Abstract
Purpose
Endoscopic nipple-sparing mastectomy (E-NSM) has been reportedly associated with smaller scars and greater patient satisfaction; however, long-term results of this procedure have not been made. The purpose of this retrospective study was to investigate the local recurrence (LR) rate and factors associated with it after E-NSM and to examine the oncologic safety of this procedure.
Methods
We reviewed the medical records of a total of 421 breasts in 404 patients who underwent E-NSM to investigate the LR rate and the factors associated with it. The clinico-pathological features and the treatment and outcomes of the patients with LRs were also examined.
Results
Eleven breasts (2.6 %) in 11 patients presented with LR as the first site of recurrence after a median follow-up time of 61 months. Among the 11 LRs, 9 patients presented with LR only, 1 patient exhibited regional lymph node recurrence, and 1 patient exhibited distant metastasis. The median time from surgery until LR was 25 months. Eight LRs developed near the original tumor site. The risk factors for LR in a multivariate analysis were a younger age of less than 40 years (p = 0.02), Stage III tumor (p = 0.01), and an inadequate surgical margin (p = 0.001). After the treatment, 6 patients had no evidence of disease, 2 patients died from metastatic disease, 2 patients experienced repeat LR, and the remaining patient who rejected excision exhibited a persistent LR.
Conclusions
E-NSM is an oncologically safe procedure and an acceptable method in selected patients requiring a mastectomy.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
A nipple-sparing mastectomy (NSM) is a procedure that can be applied as part of a skin-sparing mastectomy (SSM), in which the nipple-areola complex is preserved for cosmetic reasons [1]. The indications for NSM are clinically decided after considering several risk factors for nipple involvement, including a central tumor location, a large tumor size, nodal positivity, severe lymph-vascular invasion (LVI), and multicentricity or multifocality [2–5]. Therefore, NSM tends to be offered to relatively earlier stage breast cancer patients. Regarding the oncologic safety of NSM, many studies have reported a low rate of recurrence. In their systematic review, Endara et al. [6] reported that local recurrence (LR) rate was 1.8 % (range, 0–19 %) and that the distant metastasis rate was 2.2 % (range, 0–44 %) with a follow-up period ranging from 0.2 to 210 months.
The addition of endoscopy to NSM for patients with breast cancer was introduced in the early 1990s and is reportedly associated with smaller scars and greater patient satisfaction [7–9]. However, few reports on endoscopic NSM (E-NSM) have been made, and most of these studies had relatively short follow-up periods and small patient samples [7–11].
The purpose of this retrospective study was to investigate the LR rate and the factors associated with it after E-NSM and to examine the oncologic safety of this procedure.
Materials and methods
The indications for E-NSM at our institution consist of breast cancer for which skin, nipple-areola complex, or muscle involvement is not suspected based on a physical examination, mammography, or magnetic resonance imaging (MRI) examination. If a suspicious intraductal extension was observed near the nipple (as assessed using MRI), a frozen-section biopsy of the base of the nipple revealed tumor involvement, or a bloody nipple discharge was observed, then the major ducts within the nipple were cored at the time of the operation. If malignant cells in the cored duct were identified in the final pathological assessment, the nipple was removed during a second scheduled surgery. Before undergoing E-NSM, the patients were thoroughly informed of the possible risks and advantages related to the preservation of the nipple-areola complex and the use of an endoscopic technique, and E-NSM was performed for patients who consented to undergo this procedure.
Surgical procedure
Preoperative markings were made on the skin over the area of the resection. The level of the nipple was also marked in the midline of the body with the patient in a standing position. After induction of general anesthesia, the patient was placed in the flat supine position with the arm abducted to 90° (Fig. 1). Video monitors were set on both sides of the patient. Figure 2 shows the tools used for this surgery.
Sentinel lymph node (SLN) biopsy
When performing the SLN biopsy, it was carried out using the blue dye and radioisotope method. A small skin incision was made along the skin crease of the axilla. Dissection of the subcutaneous tissue was carried out, down to the lateral border of the pectoralis major muscle. The stained lymph node was found by following the blue lymphatic channels, and the SLN was removed under direct vision. SLN biopsy was also carried out with gamma probe guidance. If the patient was found to have a positive sentinel lymph node, a complete axillary node dissection was performed after extension of the incision.
Dissection of the reverse side of the breast
The dissection of the fascia off the anterior surfaces of the pectoralis major muscle was carried out as far as possible under direct vision. Then, a Vein Retractor (Karl Storz, Tuttlingen, Germany) was placed into the space, and the dissection of the pectoralis fascia was continued endoscopically by pushing the Vein Retractor along the muscle fibers or cutting the fascia using Powerstar bipolar scissors (Johnson & Johnson, NJ, USA) or electrocautery with the Vein Retractor pulled up to create a sufficient working space (Fig. 3). Arterial perforators were coagulated as needed. This process was repeated distally and proximally until the breast tissue along with the pectoral fascia was dissected off the major pectoral muscle up to a level below the clavicle superiorly, up to the inframammary fold inferiorly, and to the parasternal line medially. On the lateral side, the breast was dissected from the serratus fascia under endoscopic vision.
Create a skin flap
A periareolar semicircular incision was made, and gentle retraction of the flaps was established with skin hooks. In cases where nipple coring was indicated, the major ducts within the nipple were removed. Then, flaps were raised in the subdermal plane. Mamma retractor/light guide (FOUR MEDICS, Tokyo, Japan) was used to provide illumination and gentle traction on the skin flaps for exposure. When the dissection of the skin flaps reached the standard boundaries of the breast, the previously dissected reverse side of the breast was exposed by cutting the subcutaneous tissue toward the chest wall in all directions. The excised breast tissue was pulled out through the periareolar incision or the axillar incision. The thickness of the flaps varied with body habitus; however, it was approximately 5 mm.
Tissue expander placement
When performing the immediate reconstruction after E-NSM, it was carried out using the tissue expander. First, the pectoralis major muscle was elevated from its lateral border off of the chest wall. In the inferior aspect, the pectoralis major muscle was elevated together with the anterior rectus sheath to a level 1 cm below the contralateral inframammary fold. Then, serratus fascia was incised along the lateral border of the pectoralis muscle and elevated with serratus anterior muscle. The expander was placed in the submuscular pocket, and the lateral border of the pectoralis major muscle was sutured to the elevated serratus fascia.
Skin closure
The base of the nipple and the major pectoral muscle were sutured at the level of the marking made earlier on the midline of the body. After placement of two closed-suction drains, the incision was closed.
Between January 2003 and June 2011, E-NSM was performed for a total of 440 breasts in 423 consecutive female patients with primary operable breast cancers at our institution. Of these patients, we excluded 19 patients from the study because the patients were not followed for at least 6 months after the operation in 16 patients (3.8 %), and the nipples were removed because of occult nipple involvement in the cored duct in the remaining 3 patients (0.7 %). The reasons of nipple coring in the 3 patients were suspicious findings on MRI or bloody nipple discharge in 2 patients, and malignant cells on the base of the nipple in the frozen-section biopsy in the remaining one patient. The carcinoma remain rate in the nipple side in the patients with positive biopsy result on the base of the nipple in frozen-section biopsy was 7.1 % (1/14).
Therefore, we reviewed the medical records of a total of 421 breasts in 404 patients who underwent E-NSM to investigate the LR rate and the factors associated with it. The clinico-pathological features and the treatment and outcomes of the patients with LRs were also examined.
A local recurrence was defined as cancer recurring on the chest wall of previously operated breast area, including muscle, subcutaneous fat, nipple-areola complex, or skin. An inadequate margin was defined as malignant cells within 2 mm of a resection margin. Our institutional review board required neither the patients’ approval nor the patients’ informed consent for a review of their medical records to be made.
Statistical analysis
For the univariate analysis, the Fisher exact test was used to assess the association between LR and the patients’ characteristics including age, nodal positivity, stage, the histological type of the tumors, surgical margin, ER status, HER2new status, nuclear grade, LVI, chemotherapy, hormonal therapy, and radiotherapy. For the multivariate analysis, a multiple logistic regression was performed to assess the associations between LR and the variables. For all the analyses, a p value of <0.05 was considered statistically significant.
Results
Of the 421 breasts in 404 patients, 14 breasts in 14 patients (3.5 %) developed distant metastasis, 11 breasts (2.6 %) in 11 patients presented with LR, and 8 breasts (1.9 %) in 8 patients experienced regional lymph node metastasis as the first site of recurrence after a median follow-up time of 61 months (range, 7.2–139 months). Among the 11 LRs, 9 patients presented with LR only, 1 patient exhibited regional lymph node recurrence, and 1 patient exhibited distant metastasis.
The characteristics of the patients and the association with LR are listed in Table 1. The LR rate of patients under 40 years of age was higher than that of patients over 40 years (6/92, 6.5 % vs. 5/329, 1.5 %, p = 0.02). Of all 421 breasts in 404 patients, the tumors were classified as Stage 0 in 117 breasts (28 %), Stage I in 149 breasts (35 %), Stage II in 141 breasts (33 %), and Stage III in 14 breasts (3.3 %). The LR rate of the patients who were classified as Stage III was higher than that of the other patients classified as Stage 0, I, or II (2/14, 14 %. vs. 9/398, 2.3 %, p = 0.05). Axillary lymph node dissection was performed for 117 breasts (28 %) in 117 patients. Pathologically, the tumor was diagnosed as a ductal carcinoma in situ (DCIS) in 117 breasts, invasive ductal carcinoma in 265 breasts, invasive lobular carcinoma in 20 breasts, mucinous carcinoma in 10 breasts, apocrine carcinoma in 3 breasts, medullary carcinoma in 2 breasts, matrix-producing carcinoma in 2 breasts, and spindle cell carcinoma in 2 breasts. The LR rate of mucinous carcinoma was higher than that of all the other histological subtypes (20 %, 2/10, vs. 2.2 %, 9/411, p = 0.03). In the final pathological assessment, an inadequate margin was observed in 9 % (38/421) of the E-NSM cases. Among the 38 breasts in 38 patients with inadequate margin, radiotherapy was administered for 2 patients, additional resection was performed for 2 patients, and remaining 34 patients were followed without additional treatment. In the two patients who underwent additional resection, residual carcinoma was not observed in the specimen. The LR rate of breasts with an inadequate margin was higher than that of breasts with a negative margin (13 %, 5/38, vs. 1.6 %, 6/383, p = 0.001). LR did not occur in the four patients with inadequate margin who underwent additional treatment.
There were no significant associations between LR and the lymph node status, ER status, HER2 status, nuclear grade, LVI, chemotherapy, hormonal therapy, or radiotherapy. The factors that were associated with LR in a multivariate analysis were a younger age of less than 40 years (p = 0.02), a Stage III tumor (p = 0.01), and an inadequate surgical margin (p = 0.001). The clinico-pathological features of the primary tumors in the patients with LRs are shown in Table 2.
Among the 11 LRs, 8 LRs developed within the native skin or subcutaneous tissues adjacent to the primary tumor site, 2 presented in subcutaneous tissue under the nipple-areola complex, and the remaining one recurred in the subcutaneous tissue of the affected side chest at a distinctly different location from the primary tumor. The clinico-pathological features of the recurrent tumor and the treatment and outcomes of the patients with LRs are shown in Table 3. Of the 11 LRs, 9 recurrences (82 %) were detected by breast self-examination, and the other two (18 %) were detected by follow-up imaging. The median time from surgery until LR was 25 months (range, 5–105 months).
The basic treatment for LR was performed by excision or excision following radiotherapy if not previously administered with or without hormonal therapy and/or chemotherapy when the LR was the only site of disease; however, 2 of the 9 patients whose LR was the only site of disease rejected excision. One of these patients underwent hormone therapy, while the other did not receive any further treatment. One patient whose LR occurred with a simultaneous axillary lymph node recurrence underwent excision of the LR and axillary lymph node followed by chemotherapy, while one patient whose LR was associated with distant metastasis underwent chemotherapy without excision.
Of the 11 patients with LR, 2 of the 5 patients who developed LR within 2 years died from metastatic disease. Among the other 9 patients who were alive, 6 patients had no evidence of disease after the treatment for LR, 2 patients experienced repeat LR, and the remaining patient who rejected excision and underwent hormonal therapy exhibited a persistent LR. In the 2 patients who experienced repeat LR, the LRs occurred 19 months after the treatment for the first LR in one patient, and 7, 27, and 60 months after the treatment for the first LR in the other. Re-excisions at each time of LR revealed mucinous carcinoma.
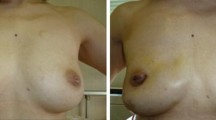
During the study period, immediate breast reconstruction using a tissue expander was performed for only 32 breasts (7.6 %) in 29 patients. Our breast reconstructions with mammary prosthesis were usually performed 3–5 years after the initial operation (Fig. 4).
a Appearance of the breast after E-NSM for left breast cancer. b Breast reconstruction with mammary prosthesis was performed 4 years after E-NSM. Postoperative image 2 years after reconstruction
Discussion
A number of studies have demonstrated the oncologic safety of NSM. Although Benediktsson et al. [12] reported that the frequency of LR was 8.5 % among patients who received radiotherapy and 28.4 % among nonirradiated patients with a mean follow-up period of 156 months, they suggested that the high LR rate among the nonirradiated patients was a result of inadequate surgery. In contrast, many studies have reported that the LR rate after NSM ranges from 1 to 12 %, with mean follow-up periods ranging from 10 to 76 months [6].
On the other hand, few reports have discussed the oncologic safety of E-NSM. Ito et al. [10] reported no LRs (0/33) with a mean follow-up period of 51.2 months (range, 16–86 months). Our previous study showed no LRs (0/87) with a median follow-up period of 52 months (range, 16–80 months) [11]. In the current study, the frequency of LRs was 2.6 % (11/421) with a median follow-up period of 61 months (range, 7.2–139 months). The relatively high LR rate in the study might be related to the larger number of patients and the longer follow-up period. Because the LR rate of 2.6 % was still comparable to the rate of NSM studies that did not use endoscopy, E-NSM seems to be a safe oncologic treatment option for patients with breast cancer.
Patients with a tumor size of more than 5 cm and/or 4 positive axillary nodes have been identified as having a high risk for locoregional recurrence after mastectomy; therefore, subsequent radiotherapy is recommended. Several studies have described the significance of other factors on the risk of LR after a conventional mastectomy including an inadequate margin, multicentric disease, a younger age, a higher stage, severe LVI, a high grade, and a triple negative subtype [13–15]. Recent reports of SSM and NSM described the significance of additional factors including HER2 overexpression, absence of estrogen receptors, luminal B subtype, and high Ki67 [16–19]. These reports indicated that tumors with an aggressive biology or progressive disease tend to be associated with a high risk of LR. In the current study, Stage III, a younger age (<40 years), and an inadequate margin were predictors of LR in a multivariate analysis. A higher stage and a younger age may represent progressive disease or an aggressive biology, and in patients with these risk factors, local control followed by systemic therapy will be important. However, a randomized trial comparing E-NSM with conventional mastectomy should be performed to clarify whether Stage III and younger patients are candidates for E-NSM.
Compared with a conventional mastectomy, preservation of the skin envelope is associated with an increased risk of positive superficial margins, with reported rates ranging from 8.5 to 43 % [15, 17, 19]. Although Vaughan et al. [17] reported that the margin status for SSM was not significantly associated with LR, several studies reported that inadequate margins were associated with a higher rate of LR than in patients with an uninvolved margin [15, 19]. In the study, an inadequate margin was also one of the predictors of LR. To reduce the incidence of LR, careful preoperative assessment of the tumor extent using imaging and excision of the subcutaneous tissue, especially that overlying the primary tumor, by creating a thin skin flap to ensure adequate margins is important because most LRs occur in the subcutaneous tissue in the same quadrant as the initial tumor.
For those patients who exhibit an inadequate margin after a surgery, additional radiotherapy may be effective for select patients. Truong et al. [20] recommended radiotherapy for patients with a positive margin plus at least one of the following factors: an age of 50 years or younger, a tumor size greater than 2 cm, grade 3 histology, or LVI. However, complications associated with breast reconstruction, resulting in a poorer cosmetic outcome, are a concern [21]. Regarding the benefit of an additional resection for patients with an inadequate margin, Sheikh et al. [19] reported that there were no differences between SSM and a mastectomy with regard to the rate of locoregional recurrence after additional tissue was removed at the site of a positive margin intraoperatively. Cao et al. [22] obtained additional superficial margin specimens from above the tumor intraoperatively and found that 20 % of the SSMs with a positive margin had residual carcinoma. A predictor of residual carcinoma in their study was extensive DCIS and thicker additional superficial samples. For the patients with inadequate margin, re-excision might be feasible by removing the appropriate soft tissue, skin, or muscle, if needed, to ensure negative margins.
LR after mastectomy is considered to be a marker of distant metastasis, which has an impact upon survival. Several studies have reported 10-year disease-free survival rates of 7 to 17 % after an initial LR [23]. Haffty et al. [24] reported that the distant metastasis free rates and long-term survival rates were lower among patients in whom an LR developed within 2 years after conventional mastectomy. Several studies of SSM have shown various rates of distant metastasis after LR: 0 % (35 months follow-up after LR) [25], 46 % (30 months) [17], and 77 % (78 months) [26]. On the other hand, a long-term study reported by Benediktsson et al. [12] showed that the occurrence of LR after NSM does not significantly affect OS. In the present study, the rate of distant metastasis after LR was 18 %. Two of the 5 patients in whom LR occurred within 2 years died with distant metastasis, whereas the other 6 patients in whom the LR occurred after 2 years survived without evidence of distant metastasis. The poorer outcomes of the patients who suffered an early LR may reflect aggressive tumor biology. Although the follow-up periods were relatively short, previous reports and our study indicated that LR after NSM is not always associated with systemic relapse or a poor prognosis. One of the reasons might be that NSM tends to be performed for relatively earlier stage breast cancer patients who are less likely to experience distant recurrence.
At our institution, the indications for sparing the nipple are decided based on a physical examination, mammography, and MRI examination without consideration of the reported risk factors of occult nipple involvement, including a central tumor location, a large tumor size, LVI, and multicentricity or multifocality [2–5]. The two studies that performed preoperative assessments using MRI reported occult nipple involvement in 3 and 2.2 %, respectively [11, 27]. In the current study, the rate of occult nipple involvement was also low (0.7 %). The low rate of nipple involvement shows the usefulness of MRI for selecting candidates for NSM [28, 29].
Our study had several limitations: first, evidence obtained from retrospective studies is statistically weak. A randomized trial comparing E-NSM with the conventional method should be performed in a subset of patients who meet the indications for both techniques to investigate the safety of E-NSM. Second, the follow-up period was still inadequate. Additional careful follow-up is needed to ascertain the long-term results and prognosis of this procedure.
In conclusion, the risk factors for LR after E-NSM were a younger age of less than 40 years, Stage III tumor, and an inadequate surgical margin. The LR rate of 2.6 % was comparable to those of previous studies examining NSM, indicating the safety of this procedure. According to our data, E-NSM is an acceptable method for selected patients requiring a mastectomy.
References
Didier F, Radice D, Gandini S, Bedolis R, Rotmensz N, Maldifassi A, et al. Does nipple preservation in mastectomy improve satisfaction with cosmetic results, psychological adjustment, body image and sexuality? Breast Cancer Res Treat. 2009;118:623–33.
Lambert PA, Kolm P, Perry RR. Parameters that predict nipple involvement in breast cancer. J Am Coll Surg. 2000;191:354–9.
Cense HA, Rutgers EJ, Lopes CM, Van Lanschot JJ. Nipple-sparing mastectomy in breast cancer: a viable option? Eur J Surg Oncol. 2001;27:521–6.
Simmons RM, Brennan M, Christos P, King V, Osborne M. Analysis of nipple/areolar involvement with mastectomy: can the areola be preserved? Ann Surg Oncol. 2002;9:165–8.
Vlajcic Z, Zic R, Stanec S, Lambasa S, Petrovecki M, Stanec Z. Nipple-areola complex preservation: predictive factors of neoplastic nipple-areola complex invasion. Ann Plast Surg. 2005;55:240–4.
Endara M, Chen D, Verma K, Nahabedian MY, Spear SL. Breast reconstruction following nipple-sparing mastectomy: a systematic review of the literature with pooled analysis. Plast Reconstr Surg. 2013;132:1043–54.
Kitamura K, Ishida M, Inoue H, Kinoshita J, Hashizume M, Sugimachi K. Early results of an endoscope-assisted subcutaneous mastectomy and reconstruction for breast cancer. Surgery. 2002;131:324–9.
Ho WS, Ying SY, Chan AC. Endoscopic-assisted subcutaneous mastectomy and axillary dissection with immediate mammary prosthesis reconstruction for early breast cancer. Surg Endosc. 2002;16:302–6.
Nakajima H, Sakaguchi K, Mizuta N, Hachimine T, Ohe S, Sawai K. Video-assisted total glandectomy and immediate reconstruction for breast cancer. Biomed Pharmacother. 2002;56:205–8.
Ito K, Kanai T, Gomi K, Watanabe T, Ito T, Komatsu A, et al. Endoscopic-assisted skin-sparing mastectomy combined with sentinel node biopsy. ANZ J Surg. 2008;78:894–8.
Sakamoto N, Fukuma E, Higa K, Ozaki S, Sakamoto M, Abe S, et al. Early results of an endoscopic nipple-sparing mastectomy for breast cancer. Ann Surg Oncol. 2009;16:3406–13.
Benediktsson KP, Perbeck L. Survival in breast cancer after nipple-sparing subcutaneous mastectomy and immediate reconstruction with implants: a prospective trial with 13 years median follow-up in 216 patients. Eur J Surg Oncol. 2008;34:143–8.
Katz A, Strom EA, Buchholz TA, Theriault R, Singletary SE, McNeese MD. The influence of pathologic tumor characteristics on locoregional recurrence rates following mastectomy. Int J Radiat Oncol Biol Phys. 2001;50:735–42.
Jaqsi R, Raad RA, Goldberg S, Sullivan T, Michaelson J, Powell SN, Taqhian AG. Locoregional recurrence rates and prognostic factors for failure in node-negative patients treated with mastectomy: implications for postmastectomy radiation. Int J Radiat Oncol Biol Phys. 2005;62:1035–9.
Childs SK, Chen YH, Duggan MM, Golshan M, Pochebit S, Wong JS, Bellon JR. Surgical margins and the risk of local-regional recurrence after mastectomy without radiation therapy. Int J Radiat Oncol Biol Phys. 2012;84:1133–8.
Carlson GW, Styblo TM, Lyles RH, Jones G, Murray DR, Staley CA, Wood WC. The use of skin sparing mastectomy in the treatment of breast cancer: the Emory experience. Surg Oncol. 2003;12:265–9.
Vaughan A, Dietz JR, Aft R, Gillanders WE, Eberlein TJ, Freer P, Margenthaler JA. Scientific presentation award. Patterns of local breast cancer recurrence after skin-sparing mastectomy and immediate breast reconstruction. Am J Surg. 2007;194:438–43.
Petit JY, Veronesi U, Orecchia R, et al. Risk factors associated with recurrence after nipple-sparing mastectomy for invasive and intraepithelial neoplasia. Ann Oncol. 2012;23:2053–8.
Sheikh F, Rebecca A, Pockaj B, Wasif N, McCullough AE, Casey W, et al. Inadequate margins of excision when undergoing mastectomy for breast cancer: which patients are at risk? Ann Surg Oncol. 2011;18:952–6.
Truong PT, Olivotto IA, Speers CH, Wai ES, Berthelet E, Kader HA. A positive margin is not always an indication for radiotherapy after mastectomy in early breast cancer. Int J Radiat Oncol Biol Phys. 2004;58:797–804.
Forman DL, Chiu J, Restifo RJ, Ward BA, Haffty B, Ariyan S. Breast reconstruction in previously irradiated patients using tissue expanders and implants:a potentially unfavorable result. Ann Plast Surg. 1998;40:360–3.
Cao D, Tsangaris TN, Kouprina N, Wu LS, Balch CM, Vang R, Argani P. The superficial margin of the skin-sparing mastectomy for breast carcinoma: factors predicting involvement and efficacy of additional margin sampling. Ann Surg Oncol. 2008;15:1330–40.
Medina-Franco H, Vasconez LO, Fix RJ, Heslin MJ, Beenken SW, Bland KI, Urist MM. Factors associated with local recurrence after skin-sparing mastectomy and immediate breast reconstruction for invasive breast cancer. Ann Surg. 2002;235:814–9.
Haffty BG, Hauser A, Choi DH, Parisot N, Rimm D, King B, Carter D. Molecular markers for prognosis after isolated postmastectomy chest wall recurrence. Cancer. 2004;100:252–63.
Meretoja TJ, von Smitten KA, Leidenius MH, Svarvar C, Heikkilä PS, Jahkola TA. Local recurrence of stage 1 and 2 breast cancer after skin-sparing mastectomy and immediate breast reconstruction in a 15-year series. Eur J Surg Oncol. 2007;33:1142–5.
Carlson GW, Styblo TM, Lyles RH, Bostwick J, Murray DR, Staley CA, Wood WC. Local recurrence after skin-sparing mastectomy: tumor biology or surgical conservatism? Ann Surg Oncol. 2003;10:108–12.
Wijayanayagam A, Kumar AS, Foster RD, Esserman LJ. Optimizing the total skin-sparing mastectomy. Arch Surg. 2008;143:38–45.
Sakamoto N, Tozaki M, Hoshi K, Fukuma E. Is MRI useful for the prediction of nipple involvement? Breast Cancer. 2013;20:316–22.
Friedman EP, Hall-Craggs MA, Mumtaz H, Schneidau A. Breast MR and the appearance of the normal and abnormal nipple. Clin Radiol. 1997;52:854–61.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Sakamoto, N., Fukuma, E., Teraoka, K. et al. Local recurrence following treatment for breast cancer with an endoscopic nipple-sparing mastectomy. Breast Cancer 23, 552–560 (2016). https://doi.org/10.1007/s12282-015-0600-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12282-015-0600-4