Abstract
Background
The prognostic implications of transient ischemic dilatation (TID) of the left ventricle with otherwise normal single-photon emission computed tomography myocardial perfusion imaging (MPI) remain controversial. Whether this finding may have prognostic implications only in high-risk populations, such as patients with diabetes or manifest coronary artery disease (CAD), is uncertain.
Methods
We conducted a prospective cohort study of 1,236 consecutive patients with normal 99mTc-sestamibi MPI, defined as normal perfusion (summed stress score = 0) and normal left ventricle volume and function. TID was defined as >2 standard deviations above the mean of patients with low likelihood of CAD.
Results
The study subjects were followed for 27 ± 9 months. The 76 (6%) patients with TID had a greater rate of cardiac death or myocardial infarction (MI) [4 (5.3%) vs 11 (0.6%), P = .003] independent of covariates [hazard ratio = 6.4, P = .004]. This finding was entirely derived from the subgroup of 294 patients with diabetes or CAD [4 (13.3%) with TID vs 1 (0.4%) without TID, P = .001] independent of covariates. However, TID was not predictive of cardiac death or MI among the 941 patients without diabetes or CAD. Furthermore, TID was not predictive of coronary revascularization.
Conclusions
This study confirms a benign prognosis of TID with otherwise normal MPI in patients without diabetes or CAD, but cautions against extending this conclusion to high-risk individuals, particularly those with diabetes or CAD.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Transient ischemic dilation (TID), as detected by myocardial perfusion imaging (MPI) with single-photon emission computed tomography (SPECT), refers to enlargement of the left ventricular (LV) cavity in the post-stress scintigraphic images relative to the resting images. Multiple studies have demonstrated that when observed in patients with myocardial perfusion defects, TID is a marker of severe and extensive coronary artery disease (CAD) and a predictor of increased risk for major adverse cardiac events (MACE).1-4 However, the prognostic value of TID in the setting of an otherwise normal study is uncertain, as some studies have shown increased risk, while others have not.5-8 It is possible that the clinical implications of this finding may differ among patients with varying clinical characteristics. In this study, we investigated the incremental prognostic value of TID with otherwise normal myocardial perfusion in patients within different clinical risk strata.
Design and Methods
We conducted a prospective cohort study of consecutive patients referred for a community-based, outpatient, clinically indicated SPECT MPI performed between August 15, 2007 and May 15, 2010 with a 2-year follow-up. Exclusion criteria from outcome analysis were (1) missing or invalid address, telephone, or social security numbers and (2) refusal of the referring physician to provide the investigators access to patients’ health records in order to conduct clinical follow-up.
Clinical Data
Baseline demographics, referral diagnosis, risk factors, cardiovascular history, indication for testing, and medications were tabulated prior to stress MPI. Framingham 10-year global coronary heart disease (CHD) risk estimates were calculated.9 History of CAD was defined as having prior myocardial infarction (MI), coronary artery bypass grafting (CABG) surgery, percutaneous coronary intervention (PCI), or angiographically documented obstructive coronary stenosis. The likelihood of obstructive CAD was tabulated in patients with ischemic-equivalent symptoms based on age, gender, and chest pain type (angina, atypical angina, non-anginal) according to the Diamond and Forrester criteria.10
Myocardial Perfusion Imaging
A one-day, rest/stress, 99mTechnetium-sestamibi (MIBI-MIBI) protocol was implemented, conforming to the American Society of Nuclear Cardiology guidelines.11 One of three stress modalities was chosen as clinically appropriate: exercise Bruce protocol, standard 6-minute adenosine infusion, or adenosine stress with low-level exercise.12,13 All MPI studies were acquired using an upright acquisition, dual-detector, dedicated cardiac SPECT camera (MAIcam180®, Mid-Atlantic Imaging Services, Inc., Columbia, MD). No attenuation correction was applied. Images were uniformly processed by a technologist blinded to clinical and outcome data.
Using QPS/QGS software (Cedars-Sinai Cardiac Suite; Los Angeles, CA), MPI scans were semiquantitatively interpreted by a single expert nuclear cardiologist (RD) who was blinded to patients’ clinical and outcome data. On a 17-segment model, the segmental radiotracer activity in the stress scans was scored according to the standard 5-point scale (0: normal; 1: mild; 2: moderate; 3: severe; 4: absent).14 The segmental scores were summed to generate summed stress score (SSS). Normal myocardial perfusion was defined as SSS = 0 (rather than <4) in order to evaluate the prognostic value of TID with “perfectly” normal perfusion. To ensure the reproducibility of the semiquantitative assessment of the perfusion data, a random subset of 151 scans (10% sample) were independently interpreted by two board-certified nuclear cardiologists who were blinded to the clinical and outcome data. The inter-rater interpretation agreement (normal vs abnormal) between the main reader and the two control readers was excellent (kappa = 0.82 and 0.86; P values < .001). Furthermore, the post-stress LV end-diastolic and end-systolic volumes were quantitatively tabulated and indexed to the body surface area. Abnormal LV end-diastolic volume index was defined as >2 standard deviations (SD) above the mean of the study population with normal perfusion (SSS = 0) and normal post-stress LV ejection fraction (LVEF ≥ 50%). We defined a normal MPI as normal perfusion (SSS = 0), normal post-stress LVEF ≥ 50%, and normal LV end-diastolic volume index.
Definition of TID
TID values were determined quantitatively from the ratio of the ungated (static) post-stress LV volume to the resting LV volume. As no universally accepted definition of TID for 99mTc-99m sestamibi MPI (MIBI-MIBI) with adenosine stress protocols exists, we determined the abnormal TID threshold internally for all three stress modalities used (exercise, adenosine, and adenosine with low-level exercise). Criteria for TID were prospectively defined as >2SD above the mean of the study population with low likelihood of CAD, normal perfusion (SSS = 0), and normal LV end-diastolic volume index. Low likelihood of CAD was defined, for the purpose of defining TID threshold, as having no clinical history of diabetes or CAD, Framingham 10-year global CHD risk < 10%, and achieving ≥85% of the maximum predicted heart rate (only if exercise stress modality was used).15
It is important to note that in the study patients, all of whom had no perfusion abnormality, TID was not documented in the official clinical report due to the questionable clinical utility of this finding. Thus, it is unlikely that TID data impacted the subsequent management and revascularization decisions in the clinical setting of this cohort.
Outcome Determination
Subjects were prospectively followed for events of death from any cause, cardiac death, MI, coronary angiography, PCI, and CABG surgery. Outcome assessors were blinded to MPI findings. Four methods for ascertaining outcome events were uniformly applied: (1) review of patient health records (from July 2011 through February 2012) at the referring physician offices; (2) two identical questionnaires mailed to patient residences 6 months apart (July 2011 and January 2012); (3) telephone interviews for subjects who did not complete mail surveys; and (4) Social Security Death Index (SSDI) search (April 2012) with cause of death determined from death certificates. MI events were defined by the clinical determination of the treating cardiologist.
The primary endpoint was a composite endpoint of cardiac death or nonfatal MI. The secondary endpoint was coronary revascularization.
Statistical Analysis
We determined that the available sample size of 1,236 patients with 6% TID prevalence, followed for a mean 27 months, was sufficient to attain 70% power to detect a statistically significant difference in the rate of cardiac death or MI using the Fisher’s exact test with a two-tailed α = 0.05. Power analysis assumed a 0.4% baseline annual event rate of cardiac death or MI and 6-fold increase in risk associated with TID.4
The Fisher’s exact test was used to compare dichotomous variables, which were expressed as frequency (percentage). The relative likelihoods of events were expressed as odds ratios (OR) with 95% confidence intervals (CI). The 2-tailed Student’s t test was used to compare normally distributed continuous variables, which were expressed as mean ± SD. Cox proportional hazards model method was used to compare event-free survival adjusted for Framingham 10-year CHD risk, exercise modality, and diabetic or CAD status. Although the mean age was statistically different between the TID study groups, we chose not to adjust for this covariate since age is already accounted for in the Framingham risk estimates. Proportionality of hazards assumption was confirmed by demonstrating parallel log minus log survival plots. The origin time in all survival analysis plots was the MPI date. Stepwise multivariable logistic regression was used to determine the gain in global chi-square value as an indicator of the incremental predictive value of sequentially added clinical and imaging predictors. Two-tailed P values < .05 were considered significant. The PASW 18.0 software (SPSS, Inc., Chicago, IL) was used for statistical analyses.
The study was approved by the institutional review board of Rush University Medical Center. A HIPAA waiver was applied to the chart review aspect of the methods. Subjects had the right to decline participation in the study via the mailed questionnaire.
Results
Cohort Definition
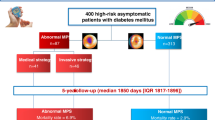
We identified 1,707 consecutive subjects referred for an outpatient, one-day, rest/stress, 99mTc sestamibi SPECT MPI. Among those, 182 subjects met one or more exclusion criteria: 84 had no valid SSN, 172 were missing a valid address or telephone number, and the managing physician of 43 patients declined to collaborate with the study. Fourteen (0.9%) subjects were lost to follow-up, none of whom were identified as deceased by SSDI. The remaining 1,511 subjects (99.1%) had complete follow-up.
A total of 275 subjects were excluded: 197 subjects with SSS ≥ 1; 31 with LVEF < 50%; and 47 with LV dilatation [end-diastolic volume index > 2SD above the mean (39.4 ± 10.8 mL/m2)]. Thus, 1,236 subjects were included in the final analysis. The baseline characteristics of the cohort were defined in Table 1.
Among the 196 patients who were excluded or lost to follow-up, there were 162 with normal MPI. Compared to the patients included in final analysis, those excluded were younger (mean 54 ± 15 vs 58 ± 12 years, P = .003), but had similar prevalence of male gender (58% vs 52%, P = .16), diabetes (22% vs 20%, P = .60), and CAD (6.2% vs 6.4%, P = 1.0). They also had similar 10-year Framingham CHD risk (12 ± 9% vs 11 ± 10%, P = .44) and TID ratio (0.94 ± 0.135 vs 0.936 ± 0.137, P = .72).
Determination of Abnormal TID Thresholds
The mean TID in the population with low likelihood of CAD and normal exercise stress MPI was 0.92 ± 0.12. The mean TID in the population with low likelihood of CAD and a normal adenosine stress MPI (with or without low-level exercise) was 1.01 ± 0.105, which was significantly greater than the mean TID associated with exercise stress MPI (P < .001). The mean TID for patients with low likelihood for CAD who underwent the standard 6-minute adenosine stress and for those who underwent adenosine infusion with low-level exercise was nearly identical (1.01 ± 0.105 and 1.01 ± 0.106, respectively; P = .88). Using a “mean + 2SD” cutoff, abnormal TID thresholds for exercise and vasodilator stress MPI were, respectively, defined as ≥1.16 and ≥1.22. Based on these values, the study population was divided into TID+ [76 (6%)] and TID− [1,160 (94%)] groups. The baseline characteristics of the study groups were similar, except that TID+ patients were older and had a higher Framingham 10-year CHD risk, prevalence of diabetes, and likelihood of undergoing a vasodilator stress (Table 1).
Outcomes
The 1,236 study subjects were followed for a mean of 27 ± 9 months for clinical events and 37 ± 8 months for mortality (clinical or SSDI). During the entire follow-up, there were 18 (1.5%) deaths, 5 (0.4%) cardiac deaths, and 6 (0.5%) nonfatal MIs. Additionally, 39 (3.2%) subjects underwent coronary angiography with 23 (1.9%) subsequent revascularizations (Table 2). The rates of coronary angiography performed within 60 days of the MPI study were similar in the TID+ and TID− groups [2 (2.6%) vs 13 (1.1%), respectively; P = .23].
The TID+ group had higher rates of the primary composite endpoint of cardiac death or MI, as well as individual endpoints of cardiac death and nonfatal MI (Table 2; Figure 1). The annualized event rate of death or MI was 2.4% in the TID+ group vs 0.4% in the TID− group. Likewise, the TID+ group had higher rates in multiple composite endpoints, including death or MI; death, MI, or revascularization; and cardiac death, MI, or revascularization (Table 2; Figure 1). The hazard of cardiac death or MI was significantly greater in the TID+ group [hazard ratio (HR) = 6.4 (CI 1.8-22.4), P = .004] after adjusting for Framingham risk, stress modality, and diabetic or CAD status, (Figure 2A). In this analysis, vasodilator stress was also predictive of the composite of cardiac death or MI, independent of TID status and Framingham risk [HR = 5.1 (CI 1.4-18.7), P = .01], whereas diabetic or CAD status was not independently predictive (P = .76). On the other hand, the rates of coronary revascularization (secondary endpoint) were not different between the TID groups (Figure 1), irrespective of covariate adjustments (Figure 3A). Only 2 revascularizations (PCIs) occurred within 60 days of MPI, both in the TID− group.
Cox proportional hazards curves of survival free of death or MI. (A) Event-free survival in the entire cohort, adjusted for Framingham 10-year CHD risk [HR = 1.01 (CI 0.95-1.1), P = .86], vasodilator vs exercise stress modality [HR = 5.1 (CI 1.4-18.7), P = .01], and diabetic or CAD status [HR = 1.2 (CI 0.3-4.8), P = .76]. (B) Event-free survival in the subgroup with diabetes or CAD, adjusted for Framingham 10-year CHD risk [HR = 1.0 (CI 0.9-1.1), P = .98] and vasodilator vs exercise stress modality [HR = 4.1 (CI 0.4-37.9), P = .21]. (C) Event-free survival in the subgroup without diabetes or CAD, adjusted for Framingham 10-year CHD risk [HR = 1.0 (CI 0.9-1.1), P = .51] and vasodilator vs exercise stress modality [HR = 6.4 (CI 1.3-32.0), P = .02]. * There were no events in the TID+ group
Cox proportional hazards curves of survival free of coronary revascularization. (A) Event-free survival in the entire cohort, adjusted for Framingham 10-year CHD risk [HR = 1.02 (CI 0.97-1.1), P = .47], vasodilator vs exercise stress modality [HR = 1.9 (CI 0.8-4.7), P = .16], and diabetic or CAD status [HR = 0.86 (0.3-2.5), P = .79]. (B) Event-free survival in the subgroups with diabetes or CAD, adjusted for Framingham 10-year CHD risk [HR = 1.0 (CI 0.9-1.04), P = .28] and vasodilator vs exercise stress modality [HR = 0.6 (CI 0.1-3.2), P = .56]. (C) Event-free survival in the subgroups without diabetes or CAD, adjusted for Framingham 10-year CHD risk [HR = 1.06 (CI 1.004-1.1), P = .04] and vasodilator vs exercise stress modality [HR = 3.2 (CI 1.2-9.1), P = .03]
Moreover, TID provided an incremental prognostic value beyond Framingham risk, CAD ,and diabetic status in predicting the composite of cardiac death or MI and the composite of death or MI, but not coronary revascularization (Figure 4).
The incremental prognostic value of TID. Stepwise multivariable logistic regression models in which outcome predictors were incrementally introduced in a stepwise fashion: 10-year Framingham CHD risk, coronary disease status, diabetic status, and TID status (present or absent). The gain in the global chi-square (χ2) value was used to determine whether each sequentially introduced predictor provides an incremental predictive value for adverse cardiac events. The goodness of fit at the final step of the regression models for all three endpoints depicted was acceptable (Hosmer and Lemeshow test P values ≥ .32)
Subgroup Analyses
Notably, all four patients who experienced a primary endpoint event (cardiac death or MI) were elderly, with premorbid diabetes and/or CAD. Three out the four patients were men or underwent vasodilator stress (Table 3). Among patients with diabetes or CAD, TID was associated with a significantly greater rate of cardiac death or MI (Table 4; Figure 5B), which was independent of covariates of Framingham risk and stress modality (Figure 2B). The annualized event rate of death or MI among patients with CAD or DM was 5.9% in the TID+ group vs 0.2% in the TID− group. Furthermore, among patients with diabetes or CAD, TID was associated with higher rates of nonfatal MI; death of any cause; death or MI; the composite of cardiac death, MI, and revascularization; and the composite endpoint of death, MI, and revascularization (Table 4; Figure 5B). The greater risk observed in the subgroup with diabetes or CAD was primarily derived from the 244 patients with diabetes mellitus (Table 4; Figure 5C). In contrast, among the 941 (76%) patients who had no history of diabetes or CAD, there was no significant difference in the adverse event rates based on TID status (Table 4; Figure 5A), irrespective of adjustment for covariates (Figure 2C). TID, on the other hand, was not associated with increased revascularization rate irrespective of diabetes or CAD status (Figure 3).
In this population with normal MPI, the rate of the primary endpoint of cardiac death or MI among subjects undergoing vasodilator stress was greater than that of subjects undergoing exercise stress (3.1% vs 0.4%; P = .001). Moreover, among the 226 patients who underwent vasodilator stress, TID was associated with a greater rate of cardiac death or MI (11.1% vs 2.0%, P = .04), even after adjusting for Framingham risk, CAD, and diabetic status [HR = 5.6 (CI 1.24-25.1); P = .03]. However, the revascularization rates were similar (Figure 5D). In the exercise stress subgroup, TID was not associated with an increase in adverse cardiac events.
Discussion
TID is generally accepted as a marker for severe multi-vessel CAD and a predictor of poor clinical outcome when seen in the presence of perfusion defects.1-4 Possible mechanisms for TID include actual ischemia-induced LV dilatation, subendocardial ischemia with a decrease in subendocardial tracer uptake leading to an appearance of LV dilatation, and post-stress stunning of the left ventricle.15-18 However, the significance of TID in patients with otherwise normal perfusion remains the subject of controversy.5-7
In this prospective study, we found that TID did predict clinical outcomes in subjects with TID with an otherwise normal 99mTc-sestamibi SPECT MPI, defined as normal myocardial perfusion (SSS = 0), LVEF, and LV volume. To put this finding in perspective, the relative risk of TID+ to the TID− patients (HR = 6.4) was similar to the relative risk of patients who received vasodilator stress compared to those who underwent exercise stress (HR = 5.1). Furthermore, the predictive value of TID depended on individual patient characteristics. TID was independently associated with a multifold increase in MACE (cardiac death or MI) in patients with diabetes or previously manifested CAD, but was not predictive in patients without these high-risk features. In fact, subjects without prior diabetes or CAD had a very low MACE risk, regardless of TID. Furthermore, TID was also associated with increased MACE risk among patients who underwent pharmacologic stress, another clinically high-risk group. We identified that all four patients who experienced cardiac death or MI were elderly and three out of four were men (Table 3). Age and gender, however, are heavily weighted in the Framingham CHD risk, which was adjusted for in the Cox regression models (Figure 2). Thus, the impact of TID on the outcome of patients with diabetes or CAD seems to be independent of age and gender. These findings should be interpreted with caution given the limited number of events observed.
Many physicians interpret a normal MPI as a “warranty” with less than 1% annual event rate. However, this low event rate is not applicable to all patients. Hachamovitch et al19 demonstrated that normal MPI in clinically high-risk patients (CAD, diabetes, pharmacologic stress, etc.) is associated with a hard event rate as high as 1.8% yearly. Therefore, it is clinically useful if TID can separate clinically high-risk patients into higher risk groups (5.9% annual event rate in our study) from those with ultralow risk (<1%) without TID, hence providing an incremental prognostic value beyond clinical and other perfusion parameters. These findings are clinically significant, as identifying TID with otherwise normal perfusion, in certain high-risk groups, may prompt the managing physician to aggressively pursue secondary risk prevention goals and consider coronary angiography in some patients.
To the best of our knowledge, this is the only large outcome study of TID in otherwise normal MPI conducted with a one-day 99mTc single-isotope (Tc-Tc) protocol, the most commonly implemented one in current practice.
Previous Studies
Our findings are similar to those reported in a landmark study by Abidov et al,5 who followed 1,560 patients with normal MPI for 2.3 years, of whom 390 had TID, and found an increase in both hard events of MI or cardiac death and soft events of coronary revascularization. The concordance between our findings and theirs is not surprising given the similarities in methods and definition of normal perfusion (SSS = 0), inclusion of patients with CAD and diabetes, and identical endpoints. In their study, however, they defined TID with dual-isotope protocol (rest 201Tl/stress 99mTc-sestamibi) as ≥1.21 (the top quartile of the study population). Moreover, their findings were primarily driven by revascularization events, whereas our findings were primarily determined by a higher rate of cardiac death or MI. In fact, we did not find a significant difference in revascularization rate between the TID groups. It is likely that the higher revascularization rate observed in their study is in part biased by the TID finding itself.5 In our study, however, TID with normal perfusion was not reported to the managing physician, explaining the non-significant effect of TID on revascularization rate.
More recently, a study by Valdiviezo et al6 reported that 28 patients with TID, but otherwise normal MPI, had a similar CAD burden to a cohort without TID, suggesting that TID noted on SPECT scans without any concomitant perfusion defects does not predict multi-vessel CAD. These investigators also followed a cohort of 593 patients with TID, but otherwise normal MPI for an average of 3.6 years and did not find any difference in survival compared to patients without TID.6 There are a few possibilities to account for the discordant results. In the study by Valdiviezo et al, dual isotope was the protocol implemented in a majority of the patients. Use of 201Tl with its poor endocardial definition and low signal-to-noise ratio could have led to small statistically random changes in the cavity size on the resting scan, thus yielding high TID ratios in patients with small left ventricles.2,3 Another source for the discrepancy may be the definition of normal perfusion, which they defined as SSS < 4 (rather than 0). This could have increased the event rate in the TID− group, biasing the study toward the null. Unlike our study, Valdiveizo et al did not study cardiac specific endpoints. Thus, noncardiac deaths could have, in part, offset an association between TID and cardiac deaths. We do not believe that including patients with CAD in the present investigation, but excluding them in the study by Valdiviezo et al, can explain the difference in the results. Having excluded CAD patients in the current study would not change its overall conclusions.
Pathophysiology
There are multiple potential explanations for increased MACE risk associated with TID among patients with CAD and diabetes. A frequently cited hypothesis is “balanced” myocardial ischemia due to diffuse CAD leading to homogenously reduced radioisotope uptake with subendocardial ischemia and/or post-stress stunning manifesting as TID on the ungated SPECT images. This hypothesis is supported by data from Fallahi et al, who demonstrated that, among diabetics with otherwise normal MPI, TID strongly correlated with severe and extensive CAD. This correlation was not found among non-diabetics.8 Furthermore, a recent report by Petretta et al20 demonstrated the incremental value of TID in predicting severe CAD in diabetics. A second plausible explanation is that TID in the setting of otherwise normal perfusion is a manifestation of diffuse microvascular disease, which may render patients more vulnerable to adverse events. A third reason, which seems most likely, is that the increased risk observed among patients with diabetes or CAD is simply a manifestation of Bayes’ theorem, analogous to the diagnostic value of stress testing in groups with different disease prevalence. Therefore, TID identifies a significant increase in MACE risk in a population with high prevalence of severe CAD and greater baseline MACE rate despite normal perfusion.4 In a low-risk group, however, TID fails to predict a significant increase in MACE rate due to very low baseline risk.
Methodological Considerations
We prospectively defined abnormal TID ratio as >2SD above the mean of the study population with low likelihood of CAD, normal myocardial perfusion, normal LV function and volume, and achieved 85% of the maximum predicted heart rate (if exercise Bruce protocol was used). Although a recent report defined abnormal TID threshold with rest/exercise stress 99mTc-sestamibi protocol,15 no published study established a cutoff for adenosine stress modality. Clearly, exercise stress TID thresholds are not applicable to adenosine stress.1,3,21 Therefore, we had to internally define thresholds for all three stress modalities used in the study, applying uniform methodology. We defined a TID threshold for rest/exercise stress 99mTc-sestamibi protocol at 1.16, which is significantly lower than associated with adenosine stress (1.22), mirroring a similar observation with dual-isotope protocols (1.36 and 1.22, respectively).3,21 It is important to note that defining TID thresholds in the present study was for the sole purpose of dichotomizing the cohort into two TID groups. Pending external validation, these thresholds are not to be used outside the context of this investigation. Furthermore, the inclusion of the subjects from whom these thresholds were derived in subsequent analyses may have provided more optimistic results than would be accomplished if externally validated TID thresholds were used.
Secondly, this study was designed as an outcome study. Thus, angiography results were not collected. However, coronary revascularization events provide an insight into subsequently identified “revascularization anatomy” CAD. In this regard, it is unique to the current study that TID with otherwise normal perfusion was not noted in the official clinical report. Therefore, it is unlikely that TID data triggered any of the subsequent medical or procedural interventions. This proposition was confirmed as all revascularizations in the TID+ group occurred late (>60 days) post-MPI, suggesting that clinical events were their trigger. This cohort, therefore, provides a unique opportunity to evaluate the unmodified impact of TID with otherwise normal MPI on a patient’s outcome and subsequent coronary revascularization.
Limitations
This study is primarily limited by the low number of events. After all, only 11 cardiac deaths or MI events were recorded, 4 of which were in the TID+ group. This limitation leaves uncertainty as to the exact expected event rate associated with TID, which is evident in the wide CI shown in Figures 1 and 4. Furthermore, the low number of hard events limited our ability to adjust for multiple covariates. Despite that, the findings were significant and consistent with prior reports.5,8 Thus, the study cautions from dismissing TID with otherwise normal perfusion in high-risk groups. Certainly, a dedicated study to evaluate the prognostic value of this imaging finding in high-risk population is warranted.
Conclusions
This investigation confirms a benign prognosis of TID with otherwise normal MPI in patients without high-risk clinical features such as diabetes or CAD. However, the study cautions against extending this conclusion to high-risk individuals.
References
Abidov A, Germano G, Berman DS. Transient ischemic dilation ratio: A universal high-risk diagnostic marker in myocardial perfusion imaging. J Nucl Cardiol 2007;14:497-500.
McLaughlin MG, Danias PG. Transient ischemic dilation: A powerful diagnostic and prognostic finding of stress myocardial perfusion imaging. J Nucl Cardiol 2002;9:663-7.
Mazzanti M, Germano G, Kiat H, Kavanagh PB, Alexanderson E, Friedman JD, et al. Identification of severe and extensive coronary artery disease by automatic measurement of transient ischemic dilation of the left ventricle in dual-isotope myocardial perfusion SPECT. J Am Coll Cardiol 1996;27:1612-20.
Doukky R, Frogge N, Balakrishnan G, Hayes K, Collado FM, Rangel MO, et al. The prognostic value of cardiac SPECT performed at the primary care physician’s office. J Nucl Cardiol 2013; in press.
Abidov A, Bax JJ, Hayes SW, Hachamovitch R, Cohen I, Gerlach J, et al. Transient ischemic dilation ratio of the left ventricle is a significant predictor of future cardiac events in patients with otherwise normal myocardial perfusion SPECT. J Am Coll Cardiol 2003;42:1818-25.
Valdiviezo C, Motivala AA, Hachamovitch R, Chamarthy M, Navarro PC, Ostfeld RJ, et al. The significance of transient ischemic dilation in the setting of otherwise normal SPECT radionuclide myocardial perfusion images. J Nucl Cardiol 2011;18:220-9.
Mandour Ali M, Bourque J, Allam A, Beller G, Watson D. The prevalence and predictive accuracy of quantitatively defined transient ischemic dilation of the left ventricle on otherwise normal SPECT myocardial perfusion imaging studies. J Nucl Cardiol 2011;18:43-1036.
Fallahi B, Beiki D, Fard-Esfahani A, Akbarpour S, Abolhassani A, Kakhki VR, et al. The additive value of transient left ventricular dilation using two-day dipyridamole 99mTc-MIBI SPET for screening coronary artery disease in patients with otherwise normal myocardial perfusion: a comparison between diabetic and non-diabetic cases. Hell J Nucl Med 2010;13:246-52.
Wilson PW, D’Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation 1998;97:1837-47.
Diamond GA, Forrester JS. Analysis of probability as an aid in the clinical diagnosis of coronary-artery disease. N Engl J Med 1979;300:1350-8.
Henzlova MJ, Cerqueira MD, Mahmarian JJ, Yao SS. Stress protocols and tracers. J Nucl Cardiol 2006;13:e80-90.
Doukky R. Pharmacologic Stress testing in myocardial perfusion imaging: Technical applications. In: Mann A, Heller GV, Hendel RC, editors. Nuclear cardiology: Technical applications. New York: McGraw-Hill; 2007. p. 107-24.
Elliott MD, Holly TA, Leonard SM, Hendel RC. Impact of an abbreviated adenosine protocol incorporating adjunctive treadmill exercise on adverse effects and image quality in patients undergoing stress myocardial perfusion imaging. J Nucl Cardiol 2000;7:584-9.
Cerqueira MD, Weissman NJ, Dilsizian V, Jacobs AK, Kaul S, Laskey WK, et al. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart: A statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation 2002;105:539-42.
Xu Y, Arsanjani R, Clond M, Hyun M, Lemley M, Fish M, et al. Transient ischemic dilation for coronary artery disease in quantitative analysis of same-day sestamibi myocardial perfusion SPECT. J Nucl Cardiol 2012;19:465-73.
Kakhki VR, Sadeghi R, Zakavi SR. Assessment of transient left ventricular dilation ratio via 2-day dipyridamole Tc-99m sestamibi nongated myocardial perfusion imaging. J Nucl Cardiol 2007;14:529-36.
van der Veen B, Kuperij N, Stokkel M. Transient ischemic dilatation ratio derived from myocardial perfusion scintigraphy: What are we looking at? J Nucl Cardiol 2010;17:207-15.
Emmett L, Ng A, Ha L, Russo R, Mansberg R, Zhao W, et al. Comparative assessment of rest and post-stress left ventricular volumes and left ventricular ejection fraction on gated myocardial perfusion imaging (MPI) and echocardiography in patients with transient ischaemic dilation on adenosine MPI: Myocardial stunning or subendocardial hypoperfusion? J Nucl Cardiol 2012;19:735-42.
Hachamovitch R, Hayes S, Friedman JD, Cohen I, Shaw LJ, Germano G, et al. Determinants of risk and its temporal variation in patients with normal stress myocardial perfusion scans: What is the warranty period of a normal scan? J Am Coll Cardiol 2003;41:1329-40.
Petretta M, Acampa W, Daniele S, Petretta MP, Nappi C, Assante R, et al. Transient ischemic dilation in SPECT myocardial perfusion imaging for prediction of severe coronary artery disease in diabetic patients. J Nucl Cardiol 2013;20:45-52.
Abidov A, Bax JJ, Hayes SW, Cohen I, Nishina H, Yoda S, et al. Integration of automatically measured transient ischemic dilation ratio into interpretation of adenosine stress myocardial perfusion SPECT for detection of severe and extensive CAD. J Nucl Med 2004;45:1999-2007.
Conflicts of interest
Rami Doukky served on the advisory board of Astellas Pharma US and received past funding investigator-initiated grant support from Astellas Pharma US.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Doukky, R., Frogge, N., Bayissa, Y.A. et al. The prognostic value of transient ischemic dilatation with otherwise normal SPECT myocardial perfusion imaging: A cautionary note in patients with diabetes and coronary artery disease. J. Nucl. Cardiol. 20, 774–784 (2013). https://doi.org/10.1007/s12350-013-9765-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12350-013-9765-4