Abstract
A field demonstration on the ultrafiltration of sugarcane juice for raw sugar production was conducted in a raw sugar mill in Guangxi Province, China. Heated limed sugarcane juice was processed using 0.05 μm ceramic membranes in a pilot plant with a design capacity of 5.5 m3/h. Results indicated that the ceramic membrane modules were satisfactory for sugarcane juice clarification, which yielded 119.13–142.43 L/(m2 h) of average flux under the volumetric concentration factor of 10–12 and produced clarified juice of superior quality with more than 1.2 unit rise in purity, 99.96 % reduction in turbidity, and 10.42 % removal of color. The membrane cleaning period was 10–25 h. Moreover, a high-quality product with high Pol, low color, and low ash content was obtained when the permeate juice was concentrated and crystallized to form raw sugar. However, further studies are necessary to investigate the methods of processing retentate for the recovery of sucrose and handling of wastewater generated during membrane cleaning, as well as the service life of the membrane.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The production of raw sugar from sugarcane generally consists of four stages: (1) crushing of sugarcane to produce raw juice, which contains sucrose and various impurities; (2) clarification, in which the juice is treated at 98–105 °C with chemicals (e.g., lime, sulfur dioxide, phosphoric acid, and flocculant) and then settled in a clarifier to remove non-sugar impurities; (3) evaporation of clarified juice to form syrup; and (4) crystallization to form raw sugar. The disadvantages of conventional processing of sugarcane juice include the inefficient removal of substances (e.g., starch, color, colloids, and other suspended matters) during clarification, which adversely affects the quality of final product (raw sugar) (Hamachi et al. 2003), as well as the use of heavy equipment and chemicals, which can lead to high operating costs and related environmental problems (Jegatheesan et al. 2009). Thus, the sugar industry needs efficient clarifying methods to improve the quality of clarified juice and reduce or eliminate the usage of chemicals. Membrane filtration is an advanced method that ameliorates these issues (Steindl 2001). Numerous laboratory-scale studies of membrane applications, as well as a few pilot-scale and industrial studies, have been conducted (Jegatheesan et al. 2012).
In a study, clarified juice (liming–sulphitation) was treated with polymeric spiral-wound membranes in a pilot plant with a capacity of 10 m3/h. The membrane modules displayed satisfactory separation with 0.9 unit of purity rise, 31 % reduction of turbidity, and 47 % removal of color in the permeate (Ghosh and Balakrishnan 2003). However, spiral-wound membranes could be fouled easily, and the average flux was low at only 7 L/(m2 h). In another report, ceramic ultrafiltration membranes with pore size of 0.02 μm were used to filter clarified juice (lime defecation) in a sugar mill (Kwok 1996). The ultrafiltration system was used for the entire 1995 crop season, during which 65 % of the total clarified juice went through the system. Although good results were obtained, several membrane breakages occurred during the early part of the cropping season and a gradual reduction in flux over the crop period was experienced, possibly because the membrane technology and automatic control system were relatively new at that time. Similarly, dead-end ceramic membranes with pore size of 0.20 μm were used to treat clarified juice (lime defecation) in an industrial-sized pilot plant (Farmani et al. 2008). However, this report only provided parameters, such as decolorization, turbidity removal, purity rise, and viscosity reduction, but not temporal variations in permeate flux. Even though dead-end filtration is easy to operate, it results in a severe membrane fouling. Even the membrane technology was relatively new in the 1990s, the use of membranes to clarify raw sugarcane juice was not feasible (Steindl 2001). The application of membrane filtration in the sugar industry in the pilot or industrial scale is mainly based on clarification of clarified juice. Lower impurity loading in clarified juice will enable higher permeate flux levels to be reached and maintained. However, significant progress has been achieved in membrane characteristics over the past two decades, which makes clarification of raw sugarcane juice with membranes possible.
In terms of manufacturing materials, membranes are mainly classified into ceramic membrane, stainless steel membrane, and organic polymer membrane. Both stainless steel membranes and ceramic membranes are inorganic membranes with good performance. Nonetheless, the cost of stainless steel membranes is three to five times as much as that of ceramic membranes (in China). Moreover, the improved performance of ceramic membranes over polymeric membranes has been established, which may represent a future direction for the application of membranes in the sugar industry (Jegatheesan et al. 2012). However, the application of ceramic membranes in clarifying raw sugarcane juice (mixed juice) for raw sugar production at the pilot or industrial scale for a longer time period is still limited (Jegatheesan et al. 2012). To solve the above-mentioned problems, a field demonstration on ultrafiltration of raw sugarcane juice for raw sugar production was conducted in a raw sugar mill. The main objectives of this work were to (1) evaluate the permeate flux and quality obtained for the membranes; (2) concentrate and crystallize permeate juice to form raw sugar; (3) evaluate the quality of final product (raw sugar); and (4) present the critical issues encountered during operation.
Materials and Methods
Sugarcane Juice and Membranes
Raw sugarcane juice (mixed juice) was collected from Fangcheng Sugar Mill (Guangxi, China). Table 1 summarizes the average juice properties recorded in the 2014–2015 crushing season. The juice was filtered through a 100-mesh stainless steel screen to remove large fibers. The juice was then treated with milk of lime and mixed well by a stirrer to raise the pH from 5.4–5.8 to 7.4–7.6. The limed juice was heated by tube-type heat exchangers to raise the temperature from 30–35 °C to 85–95 °C. The heated limed juice was used as feed for the experiments. Ceramic membranes were provided by Jiangsu Jiuwu Hi-Tech Co., Ltd. Details of the membrane characteristics are presented in Table 2.
Ultrafiltration Demonstration Plant
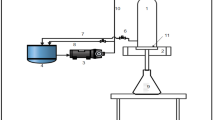
The demonstration was conducted at the Fangcheng Sugar Mill (Guangxi, China). A multistage ceramic ultrafiltration membrane system with capacity of 5.5 m3/h as the experimental setup was designed by Jiangsu Jiuwu Hi-Tech Co., Ltd. (Fig. 1). Ultrafiltration installation is composed of five-stage series ceramic membranes (I, II, III, IV, and V) using a feed and bleed continuous system (the concentrate of one stage is the feed of the next stage), in which each stage consists of two modules (LP and HP modules). Each module contains 12 membrane tubes. The total membrane area of the plant was calculated as 43.49 m2. To ensure the consistent operating performance of the system throughout the experiment, one of the five stages was used for cleaning and served as a replacement at any time. The heated limed sugarcane juice was pumped into the feed tank and then pumped into the membrane modules. Under pressure, the sugarcane juice was radially permeated through the membrane for clarification. The juice was then passed from one stage to the next with the circulation pumps providing the desired velocity in the channels. The retentate leaving the last stage was collected in the retentate tank, and the permeate juice was collected by the permeate tank. The volumetric concentration factor (VCF) was controlled between 10 and 12 by the inlet (V1) and outlet valve (V2). The unit was equipped with suitable instrumentation to measure flow, temperature, and pressure. The permeate clarified by the membrane was concentrated from 16.0–17.0 °Brix to 60.0–65.0 °Brix by a falling film evaporator. The concentrated sugarcane juice was also referred to as syrup. The syrup was crystallized in a vacuum boiling pan by seeding with crystalline sugar. Sugar crystals were then separated from the massecuite by centrifugation. The final product (raw sugar) was obtained after drying.
Operational data, such as flow rates, pressure, and temperature, were monitored and recorded at 15–30 min intervals. Samples from the feed, permeate, syrup, and final product (raw sugar) were collected once in 4–8 h for analysis. The pilot plant ran continuously for 56 days throughout the 2014–2015 crushing season.
Membrane Cleaning
The membranes were cleaned when the permeate flux declined to 70 L/(m2 h). The cleaning process varied according to the feed property. For the sugarcane juice, (1) it was first rinsed with clean industrial water for four to five times to remove all juice from the system until the resulting rinse was clear (the first rinse was pumped into the retentate tank). (2) The membrane was then rinsed for 1–2 h with a mixed solution of 1 % NaOH and 0.5 % NaOCl at 60–80 °C. The solution was drained, and the membrane was rinsed with water again until the pH approximated 7.0. (3) The membrane was finally rinsed with 0.5 % HNO3 solution for 10–20 min. Membrane cleaning was conducted at a low transmembrane pressure and high cross-flow velocity to ensure optimal scouring of the membrane surface. During chemical cleaning, the permeate valve was closed for the first half of the cleaning duration to remove foulants from the membrane surface. For the remainder of the cleaning duration, the permeate valve was opened to remove foulants trapped under the membrane layer and support.
Analysis
Brix, a measure of refractometric dry substance, was measured using a digital refractometer (PAL-1, Atago) (Jegatheesan et al. 2012). Pol is a measurement of the total polarized substance in the samples, and it represents the sucrose content in the samples. Pol was measured by a polarimeter (WZZ-2SS, SHANGHAI JINGKE) (Jegatheesan et al. 2009). The purity of the samples was calculated as follows (Jegatheesan et al. 2012):
Color, a measurement of the colored substances in the samples, was measured by a spectrophotometer (722 N, SHANGHAI JINGKE) (Ghosh and Balakrishnan 2003). The turbidity (NTU) of sugarcane juice was measured by a digital turbidity meter (WGZ-4000B, SHANGHAI XINRUI). The pH was measured by a digital pH meter (PHS-3C, P.R. China).
Reducing sugar content is a significant evaluation index of the quality of raw sugar. The reducing sugar content in raw sugar was measured according to Ofner’s method (Mcdonald and Turcotte 1946). Ash is a measurement of the inorganic contents in raw sugar. The conductometric method (Ghosh and Balakrishnan 2003) was used to measure ash by a conductivity meter (DDSJ-308A, SHANGHAI JINGKE). The turbidity (MAU) of raw sugar was measured according to GS-21 method (Ghosh and Balakrishnan 2003) using a spectrophotometer (722 N, SHANGHAI JINGKE). The gravimetric method was used to measure the amount of water-insoluble impurities in raw sugar. A certain mass of raw sugar was weighed and dissolved. The sugar solution was then filtered through a pore size less than 40 μm. The residues were used to measure the water-insoluble impurity content in raw sugar after drying.
Results and Discussion
Capacity and Flux
The capacity and permeate flux are two essential parameters to evaluate membrane separation. Supplementary Fig. S1 describes the capacity and permeate flux of the demonstration plant during operation in the 2014–2015 crushing season. Each data point represents the average flux (or capacity) obtained in 1 day (24 h). As shown in Supplementary Fig. S1, throughout the trials, the daily average flux was 119.13–142.43 L/(m2 h) and the capacity of the demonstration was 4.6–5.5 m3/h under the transmembrane pressure of 1.0–2.5 bar (LP = 1.0–1.5 bar, HP = 2.0–2.5 bar), cross-flow velocity of 4–5 m/s, feed temperature of 85–95 °C, and volumetric concentration factor of 10–12. These results indicated that the flux and capacity obtained were suitable for industrial production.
Quality of Clarified Sugarcane Juice
The quality of clarified juice is another significant parameter used to assess membrane filtration. The typical quality parameters of the feed and permeate streams are indicated in Table 3. All the different operating times produced high-quality filtered juice with more than 1.2 unit increase in purity, 99.96 % reduction in turbidity, and 10.42 % removal of color. These results demonstrated a remarkable improvement compared with the clarified juice obtained by lime defecation or liming–sulphitation. In addition to small amounts of lime, reducing turbidity, removing color, and rising purity were possible with ceramic membrane filtration without the addition of any other chemical substances.
Membrane Cleaning Period
The dirty membrane was successively cleaned with (1) clean industrial water, (2) a 1 % NaOH + 0.5 % NaOCl solution (60–80 °C), and (3) a 0.5 % HNO3 solution. The membrane filtration system was divided into five stages. The cleaning period of each stage is indicated in Table 4, which shows that the latter stage membrane had a shorter cleaning period. For instance, the cleaning period was 20–25 h in the first-stage membrane, but it was shortened to 10–12 h in the fifth stage (the last stage). This phenomenon was due to the fact that the membrane fouling degree was related to the concentration of impurities in the feed. The higher the concentration of impurities in the feed, the easier the membrane was fouled and the faster the permeate flux declined. During the filtration of sugarcane juice by the system, sugarcane juice was initially processed by the first stage. The retentate from the first stage was processed successively at the second and third stages and finally entered the fourth stage (one of the five stages was used for cleaning and functioned as a replacement during the operation). The VCFs of retentate from the first, second, third, and fourth stages were 1.1–1.8, 1.5–3.0, 3.5–6.0, and 10–12, respectively. Therefore, for the latter stage membrane, the membrane was easily fouled at higher concentrations of impurities in the feed. A membrane with a latter stage demonstrated a short cleaning period.
Raw Sugar Quality
The permeate clarified by the ceramic membranes was concentrated with a falling film evaporator to form syrup of 60–65 °Brix. The syrup was crystallized in a vacuum boiling pan by seeding with crystalline sugar. Sugar crystals were then separated from the massecuite by centrifugation. During centrifugation, water was added to assist in removing the mother liquor adhered to the crystals. This step improved the rate of purging and quality of sugar, but some sugar crystals were dissolved and affected the purity of molasses. Upon comprehensive consideration, the time of water addition should be controlled at the range of 4–5 s during centrifugation. The final product (raw sugar) was obtained after drying. Raw sugar quality is a critical factor because it determines the value of raw sugar and affects the performance of refineries (Jansen 2010). The typical quality parameters of raw sugar are presented in Table 5. High-quality raw sugar with high Pol, low color, and low ash content was obtained from the trials. After remelting, this high-quality raw sugar to produce refined sugar, affination, and carbonation (or phosphoric floating) was omitted. The raw sugar after remelting and filtering could be used in decolorization and crystallization, thereby reducing costs and preventing environmental problems caused by the excessive use of chemicals in carbonation or phosphoric floating. This sugar may serve as an edible product to be sold in China or in other countries.
A considerable number of studies have been conducted to investigate the application of membrane filtration to clarify sugarcane juice (Kwok 1996; Ghosh and Balakrishnan 2003; Wittwer 1999; Steindl and Doyle 1999; Kochergin et al. 2000; Ghosh et al. 2000; Jegatheesan et al. 2009; Priscilla et al. 2014). Supplementary Table S1 lists the experimental results of some previous studies, as well as those of the present study. The capacities of pilot and industrial studies reported by Kwok (1996), Ghosh and Balakrishnan (2003), Wittwer (1999), and the present study were closer to actual production, and the experiments conducted by different researchers all produced high-quality permeate juice or sugar. The experimental results reported by Kwok (1996), Ghosh and Balakrishnan (2003), and Wittwer (1999) were based on clarification of clarified juice, in which conventional raw sugarcane juice treatment should be retained as pretreatment for membrane filtration. However, in the present study, raw sugarcane juice (mixed juice) was processed directly using ceramic membranes, which is a remarkable improvement compared with the techniques used in previous studies. Based on Supplementary Table S1, the performance of inorganic membranes (ceramic membranes or stainless steel membranes) was better than that of organic polymer membranes with higher permeate flux.
Critical Issues Encountered During Operation
This study obtained positive results. However, further investigations are necessary to solve the following critical issues encountered during operation: (1) recovery of sucrose from retentate, which contains large amounts of impurities and a considerably high sucrose content; (2) treatment of wastewater generated from cleaning the membrane; and (3) investigation on the service life of membrane, which is a significant factor to assess membrane application.
Conclusions
Ultrafiltration of raw sugarcane juice for raw sugar production was successfully demonstrated in a raw sugar mill. Heated limed sugarcane juice was processed using 0.05 μm ceramic membranes in a pilot plant with a design capacity of 5.5 m3/h. The pilot plant ran continuously for 56 days throughout the 2014–2015 crushing season. The ceramic membrane modules displayed satisfactory performance, yielding 119.13–142.43 L/(m2 h) of average flux and producing permeate juice of superior quality. A high-quality product was obtained when the permeate juice was concentrated and crystallized to form raw sugar.
References
Farmani, B., M.H. Haddadekhodaparast, J. Hesari, and S. Aharizad. 2008. Determining optimum conditions for sugarcane juice refinement by pilot plant dead-end ceramic micro-filtration. Journal of Agricultural Science and Technology 10: 351–357.
Ghosh, A.M., and M. Balakrishnan. 2003. Pilot demonstration of sugarcane juice ultrafiltration in an Indian sugar factory. Journal of Food Engineering 58: 143–150.
Ghosh, A.M., M. Balakrishnan, M. Dua, and J.J. Bhagat. 2000. Ultrafiltration of sugarcane juice with spiral wound modules: On-site pilot trials. Journal of Membrane Science 174: 205–216.
Hamachi, M., B.B. Gupta, and R. Ben-Aim. 2003. Ultrafiltration: a means for decolorization of cane sugar solution. Separation and Purification Technology 30: 229–239.
Jansen, T.M. 2010. Raw sugar quality from a refiner’s perspective. International Sugar Journal 112: 250–256.
Jegatheesan, V., L. Shu, G. Keir, and D.D. Phong. 2012. Evaluating membrane technology for clarification of sugarcane juice. Reviews in Environmental Science and Bio/Technology 11: 109–124.
Jegatheesan, V., D.D. Phong, L. Shu, and R. Ben-Aim. 2009. Performance of ceramic micro- and ultrafiltration membranes treating limed and partially clarified sugar cane juice. Journal of Membrane Science 327: 69–77.
Kochergin, V., M. Kearney, W. Jacob, L. Velasquez, J. Alvarez, and C. Baez-Smith. 2000. Chromatographic desugarisation of syrups in cane mills. International Sugar Journal 102: 568–578.
Kwok, R.J. 1996. Ultrafiltration/Softening of clarified juice: the door to direct refining and molasses desugarisation in the cane sugar industry. Proceedings of the Annual Congress South African Sugar Technologists’ Association 70: 166–170.
Mcdonald, E.J., and A.L. Turcotte. 1946. Study of Ofner’s method for the determination of invert sugar. Journal of Research of the National Bureau of Standards 37: 429–434.
Priscilla, D.S.G., D.S.G. Paola, T.D.D.B. Sueli, and C.P. Nehemias. 2014. Pretreatment with ceramic membrane microfiltration in the clarification process of sugarcane juice by ultrafiltration. Acta Scientiarum Technology 36: 303–306.
Steindl, R.J. 2001. Membrane filtration technology in the cane sugar industry. Proceedings of International Society of Sugar Cane Technologists 24: 3–10.
Steindl, R.J., and C.D. Doyle. 1999. Applications and benefits of membrane filtration for the Australian sugar industry. Proceedings of the Australian Society of Sugar Cane Technologists 21: 406–411.
Wittwer, S. 1999. Applications for stainless steel crossflow membranes in sugar processing. Symposium on Advanced Technology for Raw Sugar and Cane and Beet Refined Sugar Production, September 9–10.
Funding
This study was funded by Guangxi Science and Technology Development Program (Grant Number: 14122003-6).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Li, W., Ling, GQ., Shi, CR. et al. Pilot Demonstration of Ceramic Membrane Ultrafiltration of Sugarcane Juice for Raw Sugar Production. Sugar Tech 19, 83–88 (2017). https://doi.org/10.1007/s12355-016-0434-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12355-016-0434-1