Abstract
Background
Eligibility of nipple-sparing mastectomy has been expanded. The purpose of this study was to evaluate interobserver agreement regarding magnetic resonance imaging (MRI) descriptors important in determining eligibility for mastectomy, and to investigate the significance of enhancement extending to the areola concerning nipple–areolar complex (NAC) involvement.
Methods
Fifty-one cases with histologically confirmed NAC involvement and 54 cases with negative NAC were enrolled. Two radiologists assessed the following factors: lesion morphology (mass or non-mass enhancement); intra-nipple bright signal; enhancement extending to the areola; abnormal nipple enhancement; and tumor–nipple distance. Factors that showed a significant association with outcome in the univariate analysis were assessed by means of multivariate analysis using a logistic regression model. Interobserver agreement between observers was assessed by calculating κ values (dichotomous variables), or intraclass correlation coefficients (ICCs; continuous variables).
Results
In multivariate analysis of the results from the two observers, tumor–nipple distance (observer 1: odds ratio [OR] 0.93; 95% confidence interval [CI] 0.88–0.99; observer 2: OR 0.89; 95% CI 0.83–0.95) and enhancement extending to the areola (observer 1: OR 17.9; 95% CI 1.97–162.2; observer 2: OR 24.0; 95% CI 2.62–219.7) were found to be significant predictors of NAC involvement. A substantial agreement (κ = 0.64–0.71) for every dichotomous variable and an almost perfect agreement (ICC = 0.86) for continuous variable were observed.
Conclusions
Findings of breast MRI for NAC preservation had good interobserver agreement. Enhancement extending to the areola, together with tumor–nipple distance, was significant factors for NAC involvement.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nipple-sparing mastectomy (NSM), a modified mastectomy in which breast tissue, is removed, while the skin and nipple–areolar complex (NAC) is spared, and is reportedly a safe procedure in properly selected breast cancer patients with low locoregional recurrence [1,2,3,4]. The reported locoregional recurrence rate of NSM ranges from 0.8 to 2.6% [1,2,3]. A retrospective study concluded that there was no significant difference in the cancer recurrence rate or survival rate between NSM and skin-sparing mastectomy, a total mastectomy with skin preservation and NAC resection [4]. Several studies have reported that patient satisfaction after NSM was high [5], especially when compared with skin-sparing mastectomy [6]. This procedure has been adopted not only in cases with earlier stage breast cancers, but also in cases with more locally advanced diseases [7, 8]. Because eligibility has been expanded, relevant preoperative evaluation concerning tumor involvement of the nipple and subareolar area are important in the selection of appropriate candidates.
Breast magnetic resonance imaging (MRI) has been widely used for preoperative assessment of NSM candidates. The previous studies have revealed several findings regarding preoperative breast MRI associated with NAC involvement. Non-mass enhancement (NME) as opposed to mass enhancement [9], presence of enhancement of the ipsilateral nipple [9,10,11], or shorter distance between lesion and the nipple [12] have reportedly been associated with NAC involvement; in particular, several cut-off values of tumor–nipple distance have been suggested, ranging from 5 to 20 mm [12,13,14,15]. Interobserver variability regarding breast MRI descriptors has been investigated in the previous studies with variable results [16,17,18,19]. Interobserver agreement, which is also important for reliable assessment of whether or not NSM, can be safely performed, and has not been investigated to date. Furthermore, an enhancing structure probably related to a malignant lesion, which extends to the areola instead of the nipple is occasionally detected. This finding can reflect pathological extension of disease and thereby can prevent NAC reservation, although it has not been discussed in the previous studies.
The aim of our study was to evaluate the reproducibility of MRI descriptors relevant to NSM between observers. In addition, we investigated the significance of enhancement extending to the areola, and other MRI findings, as factors for the determination of patient eligibility for NSM.
Materials and methods
Our institutional review board approved this retrospective study and waived the requirement for informed patient consent. We reviewed our database for operations performed at our institution between March 2010 and July 2013; 699 mastectomy cases were identified. In this study, positive NAC involvement was defined as being present in patients who underwent total mastectomy or skin-sparing mastectomy. Histopathological reports of total mastectomy or skin-sparing mastectomy specimens were reviewed, and NAC involvement was considered positive if it was clearly stated as being present by the pathologist at the time of the postoperative pathological examination. Based on the pathological results, there were 56 patients who did not undergo primary systemic therapy and were free from locoregional recurrence or metastasis for ≥ 2 years after surgery. The presence of tumor involvement of the NAC was definitively confirmed histopathologically (NAC-positive group). On the other hand, there were 227 out of total 699 patients who did not receive primary systemic therapy; a negative margin was confirmed histopathologically using NSM specimens, and there was no locoregional recurrence or metastasis for ≥ 2 years after surgical treatment. Postoperatively, pathologists searched the main ducts and surrounding areas with the guidance of marks that had been made by the surgeon before submitting the resected specimens. When the pathologists did not find any tumor on the surface of the specimen, they considered the NAC to be free of tumor involvement. From this population, one-by-one matching against the NAC-positive group based on age and disease T stage was conducted; 56 cases involving negative NAC margins were selected (NAC negative group). After extraction, medical record review of these cases revealed that no preoperative breast MRI was performed for 5/56 cases in NAC-positive group, while cancer was not visible on preoperative breast MRI in 2/56 cases in NAC negative group. These seven cases were excluded, and the remaining 105 cases (NAC-positive group, 51; NAC negative group, 54) were enrolled (Supplemental Fig. 1). The largest dimension of lesions on MRI, which had been measured at the time of clinical care, ranged from 6 to 52 mm (mean, 21.4 mm).
MRI technique
MRI was performed using a 1.5-T system (Avanto, Siemens Healthcare, Erlangen, Germany). A body coil was used for transmission, and a double breast coil (16-channel breast array coil) was used for MRI analysis. Dynamic MRI using a 3D fat-suppressed volumetric interpolated breath-hold examination sequence with parallel acquisition was performed before and three times after injection of a bolus of gadopentetate dimeglumine (0.1 mmol/kg; Magnevist, Bayer HealthCare AG, Wuppertal, Germany) at a rate of 2 mL/s, followed by a 20 mL saline flush administered with an automatic injector. Both breasts were scanned in the coronal plane on first-, second-, and third-phase dynamic images acquired at 30 s, 1.5, and 4.5 min after contrast injection, respectively. The parameters for dynamic MRI were as follows: 5.2/2.3; flip angle, 12°; field of view, 33 cm; matrix, 448 × 318; receiver bandwidth, 430 Hz per pixel; interpolated slice thickness, 0.9 mm; partitions, 144; and time of acquisition, 60 s. The right and left breasts were scanned in the sagittal plane using the volumetric interpolated breath-hold examination sequence without parallel acquisition at 2.5 and 3.5 min after contrast injection, namely, between the second- and third-phase coronal images (4.0/2.2; flip angle, 15°; field of view, 16 cm; matrix, 256 × 256; receiver bandwidth, 390 Hz per pixel; interpolated slice thickness, 1.2 mm; partitions, 80; time of acquisition, 60 s). In addition, bilateral sagittal fat-suppressed T2-weighted images and coronal diffusion-weighted images were obtained before the administration of the contrast material.
Observer study
Two radiologists (observer 1: A.S. with 15 years of experience in breast imaging [approximately 3300 breast MRI examinations]; observer 2: T.I. with 15 years of experience in breast imaging [approximately 7200 breast MRI examinations]) independently reviewed the preoperative breast MRIs. The side and quadrant of the breast, where the lesion was located, were indicated to the observers, but they were blinded to all other clinical or pathological information. They assessed the following factors: morphology of the lesion (mass or NME); bright signal within the ipsilateral nipple on pre-contrast T1-weighted images (intra-nipple bright signal, positive or negative); and enhancement extending to the areola (not the nipple, positive or negative); abnormal enhancement of the ipsilateral nipple in comparison with the contralateral nipple (abnormal nipple enhancement, positive or negative). The overall morphology of the lesion was identified according to its predominant morphology. That is, if a lesion looked like it was predominantly a mass, the observers were asked to define it a ‘mass’ even if there was a component that looked like a-NME, and vice versa. Abnormal nipple enhancement was considered positive regardless of its shape or size when it was conspicuous compared with that in of the contralateral nipple. In contrast, even when nipple enhancement was observed in the ipsilateral nipple, abnormal nipple enhancement was considered negative if the nipple was comparable to that in the contralateral nipple. In this case, the enhancement in the nipple was considered normal. Enhancement extending to the areola was considered positive if relevance between such enhancement and the main lesion was suspected by the observer (Fig. 1). The areola was identified by the thickening of skin around the nipple. Focus, a small enhancing lesion (generally < 5 mm), was not included as a type of morphology of the lesion in this study, because all the lesions measured larger than 5 mm. In addition, the distance between the base of the nipple and the lesion (tumor–nipple distance) was measured. All the image sequences from each MRI study were provided to the observers, and they were allowed to evaluate factors using all of these images as well as reformatted images.
Statistical analysis
In univariate analysis, differences in MRI findings between the two groups evaluated by each observer were analyzed using the Mann–Whitney U Test (for continuous variables) or the Chi-squared test (for dichotomous variables). Factors that showed a significant association with outcome in the univariate analysis were also assessed by means of multivariate analyses using a logistic regression model with forward stepwise modeling and likelihood ratio tests. The positive predictive value (PPV), negative predictive value (NPV), and their 95% confidence intervals (CIs) for each MRI finding that showed a significant association with outcome in the univariate analysis were recorded. Receiver operating characteristic (ROC) curves with statistical significance were calculated for the tumor–nipple distance, and the optimal cut-off values were determined based on the highest combined specificity and sensitivity pair using ROC curves for each observer’s results. Their average value as well as the sensitivity, specificity, PPV, and NPV were then calculated.
Sensitivity and specificity based on factors that were identified as significant predictors by the multivariate analysis of both two observers’ results were also calculated using the ROC curve. At this point, the average value of the optimal tumor–nipple distance was adopted if this finding had been confirmed as a significant predictor by the multivariate analysis of both observers’ results.
Interobserver agreement of MRI findings between the two observers was assessed by calculating κ values for dichotomous variables, or intraclass correlation coefficients (ICCs) for continuous variables.
Statistical analyses were performed using SPSS statistics 23 (IBM Corp., Armonk, NY, USA). A p value of < 0.05 was considered to indicate a statistically significant difference. The κ values and ICCs were classified as slight (< 0.20), fair (0.21–0.40), moderate (0.41–0.60), substantial (0.61–0.80), or almost perfect (0.81–1.0).
Results
Study subjects
The clinical characteristics of the two groups are detailed in Table 1. No significant difference was observed between the two groups concerning most of the clinical and pathological factors, with the exception of the progesterone receptor status (p = 0.01).
Relationship between MRI findings and NAC involvement
In univariate analyses, NME as opposed to mass (observer 1, p = 0.042; observer 2, p = 0.046), presence of enhancement extending to the areola (p < 0.001 for observers 1 and 2) and abnormal nipple enhancement (p < 0.001 for observers 1 and 2; Fig. 2) were significantly more frequent in the NAC-positive group than in the NAC negative group; tumor–nipple distance (p < 0.001 for observers 1 and 2) was significantly shorter in the NAC-positive group than in the NAC negative group (Fig. 3), based on the interpretation by both observers. Results of the univariate analyses, PPV, NPV, and their 95% CIs of each MRI finding with significant association for NAC involvement are shown in Table 2. When these variables were subjected to multivariate analysis, tumor–nipple distance (odds ratio [OR] 0.93; 95% confidence interval [CI] 0.88–0.99), enhancement extending to the areola (OR 17.9; 95% CI 1.97–162.2) and abnormal nipple enhancement (OR 18.7; 95% CI 1.97–177.3) were found to be significant predictors of NAC involvement based on the interpretation of observer 1; whereas NME morphology compared with mass (OR 4.2; 95% CI 1.11–15.9), tumor–nipple distance (OR 0.89; 95% CI 0.83–0.95) and enhancement extending to the areola (OR 24.0; 95% CI 2.62–219.7) were found to be significant predictors of NAC involvement based on the interpretation of observer 2 (Table 3).
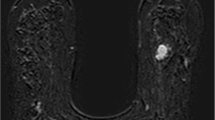
57-year-old woman with invasive carcinoma of no special type in the left breast. A sagittal contrast-enhanced T1-weighted image is shown. Abnormal nipple enhancement (arrow) was considered positive by both observers. Skin-sparing mastectomy was performed and nipple–areolar complex involvement was detected histopathologically
47-year-old woman with invasive carcinoma of no special type (arrowheads) in the left breast. A sagittal contrast-enhanced T1-weighted image is shown. Enhancement extending to the areola and abnormal nipple enhancement were both negative, and measured tumor–nipple was 19 mm (observer 1) and 20 mm (observer 2). Nipple-sparing mastectomy was performed and a negative nipple–areolar complex margin was confirmed histopathologically
Optimal cut-off value of tumor–nipple distance
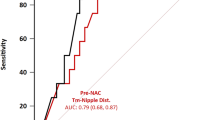
ROC curve analyses revealed an area under the ROC curve of 0.88 (95% CI 0.81–0.94) and 0.87 (95% CI 0.80–0.94) based on the interpretations of observers 1 and 2, respectively (Supplemental Fig. 2). The highest combined specificity and sensitivity pair (observer 1: sensitivity 88.9%, specificity 76.5%; observer 2: sensitivity 88.9%, specificity 78.4%) was obtained at the cut-off values of 5.5 mm (observer 1) and 4.5 mm (observer 2). When the cut-off values were set to 5 mm, the average value of the two observers’ results, PPV, NPV and their 95% CIs for NAC involvement were 88.1% (95% CI 78.3–97.9%), 77.8% (95% CI 67.5–88.0%) for observer 1, and 87.0% (95%CI 77.2–96.7%), 81.4% (95% CI 71.4–91.3%), respectively.
The sensitivity and specificity when the tumor–nipple distance of 5 mm and enhancement extending to the areola were used as predicting factors for NAC involvement. They were, respectively, 80.4, and 88.9% for observer 1 and 84.3 and 88.9% for observer 2. The area under the ROC curve and its 95% CIs were, respectively, 0.87 (0.80–0.94) for observer 1 and 0.88 (0.82–0.95) for observer 2 (Supplemental Fig. 3).
Intraobserver agreement of findings
A substantial agreement (κ = 0.64–0.71) for every dichotomous variable and an almost perfect agreement (ICC = 0.86) for continuous variables were observed between the MRI findings assessed by the two observers (Table 4).
Discussion
This retrospective study investigated observer agreement regarding MRI findings related to patient eligibility for NSM, and the significance of the preoperative breast MRI findings. Substantial agreement for dichotomous variables and an almost perfect agreement for continuous variable were observed between the interpretations of the two observers. In addition, multivariate analysis of MRI data based on the interpretation of the two observers revealed several findings, including that tumor–nipple distance and enhancement extending to the areola were significant predictors for NAC involvement.
One-by-one matching was performed in the recruitment of study subjects. Hence, we built a study cohort relatively enriched with NAC involvement (48.6%; 51/105 cases), relative to the previous studies, in which NAC involvement was positive in a smaller proportion of the population (7.7–27.7%) [9, 10, 13, 14, 20, 21]. Although a total of 7/112 (6.3%) cases were excluded from further analysis after choosing the cases, we succeeded in having two study groups with no statistical differences concerning most of the clinical and pathological factors.
In this study, morphology of the lesion (mass vs NME) showed a significant association with NAC involvement, based on univariate analysis of the results from both observers. This finding is consistent with a previous study [9], although MRI interpretation was conducted by a single radiologist, in contrast to the current study. Interobserver agreement concerning the morphology of the lesion was assessed in a previous study involving a cohort comprising probable benign lesions [17]; the κ value (0.53) was relatively lower than that in our study (0.71). This is possibly because “focus”, a type of morphology which represents a small enhancing lesion (generally < 5 mm) [22], was included in their study; in contrast, in our study, all the lesions measured larger than 5 mm, and the morphology of focus was not included.
Findings including abnormal nipple enhancement and tumor–nipple distance have been investigated and reported to be significantly associated with NAC involvement in the previous studies [9,10,11, 13, 14, 21]. These findings also showed a significant association with NAC involvement in our study, and we further revealed that they had substantial to almost perfect agreement between observers. From these results, all of these findings could be evaluated as findings relevant to NAC involvement, without interobserver variability. It should be noted that interobserver agreement regarding the tumor–nipple distance was almost perfect, even though the observers could measure it using any cross sectional images in addition to reformatted images. Moreover, tumor involvement not only of the nipple but also the areola will be important in the determination of patient eligibility for NSM, because both the nipple and areola are conserved in NSM. Our study revealed that enhancement extending to the areola was a significant factor for NAC involvement, with substantial interobserver agreement. Observers should be cautious regarding an abnormal enhancing structure which extends to the areola, in addition to other relevant findings in the evaluation of NAC involvement using preoperative breast MRI.
Although several studies have reported the feasibility of NSM with larger cut-off values concerning tumor–nipple distance (e.g., 20 mm) [12, 15], Ryu et al. reported that there were no significant differences between groups with a tumor–nipple distance ≥ 20 mm and those with a tumor–nipple distance ˂20 mm; they concluded that NSM can be a feasible treatment options even for cases with tumor–nipple distances ˂20 mm [23]. In addition, some studies have reported shorter cut-off values of tumor–nipple distance. For example, a study [13] that evaluated clinical and radiological predictors of NAC involvement reported a negative predictive value of 100% for MRI when the cut-off of the tumor–NAC distance was set at 10 mm. A more recent study [14] reported that negative intraoperative pathological assessment and a tumor–NAC distance of 5 mm at MRI allowed optimal discrimination for NSM. In the current study, the cut-off values were demonstrated to be 5.5 and 4.5 mm by the two observers; this appears to be consistent with the previous reports. Furthermore, almost perfect agreement in assessing tumor–nipple distance between the two observers would imply good reproducibility of this variable. Based on the results of our current study, the optimal cut-off value of the tumor–NAC distance can be estimated to be 5 mm, although this assumption will have to be validated in a future investigation. In addition, when tumor–nipple distance < 5 mm was considered together with other significant factors, NME morphology type, enhancement extending to the areola and abnormal nipple enhancement, NPV was 92.0% for observer 1 and 95.7% for observer 2. This indicates that NAC involvement would be unlikely in cases without any of these significant factors. It should be noted that none of those factors evaluated in this study was not perfect for assessing NAC involvement. False-positive cases could be due to background parenchymal enhancement, subtle inflammation of the duct, or vessels being confused with an enhanced intraductal tumor. False-negative cases could be due to indolent lesions that were not visible on MRI. Despite the false-positive and negative cases, our results suggested that preoperative breast MRI would be useful for evaluating NAC involvement, and can be assessed with good interobserver agreement.
This study had several limitations. First, 6.3% of the total cases were excluded from analyses after one-by-one matching. A confounding bias could be caused by this non-uniformity between the groups. Second, whether the results of this study in which patients were recruited by one-by-one matching can be generalized remains controversial, although the results were comparable with the previous studies [9,10,11, 14]. Although good interobserver agreement was observed for variables in this study, the two observers’ significant predictors and the prediction model determined by the multivariate analysis were not totally comparable. However, comparably high sensitivity and specificity were observed for the results of both observer 1 and observer 2 when we performed ROC curve analysis using a tumor–nipple distance of 5 mm and enhancement extending to the areola as predicting factors for NAC involvement. A prospective, multi-institutional study that adopts a consensus interpretation by multiple observers will be necessary to validate our results. In addition, the incidence of NAC necrosis, which is known as an occasional complication after NSM [24, 25], was not considered in our study. We concentrated on evaluating the relationship between preoperative MRI findings and the oncologic success of NAC preservation.
In conclusion, there was good interobserver agreement regarding the breast MRI findings that could determine factors for NAC preservation. Enhancement extending to the areola was a significant factor for NAC involvement, together with tumor–nipple distance, as demonstrated by the results of both observers.
References
Sakamoto N, Fukuma E, Teraoka K, Hoshi K. Local recurrence following treatment for breast cancer with an endoscopic nipple-sparing mastectomy. Breast Cancer. 2016;23:552–60.
Frey JD, Alperovich M, Kim JC, Axelrod DM, Shapiro RL, Choi M, et al. Oncologic outcomes after nipple-sparing mastectomy: a single-institution experience. J Surg Oncol. 2016;113:8–11.
Moo TA, Pinchinat T, Mays S, Landers A, Christos P, Alabdulkareem H, et al. Oncologic Outcomes After Nipple-Sparing Mastectomy. Ann Surg Oncol. 2016;23:3221–5.
Poruk KE, Ying J, Chidester JR, Olson JR, Matsen CB, Neumayer L, et al. Breast cancer recurrence after nipple-sparing mastectomy: one institution’s experience. Am J Surg. 2015;209:212–7.
El Hage Chehade H, Headon H, Wazir U, Carmaichael AR, Choy C, Kasem A, et al. Nipple-sparing mastectomy using a hemi-periareolar incision with or without minimal medial-lateral extensions; clinical outcome and patient satisfaction: a single centre prospective observational study. Am J Surg. 2017;213:1116–24.
Wei CH, Scott AM, Price AN, Miller HC, Klassen AF, Jhanwar SM, et al. Psychosocial and sexual well-being following nipple-sparing mastectomy and reconstruction. Breast J. 2016;22:10–7.
Burdge EC, Yuen J, Hardee M, Gadgil PV, Das C, Henry-Tillman R, et al. Nipple skin-sparing mastectomy is feasible for advanced disease. Ann Surg Oncol. 2013;20:3294–302.
Santoro S, Loreti A, Cavaliere F, Costarelli L, La Pinta M, Manna E, et al. Neoadjuvant chemotherapy is not a contraindication for nipple sparing mastectomy. Breast. 2015;24:661–6.
Piato JR, de Andrade RD, Chala LF, de Barros N, Mano MS, Melitto AS, et al. MRI to predict nipple involvement in breast cancer patients. AJR Am J Roentgenol. 2016;206:1124–30.
Cho J, Chung J, Cha ES, Lee JE, Kim JH. Can preoperative 3-T MRI predict nipple-areolar complex involvement in patients with breast cancer? Clin Imaging. 2016;40:119–24.
Moon JY, Chang YW, Lee EH, Seo DY. Malignant invasion of the nipple-areolar complex of the breast: usefulness of breast MRI. AJR Am J Roentgenol. 2013;201:448–55.
Karamchandani DM, Chetlen AL, Riley MP, Schetter S, Hollenbeak CS, Mack J. Pathologic-radiologic correlation in evaluation of retroareolar margin in nipple-sparing mastectomy. Virchows Arch. 2015;466:279–87.
D’Alonzo M, Martincich L, Biglia N, Pisacane A, Maggiorotto F, Rosa GD, et al. Clinical and radiological predictors of nipple-areola complex involvement in breast cancer patients. Eur J Cancer. 2012;48:2311–8.
Ponzone R, Maggiorotto F, Carabalona S, Rivolin A, Pisacane A, Kubatzki F, et al. MRI and intraoperative pathology to predict nipple-areola complex (NAC) involvement in patients undergoing NAC-sparing mastectomy. Eur J Cancer. 2015;51:1882–9.
Cense HA, Rutgers EJ, Lopes Cardozo M, Van Lanschot JJ. Nipple-sparing mastectomy in breast cancer: a viable option? Eur J Surg Oncol. 2001;27:521–6.
El Khoury M, Lalonde L, David J, Labelle M, Mesurolle B, Trop I. Breast imaging reporting and data system (BI-RADS) lexicon for breast MRI: interobserver variability in the description and assignment of BI-RADS category. Eur J Radiol. 2015;84:71–6.
Grimm LJ, Anderson AL, Baker JA, Johnson KS, Walsh R, Yoon SC, et al. Interobserver variability between breast imagers using the fifth edition of the BI-RADS MRI Lexicon. Am J Roentgenol. 2015;204:1120–4.
Kinkel K, Helbich TH, Esserman LJ, Barclay J, Schwerin EH, Sickles EA, et al. Dynamic high-spatial-resolution MR imaging of suspicious breast lesions: diagnostic criteria and interobserver variability. Am J Roentgenol. 2000;175:35–43.
Machida Y, Tozaki M, Shimauchi A, Yoshida T. Two distinct types of linear distribution in nonmass enhancement at breast mr imaging: difference in positive predictive value between linear and branching patterns. Radiology. 2015;276:686–94.
Steen ST, Chung AP, Han SH, Vinstein AL, Yoon JL, Giuliano AE. Predicting nipple-areolar involvement using preoperative breast MRI and primary tumor characteristics. Ann Surg Oncol. 2013;20:633–9.
Byon W, Kim E, Kwon J, Park YL, Park C. Magnetic resonance imaging and clinicopathological factors for the detection of occult nipple involvement in breast cancer patients. J Breast Cancer. 2014;17:386–92.
D’Orsi CJ. ACR BI-RADS atlas: breast imaging reporting and data system: mammography, ultrasound, magnetic resonance imaging, follow-up and outcome monitoring, data dictionary. 5th edn. Am Coll Radiol. 2013.
Ryu JM, Nam SJ, Kim SW, Lee SK, Bae SY, Yi HW, et al. Feasibility of nipple-sparing mastectomy with immediate breast reconstruction in breast cancer patients with tumor-nipple distance less than 2.0 cm. World J Surg. 2016;40:2028–35.
Cho JW, Yoon ES, You HJ, Kim HS, Lee BI, Park SH. Nipple-areola complex necrosis after nipple-sparing mastectomy with immediate autologous breast reconstruction. Arch Plast Surg. 2015;42:601–7.
Komorowski AL, Zanini V, Regolo L, Carolei A, Wysocki WM, Costa A. Necrotic complications after nipple- and areola-sparing mastectomy. World J Surg. 2006;30:1410–3.
Acknowledgements
This study was presented at the 24th Annual Meeting of the Japanese Breast Cancer Society, Fukuoka, July 13–15, 2017 (Presentation No. OS-1-01-04).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
12282_2018_845_MOESM1_ESM.tif
Supplementary material 1 (TIFF 86029 kb) Supplemental Fig. 1 The enrollment criteria. Primary systemic therapy was not performed, and no locoregional recurrence or metastasis was detected for ≥ 2 years after surgical treatment, in eligible cases. Bt, total mastectomy; NAC, nipple–areolar complex; NSM, nipple-sparing mastectomy; SSM, skin-sparing mastectomy
12282_2018_845_MOESM2_ESM.tif
Supplementary material 2 (TIFF 86943 kb) Supplemental Fig. 2 Receiver operating characteristic (ROC) curves of tumor–nipple distance, for the prediction of nipple–areolar complex involvement
12282_2018_845_MOESM3_ESM.tif
Supplementary material 3 (TIFF 2588 kb) Supplemental Fig. 3: Receiver operating characteristic (ROC) curves of tumor–nipple distance of 5 mm and enhancement attached to the areolar, for the prediction of nipple–areolar complex involvement
About this article
Cite this article
Machida, Y., Shimauchi, A., Igarashi, T. et al. Preoperative breast MRI: reproducibility and significance of findings relevant to nipple–areolar complex involvement. Breast Cancer 25, 456–463 (2018). https://doi.org/10.1007/s12282-018-0845-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12282-018-0845-9