Abstract
Background
Transient ischemic dilation (TID) of the left ventricle seen on myocardial perfusion imaging (MPI) is sometimes used as a marker of severe coronary artery disease. The prognostic value of TID obtained using regadenoson, a selective adenosine A2A receptor agonist, as a stress agent for MPI has not been studied.
Methods
TID ratio was measured using an automated software program on consecutive patients with normal and abnormal perfusion pattern on regadenoson MPI at a single institution. An abnormal TID was defined as greater than 1.33. The primary outcome was a composite of cardiac death, non-fatal myocardial infarction (MI), and late coronary revascularization (CR, >90 days after MPI).
Results
The study population consisted of 887 patients (62 ± 12 years, 66% male, 48% diabetes, 46% prior CR, 75% with abnormal perfusion pattern, left ventricular ejection fraction—LVEF 55 ± 6%). An abnormal TID was present in 51 (6%) patients. Baseline characteristics were not different based on the presence or absence of TID. Early CR (≤90 days) was performed in 11 (22%) patients with vs 92 (11%) patients without TID (P = .04). During a mean follow-up of 29 ± 19 months, the primary outcome occurred in 271 (31%) patients (22% cardiac death, 6% MI, 9% late CR). TID was associated with increased risk of the primary outcome (log-rank P = .017), an association largely driven by late CR. In a Cox proportional model adjusted for multiple variables including perfusion defect size (PDS) and LVEF, the hazard ratio for TID was 1.92 (95% CI 1.20-3.08, P = .007). In the subset of patients with normal perfusion pattern, there was no association between TID and outcomes.
Conclusions
TID on regadenoson MPI carries important prognostic information that is independent from PDS and LVEF, but this association is restricted to patients with abnormal perfusion on imaging.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Background
Transient ischemic dilation (TID) of the left ventricle on single-photon emission computed tomography myocardial perfusion imaging (MPI) is sometimes used as a marker of severe coronary artery disease (CAD), and has been shown to have important prognostic value that is incremental to that provided by the perfusion pattern alone.1-10 TID, usually presented as a ratio, describes an increase in left ventricular (LV) cavity size on post-stress compared to rest images. Multiple mechanisms have been proposed to explain TID observed during nuclear stress testing including diffuse subendocardial ischemia leading to an apparent increase in LV endocardial cavity size, myocardial stunning, a true increase in the LV size, or a combination of these mechanisms.11,12 The assessment of TID is dependent on multiple factors including the stress modality, nuclear tracer, and imaging protocol used and other technical factors related to imaging.13
Regadenoson, a selective adenosine A2A receptor agonist, is the most frequently used pharmacologic stress agent for MPI in the United States.14 The perfusion pattern and perfusion defect size (PDS) obtained with regadenoson MPI have been shown to provide powerful diagnostic and prognostic information that can inform patient management.15-20 Recent reports have evaluated the diagnostic value of TID with regadenoson MPI, but its prognostic value has not been studied.9,21 Further, recent studies have suggested that TID may carry prognostic information in some populations but not others.10 In particular, it has been suggested that TID may be prognostically relevant only in patients with an abnormal perfusion pattern.7 Using an automated software program to measure TID ratio, we report here on the prevalence of TID in patients undergoing regadenoson technetium-99m sestamibi MPI, its association with future adverse cardiovascular outcomes, and its relevance in those with and without perfusion defects on imaging.
Methods
Study Cohort
The study population consisted of patients who underwent regadenoson MPI at the University of Alabama at Birmingham Nuclear Cardiology Laboratory between July 2008 and January 2010. The overall cohort has been comprehensively described in a previous report.19 In order to evaluate the prognostic relevance of TID in relation to the perfusion pattern, 700 consecutive patients with normal perfusion and normal LV ejection fraction (LVEF ≥50%) and 700 consecutive patients with abnormal perfusion on regadenoson MPI were included. Medical records were queried for patient demographics (age, gender, race), co-morbidities (diabetes, hypertension, hyperlipidemia, end-stage renal disease), and history of prior coronary revascularization (CR). MPI images were retrieved and reprocessed for automated evaluation of PDS, LVEF, and TID ratio. Of these patients, stress and rest images were available for analysis in 667 (95%) patients with abnormal perfusion and 220 (31%) with normal perfusion and these constituted the study cohort. The predominant cause for excluding cases from our cohort was the unavailability of rest images since our laboratory uses stress-only images to decrease radiation exposure when possible.22 The study was approved by the institutional review board for human research at the University of Alabama at Birmingham.
Myocardial Perfusion Imaging
Regadenoson MPI was performed using standard protocols approved by the American Society of Nuclear Cardiology.23 Details related to stress testing, image acquisition, and interpretation have been previously described.17,19 Same-day stress-rest imaging protocol was used in the vast majority of patients with rest images performed if the stress images were abnormal or if there was uncertainty in the interpretation of the stress images. All stress tests were performed in the absence of accompanying exercise. Regadenoson was administered as a 400 µg fixed intravenous bolus followed by a 5 mL saline flush. Technetium-99m sestamibi was injected 10-20 s later. Single-photon emission computed tomography gated images were acquired using a dual-head detector with an elliptical 180° acquisition (45° RAO to 45° LAO) and 8-16 frames per R-R cycle. Image analysis was performed using automated software (Corridor4DM) which determined the presence, extent, and severity of PDS (expressed as % of LV myocardium) and whether the defect was fixed or reversible at rest. This analysis was performed in a manner blinded to the current hypothesis and has been reported separately.19 LV volumes were measured at end systole and end diastole for calculating LVEF. The same software calculated TID ratio as the ratio of LV volume on the sum of the post-stress gated images to that on the sum of the rest gated images. The automated analysis was performed with visual supervision by readers who were blinded to subsequent events as previously described.19
Outcomes
The primary outcome was a composite of cardiac death, non-fatal myocardial infarction (MI), and late CR. Secondary outcomes were the individual components of the composite outcome. Outcomes were ascertained by review of patients’ health records and adjudicated by a blinded reviewer. Scripted telephonic interviews were used for subjects whose follow-up was less than 2 years. In addition, death was verified against the Social Security Death Index database. When the cause of death was not known, the death was categorized as cardiac. CR, which included both coronary artery bypass grafting and percutaneous coronary intervention, was categorized as early (≤90 days from index MPI) or late (>90 days). Only late CR was included in the primary outcome, but early CR was evaluated separately as a secondary outcome since early CR may have been driven by the MPI findings including TID.
TID Cutoff
We measured TID in patients with normal perfusion and normal LVEF on regadenoson MPI who did not undergo early or late CR and did not succumb to non-fatal MI or cardiac death during follow-up. The TID cutoff was defined as the value that encompasses 95% of this cohort. Patients with a measured TID ratio higher than this cutoff were considered to have abnormal TID. An alternative cutoff that encompasses 90% of the low-risk cohort was also assessed.
Statistical Analysis
Statistical analysis was performed using SPSS version 17 for Windows (SPSS Inc., Chicago, Illinois). Continuous variables were presented as mean ± SD and compared between the groups using the unpaired t test or Mann-Whitney U test, as appropriate. Discrete variables were presented as frequencies and percentages and compared between the groups using the χ2 test. Survival curves were constructed for patients with and without TID using the Kaplan-Meier method, and differences between survival curves were estimated by the log-rank test. Survival analysis treated the time of MPI as “time 0.” In order to estimate the independent risk associated with TID, a Cox regression model was constructed adjusting for demographics (age, gender, race), co-morbidities (diabetes, hypertension, dyslipidemia, end-stage renal disease), prior CR, and MPI findings (PDS and LVEF). Estimated risks were reported as hazard ratios and the correspondent 95% confidence interval. All tests were 2-tailed, and a P value of <.05 was considered statistically significant.
Results
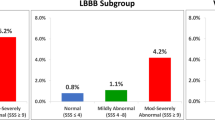
The mean TID ratio in the population that we used to derive the TID cutoffs (n = 188) was 1.04 ± 0.19 (median 1.02, interquartile range 0.94-1.13, non-Gaussian distribution). Of these patients, 90% had a TID less than 1.23 and 95% less than 1.33. We therefore defined the presence of TID in our study as a TID ≥1.33, and an alternative cutoff of TID ≥1.23 was used for comparison. Our study cohort consisted of 887 patients with a mean age of 62 ± 12 years, of which 66% are male, 66% are Caucasian, 48% have diabetes, 29% have end-stage renal disease, and 46% have a prior history of CR. Of these patients, 764 (86%) had normal TID (<1.23), 72 (8%) had an intermediate TID (1.23-1.32), and 51 (6%) had abnormal TID (≥1.33). The characteristics of the cohort according to TID status are detailed in Table 1. There were no major differences between the groups aside from a higher TID ratio and higher prevalence of ischemia in patients with TID. The characteristics of the patients according to the presence or absence of perfusion defects on imaging are shown in Table 2 demonstrating significant differences between these groups. There was a trend towards higher TID ratio in patients with abnormal myocardial perfusion and a numerically higher proportion of patients with abnormal TID in this group, but this did not reach statistical significance (Table 2).
In the first 90 days after index MPI, 103 patients underwent CR (early CR, 12%). Early CR was performed in 92 (11%) patients without TID vs 11 (22%) patients with TID (P = .04). Almost all (102 out of 103) patients who underwent early CR had abnormal perfusion on MPI.
During a mean follow-up of 29 ± 19 months, 271 (31%) patients experienced the primary outcome. There were 293 all-cause deaths out of whom 193 (22%; 114 with cause of death unknown and 79 with cardiac cause of death) were categorized as cardiac deaths, 53 (6%) non-fatal MIs, and 75 (9%) late CRs. The primary outcome occurred significantly more frequently in patients with than without TID (Figure 1, log-rank P = .017). The cumulative incidences of the primary outcome in patients without TID at 6, 12, 24, and 36 months were 6%, 9%, 17%, and 23%, respectively. The incidences for patients with TID at the same time intervals were 12%, 18%, 26%, and 44%, respectively. There were no significant differences between the two groups in cardiac deaths or non-fatal MIs, and therefore the difference in events was largely attributed to a higher incidence of late CR in patients with TID (Figure 1). When the cohort was dichotomized according to the presence or absence of perfusion abnormalities on MPI, the increased occurrence of the primary outcome was apparent in patients with perfusion abnormalities, whereas there was no significant difference in patients with normal perfusion (Figure 2).
Kaplan-Meier survival curve for the composite outcome of cardiac death, MI, and late CR stratified by the presence or absence of TID in patients with normal perfusion (left panel) and abnormal perfusion (right panel). In the left panel is shown a dotted line which represents patients who underwent stress-only imaging (no TID available)
We constructed a Cox proportional hazard model to estimate the hazard ratio independently associated with TID (Table 3). Adjusting for age, gender, race, co-morbidities, and prior CR, both PDS and LVEF on MPI were significantly associated with increased risk of the composite primary outcome. In addition, TID provided incremental prognostic information with a hazard ratio of 1.9 (95% confidence interval 1.2-3.1, P = .007). The model had an overall χ2 of 112 (P < .001). When the alternative cutoff of 1.23 was used for TID in the model, its association with outcomes remained statistically significant but with a smaller hazard ratio (1.39, 95% confidence interval 1.0-1.9, P = .049).
Discussion
The main aim of this study was to determine the prognostic significance of TID on regadenoson MPI. We found that TID was associated with increased risk of cardiac events during follow-up in a manner independent of baseline characteristics and other findings on MPI. Most of this association was related to increased risk for late CR with no significant difference in hard cardiac events between patients with and without TID. Importantly, TID on regadenoson MPI did not seem to associate with outcomes in patients with no perfusion defects on MPI.
TID ratio is a continuous variable posing challenges to what should be considered an abnormal TID in clinical practice. This is compounded by the dependence of TID ratio on several imaging-related factors such as the stress modality (exercise, adenosine, dipyridamole, regadenoson), tracer (technetium-99m, thallium-201, or dual isotope), 1- or 2-day protocols, camera settings, filters, and other technical factors.13 The cutoff appears to be higher in patients who undergo exercise vs adenosine stress,6,24 2-day vs 1-day protocols, and dual-isotope vs single-isotope imaging.25,26
Katz et al21 recently found a TID cutoff of 1.39 for regadenoson dual-isotope imaging, whereas Golzar et al9 had a cutoff of 1.31 for single-isotope technetium-99m regadenoson MPI. Both cutoffs were higher than those previously reported with other vasodilator stress agents which emphasize the need for stress agent-specific data. Our cutoff of 1.33 was very similar to that derived by Golzar et al 9 despite the use of different methodologies in the 2 studies. In their study, Golzar et al9 used a derivation cohort of 100 patients with low likelihood of CAD and normal perfusion on imaging. We derived our cutoff in 188 patients with normal MPI who did not have cardiac events (cardiac deaths, non-fatal MIs, or CR) during follow-up. Thus both methods avoid selection bias by not requiring the patients to have a coronary angiogram after MPI, but the study by Golzar et al defined a low-risk population based on baseline characteristics, whereas our study determined risk based on actual follow-up for events including CR. It is reassuring that both methods yielded very similar cutoffs. Further, the prevalence of TID in our cohort (6%) was very similar to that in the validation cohort (7.5%) of Golzar et al.
The association of TID on vasodilator stress MPI with adverse outcomes is well validated in multiple studies, but there is controversy with regard to its prognostic value in low-risk populations as recently reviewed by Bourque.10 For example, Chouraqui et al2 found a strong association of TID on dipyridamole MPI with severe and extensive multi-vessel CAD, while McClellan et al4 described a strong association between TID and adverse cardiac events, both in populations with high proportion of perfusion abnormalities. In contrast, Valdiviezo et al7 found no association between TID and multi-vessel disease or outcomes in a population with normal perfusion on imaging, the vast majority of whom were stressed pharmacologically. Mandour et al27 found that TID has a high predictive accuracy in patients with perfusion abnormalities, but in the setting of normal perfusion TID has poor predictive accuracy and should not be used as a marker of severe CAD. More recently, Doukky et al28 reported that in patients with normal perfusion on MPI (majority stressed with exercise), TID was associated with adverse cardiovascular events only in the subset of patients with known CAD or diabetes.
In the validation cohort of the study by Golzar et al9 which consisted of 547 patients who underwent angiography within 6 months of regadenoson MPI and included a mix of patients with normal and abnormal perfusion, TID did not identify patients with severe CAD. The authors speculated that this may be due to the temporal decline in the prevalence of significant ischemia on imaging over the last decades.29-32 Taking this into account, we enrolled in our study consecutive patients with normal and abnormal myocardial perfusion in 2 distinct cohorts to enrich our study population with high-risk scans. Indeed, the mean PDS of patients with abnormal perfusion was large (24% ± 15% of the LV) and the vast majority (95%) had moderate to large perfusion abnormalities. We found a strong association of TID with adverse cardiovascular outcomes which was independent of other factors including the size of the perfusion defect and LVEF on MPI. The presence of TID almost doubled the risk of cardiac adverse events on follow-up. This association remained statistically significant, although attenuated, when a lower cutoff of TID was used (TID ≥1.23, present in 14% of the cohort). In line with the studies discussed above, this association was only present in the subset of patients with abnormal perfusion, whereas there was no association of TID with outcomes in patients with normal perfusion (Figure 2).
Limitations
First, our study is retrospective and from a single center. Second, the association of TID with adverse cardiac events was limited to CR, whereas its association with hard cardiac events (cardiac death and non-fatal MI) was not statistically significant. Although this may be related to the declining risk of CAD as described above and the aggressive treatment of CAD in current practice, this raises the possibility that the TID on imaging may be driving the increased risk of CR rather than being a true marker of risk. We attempted to address this by looking at early and late CR using the conventional cutoff of 90 days from index MPI.19 TID appeared to inform decision making since it was associated with early CR, but it was also strongly associated with late CR. Further, the curves for late CR continued to diverge over time (Figure 1, lower right panel) suggesting that the risk persisted even remotely after imaging. Further, we did not depend on the clinical report to determine the presence of TID but reanalyzed the images using automated software to measure TID. In fact, TID was mentioned in the clinical report in a small minority of patients deemed to have TID on our analysis (8 of 51 patients, 16%), and therefore this is less likely to have influenced our findings. Third, since there is no accepted definition of TID on regadenoson MPI, we derived this from a low-risk population with normal imaging which needs to be verified in future cohorts. However, a recent study9 reported a very similar cutoff using a different methodology which supports our findings. Fourth, our population included equal number of patients with normal and abnormal perfusion rather than consecutive patients presenting for imaging which may limit the generalizability of our findings. Fifth, TID was not available on a substantial proportion of patients with normal perfusion due to the use of stress-only imaging in our laboratory. However, the risk of the primary outcome in patients who underwent stress-only imaging was comparable (Figure 2, dotted line) to that of patients who did not. Lastly, the number of patients with normal perfusion who had TID is small and therefore the findings in the normal perfusion cohort should be confirmed in larger studies. However, as discussed above our findings are in agreement with previous reports which support our conclusions.7,10,27
New Knowledge Gained
TID on regadenoson MPI is independently associated with a twofold increased risk of adverse cardiac events, but this association is limited to patients with abnormal perfusion on MPI and is largely related to increased risk of late CR.
Abbreviations
- CAD:
-
Coronary artery disease
- CR:
-
Coronary revascularization
- HR:
-
Hazard ratio
- LV:
-
Left ventricle
- LVEF:
-
Left ventricular ejection fraction
- MI:
-
Myocardial infarction
- MPI:
-
Myocardial perfusion imaging
- PDS:
-
Perfusion defect size
- TID:
-
Transient ischemic dilation
References
Stolzenberg J. Dilatation of left ventricular cavity on stress thallium scan as an indicator of ischemic disease. Clin Nucl Med 1980;5:289-91.
Chouraqui P, Rodrigues EA, Berman DS, Maddahi J. Significance of dipyridamole-induced transient dilation of the left ventricle during thallium-201 scintigraphy in suspected coronary artery disease. Am J Cardiol 1990;66:689-94.
Lette J, Bertrand C, Gossard D, Ruscito O, Cerino M, McNamara D, et al. Long-term risk stratification with dipyridamole imaging. Am Heart J 1995;129:880-6.
McClellan JR, Travin MI, Herman SD, Baron JI, Golub RJ, Gallagher JJ, et al. Prognostic importance of scintigraphic left ventricular cavity dilation during intravenous dipyridamole technetium-99m sestamibi myocardial tomographic imaging in predicting coronary events. Am J Cardiol 1997;79:600-5.
Abidov A, Bax JJ, Hayes SW, Hachamovitch R, Cohen I, Gerlach J, et al. Transient ischemic dilation ratio of the left ventricle is a significant predictor of future cardiac events in patients with otherwise normal myocardial perfusion SPECT. J Am Coll Cardiol 2003;42:1818-25.
Abidov A, Bax JJ, Hayes SW, Cohen I, Nishina H, Yoda S, et al. Integration of automatically measured transient ischemic dilation ratio into interpretation of adenosine stress myocardial perfusion SPECT for detection of severe and extensive CAD. J Nucl Med 2004;45:1999-2007.
Valdiviezo C, Motivala AA, Hachamovitch R, Chamarthy M, Navarro PC, Ostfeld RJ, et al. The significance of transient ischemic dilation in the setting of otherwise normal SPECT radionuclide myocardial perfusion images. J Nucl Cardiol 2011;18:220-9.
Petretta M, Acampa W, Daniele S, Petretta MP, Nappi C, Assante R, et al. Transient ischemic dilation in SPECT myocardial perfusion imaging for prediction of severe coronary artery disease in diabetic patients. J Nucl Cardiol 2013;20:45-52.
Golzar Y, Olusanya A, Pe N, Dua SG, Golzar J, Gidea C, et al. The significance of automatically measured transient ischemic dilation in identifying severe and extensive coronary artery disease in regadenoson, single-isotope technetium-99m myocardial perfusion SPECT. J Nucl Cardiol 2015;22:526-34.
Bourque JM. Contemporary relevance of TID: Based on the company it keeps. J Nucl Cardiol 2015;22:535-8.
Iskandrian AS, Heo J, Nguyen T, Lyons E, Paugh E. Left ventricular dilatation and pulmonary thallium uptake after single-photon emission computer tomography using thallium-201 during adenosine-induced coronary hyperemia. Am J Cardiol 1990;66:807-11.
Takeishi Y, Tono-oka I, Ikeda K, Komatani A, Tsuiki K, Yasui S. Dilatation of the left ventricular cavity on dipyridamole thallium-201 imaging: A new marker of triple-vessel disease. Am Heart J 1991;121:466-75.
McLaughlin MG, Danias PG. Transient ischemic dilation: A powerful diagnostic and prognostic finding of stress myocardial perfusion imaging. J Nucl Cardiol 2002;9:663-7.
2013 Nuclear Cardiology Trend Survey. J Nucl Cardiol 2014;21:S5-88.
Iskandrian AE, Bateman TM, Belardinelli L, Blackburn B, Cerqueira MD, Hendel RC, et al. Adenosine versus regadenoson comparative evaluation in myocardial perfusion imaging: Results of the ADVANCE phase 3 multicenter international trial. J Nucl Cardiol 2007;14:645-58.
Cerqueira MD, Nguyen P, Staehr P, Underwood SR, Iskandrian AE. Effects of age, gender, obesity, and diabetes on the efficacy and safety of the selective A2A agonist regadenoson versus adenosine in myocardial perfusion imaging integrated ADVANCE-MPI trial results. JACC Cardiovasc Imaging 2008;1:307-16.
Iqbal FM, Hage FG, Ahmed A, Dean PJ, Raslan S, Heo J, et al. Comparison of the prognostic value of normal regadenoson with normal adenosine myocardial perfusion imaging with propensity score matching. JACC Cardiovasc Imaging 2012;5:1014-21.
Ghimire G, Hage FG, Heo J, Iskandrian AE. Regadenoson: A focused update. J Nucl Cardiol 2013;20:284-8.
Hage FG, Ghimire G, Lester D, McKay J, Bleich S, El-Hajj S, et al. The prognostic value of regadenoson myocardial perfusion imaging. J Nucl Cardiol 2015;. doi:10.1007/s12350-014-0050-y.
Farzaneh-Far A, Shaw LK, Dunning A, Oldan JD, O’Connor CM, Borges-Neto S. Comparison of the prognostic value of regadenoson and adenosine myocardial perfusion imaging. J Nucl Cardiol 2015;. doi:10.1007/s12350-015-0155-y.
Katz JS, Ruisi M, Giedd KN, Rachko M. Assessment of transient ischemic dilation (TID) ratio in gated SPECT myocardial perfusion imaging (MPI) using regadenoson, a new agent for pharmacologic stress testing. J Nucl Cardiol 2012;19:727-34.
Iskandrian AE. Stress-only myocardial perfusion imaging a new paradigm. J Am Coll Cardiol 2010;55:231-3.
Henzlova MJ, Cerqueira MD, Hansen CL, Taillefer R, Yao SS. Stress protocols and tracers. ASNC imaging guidelines for nuclear cardiology procedures. J Nucl Cardiol 2009;. doi:10.1007/s12350-009-9062-4.
Mazzanti M, Germano G, Kiat H, Kavanagh PB, Alexanderson E, Friedman JD, et al. Identification of severe and extensive coronary artery disease by automatic measurement of transient ischemic dilation of the left ventricle in dual-isotope myocardial perfusion SPECT. J Am Coll Cardiol 1996;27:1612-20.
Abidov A, Germano G, Berman DS. Transient ischemic dilation ratio: A universal high-risk diagnostic marker in myocardial perfusion imaging. J Nucl Cardiol 2007;14:497-500.
Iskandrian AE, Garcia EV. Nuclear cardiac imaging: Principles and applications. Oxford: Oxford University Press; 2008. p. 732.
Ali MAM, Bourque JM, Allam AH, Beller GA, Watson DD. The prevalence and predictive accuracy of quantitatively defined transient ischemic dilation of the left ventricle on otherwise normal SPECT myocardial perfusion imaging studies. J Nucl Cardiol 2011;18:1036-43.
Doukky R, Frogge N, Bayissa YA, Balakrishnan G, Skelton JM, Confer K, et al. The prognostic value of transient ischemic dilatation with otherwise normal SPECT myocardial perfusion imaging: A cautionary note in patients with diabetes and coronary artery disease. J Nucl Cardiol 2013;20:774-84.
Rozanski A, Gransar H, Hayes SW, Min J, Friedman JD, Thomson LE, et al. Temporal trends in the frequency of inducible myocardial ischemia during cardiac stress testing: 1991 to 2009. J Am Coll Cardiol 2013;61:1054-65.
Iskandrian AE, Hage FG. Declining frequency of ischemia detection using stress myocardial perfusion imaging. J Am Coll Cardiol 2013;61:1066-8.
Beller GA. Decrease in the frequency of stress-induced ischemia over the past two decades. J Nucl Cardiol 2013;20:322-3.
Duvall WL, Rai M, Ahlberg AW, O’Sullivan DM, Henzlova MJ. A multi-center assessment of the temporal trends in myocardial perfusion imaging. J Nucl Cardiol 2015;22:539-51.
Disclosures
Drs. Hage and Iskandrian have received research grants from Astellas Pharma USA.
Author information
Authors and Affiliations
Corresponding author
Additional information
See related editorial, doi:10.1007/s12350-015-0299-9.
Davis Lester and Stephanie El-Hajj have contributed equally.
All editorial decisions for this article, including selection of reviewers and the final decision, were made by guest editor Alberto Cuocolo, MD.
Rights and permissions
About this article
Cite this article
Lester, D., El-Hajj, S., Farag, A.A. et al. Prognostic value of transient ischemic dilation with regadenoson myocardial perfusion imaging. J. Nucl. Cardiol. 23, 1147–1155 (2016). https://doi.org/10.1007/s12350-015-0272-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12350-015-0272-7