Abstract
Background
Indications for nipple sparing mastectomy (NSM) is extending to post-neoadjuvant chemotherapy (NAC) setting. Eligibility for NSM with an optimum tumor-nipple distance (TND) after NAC is unclear. We examined predictive factors for nipple tumor involvement in patients undergoing total mastectomy following NAC.
Methods
Clinical and pathological data from prospectively collected medical records of women with invasive breast carcinoma, who were undergone NAC and total mastectomy with sentinel lymph node biopsy and/or axillary lymph node dissection were analyzed. PreNAC and postNAC magnetic resonance imaging (MRI) views were examined and a cut-off TND value for predicting the negative nipple tumor status was determined.
Results
Among 180 women, the final mastectomy specimen analysis revealed that 12 (7%) had nipple involvement as invasive carcinoma. Patients with nipple involvement had more postNAC multifocal/multicentric tumors (p: 0.03), larger tumors on preNAC and postNAC images (p: 0.002 and p < 0.001), shorter median TNDs on preNAC and postNAC images (7 mm-IQR 1.5–14, p: 0.005 and 8.5 mm-IQR 3–15.5, p < 0.001, respectively), more nipple retraction on preNAC and postNAC images (p: 0.007 and p: 0.006) and more nipple areola complex skin thickening (> 2mm) on preNAC and postNAC images (p < 0.001 and p: 0.01). The best likelihood ratios (LR) belonged to the postNAC positivity of the < 20 mm TND, with a + LR of 3.40, and − LR of 0.11 for nipple involvement. PreNAC positivity of the < 20 mm TND also had a similar − LR of 0.14.
Conclusion
A TND-cut-off ≥ 2 cm on preNAC and postNAC MRI was shown to be highly predictive of negative nipple tumor involvement.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Neoadjuvant chemotherapy (NAC) was introduced in early 70’s as the standard care particularly for patients with locally advanced breast cancer to reduce the tumor burden and make it operable [1]. Contemporary practice in NAC use was shifted from locally advanced cases to treat early breast cancer, thereby making breast conservation feasible in patients who would otherwise undergo mastectomy due to large tumor size compared to small breast mount [2].
The demonstrated oncologic safety and enhanced cosmetic outcomes resulted in a dramatic increase in the use of nipple/skin sparing mastectomies (NSM/SSM), which were previously saved for risk-reducing setting [3, 4]. The downstaging power of multimodal NAC had broadened the surgical options and NSM/SSM are nowadays offered to selected patients even with locally advanced disease after NAC [5, 6]. In upfront surgery setting, NSM is offered to patients with tumors that does not clinically involve the nipple areola complex. However, selecting the eligible patients for NSM after NAC is still a major concern [3, 7, 8].
Accurate determination of nipple involvement at radiologic imaging is critical to identifying appropriate candidates and preventing local recurrence for NSM after NAC. Magnetic resonance imaging (MRI) of the breast is widely used to evaluate the extent of the tumor before the initiation and after finalizing the NAC. Moreover, breast MRI is more accurate than digital mammography and ultrasound to predict nipple involvement with higher specificity and negative predictive value [9, 10].
Estimating tumor-nipple distance (TND) with MRI is crucial to select eligible patients for NSM after NAC. In post-neoadjuvant setting, cut-off value for TND in preoperative imaging to predictive nipple involvement is still unclear. In this study, we examined the preNAC (before neoadjuvant chemotherapy) and postNAC (after neoadjuvant chemotherapy) MRI of patients who were undergone total mastectomy to set a cut-off TND value for predicting the negative nipple status.
Methods
Clinical and pathological data from prospectively collected medical records of women with invasive breast carcinoma, who were undergone NAC and total mastectomy with sentinel lymph node biopsy and/or axillary lymph node dissection between 2015 and 2020 were analyzed after ethical approval by the Institutional Review Board. Study was designed as a single-center study involving two Cohorts as patients with pathological nipple involvement versus non after the completion of NAC. Women with clinical T1–T3 tumors and available preNAC and postNAC MRIs were included in the study. Patients with clinical T4 tumors, distant metastasis, preNAC clinical and/or pathologic nipple involvement, Paget’s disease, nipple/skin sparing mastectomies, poor quality, or missing breast MRIs were excluded from the study. We, also excluded patients who could not finalize the intended neoadjuvant protocol due to toxicity.
Pathologic data such as histopathology, subtype (Luminal type, Her2( +), triple negative), grade (low/moderate, high), clinical T stage, Ki-67 status, clinical N stage, pathologic N stage, and total number of involved axillary lymph nodes in final pathology were collected.
All the preNAC and postNAC MRI views were reviewed by a dedicated breast radiologist who was blind to the final nipple pathologies. On MRI, largest tumor diameter, presence of multifocality/multicentricity, and nipple retraction were documented. TND (tumor-nipple distance) was measured as closest distance of nipple to the mass and non-mass contrast enhancements. The TNDs were stratified as < 10 mm and < 20 mm. Both on preNAC and postNAC MRI of the patients, nipple areola complex skin thickness was measured and values >2 mm were interpreted as thickened nipple areola complex.
As a routine procedure in pathology protocol, after proper fixative procedure, mastectomy specimens were conventionally painted from posterior with Indian ink and sectioned at approximately 5–10 mm intervals in the sagittal plane. Nipple areola complex was examined macroscopically for gross tumor involvement. The entire nipple was removed and dissected for further examination. The nipple was assessed for presence of in situ and/or invasive carcinoma by coronal sections. If necessary, additional sections or immunohistochemical stains were performed for diagnosis. Sagittal section through the skin of the nipple was taken to exclude occult Paget’s disease. The level (papillae, skin level, or base of the nipple margin) at which the nipple was involved by malignancy was also reported.
Clinical, pathologic, and radiologic variables were compared according to the involvement of nipple by invasive cells (nipple involved versus non-involved). The Chi-square or Fisher’s exact tests was used for comparison of categorical variables. The student t-test or Mann–Whitney U test was used for comparison of continuous variables. The predictive utility measures of TNDs for estimating nipple involvement were determined at two different distances (< 10 mm and < 20 mm) according to the literature. Accuracy, sensitivity, specificity, positive likelihood ratios (+ LR), and negative likelihood ratios (− LR) were calculated using contingency tables with their corresponding 95% confidence intervals (CIs). The accuracies of TNDs in predicting nipple involvement were calculated by the area under the curves (AUCs) of their receiver operating characteristics (ROC) curves, AUCs were compared with the DeLong’s method, and the difference in AUCs and their CIs were calculated using Binomial method. Youden J-Index was used to estimate each TND threshold value and presented on dot diagrams. MedCalc Statistical Software version 20.027 (MedCalc Software, Ostend, Belgium; https://www.medcalc.org; 2022) was used for all statistical analysis.
Results
Medical records between 2015 and 2020 demonstrated 241 women who had undergone total mastectomy without immediate reconstruction following NAC. Of these women, 211 women had preNAC and postNAC MRIs which were suitable for revisit. We excluded T4 tumors, ones with clinical nipple involvement and those having low quality MR images, hence 180 eligible women were included in the final analysis.
In terms of clinical and pathological features, median age was 50 (IQR 41.8–60), majority of the patients had ductal histology (n: 144, 80%), luminal B subtype (n: 103, 57%), clinical T3 tumors (n: 96, 53.3%), high grade tumors (n: 124, 69%) and high (≥ 20%) Ki-67 levels (n: 134, 75%) (Table 1). The mastectomy specimen analysis revealed that 12 (7%) patients had nipple involvement as invasive carcinoma.
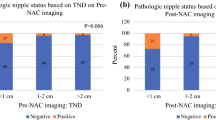
There were 69 (38%) pathologic complete responders in the study group. We further revisited their preNAC MR images and their TNDs. TND was < 10 mm in 11 (16%) patients, ≥ 10–< 20 mm in 27 (39%) patients, and ≥ 20 mm in 34 (45%) patients (p: 0.12).
When the pathologic findings were compared between the patients with positive and negative nipple involvement, it was shown that postNAC number of involved axillary lymph nodes was significantly associated with nipple involvement (p: 0.009). Univariate analysis of MRI signs showed that patients with nipple involvement had more postNAC multifocal/multicentric tumors (p: 0.03), larger tumors on preNAC and postNAC images (p: 0.002 and p < 0.001), shorter median TNDs on preNAC and postNAC images (7 mm-IQR 1.5–14, p: 0.005 and 8.5 mm-IQR 3–15.5, p < 0.001, respectively), more nipple retraction on preNAC and postNAC images (p: 0.007 and p: 0.006), and more nipple areola complex skin thickening on preNAC and postNAC images (p < 0.001 and p : 0.01).
Multivariate analysis to interpret the influence of factors on pathological nipple involvement revealed that statistically significant predictors were preNAC nipple areola complex skin thickening (> 2 mm) (OR 6.09, 95% CI 1.27–29.18, p: 0.024) and presence of PreNAC nipple retraction on MRI (OR 5.07, 95% CI 1.41–18.19, p: 0.013) (Table 2).
The predictive utility measures of pre- and postNAC TNDs for nipple involvement using < 10 and < 20 mm thresholds are presented in Table 3. The best LRs belonged to the postNAC positivity of the < 20 mm TND, with a + LR of 3.40, and − LR of 0.11 for nipple involvement. PreNAC positivity of the < 20 mm TND also had a similar − LR of 0.14. The accuracies of the TNDs were compared with the help of the AUCs of their ROC curves. The difference in AUCs of TNDs was 0.05 (95% CI − 0.02, 0.13) and not statistically significant (p = 0.126; Fig. 1), revealing similar predictive utilities for TNDs. Dot diagram of pre and postNAC tumor to nipple distance values of each patient according to their final nipple status are presented in Fig. 2.
Discussion
In this study, we analyzed the findings on breast MRI of patients on preNAC and postNAC settings to determine an optimum TND cut-off for achieving a tumor-free nipple. We found that using ≥ 2 cm TND-cut-off on both preNAC and postNAC MRI can rule out nipple positivity (< 20 mm TND, -LR:0.14 for preNAC and <20 mm TND, -LR: 0.11 for postNAC).
During the last decade, NSM with immediate breast reconstruction (IBR) in primary surgery setting has been proposed to be an alternative to simple mastectomy with acceptable postoperative complication rates [11]. The conservation of NAC has been shown to improve cosmesis and psychosexual well-being [12, 13]. Although lack of randomized trials evaluating oncologic safety of NSM with IBR on primary setting, the data are promising. Previous studies have shown acceptably low rates of cancer recurrence at the NAC after NSM (0–3.7%) [6]. For overall survival, the hazard ratio (HR) for NSM compared to non-NSM procedures was found to be 0.72 [11].
Determining the eligibility for NSM relies on preoperative clinical and radiological features of the cancerous breast. Breast MRI is known to be the most sensitive imaging modality for assessment of disease extent [9]. Current paradigms for NSM and the role of breast MRI to evaluate nipple invasion are mostly based on studies for upfront surgery setting. It was shown that contrast enhancement of nipple areola complex on MRI showed nipple involvement with a sensitivity of 93.8% and specificity of 85.7% [14]. Also, there is still ongoing discussion on optimal TND cut-off to preserve the nipple in patients undergoing NSM for breast cancer. Controversy still exists for ideal TND because skipped lesions remaining under the nipple may potentially appear as local recurrence and compromise the oncological safety of this procedure. A meta-analysis on predictive factors of nipple involvement in breast cancer included 27 studies and stratified patients into 4 groups based on the TND (< 2 vs. > 2 cm, < 2.5 vs. > 2.5 cm, < 3 vs. > 3 cm and < 4 vs. > 4 cm) and they found that highest pooled relative ratio (RR) was found in the subgroup “TND < 2.5 vs. > 2.5 cm” and concluded as TND = 2.5 cm may be considered for patient selection for NSM (RR 3.65, 1.42–9.33) [15]. There are previous trials examining the oncological safety of shorter TND in NSM. Frey et al. showed that TND ≤ 1 cm trended towards higher rates of locoregional recurrence (25%) compared to TND > 1 cm (2.4%). However there were only four patients in their series having TND ≤ 1 cm which is not sufficient to rationalize their high rate of nipple recurrence [16]. D’Alanzo et al. and Ponzone et al. showed that MRI can predict NAC involvement with cut-off TND at 1 cm with a sensitivity of 100% vs. 71% and specificity of 66% vs. 63%, respectively [17, 18]. Unfortunately, these studies have not yet been replicated in the neoadjuvant setting. We conducted our study on patients undergoing NAC and TND was measured as closest distance of nipple to the mass or the non-mass contrast enhancements. We showed that breast MRI has the 91.75 sensitivity and 57.% specificity in preNAC setting, 91.6% sensitivity and 73% specificity in postNAC setting with a TND using ≥ 2 cm TND-cut-off.
The significance of non-mass contrast enhancement extension to the nipple at MRI is also studied and it’s found that non-mass contrast enhancement extension has diagnostic accuracy of 88% in identifying tumor involvement of the nipple [19]. A current study focused on feasibility of NSM when non-mass contrast enhancement extension to the nipple resolves after neoadjuvant chemotherapy (NAC) and they showed that resolution was radiologically demonstrated in 70.5% of the cases. They also found that among the women in whom the non-mass contrast enhancement extension to the nipple resolved after NAC, the rate of pathology confirmed tumor invasion of the nipple was 2.6% [20]. We had 69 (38%) patients with pathologic complete response and 16% of them had TND < 10 mm with non-mass enhancement extension to the nipple. We can hypothesize that some of the patients in this group had nipple involvement which reversed after NAC.
In healthy breasts, skin thickening is approximately 0.5–2 mm at MR imaging and mammography [21, 22]. Pathologic nipple enhancement can be seen as nodular or irregular enhancement along the posterior borders and may be presented as nipple retraction on breast MRI [23]. We found that nipple areola complex skin thickening > 2 mm (OR 6.09; 95% CI 1.27–29.18; p: 0.024) and presence of nipple retraction (OR 5.07; 95% CI 1.41–18.19, p : 0.013) in preNAC MRI was associated with nipple involvement in final pathologic examination in multivariate analysis. Thirty-six patients had nipple retraction on preNAC MRI and 8 (32.3%) of them had nipple involvement on final pathology. Likewise, 27 patients had nipple retraction on postNAC MRI and 5 (18.5%) patients had nipple involvement on final pathology. Since we try to adapt the NSM for women following NAC, presence of nipple retraction on index MRI seems to be important to predict pathologic nipple involvement in a considerable number (32.3%) of the patients.
Contemporary nipple sparing mastectomy technique involves routine intraoperative subareolar tissue biopsies and frozen examination to disclose occult nipple involvement. In primary surgery setting, occult involvement of the nipple reported to be in less than 5% of the cases which is associated with TND, tumor size, grade, and nodal positivity [24]. Intraoperative frozen (IOF) evaluation of subareolar tissue aids for immediate decision for salvage of nipple areola complex and optimization of reconstructive planning. However, the IOF has a potential for overestimation in such cases as ductal hyperplasia, sclerosing adenosis, intracystic papilloma, lobular carcinoma in situ, and fat necrosis that leads to unnecessary nipple areola complex resection. Also, IOF was shown to have possible false negativity and lower estimation in lesions such as invasive lobular carcinoma, ductal carcinoma in situ and changes caused by neoadjuvant chemotherapy [25]. We have found that preNAC and postNAC breast MRI have impressive − LR in ≥ 2 cm TND-cut-off to predict negative nipple involvement which may eliminate unnecessary IOF examination in select cases.
Our study is limited by the retrospective nature of the chart review. We dichotomized the mastectomy patients according to final nipple tumor involvement and we did not revisit the pathology specimens and relied on the pathology reports. The strength of our study is the inclusion of non-mass enhancement extension on preNAC and postNAC MRI to calculate the optimal TND.
In conclusion, we found that ≥ 2 cm TND-cut-off in preNAC and postNAC breast MRI was associated with a higher likelihood of having negative nipple involvement. Presence of preNAC nipple retraction, preNAC nipple areola complex skin thickening (> 2 mm), postNAC persistence of multicentricity in breast MRI were found to be strong predictors of positive nipple involvement in permanent pathologic analysis.
Since NAC is a game changer achieving radiologic and pathologic complete response in considerable number of patients, we need long-term oncological outcomes to choose appropriate candidates for NSM after NAC particularly in patients who have shorter TND in index imaging and have radiologic complete response following NAC.
Data availability
The data of the study are available from the corresponding author upon request.
References
Rubens RD, Sexton S, Tong D, Winter PJ, Knight RK, Hayward JL (1980) Combined chemotherapy and radiotherapy for locally advanced breast cancer. Eur J Cancer 16(3):351–356. https://doi.org/10.1016/0014-2964(80)90352-7
Wolmark N, Fau WJ, Fau ME, Bryant J, Fisher B (2001) Preoperative chemotherapy in patients with operable breast cancer: nine-year results from National surgical adjuvant breast and bowel project B-18. J Natl Cancer Inst Monogr. https://doi.org/10.1093/oxfordjournals.jncimonographs.a003469
Bartholomew AJ, Dervishaj OA, Sosin M, Kerivan LT, Tung SS, Caragacianu DL et al (2019) Neoadjuvant chemotherapy and nipple-sparing mastectomy: timing and postoperative complications. Ann Surg Oncol 26(9):2768–2772. https://doi.org/10.1245/s10434-019-07418-4
Headon HL, Kasem A, Mokbel K (2016) The oncological safety of nipple-sparing mastectomy: a systematic review of the literature with a pooled analysis of 12,358 procedures. Arch Plast Surg 43(4):328–338. https://doi.org/10.5999/aps.2016.43.4.328
Burdge EC, Yuen J, Hardee M, Gadgil PV, Das C, Henry-Tillman R et al (2013) Nipple skin-sparing mastectomy is feasible for advanced disease. Ann Surg Oncol 20(10):3294–3302. https://doi.org/10.1245/s10434-013-3174-4
Galimberti V, Vicini E, Corso G, Morigi C, Fontana S, Sacchini V et al (2017) Nipple-sparing and skin-sparing mastectomy: review of aims, oncological safety and contraindications. Breast 34(Suppl 1):S82–S84. https://doi.org/10.1016/j.breast.2017.06.034
Santoro S, Loreti A, Cavaliere F, Costarelli L, La Pinta M, Manna E et al (2015) Neoadjuvant chemotherapy is not a contraindication for nipple sparing mastectomy. Breast 24(5):661–666. https://doi.org/10.1016/j.breast.2015.08.001
Agresti R, Sandri M, Gennaro M, Bianchi G, Maugeri I, Rampa M et al (2017) Evaluation of local oncologic safety in nipple-areola complex-sparing mastectomy after primary chemotherapy: a propensity score-matched study. Clin Breast Cancer 17(3):219–231. https://doi.org/10.1016/j.clbc.2016.12.003
Li H, Yao L, Jin P, Hu L, Li X, Guo T et al (2018) MRI and PET/CT for evaluation of the pathological response to neoadjuvant chemotherapy in breast cancer: a systematic review and meta-analysis. Breast 40:106–115. https://doi.org/10.1016/j.breast.2018.04.018
Londero V, Bazzocchi M, Del Frate C, Puglisi F, Di Loreto C, Di Loreto C, Francescutti G et al (2004) Locally advanced breast cancer: comparison of mammography, sonography and MR imaging in evaluation of residual disease in women receiving neoadjuvant chemotherapy. Eur Radiol 14(8):1371–1379. https://doi.org/10.1007/s00330-004-2246-z
Mota BS, Riera R, Ricci MD, Barrett J, de Castria TB, Atallah ÁN et al (2016) Nipple- and areola-sparing mastectomy for the treatment of breast cancer. Cochrane Database Syst Rev 11(11):CD008932
Esgueva AJ, Noordhoek I, Kranenbarg EM, Espinosa-Bravo M, Mátrai Z, Zhygulin A et al (2022) Health-related quality of life after nipple-sparing mastectomy: results from the INSPIRE registry. Ann Surg Oncol 29(3):1722–1734. https://doi.org/10.1245/s10434-021-10930-1
Yoon-Flannery K, DeStefano LM, De La Cruz LM, Fisher CS, Lin LY, Coffua LS et al (2018) Quality of life and sexual well-being after nipple sparing mastectomy: a matched comparison of patients using the breast Q. J Surg Oncol 118(1):238–242. https://doi.org/10.1002/jso.25107
Moon JY, Chang YW, Lee EH, Seo DY (2013) Malignant invasion of the nipple-areolar complex of the breast: usefulness of breast MRI. Am J Roentgenol 201(2):448–455. https://doi.org/10.2214/AJR.12.9186
Zhang H, Li Y, Moran MS, Haffty BG, Yang Q (2015) Predictive factors of nipple involvement in breast cancer: a systematic review and meta-analysis. Breast Cancer Res Treat 151(2):239–249. https://doi.org/10.1007/s10549-015-3385-4
Frey JD, Salibian AA, Lee J, Harris K, Axelrod DM, Guth AA et al (2019) Oncologic trends, outcomes, and risk factors for locoregional recurrence: an analysis of tumor-to-nipple distance and critical factors in therapeutic nipple-sparing mastectomy. Plast Reconstr Surg 143(6):1575–1585. https://doi.org/10.1097/PRS.0000000000005600
D’Alonzo M, Martincich L, Biglia N, Pisacane A, Maggiorotto F, Rosa GD et al (2012) Clinical and radiological predictors of nipple-areola complex involvement in breast cancer patients. Eur J Cancer 48(15):2311–2318. https://doi.org/10.1016/j.ejca.2012.04.017
Ponzone R, Maggiorotto F, Carabalona S, Rivolin A, Pisacane A, Kubatzki F et al (2015) MRI and intraoperative pathology to predict nipple-areola complex (NAC) involvement in patients undergoing NAC-sparing mastectomy. Eur J Cancer 51(14):1882–1889. https://doi.org/10.1016/j.ejca.2015.07.001
Bae SJ, Cha YJ, Eun NL, Ji JH, Kim D, Lee J et al (2021) Diagnostic accuracy of nonmass enhancement at breast MRI in predicting tumor involvement of the nipple: a prospective study in a single institution. Radiology 301(1):47–56. https://doi.org/10.1148/radiol.2021204136
Bae SJ, Ahn SG, Park EJ, Eun NL, Kim JH, Ji JH et al (2023) Resolution of nonmass enhancement extension to the nipple at breast MRI after neoadjuvant chemotherapy: pathologic response and feasibility for nipple-sparing mastectomy. Radiology 307(2):e221777. https://doi.org/10.1148/radiol.221777
Kalli S, Freer PE, Rafferty EA (2010) Lesions of the skin and superficial tissue at breast MR imaging. Radiographics 30(7):1891–1913. https://doi.org/10.1148/rg.307105064
Liberman L, Breast MRI (2005) Diagnosis and intervention. Springer Science+Business Media Inc, New York
Nicholson BT, Harvey JA, Cohen MA (2009) Nipple-areolar complex: normal anatomy and benign and malignant processes. Radiographics 29(2):509–523. https://doi.org/10.1148/rg.292085128
Zaborowski AM, Roe S, Rothwell J, Evoy D, Geraghty J, McCartan D et al (2023) A systematic review of oncological outcomes after nipple-sparing mastectomy for breast cancer. J Surg Oncol 127:361–368. https://doi.org/10.1002/jso.27115
Morales Piato JR, Aguiar FN, Mota BS et al (2015) Improved frozen section examination of the retroareolar margin for prediction of nipple involvement in breast cancer. Eur J Surg Oncol 41:986–990. https://doi.org/10.1016/j.ejso.2015.04.019
Funding
This study did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
MUU, BMG: the conception and design of the study, or analysis and interpretation of data, drafting and revising the article. OB, AA, HK, TTA: Design of the study, interpretation of the data, revising. HA: Analysis, statistical analysis and supervision, drafting the article.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to disclose.
Ethical approval
The study was approved by Institutional Ethics Board.
Consent for publication
This paper does not contain any individual-level data.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ugurlu, M.U., Bugdayci, O., Akmercan, A. et al. Prediction of nipple involvement in breast cancer after neoadjuvant chemotherapy: Should we rely on breast MRI to preserve the nipple?. Breast Cancer Res Treat 201, 417–424 (2023). https://doi.org/10.1007/s10549-023-07041-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-023-07041-8