Abstract
The K+, Na+/H+ antiporter LeNHX2 and the regulatory kinase SlSOS2 are important determinants of salt tolerance in tomato plants and their fruit production ability. In this work, we have analyzed the effects of LeNHX2 and SlSOS2 co-overexpression on fruit production, quality in tomato plants (Solanum lycopersicum L. cv. MicroTom), and analyzed physiological parameters related to salt tolerance. Plants overexpressing LeNHX2, SlSOS2 or both were grown in greenhouse. They were treated with 125 mM NaCl or left untreated and their salt tolerance was analyzed in terms of plant biomass and fruit yield. Under NaCl cultivation conditions, transgenic tomato plants overexpressing either SlSOS2 or LeNHX2 or both grew better and showed a higher biomass compared to their wild-type plants. Proline, glucose and protein content in leaves as well as pH and total soluble solid (TSS) in fruits were analyzed. Our results indicate that salinity tolerance of transgenic lines is associated with an increased proline, glucose and protein content in leaves of plants grown either with or without NaCl. Salt treatment significantly reduced yield, pH and TSS in fruits of WT plants but increased yield, pH and TSS in fruits of transgenic plants, especially those overexpressing both LeNHX2 and SlSOS2. All these results indicate that the co-overexpression of LeNHX2 and SlSOS2 improve yield and fruit quality of tomato grown under saline conditions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Salt stress is one of the major factors that limit plant growth and crop productivity, especially in the arid and semi-arid regions where soil salinity poses a severe threat to food security. Plants respond to salt stress by osmotic adjustment, generally by increasing the concentrations of solutes such as proline and soluble sugars in order to adjust osmotic potential for a better uptake of water (Li et al. 2014; Wu et al. 2016). Proline is accumulated in leaves in order to maintain chlorophyll level and cell turgor to protect photosynthesis activity under salt stress (Silva-Ortega et al. 2008). The soluble sugars are highly accumulated in vacuoles and produce high turgor pressure that are involved in response to abiotic stresses such as salt stress by affecting osmotic potentials (Rasheed et al. 2011). Veeranagamallaiah et al. (2007) reported that plants respond and adapt to salt stress through the synthesis of specific proteins, and the synthesis of stress-induced proteins is part of the stress tolerance mechanism.
The overexpression of Na+, K+/H+ antiporters and their regulatory proteins has been evidenced as an important method to overcome the adverse effects of salt stress on tomato plants and improve plant growth as well as crop productivity (Rodríguez-Rosales et al. 2008, 2009; Gálvez et al. 2012; Huertas et al. 2012, 2013; Cagnac et al. 2020; Maach et al. 2020). The intracellular NHX transporters constitute the first Cation/Proton exchanger family studied in plants. It was shown that overexpression of this protein in various plants improves salt tolerance, indicating a role of the protein in vacuolar Na+ accumulation (Apse et al. 1999; Zhang and Blumwald 2001; Venema et al. 2002). In Arabidopsis, the SOS signal transduction pathway is responsible for Na+ homeostasis and salinity tolerance by maintaining favourable K+/Na+ ratios in the cytoplasm through the action of the plasma membrane Na+/H+ antiporter SOS1, which mediates Na+ extrusion out of the root cell and long-distance Na+ transport from roots to shoots (Shi et al. 2002; Zhu 2002). In this pathway, a calcium-binding protein, SOS3, senses cytosolic calcium changes elicited by salt stress (Ishitani et al. 2000). SOS3 physically interacts with and activates the serine/threonine protein kinase, SOS2 (Liu et al. 2000). The SOS3/SOS2 kinase complex phosphorylates and activates the plasma membrane Na+/H+ exchanger encoded by the SOS1 gene (Qiu et al. 2002; Quintero et al. 2002; Shi et al. 2002; Olías et al. 2009). Coordination of the activity between the Na+/H+ exchangers of the tonoplast and plasma membrane were suggested by Qiu et al. (2004) who observed that the mRNA levels of NHX genes were up-regulated in the sos1 mutant. Moreover, an increased fruit production under NaCl conditions was reported in tomato plants co-overexpressing the Pennisetum glaucum vacuolar Na+/H+ antiporter and Arabidopsis H+-pyrophosphatase (Bhaskaran and Savithramma 2011). Also, in Arabidopsis, the co-overexpression of NHX1 and SOS3 was demonstrated to further improve salt tolerance relative to plants overexpressing only NHX1 (Yang et al. 2009) and an increased silique production under salt stress was detected in double transgenic plants overexpressing AtNHX1 and SOS1 (Pehlivan et al. 2016). Recently, we have also reported that in tomato that the co-overexpression of LeNHX2 and SlSOS2 increases salt tolerance and fruit production (Baghour et al. 2019).
Besides conferring salt tolerance, plant antiporters play an important role in improving the organoleptic characteristics of fruits as well as those related to fruit ripening (Hanana et al. 2009; Huertas et al. 2012). Total soluble solid (TSS) and pH are important fruit quality characteristics for suitability of tomato in industrial processing (Teka 2013). In this context, the aim of the present study is to investigate the effects of LeNHX2 and SlSOS2 co-overexpression on tomato fruit production and quality as well as determining the role of sugar, proline and protein accumulation during salt stress tolerance of tomato transgenic plants overexpressing LeNHX2, SlSOS2 or both genes.
Materials and methods
Plant Material and growth conditions
Tomato plants (Solanum lycopersicum L. cv. Microtom) overexpressing LeNHX2, SlSOS2 or both genes were used in this work. T3 tomato plants homozygous and mono-locus for the transgenes LeNHX2 (Huertas et al. 2013) and SlSOS2 (Huertas et al. 2012) were cross-pollinated. After crossing, plants were grown and seeds were removed from mature fruits derived from the cross-pollinated flowers.
The flower of the SlSOS2 overexpressing plants from line L-82 were used as female parents and those from the LeNHX2 overexpressing from L-932 were used as male parents. Analysis by real time PCR of F1 plants harbouring the constructs for SlSOS2 and LeNHX2 co-overexpression showed a higher expression of both genes compared to wild-type and single transgenic lines (Baghour et al. 2019).
Seeds from WT and single transgenic plants overexpressing LeNHX2 or SlSOS2 as well as seeds derived from the crosses between the T3 homozygous LeNHX2 and SlSOS2 transgenic plants were germinated and the resulting plants were used for experiments. Tomato seeds were sown in seedbeds containing peat-moss. The seedbeds were kept in a greenhouse from Mohamed I University in Nador (Morocco) and irrigated with tap water for 7 weeks, and then the plants were transferred to 1.2 L pot (1 plant per pot) containing peat-moss and kept in a greenhouse. These plants were irrigated with tap water for 1 week and then with either 100 mL tap water or 100 mL of 125 mM NaCl three times every week for 16 weeks.
Plant analysis
Plant materials were gently removed from their substrate at 17 weeks after transplanting. The roots, leaflets, stems, and fruit were rinsed three times in distilled water after decontamination with nonionic detergent and then blotted on filter paper (Wolf 1982). Then, samples were dried in a forced air oven at 70 °C for 48 h.
Glucose and proline determination
Samples of 0.5 g of plant tissues were crushed in 5 mL 95% (v/v) ethanol. The insoluble fraction of the extract was washed twice with 5 mL of 70% ethanol. All soluble fractions were centrifuged at 3500 × g for 10 min. The supernatants were collected and stored at 4 °C for glucose and proline determinations (Irigoyen and Emerich 1992). Glucose concentration was determined spectrophotometrically at 650 nm using the colorimetric assay with the anthrone reagent (Irigoyen and Emerich 1992). The free proline content was measured spectrophotometrically at 515 nm by the method of Paquin and Lechasseur (1979).
Soluble-protein determination
Fresh plant samples (0.5 g) were crushed with cold phosphate buffer (50 mM KH2PO4, pH 7.0) and centrifuged at 12 000 × g for 15 min. The resulting supernatant was used for the determination of soluble proteins using Bradford G-250 reagent (Bradford 1976). The results are expressed as mg bovine serum albumin/g of fresh weight.
Fruit yield and quality
At the corresponding ripening stage, 16 weeks after transplanting (at full maturity stage with an orange-red to red color), all fruits were collected and weighed to determine the yield and part of samples was immediately used to determine Total soluble solid (TSS) and pH (Papadaki et al., 2017; Baghour et al., 2019; Maach et al., 2020). For these determinations, we used 5 repetitions with two plants for each treatment (10 plants for each treatment).
TSS was measured in fruits at full maturity stage with an organe-red to red color as oBrix in a few drops of the juice of mature fruit using a digital refractometers (ATAGO Co., Ltd., Tokoyo, Japan). Tests for pH were performed on homogenate of fully mature fruit using a portable pH meter.
Statistics
All data in this report were obtained from an experiment with three to five repetition for each parameter. Statistical analyses were performed with Statgraphics Plus (Statistical Graphics Corp, StatPoint Inc., Herndon, VA). Analysis of variance (ANOVA) was used to assess difference between treatments and significance level was determined at P ≤ 0.05. Significant differences according to the Duncan’s multiple range test (DMRT) are indicated with different letters in the figures and tables.
Results
To study the effects of the co-overexpression of SlSOS2 and LeNHX2 on the plant tolerance towards NaCl, we measured the fresh weights of roots, stems and leaves of wild type and transgenic plants grown under control conditions (0 mM NaCl) and in the presence of 125 mM NaCl (Fig. 1a, b). Under control conditions (no NaCl added to the culture), our data demonstrate that plants co-overexpressing SlSOS2 and LeNHX2 had significantly higher fresh weights of roots, stems and leaves (P < 0.01). Treatments with 125 mM NaCl reduced roots, stems and leaves fresh weight of wild-type, while transgenic plants expressing either LeNHX2 or SlSOS2 or both significantly showed significant increase in roots, stems and leaves fresh weight (P < 0.001; Fig. 1a, b). Phenotypic evaluation of transgenic lines co-expressing either LeNHX2 or SlSOS2 was also performed in plants grown in the absence (Fig. 1b) or the presence (Fig. 1c) of 125 mM NaCl. Under saline conditions, all transgenic plants, especially those co-expressing LeNHX2 and SlSOS2, showed a better growth compared to untransformed plants.
a Fresh Weight of wild-type, single and double transgenic plants overexpressing LeNHX2 and SlSOS2 grown either under the absence (empty bars) or the presence of NaCl (black bars) of 125 mM NaCl. Representative images of WT, single and double transgenic plants grown in the absence (b) or the presence (c) of under 125 mM NaCl. Means that have different letters at the top of each bar are significantly different at P < 0.05 according to Duncan’s multiple range test
The glucose contents were measured in leaves of WT and transgenic lines overexpressing either LeNHX2 or SlSOS2 or both grown in the presence or in the absence of NaCl (Fig. 2a). Our results showed that under the normal conditions, plants co-overexpressing LeNHX2 and SlOS2 accumulated 80% more glucose in leaves compared to the WT and single transgenic lines (P < 0.001). However, under saline condition, all transgenic plants showed an increased level of this carbohydrate. Interestingly, plants simultaneously overexpressing LeNHX2 and SlOS2 accumulated almost three times more glucose compared to wild type plants under NaCl treatment.
Effect of LeNHX2 and SlSOS2 co-overexpression on glucose (a), proline (b) and protein content (c) of tomato plants. WT and LeNXH2, SlSOS2 and LeNXH2 x SlSOS2 plants were cultivated in peat-moss for 17 weeks in the absence (empty bars) or the presence (black bars) of 125 mM NaCl. Means that have different letters at the top of each bar are significantly different at P < 0.05 according to Duncan’s multiple range test
In relation to the proline accumulation, our data demonstrate that NaCl treatments significantly increased proline levels in wild-type and transgenic lines (P < 0.001; Fig. 2b). Furthermore, all transgenic plants, especially those overexpressing LeNHX2, accumulated higher contents of proline in either normal or saline conditions.
Regarding the effect of LeNHX2 and SlSOS2 co-overexpression on protein content in tomato leaves, our results showed that under normal conditions single transgenic plants that overexpress LeNHX2 present the highest protein levels (P < 0.01; Fig. 2c). Nonetheless, under NaCl stress, increase in protein content was detected in plants overexpressing LeNHX2 alone or in double transgenic lines, compared with wild-type and single transgenic plants overexpressing SlSOS2.
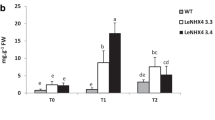
We have measured fruit production (Fig. 3a), total soluble solids (Fig. 3b) and pH (Fig. 3c) in NaCl-treated and untreated wild-type and transgenic plants. Interestingly, the effect of LeNHX2 and SlSOS2 co-overexpression on fruit yield (g/plant) is particularly evident under salt treatment (P < 0.001).
Effect of LeNHX2 and SlSOS2 co-overexpression on yield (a), TSS (b) and pH (c) of tomato plants. WT and LeNXH2, SlSOS2 and LeNXH2 x SlSOS2 plants were cultivated in peat-moss for 17 weeks in the absence (empty bars) or the presence (black bars) of 125 mM NaCl. Means that have different letters at the top of each bar are significantly different at P < 0.05 according to Duncan’s multiple range test
Under control conditions, (no NaCl added), the highest TSS levels were observed in wild-type plants (Fig. 3b). Treatment with NaCl stimulated an increase of TSS values in all transgenic plants (P < 0.001), reaching the highest value in fruits of double transgenic plants with an increase of 39% relative to WT fruits.
In relation to fruit pH, our results showed no significant differences between NaCl-untreated wild-type and transgenic plants (Fig. 3c). Relative to the fruit pH of untreated tomato plants, NaCl application significantly increased the pH of fruits from LeNHX2, SlSOS2 and LeNXH2SlSOS transgenic plants while decreased the pH of fruits of wild-type plants (P < 0.001).
Discussion
The effect of overexpression of ion transport genes on plant's salt tolerance could be further enhanced when more than one of these genes was overexpressed. It has been reported in Arabidopsis that overexpression of the vacuolar Na+/H+ antiporter AtNHX1 or the plasma membrane Na+/H+ antiporter SOS1 improve salt tolerance in transgenic plants, but the improved salt tolerance is limited to NaCl concentrations lower than 200 mM (Pehlivan et al. 2016). LeNHX2 is a K+, Na+/H+ antiporter and therefore participates in Na+ accumulation in cell compartments when plants are grown under saline conditions. Intracellular Na+ and K+ accumulation could promote water influx to cell compartments and thus contribute to the increased fresh weight of transgenic plants grown in the presence of NaCl. Plants co-overexpressing the LeNHX2 and SlSOS2 genes showed an improved growth performance with higher root, stem, and leaf fresh weights under salt stress compared to WT and single transgenic plants (Fig. 1a–c). These results are in accordance with previous studies on the effects of co-overexpression of several ion transport genes on salt tolerance and biomass production in Arabidopsis, cotton and tomato (Zhao et al. 2006; Shen et al. 2015). Shen et al. (2015) have reported that transgenic cotton plants expressing the Arabidopsis vacuolar Na+/H+ antiporter gene AtNHX1 and AVP1 produced significantly higher biomass compared to WT and single transgenic plants under salt stress conditions. Gouiaa et al. (2012) showed that tobacco plants co-expressing the wheat Na+/H+ antiporter TNHXS1 and H+-pyrophosphatase TVP1 produced significantly higher number of leaves compared to monotransgenic lines and the WT plants. Moreover, a possible explanation of the increased fresh weight of double transgenic plants under control (no NaCl added) conditions could be related to a better K+ nutrition of double transgenic plants allowing for a better plant growth. All these reports indicate that expression of multiple salt-related genes could further improve salt tolerance and enhance biomass production.
Soluble sugars have been reported to increase in leaves under salinity conditions in order to maintain the osmotic adjustment in the plant (Yu et al. 2015; Pérez-Jiménez and Pérez-Tornero 2020; Shafiq et al. 2020). Our data indicate that salinity stress enhances leaf glucose accumulation both in WT and transgenic plants (Fig. 2a), and this increase is more pronounced in the transgenic lines. These results are in agreement with those reported in sugarcane by Gandonou et al. (2011) and in Populus by Watanabe et al. (2000) showing an increased level of soluble sugars as a result of salt stress enhances the plant tolerance to salt stress.
Proline is an osmoprotective agent and an important compatible solute that accumulates in plants under different types of stresses such as drought, cold, heat and salinity (Maach et al. 2020). The accumulation of this amino acid under salinity stress is an important mechanism in maintenance of the osmotic adjustment and salt tolerance (Kahlaoui et al. 2018; Ahanger et al. 2020). The higher accumulation of proline in all transgenic tomato plants analyzed in this work (Fig. 2b), especially in plants overexpressing LeNHX2 together with SlSOS2 could represent an adaptive mechanism to salt stress. Our results shows agreement with Bhaskaran and Savithramma (2011) who observed a significant increase in proline content in transgenic tomato plants co-expressing Pennisetum glaucum PgNHX1 and Arabidopsis AVP1 compared to WT and single transgenic lines expressing either PgNHX1 or AVP1. Contrary to what was expected, proline and protein content in the co-overexpressing plants are lower compared to those overexpressing LeNHX2, under both normal and saline conditions. These results could be explained by the interaction between SlSOS2 and LeNHX2 proteins. More recently, Dong et al (2021) have reported that PgNHX proteins shared the same putatively interactive protein AVP1, HKT1, SOS2 and SOS3. Similarly, Pehlivan et al. (2016) have reported that although the SOS2 can regulate AtNHX1’s activity, there does not appear to be a synergy between overexpression of AtNHX1 and SOS3.
Protein accumulation has been reported to play an important role for cell survival and membrane stabilization under saline conditions (Goudarzi and Pakniyat 2009). The increase in the soluble protein content can be the result of the enhanced de novo synthesis of proteins for cell protection (Teixeira et al. 2005). In our study, the soluble proteins significantly increased in single and double transgenic plants overexpressing LeNHX2 alone or together with SlSOS2 (Fig. 2c). Previously, Gandonou et al. (2011) and Kahlaoui et al. (2018) showed that the content of soluble proteins increases in leaves and roots of salt tolerant cultivars whereas it decreases in the sensitive ones, suggesting that these components could play a key role in sugarcane and tomato salt tolerance. All these results are consistent with the previous findings that salt resistant plants are able to respond and adapt to salt stress through the synthesis of stress-induced proteins as a tolerance mechanism (Veeranagamallaiah et al. 2007; Maach et al. 2020).
The high expression level of some NHX isoforms in known sinks for potassium like fruits or flowers, where growth is dependent on cell expansion, point to a role of these isoforms in vacuolar K+ accumulation (Rodríguez-Rosales et al. 2009). Silencing of the gene in tomato has a severe effect on growth and fruit and seed Production (Rodríguez-Rosales et al. 2008). It has been shown that potassium plays a vital role in photoassimilate transport from source to sink (Schobert and Tschesche 1978; Lalonde et al. 2003) and thus improved potassium homeostasis could enhance fruit production. In this study, tomato plants overexpressing both LeNHX2 and SlSOS2 showed a better yield compared to plants overexpressing only one gene (Fig. 3a). Our results have been supported by the observations of Pehlivan et al. (2016) who reported that co-overexpression of AtNHX1 and SOS1 could significantly reduce yield loss in Arabidopsis plants grown under 250 mM NaCl. Moreover, Shen et al. (2015) observed an increase in yield of transgenic cotton plants co-expressing the Arabidopsis vacuolar Na+/H+ antiporter gene AtNHX1 and H+-pyrophosphatase gene AVP1 when cultivated under salt stress. Our results clearly show that plants ovexpressing LeNHX2, SlSOS2 or both genes grow better and produce more fruits than WT plants both under control and NaCl irrigation conditions (Fig. 1a, 3a). In addition, results in Figs. 1a and 3a show that compared to plants irrigated with water, adding 125 mM NaCl to the irrigation water improves growth of single and double transgenic plants without affecting fruit yield. Furthermore, the co-overexpression of LeNHX2 and SlSOS2 improves fruit quality of tomato plants grown under saline conditions. All these results support the potential biotechnological interest of overexpressing LeNHX2, SlSOS2 or both to enhance tomato fruit yield by plants cultivated either under saline or not saline irrigation conditions. In addition, TSS is an important quality criterion reflecting concentration of sugars in fruits (Flores et al. 2010). Shabani et al. (2013) have reported in tomato that salt treatment decreased fruit juice pH and increased TSS concentrations. We have observed that high level of NaCl treatment reduced TSS and pH value in WT plants (Fig. 3b, c), while transgenic plants, especially those co-overexpressing LeNHX2 and SlSOS2 reached higher levels of these two parameters suggesting that the co-expression of these two genes have improved fruit quality. The high levels of TSS observed in fruits of transgenic lines co-overexpressing LeNHX2 and SlSOS2 is probably due to a high transport of sugars from leaves to fruits. In this respect, Yin et al. (2010) reported that salinity stress enhances carbohydrates accumulation from the source leaves to the fruit of tomato plants. In relation to the pH of fruit juice, as reported by Mitchell et al. (1991) and Coban et al. (2020), we have observed that salinity significantly reduced the pH of fruits from wild-type plants. However, transgenic plants, especially those co-overexpressing LeNHX2 and SlSOS2, showed an increase in pH values of fruits in plants cultivated under NaCl stress. Similarly, Maach et al. (2020) have reported that the overexpression of LeNHX4 antiporter improved the fruit quality in tomato by TSS and pH under salt stress.
Conclusions
In this work, we have demonstrated that co-overexpression of the antiporter LeNHX2 and the regulatory kinase SlSOS2 confer salt tolerance and improves fruit yield and quality by increasing fruit pH and total soluble solid. Proline, glucose and protein accumulation in leaves of transgenic plants under either normal or stress conditions could be related to the better salt tolerance of these plants relative to WT tomato. Finally, our results suggest that multiple-gene co-expression results in higher salt tolerance.
References
Ahanger MA, Mir RA, Alyemeni MN, Ahmad P (2020) Combined effects of brassinosteroid and kinetin mitigates salinity stress in tomato through the modulation of antioxidant and osmolyte metabolism. Plant PhysiolBiochem 147:31–42. https://doi.org/10.1016/j.plaphy.2019.12.007
Apse MP, Aharon GS, Snedden WA, Blumwald E (1999) Salt tolerance conferred by overexpression of a vacuolar Na+/H+antiport in Arabidopsis. Science 285(5431):1256–1258
Baghour M, Gálvez FJ, Sánchez ME, Aranda MN, Venema K, Rodríguez-Rosales MP (2019) Overexpression of LeNHX2 and SlSOS2 increases salt tolerance and fruit production in double transgenic tomato plants. Plant PhysiolBiochem 135:77–86. https://doi.org/10.1016/j.plaphy.2018.11.028
Bhaskaran S, Savithramma DI (2011) Co-expression of Pennisetum glaucum vacuolar Na+/H+ antiporter and Arabidopsis H+-pyrophosphatase enhances salt tolerance in transgenic tomato. J Exp Bot 62:5561–5570. https://doi.org/10.1002/yea.3450
Bradford N (1976) A rapid and sensitive method for the quantitation microgram quantities of a protein isolated from red cell membranes. Anal Biochem 72:248–254. https://doi.org/10.1016/0003-2697(76)90527-3
Cagnac O, Baghour M, Jaime-Pérez N, Aranda-Sicilia MN, Sánchez-Romero ME, Rodríguez-Rosales MP, Venema K (2020) Deletion of the N-terminal domain of the yeast vacuolar (Na+, K+)/H+antiporter Vnx1p improves salt tolerance in yeast and transgenic Arabidopsis. Yeast 37:173–185. https://doi.org/10.1002/yea.3450
Coban A, Akhoundnejad Y, Dere S, Dasgan HY (2020) Impact of salt-tolerant rootstock on the enhancement of sensitive tomato plant responses to salinity. HortScience 55:1–5. https://doi.org/10.21273/HORTSCI14476-19
Dong J, Liu C, Wang Y, Zhao Y, Ge D, Yuan Z (2021) Genome-wide identification of the NHX gene family in Punica granatum L. and their expressional patterns under salt stress. Agronomy 11:264. https://doi.org/10.3390/agronomy11020264
Flores FB, Sanchez-Bel P, Estan MT, Martinez-Rodriguez MM, Elena Moyano E, Morales B, Campos JF, Garcia-Abellan JO, Egea MI, Fernandez-Garcia N, Romojaro F, Bolarín MC (2010) The effectiveness of grafting to improve tomato fruit quality. SciHortic 125:211–217. https://doi.org/10.1016/j.scienta.2010.03.026
Gálvez FJ, Baghour M, Hao G, Cagnac O, Rodríguez-Rosales MP, Venema K (2012) Expression of LeNHX isoforms in response to salt stress in salt sensitive and salt tolerant tomato species. Plant PhysiolBiochem 51:109–115. https://doi.org/10.1016/j.plaphy.2011.10.012
Gandonou CB, Bada F, Abrini J, Skali-Senhaji N (2011) Free proline, soluble sugars and soluble proteins concentration as affected by salt stress in two sugarcane (Saccharum sp.) cultivars differing in their salt tolerance. Int J BiolChemSci 5:2441–2453. https://doi.org/10.4314/ijbcs.v5i6.23
Goudarzi M, Pakniyat H (2009) Salinity causes increase in proline and protein contents and peroxidase activity in wheat cultivars. J ApplSci 9:348–353. https://doi.org/10.3923/jas.2009.348.353
Gouiaa S, Khoudi H, Leidi EO, Pardo JM, Masmoudi K (2012) Expression of wheat Na+/H+antiporter TNHXS1 and H+-pyrophosphataseTVP1 genes in tobacco from a bicistronic transcriptional unit improves salt tolerance. Plant MolBiol 79:137–155. https://doi.org/10.1007/s11103-012-9901-6
Hanana M, Cagnac O, Zarrouk M, Blumwald E (2009) Rôlesbiologiques des antiportsvacuolaires NHX: acquis et perspectives d’améliorationgénétique des plantes. Botanique 87:1023–1035. https://doi.org/10.1139/B09-073
Huertas R, Olias R, Eljakaoui Z, Galves FJ, Jun Li, De Morales P, Belver A, Rodriguez-Rosales MP (2012) Overexpression of SlSOS2 (SlCIPK24) confers salt tolerance to transgenic tomato. Plant Cell Environ 35:1467–1482. https://doi.org/10.1111/j.1365-3040.2012.02504.x
Huertas R, Rubio L, Cagnac O, García-Sánchez MJ, Alché JD, Venema K, Fernández JA, Rodríguez-Rosales MP (2013) The K+/H+antiporter LeNHX2 increases salt tolerance by improving K+ homeostasis in transgenic tomato. Plant Cell Environ 36:2135–2149. https://doi.org/10.1111/pce.12109
Irigoyen JI, Emerich DW (1992) Water stress induced changes in concentration of praline and total soluble sugars in modulates alfalfa (Medicago sativa) plants. Physiol Plant 84:55–60. https://doi.org/10.1111/j.1399-3054.1992.tb08764.x
Ishitani M, Liu J, Halfter U, Kim C-S, Shi W, Zhu J-K (2000) SOS3 function in plant salt tolerance requires N-myristoylation and calcium binding. Plant Cell 12:1667–2167. https://doi.org/10.1105/tpc.12.9.1667
Kahlaoui B, Hachicha M, Misle E, Fidalgo F, Teixeira J (2018) Physiological and biochemical responses to the exogenous application of proline of tomato plants irrigated with saline water. J Saudi SocAgricSci 17:17–23. https://doi.org/10.1016/j.jssas.2015.12.002
Lalonde S, Tegeder M, Throne-Holst M (2003) Phloem loading and unloading of sugars and amino acids. Plant Cell Environ 26(1):37–56. https://doi.org/10.1046/j.1365-3040.2003.00847.x
Li L, Zhang H, Zhang L, Zhou Y, Yang R, Ding C, Wang X (2014) The physiological response of Artemisia annua L. to salt stress and salicylic acid treatment. PhysiolMolBiol Plants 20(2):161–169. https://doi.org/10.1007/s12298-014-0228-4
Liu J, Ishitani M, Halfter U, Kim C-S, Zhu J-K (2000) The Arabidopsis thaliana SOS2 gene encodes a protein kinase that is required for salt tolerance. ProcNatlAcadSci USA 97:3730–3734. https://doi.org/10.1073/pnas.97.7.3730
Maach M, Baghour M, Akodad M, Gálvez FJ, Sánchez ME, Aranda MN, Venema K, Rodríguez-Rosales MP (2020) Overexpression of LeNHX4 improved yield, fruit quality and salt tolerance in tomato plants (Solanum lycopersicum L.). MolBiol Rep 47:4145–4153. https://doi.org/10.1007/s11033-020-05499-z
Mitchell JP, Shennan C, Grattan SR, May DM (1991) Tomato fruit yields and quality under water deficit and salinity. J Am Soc Hortic Sci 116:215–221. https://doi.org/10.21273/JASHS.116.2.215
Olías R, Eljakaoui Z, Li J, De Morales PA, Marin-Manzano MC, Pardo JM, Belver A (2009) The plasma membrane Na+/H+ antiporter SOS1 is essential for salt tolerance in tomato and affects the partitioning of Na+ between plant organs. Plant Cell Environ 32:904–916. https://doi.org/10.1111/j.1365-3040.2009.01971.x
Papadaki AM, Bletsos FA, Eleftherohorinos IG, Menexes G, Lagopodi AL (2017) Effectiveness of seven commercial rootstocks against verticillium wilt and their effects on growth, yield, and fruit quality of tomato. Crop Protec 102:25–31. https://doi.org/10.1016/j.cropro.2017.08.006
Paquin R, Lechasseur P (1979) Observations suruneméthode de dosage de la prolinelibredans les extraits de plantes. Can J Bot 57:1851–1854. https://doi.org/10.1139/b79-233
Pehlivan N, Sun L, Jarrett P, Yang X, Mishra N, Chen L, Kadioglu SG, Zhang H (2016) Co-overexpressing a plasma membrane and a vacuolar membrane sodium/proton antiporter significantly improves salt tolerance in transgenic Arabidopsis plants. Plant Cell Physiol 57:1069–1084. https://doi.org/10.1093/pcp/pcw055
Pérez-Jiménez M, Pérez-Tornero O (2020) Improved salt-tolerance in Citrus macrophylla mutant rootstocks. Sci Hortic 259:108815. https://doi.org/10.1016/j.scienta.2019.108815
Qiu QS, Guo Y, Dietrich MA, Schumaker KS, Zhu JK (2002) Regulation of SOS1, a plasma membrane Na+/H+ exchanger in Arabidopsis thaliana, by SOS2 and SOS3. ProcNatlAcadSci USA 99:8436–8441. https://doi.org/10.1073/pnas.122224699
Qiu QS, Guo Y, Quintero FJ, Pardo JM, Schumaker KS, Zhu JK (2004) Regulation of vacuolar Na+/H+ exchange in Arabidopsis thaliana by the salt-overly-sensitive (SOS) pathway. J BiolChem 279:207–215. https://doi.org/10.1074/jbc.M307982200
Quintero FJ, Ohta M, Shi HZ, Zhu JK, Pardo JM (2002) Reconstitution in yeast of the Arabidopsis SOS signaling pathway for Na+ homeostasis. ProcNatlAcadSci USA 99:9061–9066. https://doi.org/10.1073/pnas.132092099
Rasheed R, Wahid A, Farooq M, Hussain I, Basra SM (2011) Role of proline and glycinebetainepretreatments in improving heat tolerance of sprouting sugarcane (Saccharum sp.) buds. Plant Growth Regul 65:35–45. https://doi.org/10.1007/s10725-011-9572-3
Rodríguez-Rosales MP, Jiang X, Gálvez FJ, Aranda MN, Cubero B, Venema K (2008) Overexpression of the tomato K+/H+antiporter LeNHX2 confers salt tolerance by improving potassium compartmentalization. New Phytol 179:366–377. https://doi.org/10.1111/j.1469-8137.2008.02461.x
Rodríguez-Rosales MP, Gálvez FJ, Huertas R, Aranda MN, Baghour M, Cagnac O, Venema K (2009) Plant NHX cation/proton antiporters. Plant Signal Behav 4:265–276. https://doi.org/10.4161/psb.4.4.7919
Schobert B, Tschesche H (1978) Unusual solution properties of proline and its interaction with proteins. BiohemBiophysActa 541:270–277
Shabani ES, Tabatabaei SJ, Bolandnazar S (2013) Yield, photosynthetic efficiency and quality parameters of cherry tomato as affected by Ca2+ and K+ under NaCl salinity. Int J Agric Crop Sci 5:1280–1288
Shafiq F, Iqbal M, Ashraf MA, Ali M (2020) Foliar applied fullerol differentially improves salt tolerance in wheat through ion compartmentalization, osmotic adjustments and regulation of enzymatic antioxidants. PhysiolMolBiol Plants 26(3):475–487. https://doi.org/10.1007/s12298-020-00761-x
Shen G, Wei J, Qiu X, Hu R, Kuppu S, Alud D, Blumwald E, Gaxiola R, Pyton P, Zhang H (2015) Co-overexpression of AVP1 and AtNHX1 in cotton further improves drought and salt tolerance in transgenic cotton plants. Plant MolBiol Rep 33:167–177. https://doi.org/10.1007/s11105-014-0739-8
Shi H, Quintero FJ, Pardo JM, Zhu JK (2002) The putative plasma membrane Na+/H+antiporter SOS1 controls long distance Na+ transport in plants. Plant Cell 14:465–477. https://doi.org/10.1105/tpc.010371
Silva-Ortega CO, Ochoa-Alfaro AE, Reyes-Agüero JA, Aguado-Santacruz GA, Jiménez-Bremont JF (2008) Salt stress increases the expression of P5CS gene and induces proline accumulation in cactus pear. Plant PhysiolBiochem 46:82–92. https://doi.org/10.1016/j.plaphy.2007.10.011
Teixeira J, Pereira S, Cánovas F, Salema R (2005) Glutamine synthetase of potato (Solanum tuberosum L. cv. Desiree) plants: cell-and organ-specific expression and differential developmental regulation reveal specific roles in nitrogen assimilation and mobilization. J Exp Bot 412:663–671. https://doi.org/10.1093/jxb/eri042
Teka TA (2013) Analysis of the effect of maturity stage on the postharvest biochemical quality characteristics of tomato (Lycopersicon esculentum MILL.) fruit. Int Res J Pharm App Sci 3:180–186
Veeranagamallaiah G, Chandraobulreddy P, Jyothsnakumari G, Sudhakar C (2007) Glutamine synthetase expression and pyrroline-5-carboxylate reductase activity influence proline accumulation in two cultivars of foxtail millet (Setaria italica L.) with differential salt sensitivity. Environ Exp Bot 60:239–244. https://doi.org/10.1016/j.envexpbot.2006.10.012
Venema K, Quintero FJ, Pardo JM, Donaire JP (2002) The Arabidopsis Na+/H+ exchanger AtNHX1 catalyzes low affinity Na+ and K+ transport in reconstituted liposomes. J BiolChem 277:2413–2418. https://doi.org/10.1074/jbc.M105043200
Watanabe S, Kojima K, Ide Y, Sasaki S (2000) Effects of saline and osmotic stress on proline and sugar accumulation in Populus euphratica in vitro. Plant Cell Tiss Org 63:199–206. https://doi.org/10.1023/A:1010619503680
Wolf B (1982) A comprehensive system of leaf analyses and its use for diagnosing crop nutrient status. Comm Soil Sci Plant Anal 13:1035–1059. https://doi.org/10.1080/00103628209367332
Wu N, Li Z, Wu F, Tang M (2016) Comparative photochemistry activity and antioxidant responses in male and female Populus cathayana cuttings inoculated with arbuscularmycorrhizal fungi under salt. Sci Rep 6:37663. https://doi.org/10.1038/srep37663
Yang Q, Chen ZZ, Zhou XF, Yin HB, Li X, Xin XF, Hong XH, Zhu JK, Gong Z (2009) Overexpression of SOS (salt overlay sensitive) genes increases salt tolerance in transgenic Arabidospsis. Mol Plant 2:22–31. https://doi.org/10.1093/mp/ssn058
Yin YG, Kobayashi Y, Sanuki A, Kondo S, Fukuda N, Ezura H, Sugaya S, Matsukura C (2010) Salinity induces carbohydrate accumulation and sugar-regulated starch biosynthetic genes in tomato (Solanum lycopersicum L. cv. ‘Micro-Tom’) fruits in an ABA-and osmotic stress-independent manner. J Exp Bot 61(2):563–574. https://doi.org/10.1093/jxb/erp333
Yu J, Sun L, Fan N, Yang Z, Huang B (2015) Physiological factors involved in positive effects of elevated carbon dioxide concentration on Bermuda grass tolerance to salinity stress. Environ Exp Bot 115:20–27. https://doi.org/10.1016/j.envexpbot.2015.02.003
Zhang H, Blumwald E (2001) Transgenic salt-tolerant tomato plants accumulate salt in foliage but not in fruit. Nat Biotechnol 19:765–768. https://doi.org/10.1038/90824
Zhao FY, Zhang XJ, Li PH, Zhao YX, Zhang H (2006) Co-expression of the Suaeda salsa SsNHX1 and Arabidopsis AVP1 confer greater salt tolerance to transgenic rice than the single SsNHX1. Mol Breed 17:341–353. https://doi.org/10.1007/s11032-006-9005-6
Zhu JK (2002) Salt and drought stress signal transduction in plants. Annu Rev Plant Biol 53:247–273. https://doi.org/10.1146/annurev.arplant.53.091401.143329
Acknowledgements
The authors wish to thank Professor Dr. Boujamaa El Kouy from the English Studies Department at Multidisciplinary Faculty of Nador for this careful reading of the paper and improving the quality of English language of our manuscript.
Funding
This work was supported by grants from National Centre for Scientific and Technical Research-CNRST and Minister for Higher Education, Scientific Research and Executive Training—Morocco (PPR/2015/21). Consejería de Economía, Innovación, Ciencia y Empresa, Junta de Andalucía, Spain (CVI-7558 to MPRR), Spanish Ministry of Economy and Competitiveness and Agencia Estatal de Investigación (BIO2015-65056-P, BIO2016-81957-REDT/AEI and Programa I-COOPB + 2013 Ref: COOPB20053).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This manuscript does not imply human participants or studies on animals.
Informed consent
Not required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Maach, M., Rodríguez-Rosales, M.P., Venema, K. et al. Improved yield, fruit quality, and salt resistance in tomato co-overexpressing LeNHX2 and SlSOS2 genes. Physiol Mol Biol Plants 27, 703–712 (2021). https://doi.org/10.1007/s12298-021-00974-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12298-021-00974-8