Abstract
High temperature strongly hampers the plant growth particularly at early growth stages. In this study, changes in some physiological and anatomical characteristics and possibility of mitigating the adversities of heat stress by soaking sugarcane nodal buds in 20 mM proline and glycinebetaine (GB) solutions have been explored. Heat stress reduced the rate of bud sprouting nonetheless soaking the setts in proline followed by GB was beneficial. In addition, heat stress reduced the bud fresh and dry weights, generated H2O2, reduced the tissue levels of K+ and Ca2+, while increased the osmolytes synthesis in a time course manner. Heat stress also delayed the emergence and expansion of new bud leaves, by restricting the number and area of mesophyll cells. It also caused poor and aberrant development and diffused appearance of mesophyll cells and vascular bundles in the bud leaves. However, soaking of buds in proline and GB solutions substantially reduced the H2O2 production, improved the accumulation of soluble sugars and protected the developing tissues from heat stress effects; although proline was more effective than GB. Correlations of various attributes indicated that soaking in GB and proline restricted the H2O2 generation, improved K+ and Ca2+ contents, and increased the concentrations of free proline, GB and soluble sugars eventually improving the heat tolerance of buds. Cost-benefit analysis showed that, considering increase in sprouting of buds, soaking in 20 mM solution of both osmoprotectants is economical.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Heat stress results from temperatures high enough to damage the plant tissues. Although variable for different plant species, temperatures in the range of 35–45°C produce heat stress effects on tropical plants (Hall 1992; Mahmood et al. 2010; Ulukan 2011). Heat sensitive plants show a great deal of labiality of the cellular membranes, thereby disrupting the vital cellular phenomena. As a result of aberrant metabolism, the production of toxic metabolites and reactive oxygen species (ROS) takes place in the injured cells (Wahid et al. 2007).
The heat stress tolerance is an intricate phenomenon involving an array of physiological and biochemical processes at whole plant as well as molecular levels (Tiroli-Cepeda and Ramos 2010). These processes inlcude curtailed water loss by partial stomatal closure, enhanced water uptake with the development of prolific root systems, and synthesis and accumulation of osmolytes (Wahid et al. 2007; Yousfi et al. 2010). ROS scavenging, stabilization of biological membranes and expression of stress proteins are amongst the vital mechanisms responsible for stress tolerance in plants (Bohnert and Sheveleva 1998; Wahid and Close 2007; Al-Ghamdi 2009).
Germination and seedling emergence from seeds and planting materials are highly sensitive to thermal stress (Grass and Burris 1995; Egli et al. 2005; Farooq et al. 2009). Heat stress seriously reduces the germination and early seedling growth in a number of plant species including sugarcane (Wahid et al. 2008, 2010). However, plant age and the duration of exposure to heat stress are important (Wahid et al. 2007). In extreme cases, heat stress accelerates the senescence, reduces crop productivity (Porter 2005) and sometimes leads to plant death (Sharma et al. 2005). The visible symptoms of heat injury include leaf rolling and folding, dehydration, chlorosis, tip burning etc. (Vollenweider and Günthardt-Goerg 2005).
Increased heat stress leads to the overproduction and accumulation of various organic and inorganic osmolytes. These osmolytes protect the plants from stresses by cellular osmotic adjustment, detoxification of ROS, protection of biological membranes and stabilisation of enzymes/proteins (Bohnert and Jensen 1996; Verbruggen and Hermans 2008). Although heat sensitive plants apparently lack this ability, heat tolerance in such plants can be improved by exogenous application of such osmoprotectants and nutrients (Sakamoto and Murata 2002; Jain et al. 2009; Rasheed et al. 2010). Seed pretreatments with the osmoprotectants such as proline and GB have proven beneficial in improving germination and growth of seedlings under optimal and sub-optimal conditions (Wahid and Shabbir 2005; Song et al. 2005; Ashraf and Foolad 2007; Farooq et al. 2008). However, for efficient induction of heat stress tolerance in sensitive species, the effective concentrations of the osmoprotectants to be applied, stage of plant growth, and protocols for the induction of stress tolerance are the key steps to be carefully followed.
Sugarcane is a premier sugar crop the world over. Although a tropical plant species and requires relatively higher temperatures for growth, sugarcane shows heat sensitivity beyond 36°C as evident from its diminished growth and water relations (Wahid et al. 2010). Despite this, heat tolerance mechanisms are relatively less understood in sugarcane. The available studies show that canopy temperature is an important factor in the growth and production of new leaves in sugarcane (Robertson et al. 1998). Heat stress applied to sugarcane reduced the Hill-reaction, chlorophyll fluorescence and electron transport at PSII (Ebrahim et al. 1998). Wahid and Close (2007) reported that, despite ample water supply to roots, water potential and its components were severely affected in sugarcane leaves under heat stress. As a heat tolerance strategy, sugarcane showed the synthesis of primary and secondary metabolites. Of the primary metabolites, free proline, GB and soluble sugars while among the secondary metabolites, carotenoids, soluble phenolics and anthocyanins had close association to heat resistance (Wahid 2007). Enzymatic antioxidants also combat heat stress induced oxidative damage in sugarcane (Jain et al. 2007).
In Pakistan sugarcane is normally propagated from nodal cuttings (setts). Sprouting of sugarcane setts is adversely affected by prevailing heat stress (Moore 1987; Wahid et al. 2010). Understanding the changes produced by heat stress and finding strategies to improve heat tolerance is, therefore, imperative. The sugarcane buds present a unique system to study the development and differentiation from immature to mature state, especially under adverse conditions like heat stress. The available literature shows that sugarcane buds have been rarely investigated for physiological and histological changes and the effectiveness of osmoprotectants in improving heat tolerance at sprouting. It is predicted that soaking with GB and proline can bring profound changes and reduce the detrimental effects of heat stress on the expanding regions of sugarcane during sprouting. This study was, therefore, undertaken to monitor the bud sprouting and determine the effectiveness of proline and GB in improving heat tolerance in sugarcane buds.
Materials and methods
Bud material
Setts of sugarcane (Saccharum sp. cv. HSF-240) with healthy looking buds were obtained from Sugarcane Research Institute (SRI), Ayub Agricultural Research Institute (AARI), Faisalabad, Pakistan. Immature buds of similar age were selected from upper five nodes of culm.
Pretreatment and sprouting of bud
Single noded setts were pretreated with water and 20 mM solutions (optimized from a range 5–40 mM in a series of experiments) each of proline and GB at 25°C for 8 h. Two experiments were conducted. In long term (3 weeks) experiment, 20 buds, per replication, were sprouted to record the data for the final sprouting percentage. In short term experiment, the changes in physiological and histological parameters were investigated. In both experiments, 20 bud chips were separately kept in a double layer of moistened towel cloth in plastic trays. Then trays were transferred to separated growth chambers (FLI, Eyelatron, Rikkakai, Japan) and sprouted at 25°C (control) and 42°C (heat stress). High temperature treatment was induced by gradually raising the growth chamber temperature from 25 to 42°C in about 5 h. Design of the experiments was completely randomized with three replications.
Sett sprouting, sampling and data recording
In long term experiment, the observations were made at an interval of 3 days to record the production of roots at the node and sprouting of buds. A bud was considered sprouted with the emergence of roots around the node and greening and swelling of buds.
In short term experiment, in view of the fact that the tissues differentiation begins quite early, the buds from all treatments were harvested at 8, 16, 24, 32, 40 and 48 h after putting them to sprout. At sampling time, the sprouting buds were excised from the bud chips with a sharp razor and immediately determined for fresh weight. For taking dry weight, the excised buds were put into paper bags and placed in an oven set at 70°C for 7 days.
For the estimation of free proline, GB, soluble sugars and hydrogen peroxide (H2O2), the freshly excised bud tissue was immediately frozen and stored at −40°C until analyzed. For the analysis of free proline according to the method of Bates et al. (1973), 0.5 g of frozen fresh bud tissue was macerated in 10 mL of aqueous sulphosalicylic acid (3%, w/v), and filtered. Two mL of filtrate was mixed with 2 mL each of acid ninhydrin and glacial acetic acid and incubated at 100°C in a water bath for 1 h. The reaction was terminated in an ice bath, extracted immediately with 4 mL of toluene after vortexing for 15–20 s. The chromophore containing free proline was aspirated, added to a test tube, warmed to room temperature and the absorbance was measured at 520 nm on a spectrophotometer (Hitachi U-2001, Tokyo, Japan). Values of unknown samples were compared with standard curve prepared from a range (10 to 50 μg 2 mL−1) of proline standards, and the amount of free proline calculated.
The GB was estimated following Grieve and Grattan (1983) method. Fresh extracts of buds were prepared by vigorously shaking in 2 N H2SO4 and refrigerated as described elsewhere (Rasheed et al. 2010). These extracts were mixed with an equal volume of periodide prepared by dissolving excess of iodine in potassium iodide solution, vortexed and kept at 4°C for 16 h. The mixture was centrifuged at 10,000×g at 4°C for 15 min, and supernatant discarded. The pellet of periodide crystals was dissolved in 10 mL of 1, 2-dichloroethane, vortexed, left at room temperature for 15–20 min and absorbance of the colored solution taken at 365 nm.
As described elsewhere (Rasheed et al. 2010), to measure glucose equivalent soluble sugars, 0.1 g of frozen bud tissue was extracted overnight in 5 mL of 0.2 M phosphate buffer (pH 7) at room temperature. Next morning, 0.1 mL of the aliquot from sample was mixed with 3 mL of freshly prepared anthrone reagent and carefully vortexed. Mixture was heated at 95°C for 15 min, cooled to room temperature under running tap water, and absorbance of the colored complex was taken at 625 nm after 20 min. A standard glucose series (0–100 μg mL−1) was prepared to compute the amount of soluble sugars in the unknown samples (Yoshida et al. 1976).
For the determination of H2O2 with the method of Velikova et al. (2000), the bud tissue (0.1 g) was homogenized in a pre-chilled mortar and pestle with 1 mL 0.1% (w/v) trichloroacetic acid. The homogenate was centrifuged at 12,000×g for 15 min and 0.5 mL of supernatant was added to 0.5 mL of 10 mM potassium phosphate buffer (pH 7.0) and 1 mL 1 M potassium iodide. The supernatant was vortexed and absorbance read at 390 nm on a spectrophotometer using water as blank. The amount of H2O2 in unknown samples was derived by comparing with a standard curve prepared from standard series (0–100 μM) of H2O2.
For the determination of K+ and Ca2+ with the method of Tendon (1993), 0.5 g of the oven dried bud tissue was digested in a mixture of concentrated HNO3 and HClO4 (3:1 ratio) on a heating block by stepwise increase in temperature to 250°C. After clearing the samples (in about 1 h), the volume was made up to 50 mL with distilled water. Analysis of K+ was carried out using flame photometer (Sherwood Model 410, Cambridge), and its exact amount computed from the standard curve prepared from standard series (0–50 mg L−1) of K+ using KCl. The quantity of Ca2+ from the extracts was estimated with atomic absorption spectrophotometer (Perkin Elmer, Model AAnalyst 3000, Norwalk, Connecticut). The unknown sample values were determined by comparing with standard curve prepared from standard series (0–50 mg L−1).
Histological studies
Bud tissue processing for microtomy was done as described by Ruzin (1999). After excising from the setts, the buds were immediately fixed in formaldehyde, acetic acid, ethanol and water (FAA; 10:5:1:4) for 48 h. The tissue for section cutting was dehydrated in graded alcoholic series 50, 70, 90, 95 and 100% for 10–15 min each. The dehydrated tissue was gradually transferred to decreasing alcoholic and increasing xylene grades (25, 50, 75 and 100% xylene; each step for 25–30 min) at room temperature. The xylenated tissue was infiltrated and embedded in paraffin wax contained in plastic molds. The trimmed paraffin blocks containing tissues were adjusted on the microtome (Shandon, Germany) for cutting 5 μm thick sections. The sections were deparaffinized with xylene and rehydrated after affixing the ribbon on the adhesive coated glass slides, and stained with toluidine blue stain (0.05% aqueous solution). The photomicrographs of the stained sections were taken on a camera equipped microscope (DG3 LaboMed, USA).
The stained sections were used to take measurements of various cells and tissues with ocular and stage micrometers at various magnifications. For the calculation of area of cells and tissues, the formulae were used from the website: http://en.wikipedia.org/wiki/Area. Maximum width of each differentiating leaf was taken from the center. The number of mesophyll cells, intervening the lower and upper epidermis, was counted across the maximum leaf width. Assuming that the mesophyll cells were ellipsoidal, their area was measured with the formula: “π × a × b”; where a and b are semi-major and semi-minor axis, respectively. The number of vascular bundles was counted in whole of the leaf, while their area was calculated as described for the area of mesophyll cells.
Cost-benefit and statistical analyses
Empirical cost-benefit analysis was made for the cost of proline and GB required for soaking 40 single noded bud chips (~3–4 cm internode on each side of the node) in a liter of solution in improving bud sprouting under control and heat stress conditions. For statistics, data of all parameters were subjected to analyses of variance (ANOVA) using COSTAT computer package (CoHort software, 2003, Monterey, California) and LSD test was applied to determine the differences in various factors and their interactions at P = 0.05 and compare the treatment means (Steel et al. 1996). Correlations were drawn between different physiological and anatomical attributes at 8 (initial) and 48 (last) time points.
Results
Bud sprouting
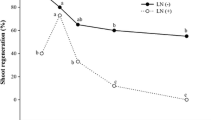
Data for bud sprouting indicated significant (P < 0.01) differences in the time intervals and soaking treatments with significant (P < 0.01) interaction of these factors. Sprouting started on day 6 in the control buds soaked in 20 mM proline and GB. On day 9, the bud sprouting was seen in all the treatments except in the unsoaked heat treated buds, which sprouted on day 12. On day 15 and 18, the bud sprouting was noted in all the treatments, but with significant (P < 0.01) differences. On day 18, presoaking in proline was the most effective followed by GB under control condition, while under heat stress proline soaking again excelled the other treatments; being at par (P > 0.05) with unsoaked control buds. Nonetheless, heat stressed buds showed lowest sprouting at all time periods (Fig. 1).
Changes in biomass of buds under heat stress
In the short term experiments on sugarcane buds, there was significant (P < 0.01) difference in the treatments and data points, but no significant (P > 0.05) interaction of these factors was evident for bud fresh and dry weights. Although there was a time course increase across all treatments, the fresh and dry weights were the lowest in heat treated followed by untreated control buds. Presoaking in 20 mM solution each of proline and GB substantially increased both these parameters, although their effectiveness was greater under heat stress. Of the two osmoprotectants, proline was more effective than GB (Table 1).
Bud physiological attributes
Although there was no difference (P > 0.05) in free proline accumulation in the time points; we noted a significant difference in the treatments, but an interaction of time points and treatments was missing (P > 0.05). For GB on the other hand, there were significant (P < 0.01) differences in the time points, treatments with an interaction (P < 0.01) of time points and various treatments for GB accumulation. Although trend did not change over the time periods, free proline accumulation was the highest in proline-soaked heat stressed buds followed by non-stressed proline-soaked buds. However, control and GB treated and heat stressed or non-stressed buds indicated a meager accumulation of free proline (Fig. 2a). A minimum GB concentration was noticed in unsoaked buds, which accumulated greatly in a time dependent manner in the GB treated buds followed by heat and GB soaked and heat treated buds. Proline-soaked control or heat stressed buds showed no change in GB accumulation compared with control buds (Fig. 2b).
For soluble sugars, data revealed significant (P < 0.01) difference in the time points and treatments with an interaction (P < 0.01) of these factors. Under control condition, the buds, irrespective of pretreatments, indicated no changes in the soluble sugar concentration. However, under heat stress both the pretreated and untreated buds showed a time-related accumulation of soluble sugars, although their accumulation was the greatest in GB-soaked followed by proline-soaked buds (Fig. 2c). For H2O2 concentration, data indicated significant (P < 0.05) difference in the time points, a non-significant (P > 0.01) one in the treatments but with an interaction (P < 0.01) of both these factors. Under control condition, the H2O2 contents did not differ much in the untreated or osmoprotectants-treated buds at all time periods. Under heat stress, however, the untreated buds indicated a linear accumulation of H2O2, while pretreatment with proline followed by GB was much effective in reducing the accumulation of H2O2 and bringing it down to the control levels. Of the osmoprotectants, proline was more effective than GB at all time points (Fig. 2d).
For K+ and Ca2+ contents, data revealed significant (P < 0.01) difference in the time points and treatments, while there was significant interaction of these factors for K+ while no interaction (P > 0.05) for Ca2+ contents of sprouting buds. Under control condition, unsoaked and soaked buds indicted no differences for their K+ contents. Heat stress caused a reduction in the K+ contents of the buds, but this reduction was lower in GB followed by proline pretreated buds, while untreated buds indicated the lowest K+ accumulation (Fig. 2e). Soaking in proline or GB solutions was effective in improving the Ca2+ contents of sugarcane buds under control or heat stress. However, proline improved the Ca2+ contents of heat stressed buds, which was similar to that of control buds. Here, GB was more effective in improving Ca2+ contents of sprouting buds (Fig. 2f).
Histological changes in buds
Although we determined histological changes in the sprouting buds at all harvests (8, 16, 24, 32, 40 and 48 h), the photographs have been presented only those taken at 32 h time point (Fig. 3). The measurements of various cells and tissues at each time point are given in Fig. 4. For differentiation of leaves, data indicated significant differences in time points but not among treatments, while interaction of these factors was not evident (P > 0.05). The differentiation of leaves although increased with time in all the treatments, it was the lowest in heat stressed control buds. Pretreatment with GB and proline improved the differentiation of leaves both under control and heat stress treatments in a time dependent manner. Of the two osmoprotectants, GB soaking was relatively better under control and heat stress (Fig. 4a). With significant (P < 0.01) difference in the time points and treatments and with a significant interaction of these factors, the maximum width of differentiating leaves, progressed with time in all the treatments, but applied heat stress greatly diminished this character. The differentiating leaves were the narrowest in heat treated buds, whilst pretreatment with both GB and proline increased leaf width under control and heat stress. The effectiveness of GB and proline was similar under control. However, under heat stress pretreatment with GB proved more effective in increasing leaf width than with proline (Fig. 4b).
Diagrammatic presentation of the changes in the development of various cells and tissues in the toluidine blue stained transverse sections of buds under control (left panel) and heat stress (right panel) conditions after 32 h. The buds were treated with water (control) and 20 mM solution each of proline and glycinebetaine (pretreated). MC mesophyll cells; VB vascular bundles; EL elongating bud leaves
For number and area of mesophyll cells, data indicated significant differences in the time points and treatments; however, interaction of these factors was present for the number of mesophyll cells only. At 8 and 16 h, there were no remarkable differences in various treatments for the number of mesophyll cells; however, at later time periods this number decreased under heat stress. Under control condition, GB and proline were equally effective in increasing mesophyll cell numbers, while under heat stress this number was the lowest in unsoaked buds but quite higher in proline and GB soaked buds (Fig. 4c). The area of individual mesophyll cells did not differ between soaked and unsoaked buds over time under control condition. However, under heat stress the mesophyll cell area was the lowest in the unsoaked samples while GB followed by proline was effective in improving this area at all time points (Fig. 4d).
Data analysis for the number and area of vascular bundles revealed significant (P < 0.01) differences among all the time points and various treatments with an interaction (P < 0.05) of these factors. Under control condition, the number of vascular bundles in elongating leaves was similar in soaked or unsoaked buds at all time points. Under heat stress, this number was the lowest in untreated control and proline treated buds, while GB showed a greater improvement under heat stress (Fig. 4e). Although increased in all treatments in a time course manner, the area of vascular bundles was the greatest in GB treated buds followed by proline and untreated buds under control condition. However, heat stress greatly reduced vascular bundle area; being the lowest in untreated heat stressed buds. Presoaking with GB was more effective than proline in improving the area of vascular bundles under heat stress at all time points (Fig. 4f).
Correlations
In order to find possible relationships of the physiological and structural changes, correlations were established of bud biomass, with physiological and histological characters of buds at initial (8 h) and final (48 h) time points. However, data have been given only where the correlations were significant (Table 2). At 8 h, most of the correlations were non-significant except a negative correlation of H2O2 with the width of elongating leaves and positive correlations of GB with number of differentiating leaves; free proline with number of vascular bundles per leaf; soluble sugars with number of differentiating leaves and K+ and Ca2+ with the width of differentiating leaves. However, at 48 h dry weight was negatively correlated with H2O2 concentration of buds but positively correlated with Ca2+, number of differentiating leaves, area of mesophyll cells and area of vascular bundles. K+ was positively correlated with the width of differentiating leaves, number and area of mesophyll cells and vascular bundles, while Ca2+ paralleled with number of differentiating leaves, area of mesophyll cell and area of vascular bundles (Table 2).
Cost-benefit analysis
To soak 30 single noded setts (3–4 cm internode on both sides of the node), 1 L of 20 mM solution each of proline and GB would require 2.3 g proline (US$ 0.45) and 2.34 g GB (US$ 0.50). Assuming 25–30% increase in crop stand of under heat stress over control, the soaking of setts in both osmoprotectants proved beneficial.
Discussion
Use of low molecular weight osmoprotectants has been promising one for plants grown from seeds or propagating materials (Ashraf and Foolad 2007; Wahid et al. 2007). In the long term experiment, data revealed significant influence of heat stress on the bud sprouting, while presoaking in proline and GB proved of considerable help in alleviating the adversities of heat stress, although former osmoprotectant was relatively more effective (Fig. 1). Bud sprouting is a very important aspect of sugarcane production under suboptimal growth conditions (Wahid et al. 2009). These data suggested that both these osmolytes, due to their specific membrane protective properties, can be used to improve heat tolerance in sugarcane.
A short term time course study was conducted to understand the basis of improvements in the bud sprouting under heat stress and specific role of proline and GB in this respect. Results indicated substantial reductions in fresh and dry weight under heat stress, which was related to hampered physiological activities in the bud, their restricted development and biomass accumulation. Pretreatment of buds with GB and proline had a little effect under control condition but a great improvising effect on fresh and dry weights of bud under heat stress (Table 1). Although the sugarcane bud is a vegetative and non-embryonic tissue (antonym to germinating seed; Alexander 1973), it is sensitive to stress conditions in a fashion similar to seed during germination and seedling emergence (Wahid et al. 2010).
One of the prominent effects of heat stress is the production of ROS, causing oxidative damage on the cells and tissues (Morison 1996; Wahid et al. 2007); which is quantitatively measured in terms of malondialdehyde (Gür et al. 2010; Savicka and Škute 2010). Although not measured in this study, it is most likely that H2O2 accumulation led to the membrane damage and accumulatrion of malondialdehyde. The endogenous synthesis or external supply of osmoprotectants and other chemicals have been reported to be effective in reducing the oxidative stress with the generation of ROS (Smirnoff 2005; Ashraf and Foolad 2007; Wahid et al. 2008). Other effects of heat stress include reduced concentration of essential nutrients (Wahid et al. 2007). In this study, measurement of H2O2 in buds indicated that heat stress led to a greater production of H2O2 in the untreated buds, while treated buds indicated its lower production (Fig. 2). H2O2 is a relatively longer-lived amongst the ROS is highly toxic (Gong et al. 1998; Wahid et al. 2007). These findings showed the effectiveness of both the osmoprotectants in the alleviation of oxidative damage.
Among other physiological attributes, the production of free proline, GB, soluble sugars, and changes in the accumulation of K+ and Ca2+ were monitored. It is important to notice that both GB and proline soaked buds indicated steady state levels of both these osmolytes under control or heat stress (Fig. 2), indicating that both were not metabolized rather they persisted and appeared to play a role in maintaining water economy of the sprouting buds as evident from the fresh weight of buds (Table 1). Contrarily, soluble sugars indicated a linear accumulation, which was enhanced further by pretreatment with both GB and proline. Similar was the trend for the accumulation of K+ and Ca2+ (Fig. 2) in the presoaked heat stressed buds. Both these ions play protective roles, particularly for the biological membranes of plants under heat and other stresses (Ashraf and Foolad 2007; Farooq et al. 2009). These findings revealed that soaking of buds with GB and proline augmented the accumulation of soluble sugars, K+ and Ca2+ and helped the buds to withstand heat stress while sprouting.
Immature bud is a vegetative tissue comprising a ground mass of cells. When provided with appropriate medium the buds show the differentiation of leaf primordia and leaves, which ultimately lead to the sprouting and emergence as seedling (Alexander 1973). During the sprouting of buds, expansion of differentiating leaves and establishment of vascular connections is pivotal. To our knowledge no study has so far reported the development of various tissues of sugarcane bud from immature to mature state under normal or heat stress conditions. Here the comparative developmental changes were monitored in GB and proline soaked or unsoaked sugarcane buds under heat stress (Fig. 3). These findings revealed a progressive development of various tissues including the number of differentiating leaves and their expansion, number and area of mesophyll cells, and number and area of vascular bundles (Fig. 4). Data revealed a severe effect of heat stress on these attributes while the role of pretreatment with GB and proline was well evident, albeit to varying degrees. Most important effect of heat stress was on the expansion of mesophyll cells and the establishment of vascular connections. Although heat stress hastened the differentiation of elongating leaves, the mesophyll cells became diffused in appearance and were much reduced in size. The vascular bundles in the elongating leaves were much deformed and deshaped instead of being roundish as seen in normal sprouting buds (Fig. 3). Nevertheless, soaking in GB and proline solutions markedly reversed the heat stress effects on these tissues with reduced sizes of various cells and tissues (Figs. 3, 4). Although for some attributes, the effectiveness of GB and proline nearly equaled controls, soaking in GB was more beneficial to the development of bud tissues than proline.
The establishment of correlations is an important tool to find possible associations of various parameters (Steel et al. 1996). The validity of changes produced by soaking of buds and effect of heat stress was monitored by correlating various attributes during initial and final time points. These correlations indicated that although sparingly evident at 8 h time point, were well evident at 48 h (final) time point (Table 2). These data suggested that bud soaking in 20 mM proline and GB solutions resulted in the maintenance of requisite levels of Ca2+ and K+, which was crucial for the differentiation of the leaves from the ground tissues of immature buds and increasing dry weight of the sprouting bud under heat stress. Presence of a negative correlation of H2O2 with dry weight indicated that an element of oxidative damage (Gong et al. 1998) due to heat stress was also prevalent on the buds. Positive correlations existing in the levels of K+ and Ca2+ and development of bud tissues (Table 2) are the likely reasons for improved heat tolerance of buds triggered by soaking in proline and GB solutions. This further strengthens our view that greater endogenous nutrients are pivotal for salt tolerance of sugarcane buds (Wahid et al. 2009). However, absence of any correlation of GB, proline or sugars with dry weight or differentiation of the bud tissues revealed the indirect roles of the used osmoprotectants in improving the heat tolerance of sugarcane buds rather their direct roles in producing the above reported changes.
In conclusion, bud soaking in 20 mM GB and proline solutions counteracted the effect of heat stress on the bud sprouting by enhancing the tissue levels of K+ and Ca2+, thereby maintaining the differentiation of bud tissues and increasing its dry weight. Correlation data revealed that soaking with GB and proline had indirect roles in improving bud growth under heat stress. Soaking of sugarcane buds at the used levels is economical and thus has great implication for enhancing the sugarcane plant population in a unit area in warmer climates.
References
Alexander AG (1973) Sugarcane physiology: a comprehensive study of the saccharum source-to-sink system. Elsevier Scientific Publication, Amsterdam
Al-Ghamdi AA (2009) Evaluation of oxidative stress tolerance in two wheat (Triticum aestivum) cultivars in response to drought. Int J Agric Biol 11:7–12
Ashraf M, Foolad MR (2007) Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ Exp Bot 59:206–216
Bates IS, Waldren RP, Teare ID (1973) Rapid determination of free proline for water stress studies. Plant Soil 39:205–207
Bohnert HJ, Jensen RG (1996) Metabolic engineering for increased salt tolerance. The next step. Aust J Plant Physiol 23:661–667
Bohnert HJ, Sheveleva E (1998) Plant stress adaptations, making metabolism move. Curr Opin Plant Biol 1:267–274
Ebrahim MKH, Zingsheim O, El-Shourbagy MN, Moore PH, Komor E (1998) Growth and sugar storage in sugarcane grown at temperature below and above optimum. J Plant Physiol 153:593–602
Egli DB, TeKrony DM, Heitholt JJ, Rupe J (2005) Air temperature during seed filling and soybean seed germination and vigor. Crop Sci 45:1329–1335
Farooq M, Basra SMA, Wahid A, Cheema ZA, Cheema MA, Khaliq A (2008) Physiological role of exogenously applied glycinebetaine in improving drought tolerance of fine grain aromatic rice (Oryza sativa L.). J Agron Crop Sci 194:325–333
Farooq M, Aziz T, Wahid A, Lee DJ, Siddique KHM (2009) Chilling tolerance in maize: agronomic and physiological approaches. Crop Past Sci 60:501–516
Gong M, Li YJ, Chen SZ (1998) Abscisic acid induced thermo tolerance in maize seedlings is mediated by Ca2+ and associated with antioxidant systems. J Plant Physiol 153:488–496
Grass I, Burris I (1995) Effect of heat stress during seed development and maturation on wheat (Triticum durum) seed quality. I. Seed germination and seedling vigor. Can J Plant Sci 75:821–829
Grieve CM, Grattan SR (1983) Rapid assay for the determination of water soluble quaternary ammonium compounds. Plant Soil 70:303–307
Gür A, Demirel U, Özden M, Kahraman A, Çopur O (2010) Diurnal gradual heat stress affects antioxidant enzymes, proline accumulation and some physiological components in cotton (Gossypium hirsutum L.). Afr J Biotechnol 9:1008–1015
Hall AE (1992) Breeding for heat tolerance. Plant Breed Rev 10:129–168
Jain R, Shrivastava AK, Solomon S, Yadav RL (2007) Low temperature stress-induced biochemical changes affect stubble bud sprouting in sugarcane (Saccharum spp. hybrid). Plant Growth Regul 53:17–23
Jain R, Solomon S, Shrivastava AK, Lal P (2009) Nutrient application improves stubble bud sprouting under low temperature conditions in sugarcane. Sug Tech 11:83–85
Mahmood S, Wahid A, Javed F, Basra SMA (2010) Heat stress effects on forage quality characteristics of maize (Zea mays) cultivars. Int J Agric Biol 12:701–706
Moore PH (1987) Breeding for stress resistance. In: Heinz DJ (ed) Development in crop science. II. Sugarcane improvement through breeding. Elsevier Science Publishers, Amsterdam, pp 503–542
Morison JIL (1996) Global environment change impacts on crop growth and production in Europe. Implications of global environmental change for crops in Europe. Aspects Appl Biol 45:62–74
Porter JR (2005) Rising temperatures are likely to reduce crop yields. Nature 436:174
Rasheed R, Wahid A, Ashraf M, Basra SMA (2010) Role of proline and glycinebetaine in improving chilling stress tolerance in sugarcane buds at sprouting. Int J Agric Biol 12:1–8
Robertson MJ, Bonnett GD, Hughes RM, Muchow RC, Campbell JA (1998) Temperature and leaf area expansion of sugarcane: integration of controlled-environment, field and model studies. Aust J Plant Physiol 25:819–828
Ruzin SE (1999) Plant microtechnique and microscopy. Oxford University Press, New York
Sakamoto A, Murata N (2002) The role of glycine betaine in the protection of plants from stress: clues from transgenic plants. Plant Cell Environ 25:163–171
Savicka M, Škute N (2010) Effects of high temperature on malondialdehyde content, superoxide production and growth changes in wheat seedlings (Triticum aestivum L.). Ekologija 56:26–33
Sharma P, Sharma N, Deswal R (2005) The molecular biology of the low-temperature response in plants. BioEssays 27:1048–1059
Smirnoff N (2005) Antioxidants and reactive oxygen species in plants. Blackwell Publishing, Oxford
Song SQ, Lei YB, Tian XR (2005) Proline metabolism and cross-tolerance to salinity and heat stress in germinating wheat seeds. Russ J Plant Physiol 52:793–800
Steel RGD, Torrie JH, Dickey DA (1996) Principles and procedures of statistics: a biometrical approach, 3rd edn. McGraw Hill Co, New York
Tendon HLS (1993) Methods of analysis of soil plants water and fertilizers. Fertilization Development and Consultation Organisation, New Delhi
Tiroli-Cepeda AO, Ramos CHI (2010) Heat causes oligomeric disassembly and increases the chaperone activity of small heat shock proteins from sugarcane. Plant Physiol Biochem 48:108–116
Ulukan H (2011) Responses of cultivated plants and some preventive measures against climate change. Int J Agric Biol 13:292–296
Velikova V, Yordanov I, Edreva A (2000) Oxidative stress and some antioxidant systems in acid rain treated bean plants. Protective role of exogenous polyamines. Plant Sci 151:59–66
Verbruggen N, Hermans C (2008) Proline accumulation in plants: a review. Amino Acids 35:753–759
Vollenweider P, Günthardt-Goerg MS (2005) Diagnosis of abiotic and biotic stress factors using the visible symptoms in foliage. Environ Pollut 137:455–465
Wahid A (2007) Physiological implications of metabolites biosynthesis in net assimilation and heat stress tolerance of sugarcane (Saccharum officinarum) sprouts. J Plant Res 120:219–228
Wahid A, Close TJ (2007) Expression of dehydrins under heat stress and their relationship with water relations of sugarcane leaves. Biol Plant 51:104–109
Wahid A, Shabbir A (2005) Induction of heat stress tolerance in barley seedlings by pre-sowing seed treatment with glycinebetaine. Plant Growth Regul 46:133–141
Wahid A, Gelani S, Ashraf M, Foolad MR (2007) Heat tolerance in plants: an overview. Environ Exp Bot 61:199–223
Wahid A, Sehar S, Perveen M, Gelani S, Basra SMA, Farooq M (2008) Seed pretreatment with hydrogen peroxide improves heat tolerance in maize at germination and seedling growth stages. Seed Sci Technol 36:633–645
Wahid A, Sabir H, Farooq M, Ghazanfar A, Rasheed R (2009) Role of nodal bud and sprout tissue nutrients in sprout establishment, growth and salt tolerance of sugarcane. Crop Past Sci 60:453–462
Wahid A, Farooq M, Rasheed R, Gelani S, Rasul E (2010) Sugarcane under thermal stress: some biotechnological considerations. In: Kumar Ashwani (ed) Plant genetic transformation and molecular markers. Pointer Publishers, Jaipur, pp 109–123
Yoshida S, Forno DA, Cock JH, Gomez KA (1976) Laboratory Manual for Physiological Studies of Rice. Los Banos, Philippines
Yousfi N, Slama I, Ghnaya T, Savouré A, Abdelly C (2010) Effects of water deficit stress on growth, water relations and osmolyte accumulation in Medicago truncatula and M. laciniata populations. Comp Rend Biol 333:205–213
Acknowledgments
The financial support of Higher Education Commission (HEC), Islamabad, Pakistan under Indigenous Ph.D. Fellowship Program (5000 Fellowships) Batch-II to first author is acclaimed. Supply of sugarcane material by SRI, Faisalabad, Pakistan and microtomy and photography facilities by Prof. Ahrar Khan and Prof. Zargham Khan, Department of Pathology, University of Agriculture, Faisalabad, Pakistan are duly acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rasheed, R., Wahid, A., Farooq, M. et al. Role of proline and glycinebetaine pretreatments in improving heat tolerance of sprouting sugarcane (Saccharum sp.) buds. Plant Growth Regul 65, 35–45 (2011). https://doi.org/10.1007/s10725-011-9572-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10725-011-9572-3