Singh, L.M. Cunha, J. Food Sci. 62, 1039–1042 (1997)
A. Chiralt, N. Martínez-Navarrete, J. Martínez-Monzó, P. Talens, G. Moraga, A. Ayala, P. Fito, J. Food Eng. 49, 129–135 (2001)
M. Dalla Rosa, D. Torreggiani, Industrial application of osmotic dehydration/treatments of food, eds. M. Dalla Rosa, W.E.L. Spiess, Forum, (2000)
A. Gianotti, G. Sacchetti, M.E. Guerzoni, M. Dalla Rosa, J. Food Eng. 49, 265–270 (2001)
S. Li, L.C. Dickinson, P. Chinachoti, J. Agric, Food Chem. 46, 62–71 (1998)
P. Cornillon, Lebensm. Wiss. Technol. 33, 261–267 (2000)
L. Venturi, P. Rocculi, C. Cavani, G. Placucci, M. Dalla Rosa, M.A. Cremonini, J. Agric, Food Chem. 55, 10572–10578 (2007)
N. Aktaş, Y. Tülek, H.Y. Gökalp, J. Therm, Anal. Calorim. 50, 617–624 (1997)
D. Simatos, M. Faure, E. Bonjour, M. Couach, in Water relation in foods, ed. by R.D. Duckworth (Academic, London, 1975)
J. Wolfe, G. Bryant, K.L. Koster, Cryoletters 23, 157–166 (2002)
I. Escriche, R. Garcia-Pinchi, A. Andrés, P. Fito, J. Food Process Eng. 23, 191–205 (2000)
U. Tylewicz, P. Rocculi, E. Cocci, M. Dalla Rosa, Ind. Aliment. 48, 1–5 (2009)
AOAC International. Official Methods of Analysis of AOAC International, 17th edn. 920.15 (2002)
P. Fito, A. Chiralt, in Food Engineering 2000, ed. by P. Fito, E. Ortega, G. Barbosa (Chapman & Hall, New York, 1997), pp. 231–252
E. Palou, A. Lopez-Malo, A. Argaiz, J. Welti, Dry. Technol. 12, 965–978 (1994)
G. Sacchetti, A. Gianotti, M. Dalla Rosa, J. Food Eng. 49, 163–173 (2001)
M. Peleg, J. Food Sci. 53, 1216–1217 (1988)
F.X. Quinn, E. Kampff, G. Smyth, V.J. McBrierty, Macromol. 21, 3191–3198 (1988)
C. Vial, S. Guilbert, J.L. Cuq, Sci. Aliment. 11, 63–84 (1991)
H.N. Lazarides, E. Katsanidis, A. Nickolaidis, J. Food Eng. 25, 151–166 (1995)
H. Cao, M. Zhang, A. Mujumdar, W. Du, J. Sun, Dry. Technol. 24, 89–94 (2006)
B. Bchir, S. Besbes, H. Attia, C. Blecker, Int. J. Food Sci. Technol. 44, 2208–2217 (2009)
A.M. Tocci, R.H. Mascheroni, J. Food Eng. 88, 20–27 (2008)
B.P. Hills, B. Remigereau, Int. J. Food Sci. Technol. 32, 51–61 (1997)
B.P. Hills, K.P. Nott, Appl. Magn. Reson. 17, 521–535 (1999)
W.G. Hopkins, Introduction to plant physiology (Wiley, New York, 2008), pp. 23–38
J.M. Aguilera, A. Chiralt, P. Fito, Trends Food Sci. Technol. 14, 432–437 (2003)
Acknowledgements
The authors acknowledge Dr. Patricio Santagapita, of the Departamento de Industrias, Facultad de Ciencias Exactas y Naturales, Universidad de Buenos Aires, for his critical reading of the manuscript and highlighting suggestions, and Dr. Emiliano Cocci of our department for his help in the set up of the osmosis pilot plant.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tylewicz, U., Panarese, V., Laghi, L. et al. NMR and DSC Water Study During Osmotic Dehydration of Actinidia deliciosa and Actinidia chinensis Kiwifruit. Food Biophysics 6, 327–333 (2011). https://doi.org/10.1007/s11483-011-9210-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11483-011-9210-7