Abstract
Cerebral pericytes are perivascular cells that stabilize blood vessels. Little is known about the plasticity of pericytes in the adult brain in vivo. Recently, using state-of-the-art technologies, including two-photon microscopy in combination with sophisticated Cre/loxP in vivo tracing techniques, a novel role of pericytes was revealed in vascular remodeling in the adult brain. Strikingly, after pericyte ablation, neighboring pericytes expand their processes and prevent vascular dilatation. This new knowledge provides insights into pericyte plasticity in the adult brain.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the 19th century, a French scientist, Charles-Marie Benjamin Rouget, reported the presence of a population of contractile cells in small blood vessels, referred to as Rouget cells [1]. Later, in the 20th century, a German scientist, Karl Wilhelm Zimmermann, renamed these cells “pericytes” due to their distinct anatomical position around the vasculature [2]. The word pericyte comes from “peri” meaning “around” and “cyte” from Latin, which has origins from the Greek “kytos” (cell), properly illustrating a cell encircling a blood vessel [3]. Until the end of the 20th century, pericytes were identified still based mainly on their anatomical location and morphology. Pericytes have long processes surrounding blood vessel walls and are widely dispersed in all tissues [4]. They encircle endothelial cells, and communicate with them along the length of the blood vessels by paracrine signaling and physical contact [5]. In the brain, the ratio of endothelial cells to pericytes is ~3:1 [6, 7], implying an immense importance of cerebral pericytes.
Formerly, the accurate distinction of pericytes from other perivascular cells was impossible, as light and electron microscopy were the only technologies able to visualize these cells, limiting the information acquired. This resulted in the illusory notion that pericytes are merely inert supporting cells, limited exclusively to the physiological function of vascular stability. Already in the 21th century, the combination of fluorescent and confocal microscopy with genetic tools, such as fate lineage tracing, enabled the discovery of novel and unexpected roles for pericytes in health and disease [8]. Recently, quickly expanding insights into the pathophysiological functions of pericytes have attracted the attention of many researchers.
Pericytes participate in blood vessel development, maturation, and permeability, as well as contributing to their normal architecture [9, 10]. They regulate blood flow [11, 12], and affect coagulation [13]. Pericytes also collaborate with astrocytes, neurons, and endothelial cells, forming the neurovascular unit [12, 14, 15], to regulate maintenance of the functional integrity of the blood brain barrier [16,17,18,19,20,21]. This may occur via pericyte-derived molecules, such as platelet-derived growth factor subunit B (PDGFB)/PDGF receptor-beta (PDGFRβ) signaling, which is indispensable for the formation and maturation of this barrier [22]. In addition, pericytes perform several immune functions [23], regulate lymphocyte activation in the retina [24, 25], attract innate leukocytes to exit through sprouting blood vessels in the skin [26], and contribute to the clearance of toxic cellular byproducts, having direct phagocytic activity in the brain [27]. Interestingly, following white matter demyelination, pericytes promote the differentiation of oligodendrocyte progenitors involved in central nervous system regeneration via a2-chain of laminin [28]. Pericytes may also behave as stem cells in several tissues [29], generating other cell populations, as well as regulating the behavior of other stem cells, as hematopoietic stem cells in their niches [30,31,32,33,34]. Note that pericytes from distinct peripheral tissues may have various properties, and may differ from those in brain. Increasing evidence also shows that brain pericytes alter their traits following stimuli and develop stemness, demonstrating their plasticity [35,36,37,38,39].
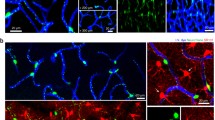
Pericytes exhibit structural plasticity during embryonic cerebral development, participating in vascular remodeling [40]. Understanding pericyte behavior in the adult brain is a central question in neuroscience, as these cells may play central roles in the pathogenesis of neurodegenerative disorders. Nevertheless, whether pericytes participate in vascular remodeling in the adult brain remains unknown. Now, in a recent article in Cell Reports, Berthiaume and colleagues investigated the behavior of pericytes in the adult mouse brain [41]. The authors revealed pericyte plasticity in the adult brain by using elegant state-of-the-art techniques, including two-photon microscopy in combination with sophisticated Cre/loxP in vivo tracing technologies. Berthiaume and colleagues imaged, at high-resolution over several weeks, cerebral pericytes in NG2-CreER/TdTomato, Myh11-CreER/TdTomato, and PDGFRβ-Cre/YFP mice. These experiments unveiled that pericytes comprise a quasi-continuous, non-overlapping network along the entire length of blood vessels. Interestingly, the pericyte prolongations were not stable in length, extending or retracting during the period of analysis. Then, the authors explored the effect of pericyte death on its neighboring pericytes. After pericyte ablation, using targeted two-photon irradiation, Berthiaume and colleagues showed that adjacent pericytes extend their processes into the uncovered area, covering the exposed blood vessel [41]. Strikingly, neighboring pericytes are able to reverse the vascular dilatation that occurs after pericyte depletion [41] (Fig. 1). Thus, this longitudinal imaging study demonstrated pericyte plasticity in the adult brain.
Cerebral pericyte plasticity in response to neighbor ablation. Pericytes are present around blood vessels in the brain. The study of Berthiaume and colleagues now suggests a novel role for pericytes in vascular remodeling in the adult brain [41]. After pericyte ablation, using targeted two-photon irradiation, adjacent pericytes extend their processes to cover the exposed endothelial bed, and reverse the vascular dilatation that occurs after pericyte depletion. Future studies will reveal in detail the cellular and molecular mechanisms involved in this process in the brain microenvironment.
Here, we consider these findings, and evaluate recent advances in our knowledge of pericyte biology in the brain.
Perspectives and Future Directions
Pericyte Heterogeneity in the Brain
Pericytes are heterogeneous regarding their distribution, phenotype, marker expression, origin, and function [42]. In the past century, pericytes were distinguished into three types based on their mural location and morphology: pre-capillary, mid-capillary, and post-capillary [2, 43]. Berthiaume and colleagues exclusively studied pericytes surrounding mid-capillary regions [41]. Thus, it remains unknown whether pre-capillary and post-capillary pericytes present different behavior. This should be taken into consideration in future work; discovering specific markers for pre-capillary and post-capillary pericytes will help to address this question. Separately analyzing the behavior of pericytes from different locations in the blood vessels may reveal their functional heterogeneity. Pericyte heterogeneity is also based on their molecular marker expression profiles. Capillary pericytes express desmin but are commonly negative for α smooth muscle actin (αSMA), while venular pericytes express both desmin and αSMA proteins [44]. Moreover, Kir6.1 is highly expressed in a subset of brain pericytes, but is undetectable in others [45]. In addition, arteriolar pericytes that do not express the leptin receptor (LEPR) have been described to be distinct from sinusoidal pericytes in the bone marrow that express LEPR [31, 46]. Also, both nerve/glial antigen 2-positive (NG2+) and -negative (NG2−) pericytes have been described in the skin [26]. Furthermore, pericytes positive and negative for glutamate aspartate transporter and the cytoskeletal protein Nestin have been described in the spinal cord [47, 48].
Cerebral pericytes also differ in their embryonic origins [42]. While pericytes in coleomic organs are mesoderm-derived [7], most cephalic pericytes are of neuroectodermal origin [49], and recent studies have shown that a subpopulation of pericytes in the embryonic brain may derive from hematopoietic progenitors [42, 50]. All these descriptive characteristics in which pericytes differ are also important regarding their functions, as pericytes in distinct locations [30], with different marker expression profiles [51], and from varying origins [42] differ in their functions. For instance, after brain injury, while one subset participates in scar tissue formation [52], another is capable of generating new blood vessels [53]. Thus, subsets of pericytes can contribute to distinct pathological conditions in varying ways. Importantly, similar analysis as done by Berthiaume et al. (2018), should be performed on cerebral pericytes from other blood vessels and in distinct brain regions, as their behavior may differ.
Pericyte Markers
Several molecular markers help to identify pericytes, such as PDGFRβ, NG2, proteoglycan (CSPG4), myosin heavy chain 11 (Myh11), aminopeptidase N (CD13), αSMA, regulator of G protein signaling 5, desmin, vimentin, ATP-binding cassette, subfamily C (CFTR/MRP), member 9 (SUR2), alkaline phosphatase, CD146, CD133, endosialin, potassium inwardly-rectifying channel, subfamily J, member 8 (Kir6.1), Tbx18, vitronectin, and interferon-induced transmembrane protein 1 (Ifitm1), among others (Table 1) [29, 48, 54,55,56,57,58,59]. The difficulty now is generating markers, antibodies, and mice to take advantage of this information. Unfortunately, there is no single molecular marker yet that can be used to unequivocally and exclusively label the whole population of pericytes. Berthiaume and colleagues imaged labeled pericytes in NG2-CreER/TdTomato, Myh11-CreER/TdTomato, and PDGFRβ-Cre/YFP mice. Although NG2, Myh11, and PDGFRβ proteins can be expressed in pericytes, none of them is specific to pericytes, or labels all pericytes, as oligodendrocyte progenitors also express NG2 [60]. Under special conditions, microglia may also express NG2 [61]. Interestingly, pericytes that do not express NG2 proteoglycan also exist [26]. In addition, Myh11 labels vascular smooth muscle cells, and is expressed only in a subgroup of pericytes [30]. PDGFRβ expression also is not restricted to pericytes. Several stromal cells such as vascular smooth muscle cells [62, 63] and fibroblasts [64] express this cell-surface tyrosine kinase receptor [7]. Note that Berthiaume and colleagues used PDGFRβ-Cre/YFP mice, in which all the cells derived from PDGFRβ-expressing cells are also labeled with fluorescence [41]. Since PDGFRβ is broadly expressed throughout the embryo in embryonic stages in several cell types, PDGFRβ-Cre/YFP mice are not the best mouse model for analyzing pericyte behavior, as several other cell populations may be labeled at the same time [29, 65]. Therefore, as PDGFRβ expression is more restricted in adult animals, the use of PDGFRβ-CreER/YFP mice instead would be more appropriate for the study of PDGFRβ-expressing pericytes in the brain [66]. Importantly, not all cells in perivascular locations are necessarily pericytes [67]. Besides pericytes, other cells surrounding blood vessels have been described, including fibroblasts [68], macrophages [69, 70], microglia [71], adventitial cells [72], and vascular smooth muscle cells [73]. Altogether, this raises the possibility that some of the observations by Berthiaume et al. (2017) are from a different, non-pericytic, cell type. Currently, the state-of-the-art identification of pericytes in tissue preparations relies on a combination of anatomical localization (covering endothelial cells and underlying the basal lamina), morphology, and the co-expression of at least two pericytic molecular markers. The discovery of a single molecular marker specific to all cerebral pericytes will facilitate the study of the behavior of these cells in the brain.
Pericytes as Stem Cells
In the last decade, the potential of pericytes to contribute to tissue regeneration/homeostasis as tissue-resident progenitors has been established by numerous studies [74,75,76]. Pericytes not only participate in the formation of new blood vessels [53], but their ability to differentiate into the neural lineage has also been demonstrated [48, 77,78,79,80,81,82,83]. Therefore, pericytes are expected to be able to proliferate and multiply when activated. Surprisingly, Berthiaume and colleagues show that pericytes are not activated to proliferate in response to the death of one adjacent pericyte; instead, they extend prolongations onto the uncovered endothelium [41]. It remains unknown whether pericytes continue to behave similarly when more adjacent pericytes are ablated. Is the elimination of adjacent pericytes unable to activate pericytic multiplication? Does this depend on the number of pericytes that die? Also, a question arises regarding the plasticity of pericytes in vivo. Are cerebral pericytes able to form other pericytes in the adult brain? This could be tested by time-lapse high-resolution imaging analysis of lineage tracing in pericyte-specific mouse models.
Pericyte Communication with Other Tissue Components in Their Microenvrionment
Which signaling molecules are needed to activate the extension of pericyte processes into the adjacent endothelial bed? And which signaling molecules are important for these pericytes to reverse the vasodilation that occurs after pericyte death [84]? Although Berthiaume and colleagues have revealed how pericytes respond to the deletion of a neighboring pericyte, they did not explore the molecular and cellular mechanisms involved in this process. A recent study in the spinal cord has shown that expression of the enzyme aromatic L-amino acid decarboxylase is important for pericyte-induced vasoconstriction after spinal cord injury [85, 86]. Is this enzyme also important in the cerebral pericyte after ablation of its neighbor? In addition to studies of genetic mouse models, transcriptomic and single-cell analysis of pericytes after ablation of their neighbors will help us to understand the molecular mechanisms involved in those processes in the brain microenvironment.
Moreover, it is still not understood whether any other cells are involved in this behavior. Do endothelial cells cross-talk to communicate that they have become uncovered? In addition, as discussed above, there are several other perivascular cells in the cerebral vasculature. How other perivascular cells participate in this process remains to be elucidated. Interestingly, after irradiation of single pericytes [41], what happens to their remnants? Does the cellular debris cause damage to neighboring cells and inflammation? Or are the cellular remnants important for adjacent pericytes to extend their prolongations in this area? Moreover, as macrophages usually engulf cellular debris, do they communicate with neighboring pericytes, activating them to expand their processes? The relationships between brain pericytes and microglia/macrophages have been addressed in recent studies. Interestingly, during development, macrophages may generate pericytes in the brain [50, 87, 88], while after stroke, pericytes may form macrophages [35, 36, 89].
It well known that pericytes produce several signaling molecules as well as responding to various signals and communicating with other cells, such as endothelial cells [90,91,92]. Interestingly, some evidence suggests direct communication with astrocytes as well. Astrocyte-derived glutamate may regulate gene transcription in pericytes [93]. Also, astrocytes may influence pericyte behavior by altering PDGFβ signaling in which pericytes play a key role [94, 95]. In contrast, very little is known about cross-talk within the population of pericytes. Future studies will need to explore how pericytes communicate with their peers.
Pericytes in Disease
Berthiaume and colleagues imaged pericytes in the healthy adult mouse brain [41]. Are the normal functions of the pericytes changed when one cell occupies the space of two? Interestingly, the authors followed pericyte behavior for several weeks up to almost 2 months [41]. It remains unknown whether this pericyte behavior is a temporary solution before the lost pericytes are regenerated. Is this process reversed after a longer period of time? Importantly, as the interruption of pericyte contact with endothelial cells may lead to endothelial hyperplasia [10], the brain vasculature should be followed for a longer time. Also, it remains unknown whether pericytes behave similarly during different life stages, such as embryonic development, the postnatal period, and aging. Furthermore, it remains to be studied how pericytes respond to the ablation of their neighbors in various brain diseases, in which it is well accepted that their dysfunction plays pivotal roles (Table 2), such as Alzheimer’s disease [96,97,98,99], amyotrophic lateral sclerosis [100, 101], diabetic retinopathy [102], cerebral autosomal-dominant arteriopathy with subcortical infarcts and leukoencephalopathy [103], epilepsy [104], human immunodeficiency virus-related dementia [105], brain tumors [106], primary familial brain calcification [107], and diabetes-related microangiopathy [108]. As pericyte degeneration also causes breakdown of the blood-brain barrier, leading to the entrance of blood-derived toxic substances into the central nervous system [16], future studies should explore how neighboring pericytes act to protect the brain against such toxicity. Recently, Arango-Lievano and colleagues have used the two-photon microscopy approach to track changes in perivascular cells during pathophysiological progression in the epileptic brain [109].
Modern Technologies to Study Pericyte Biology
Methods to eliminate pericytes from the tissue microenvironment, enabling analysis of the functioning of a tissue without pericytes, may lead to advances in our understanding of the role of pericytes in specific organs. Multiple pharmacological drugs to induce apoptotic cell death are accessible. Nevertheless, most lack spatiotemporal and cell-type specificity [110,111,112,113]. Modern state-of-the-art experimental approaches for specific cell ablation in vivo have been created, including genetically-encoded death receptors [113,114,115,116], two-photon thermal ablation [113, 117,118,119,120], chromophore-assisted light inactivation [113, 121,122,123,124,125,126,127], and more recently two-photon chemical apoptotic targeted ablation [113]. Unfortunately, every tool has limitations, for instance, prolonged illumination requirements, non-specific tissue damage from the spilling of cellular debris, induction of local inflammation, and the need to efficiently and accurately deliver the dyes or genetic materials for targeted cell killing. Ideally, these limitations can be overcome by combining different methodologies to answer the same questions. Thus, in the future, comparing distinct methods for precise ablation of pericytes in the brain should be used to achieve a complete understanding of pericyte behavior in the central nervous system.
Conclusion
The study by Berthiaume and colleagues reveals a novel and important behavior of cerebral pericytes in response to ablation of adjacent pericytes. However, our understanding of cross-talk between different cell types present in the brain vascular microenvironment remains limited, and the complexity of these interactions in distinct physiological and pathological conditions should be elucidated in future studies. The huge challenge that we face now is how to translate animal research to humans.
References
Rouget C. Mémoire sur le développement, la structure et les proprietés physiologiques des capillaires sanguins et lymphatiques. Arch de Phys 1873, 5: 603.
Zimmermann KW. Der feinere Bau der Blutkapillaren, vol 68., Berlin: Springer, 1923: 29–109.
Schrimpf C, Teebken OE, Wilhelmi M, Duffield JS. The role of pericyte detachment in vascular rarefaction. J Vasc Res 2014, 51: 247–258.
Hirschi KK, D’Amore PA. Pericytes in the microvasculature. Cardiovasc Res 1996, 32: 687–698.
Diaz-Flores L, Gutierrez R, Varela H, Rancel N, Valladares F. Microvascular pericytes: a review of their morphological and functional characteristics. Histol Histopathol 1991, 6: 269–286.
Shepro D, Morel NM. Pericyte physiology. FASEB J 1993, 7: 1031–1038.
Armulik A, Genove G, Betsholtz C. Pericytes: developmental, physiological, and pathological perspectives, problems, and promises. Dev Cell 2011, 21: 193–215.
Birbrair A, Zhang T, Wang ZM, Messi ML, Mintz A, Delbono O. Pericytes at the intersection between tissue regeneration and pathology. Clin Sci (Lond) 2015, 128: 81–93.
Enge M, Bjarnegard M, Gerhardt H, Gustafsson E, Kalen M, Asker N, et al. Endothelium-specific platelet-derived growth factor-B ablation mimics diabetic retinopathy. EMBO J 2002, 21: 4307–4316.
Hellstrom M, Gerhardt H, Kalen M, Li X, Eriksson U, Wolburg H, et al. Lack of pericytes leads to endothelial hyperplasia and abnormal vascular morphogenesis. J Cell Biol 2001, 153: 543–553.
Pallone TL, Zhang Z, Rhinehart K. Physiology of the renal medullary microcirculation. Am J Physiol Renal Physiol 2003, 284: F253–266.
Kisler K, Nelson AR, Montagne A, Zlokovic BV. Cerebral blood flow regulation and neurovascular dysfunction in Alzheimer disease. Nat Rev Neurosci 2017, 18: 419–434.
Kim JA, Tran ND, Li Z, Yang F, Zhou W, Fisher MJ. Brain endothelial hemostasis regulation by pericytes. J Cereb Blood Flow Metab 2006, 26: 209–217.
Mishra A, Reynolds JP, Chen Y, Gourine AV, Rusakov DA, Attwell D. Astrocytes mediate neurovascular signaling to capillary pericytes but not to arterioles. Nat Neurosci 2016, 19: 1619–1627.
Azevedo PO, Lousado L, Paiva AE, Andreotti JP, Santos GSP, Sena IFG, et al. Endothelial cells maintain neural stem cells quiescent in their niche. Neuroscience 2017, 363: 62–65.
Bell RD, Winkler EA, Sagare AP, Singh I, LaRue B, Deane R, et al. Pericytes control key neurovascular functions and neuronal phenotype in the adult brain and during brain aging. Neuron 2010, 68: 409–427.
Thanabalasundaram G, Schneidewind J, Pieper C, Galla HJ. The impact of pericytes on the blood-brain barrier integrity depends critically on the pericyte differentiation stage. Int J Biochem Cell Biol 2011, 43: 1284–1293.
Kamouchi M, Ago T, Kitazono T. Brain pericytes: emerging concepts and functional roles in brain homeostasis. Cell Mol Neurobiol 2011, 31: 175–193.
Guerra DAP, Paiva AE, Sena IFG, Azevedo PO, Silva WN, Mintz A, et al. Targeting glioblastoma-derived pericytes improves chemotherapeutic outcome. Angiogenesis 2018. https://doi.org/10.1007/s10456-018-9621-x.
Sena IFG, Paiva AE, Prazeres P, Azevedo PO, Lousado L, Bhutia SK, et al. Glioblastoma-activated pericytes support tumor growth via immunosuppression. Cancer Med 2018, 7: 1232–1239.
Andreotti JP, Lousado L, Magno LAV, Birbrair A. Hypothalamic neurons take center stage in the neural stem cell niche. Cell Stem Cell 2017, 21: 293–294.
Park DY, Lee J, Kim J, Kim K, Hong S, Han S, et al. Plastic roles of pericytes in the blood-retinal barrier. Nat Commun 2017, 8: 15296.
Andreotti JP, Paiva AE, Prazeres P, Guerra DAP, Silva WN, Vaz RS, et al. The role of natural killer cells in the uterine microenvironment during pregnancy. Cell Mol Immunol 2018. https://doi.org/10.1038/s41423-018-0023-1.
Tu Z, Li Y, Smith DS, Sheibani N, Huang S, Kern T, et al. Retinal pericytes inhibit activated T cell proliferation. Invest Ophthalmol Vis Sci 2011, 52: 9005–9010.
Santos GSP, Prazeres P, Mintz A, Birbrair A. Role of pericytes in the retina. Eye (Lond) 2018, 32: 483–486.
Stark K, Eckart A, Haidari S, Tirniceriu A, Lorenz M, von Bruhl ML, et al. Capillary and arteriolar pericytes attract innate leukocytes exiting through venules and ‘instruct’ them with pattern-recognition and motility programs. Nat Immunol 2013, 14: 41–51.
Castejon OJ. Ultrastructural pathology of cortical capillary pericytes in human traumatic brain oedema. Folia Neuropathol 2011, 49: 162–173.
De La Fuente AG, Lange S, Silva ME, Gonzalez GA, Tempfer H, van Wijngaarden P, et al. Pericytes stimulate oligodendrocyte progenitor cell differentiation during CNS remyelination. Cell Rep 2017, 20: 1755–1764.
Birbrair A, Borges IDT, Gilson Sena IF, Almeida GG, da Silva Meirelles L, Goncalves R, et al. How plastic are pericytes? Stem Cells Dev 2017, 26: 1013–1019.
Asada N, Kunisaki Y, Pierce H, Wang Z, Fernandez NF, Birbrair A, et al. Differential cytokine contributions of perivascular haematopoietic stem cell niches. Nat Cell Biol 2017, 19: 214–223.
Khan JA, Mendelson A, Kunisaki Y, Birbrair A, Kou Y, Arnal-Estape A, et al. Fetal liver hematopoietic stem cell niches associate with portal vessels. Science 2016, 351: 176–180.
Azevedo PO, Sena IFG, Andreotti JP, Carvalho-Tavares J, Alves-Filho JC, Cunha TM, et al. Pericytes modulate myelination in the central nervous system. J Cell Physiol 2018, 233: 5523–5529.
Borges I, Sena I, Azevedo P, Andreotti J, Almeida V, Paiva A, et al. Lung as a niche for hematopoietic progenitors. Stem Cell Rev 2017, 13: 567–574.
Alvarenga EC, Silva WN, Vasconcellos R, Paredes-Gamero EJ, Mintz A, Birbrair A. Promyelocytic leukemia protein in mesenchymal stem cells is essential for leukemia progression. Ann Hematol 2018, 97: 1749–1755.
Sakuma R, Kawahara M, Nakano-Doi A, Takahashi A, Tanaka Y, Narita A, et al. Brain pericytes serve as microglia-generating multipotent vascular stem cells following ischemic stroke. J Neuroinflammation 2016, 13: 57.
Gouveia A, Seegobin M, Kannangara TS, He L, Wondisford F, Comin CH, et al. The aPKC-CBP pathway regulates post-stroke neurovascular remodeling and functional recovery. Stem Cell Rep 2017, 9: 1735–1744.
Nakagomi T, Kubo S, Nakano-Doi A, Sakuma R, Lu S, Narita A, et al. Brain vascular pericytes following ischemia have multipotential stem cell activity to differentiate into neural and vascular lineage cells. Stem Cells 2015, 33: 1962–1974.
Takagi T, Yoshimura S, Sakuma R, Nakano-Doi A, Matsuyama T, Nakagomi T. Novel regenerative therapies based on regionally induced multipotent stem cells in post-stroke brains: their origin, characterization, and perspective. Transl Stroke Res 2017, 8: 515–528.
Tatebayashi K, Tanaka Y, Nakano-Doi A, Sakuma R, Kamachi S, Shirakawa M, et al. Identification of multipotent stem cells in human brain tissue following stroke. Stem Cells Dev 2017, 26: 787–797.
Stapor PC, Sweat RS, Dashti DC, Betancourt AM, Murfee WL. Pericyte dynamics during angiogenesis: new insights from new identities. J Vasc Res 2014, 51: 163–174.
Berthiaume AA, Grant RI, McDowell KP, Underly RG, Hartmann DA, Levy M, et al. Dynamic remodeling of pericytes in vivo maintains capillary coverage in the adult mouse brain. Cell Rep 2018, 22: 8–16.
Dias Moura Prazeres PH, Sena IFG, Borges IDT, de Azevedo PO, Andreotti JP, de Paiva AE, et al. Pericytes are heterogeneous in their origin within the same tissue. Dev Biol 2017, 427: 6–11.
Nehls V, Drenckhahn D. Heterogeneity of microvascular pericytes for smooth muscle type alpha-actin. J Cell Biol 1991, 113: 147–154.
Morikawa S, Baluk P, Kaidoh T, Haskell A, Jain RK, McDonald DM. Abnormalities in pericytes on blood vessels and endothelial sprouts in tumors. Am J Pathol 2002, 160: 985–1000.
Bondjers C, He L, Takemoto M, Norlin J, Asker N, Hellstrom M, et al. Microarray analysis of blood microvessels from PDGF-B and PDGF-Rbeta mutant mice identifies novel markers for brain pericytes. FASEB J 2006, 20: 1703–1705.
Birbrair A, Frenette PS. Niche heterogeneity in the bone marrow. Ann N Y Acad Sci 2016, 1370: 82–96.
Goritz C, Dias DO, Tomilin N, Barbacid M, Shupliakov O, Frisen J. A pericyte origin of spinal cord scar tissue. Science 2011, 333: 238–242.
Birbrair A, Zhang T, Wang ZM, Messi ML, Enikolopov GN, Mintz A, et al. Skeletal muscle pericyte subtypes differ in their differentiation potential. Stem Cell Res 2013, 10: 67–84.
Trost A, Lange S, Schroedl F, Bruckner D, Motloch KA, Bogner B, et al. Brain and retinal pericytes: origin, function and role. Front Cell Neurosci 2016, 10: 20.
Prazeres P, Almeida VM, Lousado L, Andreotti JP, Paiva AE, Santos GSP, et al. Macrophages generate pericytes in the developing brain. Cell Mol Neurobiol 2018, 38: 777–782.
Birbrair A, Zhang T, Wang ZM, Messi ML, Enikolopov GN, Mintz A, et al. Role of pericytes in skeletal muscle regeneration and fat accumulation. Stem Cells Dev 2013, 22: 2298–2314.
Birbrair A, Zhang T, Files DC, Mannava S, Smith T, Wang ZM, et al. Type-1 pericytes accumulate after tissue injury and produce collagen in an organ-dependent manner. Stem Cell Res Ther 2014, 5: 122.
Birbrair A, Zhang T, Wang ZM, Messi ML, Olson JD, Mintz A, et al. Type-2 pericytes participate in normal and tumoral angiogenesis. Am J Physiol Cell Physiol 2014, 307: C25–38.
Zeisel A, Munoz-Manchado AB, Codeluppi S, Lonnerberg P, La Manno G, Jureus A, et al. Brain structure. Cell types in the mouse cortex and hippocampus revealed by single-cell RNA-seq. Science 2015, 347: 1138–1142.
Vanlandewijck M, He L, Mae MA, Andrae J, Ando K, Del Gaudio F, et al. A molecular atlas of cell types and zonation in the brain vasculature. Nature 2018, 554: 475–480.
He L, Vanlandewijck M, Raschperger E, Andaloussi Mae M, Jung B, Lebouvier T, et al. Analysis of the brain mural cell transcriptome. Sci Rep 2016, 6: 35108.
Lousado L, Prazeres P, Andreotti JP, Paiva AE, Azevedo PO, Santos GSP, et al. Schwann cell precursors as a source for adrenal gland chromaffin cells. Cell Death Dis 2017, 8: e3072.
Silva WN, Leonel C, Prazeres PHDM, Sena IFG, Guerra DAP, Diniz IMA, et al. Role of Schwann cells in cutaneous wound healing. Wound Repair Regen 2018. https://doi.org/10.1111/wrr.12647.
Pereira LX, Viana CTR, Orellano LAA, Almeida SA, Vasconcelos AC, Goes AM, et al. Synthetic matrix of polyether-polyurethane as a biological platform for pancreatic regeneration. Life Sci 2017, 176: 67–74.
Nishiyama A, Boshans L, Goncalves CM, Wegrzyn J, Patel KD. Lineage, fate, and fate potential of NG2-glia. Brain Res 2016, 1638: 116–128.
Wohl SG, Schmeer CW, Friese T, Witte OW, Isenmann S. In situ dividing and phagocytosing retinal microglia express nestin, vimentin, and NG2 in vivo. PLoS One 2011, 6: e22408.
Lindahl P, Johansson BR, Leveen P, Betsholtz C. Pericyte loss and microaneurysm formation in PDGF-B-deficient mice. Science 1997, 277: 242–245.
Winkler EA, Bell RD, Zlokovic BV. Pericyte-specific expression of PDGF beta receptor in mouse models with normal and deficient PDGF beta receptor signaling. Mol Neurodegener 2010, 5: 32.
Ohlund D, Handly-Santana A, Biffi G, Elyada E, Almeida AS, Ponz-Sarvise M, et al. Distinct populations of inflammatory fibroblasts and myofibroblasts in pancreatic cancer. J Exp Med 2017, 214: 579–596.
Guimaraes-Camboa N, Cattaneo P, Sun Y, Moore-Morris T, Gu Y, Dalton ND, et al. Pericytes of multiple organs do not behave as mesenchymal stem cells in vivo. Cell Stem Cell 2017, 20: 345–359 e345.
Gerl K, Miquerol L, Todorov VT, Hugo CP, Adams RH, Kurtz A, et al. Inducible glomerular erythropoietin production in the adult kidney. Kidney Int 2015, 88: 1345–1355.
Prazeres PHDM, Turquetti AOM, Azevedo PO, Barreto RSN, Miglino MA, Mintz A, et al. Perivascular cell αv integrins as a target to treat skeletal muscle fibrosis. Int J Biochem Cell Biol 2018, 99:109–113.
Soderblom C, Luo X, Blumenthal E, Bray E, Lyapichev K, Ramos J, et al. Perivascular fibroblasts form the fibrotic scar after contusive spinal cord injury. J Neurosci 2013, 33: 13882–13887.
Bechmann I, Priller J, Kovac A, Bontert M, Wehner T, Klett FF, et al. Immune surveillance of mouse brain perivascular spaces by blood-borne macrophages. Eur J Neurosci 2001, 14: 1651–1658.
Silva WN, Prazeres P, Paiva AE, Lousado L, Turquetti AOM, Barreto RSN, et al. Macrophage-derived GPNMB accelerates skin healing. Exp Dermatol 2018, 27: 630–635.
Guillemin GJ, Brew BJ. Microglia, macrophages, perivascular macrophages, and pericytes: a review of function and identification. J Leukoc Biol 2004, 75: 388–397.
Crisan M, Corselli M, Chen WC, Peault B. Perivascular cells for regenerative medicine. J Cell Mol Med 2012 16: 2851–2860.
Wanjare M, Kusuma S, Gerecht S. Perivascular cells in blood vessel regeneration. Biotechnol J 2013, 8: 434–447.
Birbrair A, Delbono O. Pericytes are essential for skeletal muscle formation. Stem Cell Rev 2015, 11: 547–548.
Birbrair A, Zhang T, Wang ZM, Messi ML, Mintz A, Delbono O. Pericytes: multitasking cells in the regeneration of injured, diseased, and aged skeletal muscle. Front Aging Neurosci 2014, 6: 245.
Birbrair A, Zhang T, Wang ZM, Messi ML, Mintz A, Delbono O. Type-1 pericytes participate in fibrous tissue deposition in aged skeletal muscle. Am J Physiol Cell Physiol 2013, 305: C1098–1113.
Dore-Duffy P, Katychev A, Wang X, Van Buren E. CNS microvascular pericytes exhibit multipotential stem cell activity. J Cereb Blood Flow Metab 2006, 26: 613–624.
Birbrair A, Zhang T, Wang ZM, Messi ML, Enikolopov GN, Mintz A, et al. Skeletal muscle neural progenitor cells exhibit properties of NG2-glia. Exp Cell Res 2013, 319: 45–63.
Karow M, Sanchez R, Schichor C, Masserdotti G, Ortega F, Heinrich C, et al. Reprogramming of pericyte-derived cells of the adult human brain into induced neuronal cells. Cell Stem Cell 2012, 11: 471–476.
Andreotti JP, Prazeres PHDM, Magno LAV, Romano-Silva MA, Mintz A, Birbrair A. Neurogenesis in the postnatal cerebellum after injury. Int J Dev Neurosci 2018 67: 33–36.
Birbrair A, Sattiraju A, Zhu D, Zulato G, Batista I, Nguyen VT, et al. Novel peripherally derived neural-like stem cells as therapeutic carriers for treating glioblastomas. Stem Cells Transl Med 2017, 6: 471–481.
Birbrair A. Stem cell microenvironments and beyond. Adv Exp Med Biol 2017, 1041: 1–3.
Birbrair A, Wang ZM, Messi ML, Enikolopov GN, Delbono O. Nestin-GFP transgene reveals neural precursor cells in adult skeletal muscle. PLoS One 2011, 6: e16816.
Costa MA, Paiva AE, Andreotti JP, Cardoso MV, Cardoso CD, Mintz A, et al. Pericytes constrict blood vessels after myocardial ischemia. J Mol Cell Cardiol 2018, 116: 1–4.
Li Y, Lucas-Osma AM, Black S, Bandet MV, Stephens MJ, Vavrek R, et al. Pericytes impair capillary blood flow and motor function after chronic spinal cord injury. Nat Med 2017, 23: 733–741.
Almeida VM, Paiva AE, Sena IFG, Mintz A, Magno LAV, Birbrair A. Pericytes make spinal cord breathless after injury. Neuroscientist 2018, 24: 440–447.
Yamamoto S, Muramatsu M, Azuma E, Ikutani M, Nagai Y, Sagara H, et al. A subset of cerebrovascular pericytes originates from mature macrophages in the very early phase of vascular development in CNS. Sci Rep 2017, 7: 3855.
Yamazaki T, Nalbandian A, Uchida Y, Li W, Arnold TD, Kubota Y, et al. Tissue myeloid progenitors differentiate into pericytes through TGF-beta signaling in developing skin vasculature. Cell Rep 2017, 18: 2991–3004.
Ozen I, Deierborg T, Miharada K, Padel T, Englund E, Genove G, et al. Brain pericytes acquire a microglial phenotype after stroke. Acta Neuropathol 2014, 128: 381–396.
Paiva AE, Lousado L, Almeida VM, Andreotti JP, Santos GSP, Azevedo PO, et al. Endothelial cells as precursors for osteoblasts in the metastatic prostate cancer bone. Neoplasia 2017, 19: 928–931.
Paiva AE, Lousado L, Guerra DAP, Azevedo PO, Sena IFG, Andreotti JP, et al. Pericytes in the premetastatic niche. Cancer Res 2018, 78: 2779–2786.
Azevedo PO, Paiva AE, Santos GSP, Lousado L, Andreotti JP, Sena IFG, et al. Cross-talk between lung cancer and bones results in neutrophils that promote tumor progression. Cancer Metastasis Rev 2018. https://doi.org/10.1007/s10555-018-9759-4.
Filosa JA, Nelson MT, Gonzalez Bosc LV. Activity-dependent NFATc3 nuclear accumulation in pericytes from cortical parenchymal microvessels. Am J Physiol Cell Physiol 2007, 293: C1797–1805.
Haskew-Layton RE, Payappilly JB, Smirnova NA, Ma TC, Chan KK, Murphy TH, et al. Controlled enzymatic production of astrocytic hydrogen peroxide protects neurons from oxidative stress via an Nrf2-independent pathway. Proc Natl Acad Sci U S A 2010, 107: 17385–17390.
Vinukonda G, Dummula K, Malik S, Hu F, Thompson CI, Csiszar A, et al. Effect of prenatal glucocorticoids on cerebral vasculature of the developing brain. Stroke 2010, 41: 1766–1773.
Winkler EA, Sagare AP, Zlokovic BV. The pericyte: a forgotten cell type with important implications for Alzheimer’s disease? Brain Pathol 2014, 24: 371–386.
Halliday MR, Rege SV, Ma Q, Zhao Z, Miller CA, Winkler EA, et al. Accelerated pericyte degeneration and blood-brain barrier breakdown in apolipoprotein E4 carriers with Alzheimer’s disease. J Cereb Blood Flow Metab 2016, 36: 216–227.
Montagne A, Nikolakopoulou AM, Zhao Z, Sagare AP, Si G, Lazic D, et al. Pericyte degeneration causes white matter dysfunction in the mouse central nervous system. Nat Med 2018, 24: 326–337.
Sagare AP, Bell RD, Zhao Z, Ma Q, Winkler EA, Ramanathan A, et al. Pericyte loss influences Alzheimer-like neurodegeneration in mice. Nat Commun 2013, 4: 2932.
Winkler EA, Sengillo JD, Sullivan JS, Henkel JS, Appel SH, Zlokovic BV. Blood-spinal cord barrier breakdown and pericyte reductions in amyotrophic lateral sclerosis. Acta Neuropathol 2013, 125: 111–120.
Coatti GC, Frangini M, Valadares MC, Gomes JP, Lima NO, Cavacana N, et al. Pericytes extend survival of ALS SOD1 mice and induce the expression of antioxidant enzymes in the murine model and in IPSCs derived neuronal cells from an ALS patient. Stem Cell Rev 2017, 13: 686–698.
Geraldes P, Hiraoka-Yamamoto J, Matsumoto M, Clermont A, Leitges M, Marette A, et al. Activation of PKC-delta and SHP-1 by hyperglycemia causes vascular cell apoptosis and diabetic retinopathy. Nat Med 2009, 15: 1298–1306.
Ghosh M, Balbi M, Hellal F, Dichgans M, Lindauer U, Plesnila N. Pericytes are involved in the pathogenesis of cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy. Ann Neurol 2015, 78: 887–900.
Marchi N, Lerner-Natoli M. Cerebrovascular remodeling and epilepsy. Neuroscientist 2013, 19: 304–312.
Niu F, Yao H, Zhang W, Sutliff RL, Buch S. Tat 101-mediated enhancement of brain pericyte migration involves platelet-derived growth factor subunit B homodimer: implications for human immunodeficiency virus-associated neurocognitive disorders. J Neurosci 2014, 34: 11812–11825.
Heldin CH. Targeting the PDGF signaling pathway in tumor treatment. Cell Commun Signal 2013, 11: 97.
Keller A, Westenberger A, Sobrido MJ, Garcia-Murias M, Domingo A, Sears RL, et al. Mutations in the gene encoding PDGF-B cause brain calcifications in humans and mice. Nat Genet 2013, 45: 1077–1082.
Behl Y, Krothapalli P, Desta T, Roy S, Graves DT. FOXO1 plays an important role in enhanced microvascular cell apoptosis and microvascular cell loss in type 1 and type 2 diabetic rats. Diabetes 2009, 58: 917–925.
Arango-Lievano M, Boussadia B, De Terdonck LDT, Gault C, Fontanaud P, Lafont C, et al. Topographic reorganization of cerebrovascular mural cells under seizure conditions. Cell Rep 2018, 23: 1045–1059.
Lowe SW, Ruley HE, Jacks T, Housman DE. p53-dependent apoptosis modulates the cytotoxicity of anticancer agents. Cell 1993, 74: 957–967.
Barry MA, Behnke CA, Eastman A. Activation of programmed cell death (apoptosis) by cisplatin, other anticancer drugs, toxins and hyperthermia. Biochem Pharmacol 1990, 40: 2353–2362.
Roos WP, Kaina B. DNA damage-induced cell death by apoptosis. Trends Mol Med 2006, 12: 440–450.
Hill RA, Damisah EC, Chen F, Kwan AC, Grutzendler J. Targeted two-photon chemical apoptotic ablation of defined cell types in vivo. Nat Commun 2017, 8: 15837.
Saito M, Iwawaki T, Taya C, Yonekawa H, Noda M, Inui Y, et al. Diphtheria toxin receptor-mediated conditional and targeted cell ablation in transgenic mice. Nat Biotechnol 2001, 19: 746–750.
Buch T, Heppner FL, Tertilt C, Heinen TJ, Kremer M, Wunderlich FT, et al. A Cre-inducible diphtheria toxin receptor mediates cell lineage ablation after toxin administration. Nat Methods 2005, 2: 419–426.
Mallet VO, Mitchell C, Guidotti JE, Jaffray P, Fabre M, Spencer D, et al. Conditional cell ablation by tight control of caspase-3 dimerization in transgenic mice. Nat Biotechnol 2002, 20: 1234–1239.
Fu Y, Tucciarone JM, Espinosa JS, Sheng N, Darcy DP, Nicoll RA, et al. A cortical circuit for gain control by behavioral state. Cell 2014, 156: 1139–1152.
Orger MB, Kampff AR, Severi KE, Bollmann JH, Engert F. Control of visually guided behavior by distinct populations of spinal projection neurons. Nat Neurosci 2008, 11: 327–333.
Kirby BB, Takada N, Latimer AJ, Shin J, Carney TJ, Kelsh RN, et al. In vivo time-lapse imaging shows dynamic oligodendrocyte progenitor behavior during zebrafish development. Nat Neurosci 2006, 9: 1506–1511.
Rompolas P, Deschene ER, Zito G, Gonzalez DG, Saotome I, Haberman AM, et al. Live imaging of stem cell and progeny behaviour in physiological hair-follicle regeneration. Nature 2012, 487: 496–499.
Jay DG. Selective destruction of protein function by chromophore-assisted laser inactivation. Proc Natl Acad Sci U S A 1988, 85: 5454–5458.
Tour O, Meijer RM, Zacharias DA, Adams SR, Tsien RY. Genetically targeted chromophore-assisted light inactivation. Nat Biotechnol 2003, 21: 1505–1508.
Rajfur Z, Roy P, Otey C, Romer L, Jacobson K. Dissecting the link between stress fibres and focal adhesions by CALI with EGFP fusion proteins. Nat Cell Biol 2002, 4: 286–293.
Surrey T, Elowitz MB, Wolf PE, Yang F, Nedelec F, Shokat K, et al. Chromophore-assisted light inactivation and self-organization of microtubules and motors. Proc Natl Acad Sci U S A 1998, 95: 4293–4298.
Bulina ME, Chudakov DM, Britanova OV, Yanushevich YG, Staroverov DB, Chepurnykh TV, et al. A genetically encoded photosensitizer. Nat Biotechnol 2006, 24: 95–99.
Qi YB, Garren EJ, Shu X, Tsien RY, Jin Y. Photo-inducible cell ablation in Caenorhabditis elegans using the genetically encoded singlet oxygen generating protein miniSOG. Proc Natl Acad Sci U S A 2012, 109: 7499–7504.
Lin JY, Sann SB, Zhou K, Nabavi S, Proulx CD, Malinow R, et al. Optogenetic inhibition of synaptic release with chromophore-assisted light inactivation (CALI). Neuron 2013, 79: 241–253.
Huang FJ, You WK, Bonaldo P, Seyfried TN, Pasquale EB, Stallcup WB. Pericyte deficiencies lead to aberrant tumor vascularizaton in the brain of the NG2 null mouse. Dev Biol 2010, 344: 1035–1046.
Kunz J, Krause D, Kremer M, Dermietzel R. The 140-kDa protein of blood-brain barrier-associated pericytes is identical to aminopeptidase N. J Neurochem 1994, 62: 2375–2386.
Nehls V, Drenckhahn D. The versatility of microvascular pericytes: from mesenchyme to smooth muscle? Histochemistry 1993, 99: 1–12.
Nehls V, Denzer K, Drenckhahn D. Pericyte involvement in capillary sprouting during angiogenesis in situ. Cell Tissue Res 1992, 270: 469–474.
Cho H, Kozasa T, Bondjers C, Betsholtz C, Kehrl JH. Pericyte-specific expression of Rgs5: implications for PDGF and EDG receptor signaling during vascular maturation. FASEB J 2003, 17: 440–442.
Christian S, Winkler R, Helfrich I, Boos AM, Besemfelder E, Schadendorf D, et al. Endosialin (Tem1) is a marker of tumor-associated myofibroblasts and tumor vessel-associated mural cells. Am J Pathol 2008, 172: 486–494.
Maki T, Maeda M, Uemura M, Lo EK, Terasaki Y, Liang AC, et al. Potential interactions between pericytes and oligodendrocyte precursor cells in perivascular regions of cerebral white matter. Neurosci Lett 2015, 597: 164–169.
Kunisaki Y, Bruns I, Scheiermann C, Ahmed J, Pinho S, Zhang D, et al. Arteriolar niches maintain haematopoietic stem cell quiescence. Nature 2013, 502: 637–643.
Guerra DAP, Paiva AE, Sena IFG, Azevedo PO, Batista ML, Jr., Mintz A, et al. Adipocytes role in the bone marrow niche. Cytometry A 2018, 93:167–171.
Sena IFG, Prazeres P, Santos GSP, Borges IT, Azevedo PO, Andreotti JP, et al. Identity of Gli1+ cells in the bone marrow. Exp Hematol 2017, 54: 12–16.
Sena IFG, Borges IT, Lousado L, Azevedo PO, Andreotti JP, Almeida VM, et al. LepR+ cells dispute hegemony with Gli1+ cells in bone marrow fibrosis. Cell Cycle 2017, 16: 1–5.
Hammes HP, Lin J, Wagner P, Feng Y, Vom Hagen F, Krzizok T, et al. Angiopoietin-2 causes pericyte dropout in the normal retina: evidence for involvement in diabetic retinopathy. Diabetes 2004, 53: 1104–1110.
Braun A, Xu H, Hu F, Kocherlakota P, Siegel D, Chander P, et al. Paucity of pericytes in germinal matrix vasculature of premature infants. J Neurosci 2007, 27: 12012–12024.
Wilhelmus MM, Otte-Holler I, van Triel JJ, Veerhuis R, Maat-Schieman ML, Bu G, et al. Lipoprotein receptor-related protein-1 mediates amyloid-beta-mediated cell death of cerebrovascular cells. Am J Pathol 2007, 171: 1989–1999.
Wisniewski HM, Wegiel J, Wang KC, Lach B. Ultrastructural studies of the cells forming amyloid in the cortical vessel wall in Alzheimer’s disease. Acta Neuropathol 1992, 84: 117–127.
Yang S, Jin H, Zhu Y, Wan Y, Opoku EN, Zhu L, et al. Diverse functions and mechanisms of pericytes in ischemic stroke. Curr Neuropharmacol 2017, 15: 892–905.
Fernandez-Klett F, Potas JR, Hilpert D, Blazej K, Radke J, Huck J, et al. Early loss of pericytes and perivascular stromal cell-induced scar formation after stroke. J Cereb Blood Flow Metab 2013, 33: 428–439.
Zehendner CM, Sebastiani A, Hugonnet A, Bischoff F, Luhmann HJ, Thal SC. Traumatic brain injury results in rapid pericyte loss followed by reactive pericytosis in the cerebral cortex. Sci Rep 2015, 5: 13497.
Milesi S, Boussadia B, Plaud C, Catteau M, Rousset MC, De Bock F, et al. Redistribution of PDGFRbeta cells and NG2DsRed pericytes at the cerebrovasculature after status epilepticus. Neurobiol Dis 2014, 71: 151–158.
Klement W, Garbelli R, Zub E, Rossini L, Tassi L, Girard B, et al. Seizure progression and inflammatory mediators promote pericytosis and pericyte-microglia clustering at the cerebrovasculature. Neurobiol Dis 2018, 113: 70–81.
Acknowledgements
A. Birbrair was supported by Grants from Instituto Serrapilheira/Serra-1708-15285, Pró-reitoria de Pesquisa/Universidade Federal de Minas Gerais (PRPq/UFMG) (Edital 05/2016), the National Institute of Science and Technology in Theranostics and Nanobiotechnology (CNPq/CAPES/FAPEMIG, Process No. 465669/2014-0), FAPEMIG [Rede Mineira de Engenharia de Tecidos e Terapia Celular (REMETTEC, RED-00570-16)], and FAPEMIG [Rede De Pesquisa Em Doenças Infecciosas Humanas E Animais Do Estado De Minas Gerais (RED-00313-16)]. Akiva Mintz was supported by the National Institute of Health (1R01CA179072-01A1) and an American Cancer Society Mentored Research Scholar Grant (124443-MRSG-13-121-01-CDD).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors indicate no potential conflicts of interest.
Rights and permissions
About this article
Cite this article
Santos, G.S.P., Magno, L.A.V., Romano-Silva, M.A. et al. Pericyte Plasticity in the Brain. Neurosci. Bull. 35, 551–560 (2019). https://doi.org/10.1007/s12264-018-0296-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12264-018-0296-5