Abstract
Nipple-sparing mastectomy (NSM) has recently been increasing in popularity due to a better cosmetic outcome and quality-of-life benefit. The radiologic distance between the tumor and the nipple is independently predictive of nipple-areolar complex involvement and can assist in patient selection for NSM. However, concordance between the preoperative radiologic imaging and histologic evaluation would play a major role in making patient selection for NSM meaningful. We analyzed the pathologic-radiologic correlation for evaluation of retroareolar (RA) margin in NSM. A retrospective histologic and blinded radiologic review of 80 NSM (41 therapeutic and 39 prophylactic) performed on 45 patients was done. Histologically, the cases were divided into positive or close (invasive or in situ carcinoma within 5 mm of the RA margin) and negative (greater than 5 mm from the RA margin). Radiographically, positive cases were defined as suspicious enhancement and/or suspicious findings within 20 mm of the nipple on magnetic resonance imaging (MRI) and/or diagnostic mammography, respectively. Thirty five of 41 (85.4 %) therapeutic cases were concordant. Six cases were discordant, with 2/41 (4.9 %) discordant cases classified as positive at histology, but negative on imaging and 4/41 (9.75 %) discordant cases classified as negative at histology, but positive on imaging. Agreement between pathology and radiology was moderate [kappa coefficient 0.54 (p = 0.0003)].We conclude that there is a significant agreement between histologic and radiologic evaluation for assessment of RA margin and preoperative radiologic imaging; specifically, MRI provides valuable information and should be strongly recommended to help select patients for NSM.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nipple-sparing mastectomy (NSM) has recently been increasing in popularity due to a better cosmetic outcome and quality-of-life benefit [1–4]. Proper patient selection is imperative for disease-free margins and preservation of the nipple-areolar complex (NAC). In carefully selected patients, NSM appears to be an acceptable technique in both prophylactic and malignant settings [1–4]. There is considerable variation in inclusion criteria for NSM patient selection in the literature. Examples of such criteria include size of the tumor, distance of tumor to nipple on imaging, extent and location of disease, histopathologic features, and human epidermal growth factor receptor 2 (Her2) status [1–3, 5–7]. The radiologic distance between the tumor and the nipple is independently predictive of NAC involvement in multiple studies and can assist in patient selection for NSM [5–10]. However, in these situations, concordance between the preoperative radiologic imaging and histologic evaluation would play a major role in making patient selection for NSM meaningful.
Therefore, the aim of the present study is to analyze the pathologic-radiographic correlation (PRC) for evaluation of retroareolar (RA) margin in patients undergoing NSM.
Materials and methods
After approval from the institutional review board (IRB), a Health Insurance Portability and Accountability Act (HIPAA)-compliant retrospective review of pathology database was performed to identify all NSMs performed between July 2010 and July 2013.
An extensive histologic assessment of the NSM specimen was performed with review of conventional hematoxylin and eosin-stained sections. Involvement by invasive carcinoma with subtype, ductal carcinoma in situ (DCIS), and lobular carcinoma in situ (LCIS) was assessed along with margin status with special emphasis on the RA margin. Our usual sampling protocol for evaluation of RA region includes extensive sampling of any grossly suspicious-looking area along with two random samples of the RA quadrant. If the initial histologic examination is negative then the grossly suspicious area is entirely submitted.
The exact distance of invasive or in situ carcinoma from the RA margin was noted if less than or equal to 5 mm. Pathologically, all specimens were divided into positive/close and negative RA margins. The pathologically positive/close RA margin cases were defined as invasive or in situ carcinoma within 5 mm of the RA margin, and pathologically negative RA margin were defined as invasive or in situ carcinoma greater than 5 mm from the RA margin, as this was the standard of practice in our institution at the time of this study.
A completely blinded radiologic review was also performed for all these NSM specimens (both prophylactic and therapeutic), wherein three dedicated breast radiologists (SES, JAM, ALC) independently and blindly reviewed mammographic and magnetic resonance imaging (MRI) images from all these patients without prior knowledge of the final pathologic diagnosis or margin status. All 45 patients had undergone bilateral breast MRI, and 39 of these patients had corresponding bilateral mammograms available for review. Six of the 45 patients did not have a complete diagnostic mammogram available for review as they presented from an outside institution.
Radiographically, positive cases were defined as suspicious enhancement within 20 mm of the nipple on MRI and/or suspicious findings within 20 mm of the nipple on mammography. Tumor-to-nipple distance was measured from the base of the nipple to the enhancing subareolar mass or non-mass enhancement (NME) [11]. Dynamic contrast-enhanced first subtraction axial and non-subtracted fat-saturated axial T1-weighted magnetic resonance images were used for the evaluation of enhancement of the NAC and RA tissue. The mammographic images and MR images were reviewed independently first and then any discrepant findings among the three breast radiologists were rereviewed jointly to develop a final consensus on positive or negative radiographic RA margins. Subsequently, the radiologic images of all pathologic-radiologic discordant cases were rereviewed in retrospect by the radiologists for a second opinion after having the final pathologic data in hand. A correlation was performed between the pathologic (histologic) and radiologic findings. A kappa coefficient was estimated to measure agreement between pathologic and radiologic findings.
We acknowledge that there is no universally accepted standard cutoff for close/positive RA margin in NSM, and a cutoff of 5 mm may not be considered as a standard of care in other institutions. Hence, the raw data was also reanalyzed using an alternate definition of 1 mm or less as close margin, which may be used as a standard of care in some other institutions [12, 13].
Results
Patient characteristics
A total of 86 NSM performed in 48 patients were retrieved after retrospective review of pathology database from 2010 to July 2013. A total of 5 NSM performed in three patients were excluded from the study because these were patients referred from outside hospitals with nonavailability of outside radiologic images for review at this institution. Hence, a total of 80 NSM performed in 45 patients were included in this study.
The patients in this study were all women with ages ranging from 27 to 65 years with a mean age of 47 years.
Indications
The procedures performed were 36 bilateral NSM, four left NSM and four right NSM.
A number of 41 of 80 NSM (51.2 %) were performed owing to therapeutic indications. These consisted of 23 invasive ductal carcinoma (IDC), four invasive lobular carcinoma (ILC), two metaplastic carcinomas, one adenoid cystic carcinoma, eight DCIS, one pleomorphic LCIS, and two LCIS.
A number of 39 of 80 NSM (48.8 %) were performed because of prophylactic indications. These consisted of bilateral prophylactic NSM in four patients, contralateral prophylactic NSM in 29 patients with therapeutic NSM performed in the opposite breast owing to a biopsy-proven diagnosis of either invasive or in situ carcinoma, and two unilateral prophylactic left NSM. No invasive carcinoma or DCIS was found in prophylactic mastectomies. LCIS, classic type, was found in one prophylactic mastectomy.
Pathologic and radiologic features in therapeutic mastectomies
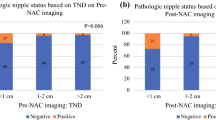
Histologically, seven of 41 cases (17.1 %) had positive findings of invasive or in situ carcinoma within 5 mm of the RA margin. These consisted of three specimens with IDC and DCIS, two specimens with DCIS, and one each with IDC and ILC with LCIS, classic type. One of these seven specimens had IDC and DCIS at the RA margin (patient 41, Table 1, Fig. 1) and another specimen had LCIS, classic type, at the margin (patient 20, Table 1), while the remaining five specimens had either invasive or in situ carcinoma within 5 mm of the RA margin, but not at the margin.
Concordant case 41. a Axial fat-saturated, T1-weighted post-contrast magnetic resonance image with focal non-mass enhancement (NME) (white arrow) extending to the nipple and involving the posterior margin of the nipple. b Corresponding photomicrograph showing cauterized invasive and in situ carcinoma (black arrow) present at the retroareolar margin (orange ink) (hematoxylin and eosin, ×100)
Radiologically, nine of 41 cases (21.95 %) were interpreted as positive by all three breast radiologists (Table 1). One patient (patient 41, Table 1, Fig. 1) with multifocal, multicentric breast carcinoma demonstrated ipsilateral clumped NME up to and involving the nipple, measured as 0 mm on MRI, a highly suspicious finding. Additionally, this was the only patient who was suspected to very likely have an extensive intraductal component by MRI. Also, this was the only patient who had positive mammographic findings, those being fine pleomorphic calcifications in a segmental distribution extending to 12 mm behind the nipple. The remaining eight cases demonstrated suspicious MRI NME within 20 mm of the nipple ipsilateral to the location of their known breast carcinoma, all with no suspicious corresponding mammographic abnormality.
A total of 35 of 41 (85.4 %) cases performed for therapeutic indications had concordance between pathologic and radiologic findings. These were further divided into:
-
Concordant negative cases—30 of 41 (73.2 %) cases with negative RA margins based on both histologic and radiologic reviews.
-
Concordant positive cases—5 of 41 cases (12.2 %) interpreted as positive on radiologic review and positive/close margins on histologic review (Fig. 1a, b and 2a, b). The histologic findings and other patient characteristics in the positive concordant cases are summarized in Tables 1 and 3. The patient who had LCIS at the margin (patient 20) had a MRI correlate in the form of NMLE, and hence, this was taken as a concordant finding [14].
Fig. 2 Concordant case 17. a Axial fat-saturated, T1-weighted post-contrast magnetic resonance image with suspicious focus (white arrow) 14 mm from the nipple seen anterior to implant. b Corresponding photomicrograph showing ductal carcinoma in situ (black arrow) seen 4 mm from cauterized retroareolar margin (orange ink) (hematoxylin and eosin, ×40)
The remaining six cases (14.6 %) were discordant. These were further divided into
-
Discordant cases classified as negative at histology, but positive on imaging—4 of 41 cases (9.75 %) (Table 1, Fig. 3a, b)
Fig. 3 Discordant case 15. a Axial fat-saturated, T1-weighted post-contrast magnetic resonance image with clumped non-mass enhancement (NME) in the central retroareolar right breast that extended in a segmental distribution from her newly diagnosed DCIS in her 11 o’clock and 12 o’clock posterior breast (not completely imaged). The NME extends to within 10 mm of her nipple (white arrow). b Photomicrograph showing negative retroareolar margin with benign breast parenchyma (orange ink) (hematoxylin and eosin, ×100)
-
Discordant cases classified as positive/close by histology, but negative on imaging—2 of 41 cases (4.9 %) (Table 2, Fig. 4a, b)
Table 2 Patients with close/positive margin on histology and negative mammography and magnetic resonance imaging (MRI) Fig. 4 Discordant case 26. a Axial fat-saturated, T1-weighted post-contrast magnetic resonance image with no suspicious enhancement seen within 20 mm from the nipple. Her primary malignancy is seen in the posterior breast (white arrow). b Photomicrograph showing neoplastic cells in lymphovascular spaces present 0.5 mm from retroareolar margin (orange ink) (hematoxylin and eosin, ×100)
-
The histologic findings and other patient characteristics seen in these cases are summarized in Tables 2 and 3.
Table 3 Additional patient and tumor characteristics in patients with positive radiologic and/or histologic findings
The latter two discordant cases (pathology positive/close, negative radiology) were rereviewed in retrospect by the breast radiologists and were confirmed as negative. One of the two cases had foci of neoplastic cells within lymphovascular spaces present within 0.5 mm of the RA margin. The rereview of radiologic images appeared to show a linear area of NME extending from the known carcinoma, but on further inspection, this area was bright on the precontrast dynamic sequence and was interpreted as proteinaceous material in the duct. There was no suspicious enhancement on subtraction images. The other case had DCIS within 4 mm of the RA margin and was interpreted as negative by all three breast radiologists, even in retrospect.
Pathologic and radiologic correlations in therapeutic mastectomies (41 cases)
There was agreement in 85.4 % of therapeutic samples. The concordance between pathology and radiology was moderate and statistically significant as indicated by a kappa coefficient of 0.54 (p = 0.0003) [15].
Pathologic and radiologic correlations in all mastectomies (80 cases)
Since a blinded radiologic review was performed for all NSM (including both prophylactic and therapeutic), concordance between pathology and radiology was also estimated for the entire set of 80 NSM. Agreement between pathology and radiology was moderate and statistically significant as indicated by a kappa coefficient of 0.59 (p < 0.0001) [15].
Pathologic and radiologic correlations in therapeutic mastectomies (41 cases) using alternate histologic cutoff of 1 mm or less as close margin
Agreement between pathology and radiology was on the threshold between moderate and substantial agreements and was statistically significant as indicated by a kappa coefficient of 0.5957 (p < 0.0001) [15].
Discussion
Over the years, surgical treatment of breast carcinoma has evolved from radical mastectomy to less invasive and more cosmetically acceptable procedures like lumpectomy and NSM, which entails preservation of the NAC [1–4].
Studies assessing the rate of nipple involvement in mastectomies have shown a wide range of occult nipple involvement in the literature varying from 0 to 53 % with some recent studies quoting this number to be around 10 to 11 % [1–3, 6, 7, 9, 16]. These findings suggest that proper patient selection for NSM is imperative even in patients who have clinically normal-appearing NAC. Several studies have shown that there are many factors that predict involvement of the NAC, and these include tumor-to-nipple distance, tumor size, type, location and extent of disease, lymph node status, lymphovascular invasion (LVI), and Her2 amplification. There is convincing evidence to suggest that suitable patients for NSM should have well-circumscribed single or multifocal tumors with an appropriate tumor-to-nipple distance. Tumors should not be poorly differentiated, should not have LVI or axillary node metastasis, and should be non-amplified for Her2. However, many of these variables either cannot be assessed or are not definitively known before the patient undergoes surgery [1–3, 5–11, 16].
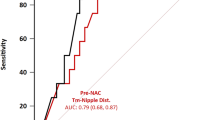
Among all of these factors, the preoperative radiologic distance between the tumor to the nipple is one of the most significant factors predicting NAC involvement as also confirmed by prior studies which have carried out multivariate analysis. Tumor-to-nipple distance is crucial for proper selection of good candidates for NSM [5–11, 16–24]. The appropriate cutoff distance in the literature varies and has been mostly reported variably as 20 mm or 10 mm, with the majority of the studies quoting 20 mm as a cutoff, based on strong evidence [1, 25–28]. Notably, a study by Billar et al. concluded that preoperative clinical and imaging abnormalities at the NAC were the only independent predictors of positive involvement of the NAC by the neoplastic cells, and a lack of these factors confers a low likelihood of positive NAC [29]. Lowen et al. [9] and Rusby et al. [10] have developed and established predictive models based on preoperative imaging, wherein the distance of the primary tumor from NAC predicted the risk of possible involvement of the nipple. The former model had a cutoff of 4.96 cm, and a distance of less than 4.96 cm predicted involvement of the NAC with a sensitivity of 82%, specificity of 62%, a positive predictive value of 20%, and a negative predictive value of 97% [9]. The latter study did not establish a specific cutoff but allowed an individualized risk prediction [10]. Both of these models have yet to be confirmed externally.
However, a caveat is that the distance of the tumor to the nipple is highly predictive for nipple involvement in well-circumscribed purely invasive carcinomas. On the contrary, tumors that are poorly circumscribed with a significant intraductal component may show a higher rate of nipple involvement even with a minimum tumor-to-nipple distance [1].
Preoperative radiologic assessment of the distance between the nipple and the tumor, usually by mammogram and MRI, aids the surgeon in the appropriate selection of candidates for NSM [8–10, 27, 28, 30]. MRI has a higher sensitivity than does mammography for the assessment of the nipple and retroareolar tumors [30]. Notably in our study also, only one of the nine patients who had positive findings on MRI showed positive mammographic findings. In our institution, the radiologists and surgeons use a radiologic cutoff of 20 mm to assess positive involvement of the NAC. Additionally, the surgeons consider tumor at the inked RA margin as “positive” and any tumor within more than 0 mm to 5 mm from RA margin in NSM as “close,” and these patients are potentially treated as positive. The RA margin is treated differently in our institution from other margins because NSM is a relatively recent technique with very limited data regarding long-term outcomes and follow-up studies in patients with close RA margins in the literature [31]. This is slightly different from other margins where tumor at the inked margin is still considered positive, however, is considered close with a distance that varies from 1 to 3 mm from the inked margin in different institutions [32–36]. In our institution, any tumor within more than 0 mm to 2 mm is considered close for non-RA margins. Although, the clinical significance of making this distinction between positive and close margin is still uncertain with some authors recommending surgical re-excision and/or radiation only in positive margins while others recommending additional therapy in both positive as well as close margins [32–36]. However, given the limited literature about positive or close RA margins in NSM, the surgeons in our institution choose to treat any patients with closer than 5 mm RA margin on final excision as positive, although we acknowledge that this may not be the standard of care in many other institutions. Hence, in this study, we also reanalyze the data using a different cutoff for close margin (1 mm or less) so that the results may be more pertinent to other institutions as well [12, 13, 31, 37].
Since the basis of preoperative positive NAC is based on radiologic imaging, the concordance of pathologic and radiologic data plays a very important role for proper patient selection and further treatment decisions in patients undergoing NSM. Needless to say, any discordance subjects the patient to an additional surgical procedure at a minimum, which increases the surgical cost as well as operative morbidity.
Hence, in this study, we aimed to study the PRC between the pathologic and radiologic findings. We found that the concordance between pathology and radiology was moderate and statistically significant. Notably, when using an alternate histologic cutoff of 1 mm or less as close margin, the agreement between pathology and radiology increases further and is on the threshold between moderate and substantial agreement.
The six discordant cases consisted of two cases classified as close or positive margins by histology, but negative on imaging. The remaining four discordant cases were classified as negative at histology, but positive on imaging. Although some of these cases could be surely be false positive cases on radiologic imaging, the remote possibility of sampling issue and missing a minute focus of neoplasm cannot be entirely excluded despite an extensive sampling protocol described above.
Another conflict in this study is the management of classic LCIS, when present on the inked RA margin in NSM (as in patient no. 20). We agree that many clinicians would not count this as a positive margin. However, this patient was treated with subsequent nipple excision. This approach is similar to the opinion of Eisenberg and colleagues who treated LCIS, including classic LCIS at the RA margin as both a “precursor” as well as a “marker” lesion and considered additional treatment as optimal given the limited follow-up data in the literature regarding outcomes of patients in which LCIS is present at the inked RA margin [37]. We completely acknowledge that other institutions may have differing views.
All the positive concordant cases and all discordant cases with positive or close pathology in our institution were subsequently treated with nipple excision, and any other additional therapy like chemotherapy or radiotherapy was customized per patient based on additional variables like lymph nodal status, tumor grade, status of other margins, and hormonal and Her2 status. Although patients with positive imaging findings were not initially good candidates for NSM, a decision to pursue NSM in these patients was based on patient preference as well as other favorable factors like size, nodal status on imaging, Her2 status, and location of the main tumor.
In summary, there is a significant agreement between pathologic and preoperative MRI evaluations for the assessment of the RA margin. The above data further reinforces that the patients with positive preoperative MRI imaging are not good candidates for NSM despite other favorable factors, and NSM should be strongly discouraged in these patients as it increases the patient morbidity and surgical costs. There were only two cases in this study with positive/close pathology and negative imaging, and one of these two cases had positive focus in the form of LVI. This further reinforces that radiologic imaging, specifically MRI, provides valuable information to help select patients for NSM, and preoperative MRI should be strongly considered in this patient population.
References
Mallon P, Feron JG, Couturaud B et al (2013) The role of nipple-sparing mastectomy in breast cancer: a comprehensive review of the literature. Plast Reconstr Surg 131:969–984
Rusby JE, Smith BL, Gui GPH (2010) Nipple-sparing mastectomy. Br J Surg 97:305–316
Li W, Wang S, Guo X et al (2011) Nipple involvement in breast cancer: retrospective analysis of 2323 consecutive mastectomy specimens. Int J Surg Pathol 19:328–334
Didier F, Radice D, Gandini S et al (2008) Does nipple preservation in mastectomy improve satisfaction with cosmetic results, psychological adjustment, body image and sexuality? Breast Cancer Res Treat 118:623–633
Brachtel EF, Rusby JE, Michaelson JS et al (2009) Occult nipple involvement in breast cancer: clinicopathologic findings in 316 consecutive mastectomy specimens. J Clin Oncol 27:4948–4954
Vlajcic Z, Zic R, Stanec S et al (2005) Nipple-areola complex preservation: predictive factors of neoplastic nipple-areola complex invasion. Ann Plast Surg 55:240–244
Kissin MW, Kark AE (1987) Nipple preservation during mastectomy. Br J Surg 74:58–61
Schecter AK, Freeman MB, Giri D et al (2006) Applicability of the nipple–areola complex-sparing mastectomy: a prediction model using mammography to estimate risk of nipple–areola complex involvement in breast cancer patients. Ann Plast Surg 56:498–504
Loewen MJ, Jennings JA, Sherman SR et al (2008) Mammographic distance as a predictor of nipple–areola complex involvement in breast cancer. Am J Surg 195:391–394
Rusby JE, Brachtel EF, Othus M et al (2008) Development and validation of a model predictive of occult nipple involvement in women undergoing mastectomy. Br J Surg 95:1356–1361
Moon JY, Chang YW, Lee EH et al (2013) Malignant invasion of the nipple-areolar complex of the breast: usefulness of breast MRI. Am J Roentgenol 201:448–455
Untch M, Gerber B, Harbeck N et al (2013) 13th St. Gallen international breast cancer conference 2013: primary therapy of early breast cancer evidence, controversies, consensus—opinion of a German team of experts (Zurich 2013). Breast Care (Basel) 8:221–229
Harbeck N, Thomssen C, Gnant M (2013) St. Gallen 2013: brief preliminary summary of the consensus discussion. Breast Care (Basel) 8(2):102–109
Strigel RM, Eby PR, Demartini WB et al (2010) Frequency, upgrade rates, and characteristics of high-risk lesions initially identified with breast MRI. AJR Am J Roentgenol 195:792–798
Landis JR, Koch GG (1977) The measurement of observer agreement for categorical data. Biometrics 33:159–174
Lagios MD, Gates EA, Westdahl PR et al (1979) A guide to the frequency of nipple involvement in breast cancer: a study of 149 consecutive mastectomies using a serial subgross and correlated radiographic technique. Am J Surg 138:135–142
Jitendra J, Chinoy RF, Vaidyat JS (1998) Prediction of nipple and areola involvement in breast cancer. Eur J Surg Oncol 24:15–16
Pirozzi PR, Rossetti C, Carelli I et al (2010) Clinical and morphological factors predictive of occult involvement of the nipple-areola complex in mastectomy specimens. Eur J Obstet Gynecol Reprod Biol 148:177–181
Lüttges J, Kalbfleisch H, Prinz P (1987) Nipple involvement and multicentricity in breast cancer: a study on whole organ sections. J Cancer Res Clin Oncol 113:481–487
Cucin RL, Guthrie RH Jr, Luterman A et al (1980) Screening the nipple for involvement in breast cancer. Ann Plast Surg 5:477–479
Wijayanayagam A, Kumar AS, Foster RD et al (2008) Optimizing the total skin-sparing mastectomy. Arch Surg 143:38–45
Verma GR, Kumar A, Joshi K (1997) Nipple involvement in peripheral breast carcinoma: a prospective study. Indian J Cancer 34:1–5
Quinn RH, Barlow JF (1981) Involvement of the nipple and areola by carcinoma of the breast. Arch Surg 116:1139–1140
Khan K, Chakraborti S, Mondal S (2010) Morphological predictors of nipple areola involvement in malignant breast tumors. Indian J Pathol Microbiol 53:232–237
de Alcantara FP, Capko D, Barry JM et al (2011) Nipple-sparing mastectomy for breast cancer and risk-reducing surgery: the Memorial Sloan-Kettering Cancer Center experience. Ann Surg Oncol 18:3117–3122
Alperovich M, Tanna N, Samra F et al (2013) Nipple-sparing mastectomy in patients with a history of reduction mammaplasty or mastopexy: how safe is it? Plast Reconstr Surg 131:962–967
D’Alonzo M, Martincich L, Biglia N et al (2012) Clinical and radiological predictors of nipple-areola complex involvement in breast cancer patients. Eur J Cancer 48:2311–2318
Steen ST, Chung AP, Han S-H et al (2013) Predicting nipple–areolar involvement using preoperative breast MRI and primary tumor characteristics. Ann Surg Oncol 20:633–639
Billar JA, Dueck AC, Gray RJ et al (2011) Preoperative predictors of nipple–areola complex involvement for patients undergoing mastectomy for breast cancer. Ann Surg Oncol 18:3123–3128
Friedman EP, Hall-Craggs MA, Mumtaz H et al (1997) Breast MR and the appearance of the normal and abnormal nipple. Clin Radiol 52:854–861
Kneubil MC, Lohsiriwat V, Curigliano G et al (2012) Risk of locoregional recurrence in patients with false-negative frozen section or close margins of retroareolar specimen in nipple-sparing mastectomy. Ann Surg Oncol 19:4117–4123
Papa MZ, Zippel D, Koller M et al (1999) Positive margins of breast biopsy: is reexcision always necessary? J Surg Oncol 70:167–171
Gage I, Schnitt SJ, Nixon AJ et al (1996) Pathologic margin involvement and the risk of recurrence in patients treated with breast-conserving therapy. Cancer 78:1921–1928
Schnitt SJ, Abner A, Gelman R et al (1994) The relationship between microscopic margins of resection and the risk of local recurrence in patients with breast cancer treated with breast-conserving surgery and radiation therapy. Cancer 74:1746–1751
Pittinger TP, Maronian NC, Poulter CA et al (1994) Importance of margin status in outcome of breast-conserving surgery for carcinoma. Surgery 116:605–608
Kunos C, Latson L, Overmoyer B et al (2006) Breast conservation surgery achieving > or = 2 mm tumor-free margins results in decreased local-regional recurrence rates. Breast J 12:28–36
Eisenberg RE, Chan JS, Swistel AJ et al (2014) Pathological evaluation of nipple-sparing mastectomies with emphasis on occult nipple involvement: the Weill-Cornell experience with 325 cases. Breast J 20:15–21
Acknowledgments
Dr. Alison Chetlen and Dr. Susann Schetter were paid research consultants for Siemens in October and November 2014. This has no relation to the current research study. The other authors do not have any sources of support or disclosure of funding.
Conflict of interest
We declare that we have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Portions of this study were presented as an abstract/poster at the USCAP 2014 meeting, San Diego.
Rights and permissions
About this article
Cite this article
Karamchandani, D.M., Chetlen, A.L., Riley, M.P. et al. Pathologic-radiologic correlation in evaluation of retroareolar margin in nipple-sparing mastectomy. Virchows Arch 466, 279–287 (2015). https://doi.org/10.1007/s00428-014-1714-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-014-1714-3