Abstract
We investigated the effects of eight different levels of salinity (0–35) on the larval development of Upogebia vasquezi, while the abundance of the larvae within the Marapanim estuary on the Amazon Coast was verified through the monthly collection of specimens between August 2006 and July 2007. This species reproduces year-round on the Amazon Coast, which is subjected to strong seasonal fluctuations in salinity due to the local precipitation regime. Upogebia vasquezi larvae developed optimally in salinity close to that of seawater (20–35), while low salinities (0, 5, and 10) did not support the survival of the larvae. Only zoeal stages I, II, and III were captured in the field and were more abundant at the higher end of the salinity gradient, in the areas closest to the adjacent coastal waters. Data from both the laboratory and the field data emphasized the low survival potential of the larvae in low salinities, and increased survival and improved development in more saline water. These results support the hypothesis that U. vasquezi undergo development on the shelf, and also suggest the possibility of an ontogenetic migration toward to adjacent coastal areas during early larval stages, as observed in other decapod species around the world.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The ability of a species to establish a viable population in a given habitat depends on the capacity of each of its developmental stages to adapt to the conditions found in this environment. In coastal aquatic ecosystems, salinity and temperature are among the principal factors limiting the distribution of organisms (Costlow et al., 1960; Charmantier, 1998). In marine invertebrates, the osmotic stress derived from inadequate salinity levels may affect development rates, leading to delays in the larval cycle, the number of stages, and a reduction in the survival of the larvae (see Costlow et al., 1960; Anger, 2001, 2003).
The salinity of coastal and estuarine environments varies seasonally, regionally, and locally, exposing resident organisms to varying levels of osmotic stress during their development. These conditions demand physiological and behavior adaptations in the early stages of development (Charmantier, 1998; Anger, 2003; Torres et al., 2011; Vuichard et al., 2013). In decapod larvae, saline stress is reflected primarily in delayed development, and reduced rates of survival, feeding, growth, and behavior (Anger, 2003).
The variation in the tolerance of decapods to the saline stress of estuarine environments during different developmental stages is reflected in migration patterns, which translocate the larvae to bodies of water with optimal conditions for the physiological needs of the organism. This influences the extent of larval dispersal into or out of estuaries, depending on the net movement and abundance of larvae at different depths (O’Connor & Epifanio, 1985). The principal processes here are the exportation from or retention of the larvae within the estuarine environment (see Wooldridge & Loubser, 1996; Paula et al., 2001; Anger, 2003). Reproduction in the estuarine crabs Ucides cordatus (Linnaeus, 1763), Uca vocator (Herbst, 1804), and Uca rapax (Smith, 1870) on the Amazon coast involves the exportation of the larvae from estuarine areas and mangroves, which they inhabit as adults, to adjacent coastal waters, with higher salinity (Diele & Simith, 2006; Simith & Diele, 2008; Simith et al., 2012, 2014). This process ensures the continuation of the parental populations, given that breeding may often occur during the rainy season—in U. cordatus, it occurs only during this period—when salinity decreases significantly, and would be lethal for the larvae. It also increases the possibility of reaching adjacent mangroves and estuaries. By contrast, studies of the larvae of the porcelain crab Petrolisthes armatus (Gibbes, 1850) indicate the adoption of retention mechanisms, not only in the equatorial region (Oliveira et al., 2013) but also in the Brazilian tropics, i.e., the southwestern Atlantic (Melo et al., 2012).
Mud shrimps of the genus Upogebia (Gebiidea) are among the most abundant decapods found in the intertidal and subtidal sediments of coastal environments around the world (Pires et al., 2013; Sakai & Turkay, 2014), but especially at lower latitudes, where the highest diversity of the Gebiidea and Axiidea can be found (Dworschak, 2005; Dworschak et al., 2012). The typical reproductive pattern in these organisms appears to be the exportation of the early larval stages from the estuary to the ocean, where the larvae develop in seawater, before returning to the estuary as megalopae (Faleiro et al., 2012). For example, the larvae of Upogebia africana (Ortmann, 1894) and U. pusilla (Petagna, 1792) present higher survival rates in saline conditions most similar to those of the open sea, adopting an exportation-type strategy (Dworschak, 1988; Paula et al., 2001; Faleiro et al., 2012; Pires et al., 2013). The larvae of U. deltaura (Leach, 1815) are also exported from estuarine waters, remaining primarily in the surface levels of the water column, where there is a greater potential for dispersal to offshore environments (Pires et al., 2013).
Experimental laboratory studies of the effects of different saline conditions on the development of decapod larvae provide valuable evidence on the reproductive strategies adopted by the different species, given that their tolerance of salinity in the laboratory generally coincides with their distribution along salinity gradients in the wild (Anger, 2003). These studies have demonstrated distinct levels of osmotic tolerance in different species, providing evidence for the discussion of the mechanisms of larval exportation or retention (e.g., Anger, 1991, 1996; Charmantier et al., 2002, Diele & Simith, 2006; Anger et al., 2008; Esser & Cumberlidge, 2011; Fowler et al., 2011; Faleiro et al., 2012; Simith et al., 2012, 2014).
Upogebia vasquezi Ngoc-Ho, 1989 has an ample geographic distribution, including Florida, the Bahamas, Mexico, Costa Rica, and Colombia, ranging as far south as Brazil (Sakai & Turkay, 2014), including the Amazon coast (Oliveira et al., 2012), where it is typically found in rocky outcrops in the vicinity of mangroves (Silva & Martinelli-Lemos, 2012). In the laboratory, the complete development of the larvae of U. vasquezi up until the first juvenile stage takes approximately 16 days and includes four zoeal and one megalopal stages (Oliveira et al., 2014). On the Amazon coast, the species reproduces year-round, with ovigerous females and juveniles being found during most of the year (Oliveira et al., 2012; Silva & Martinelli-Lemos, 2012). In the Marapanim estuary, on the Amazon coast of northern Brazil, U. vasquezi is the most dominant of the axiid or gebiidea species, accounting for approximately 82% of all individuals (Oliveira et al., 2012).
The climate of the Amazon coast is characterized by two distinct periods—the rainy season, between January and July, and the dry season, between August and December, with mean salinity of approximately 14 and 25, respectively, in the Marapanim estuary (Silva & Martinelli-Lemos, 2012). The organisms resident in the estuary are thus exposed to considerable seasonal fluctuations in salinity levels, in addition to the spatial gradient that exists between the uppermost reaches of the estuary and its mouth, adjacent to the Atlantic Ocean. The seasonal fluctuations in the salinity of the estuary’s waters is one of the principal factors determining the population and reproductive biology of the region’s decapods (Silva & Martinelli-Lemos, 2012; Oliveira et al., 2012, 2013).
The present study investigated the reproductive strategy of U. vasquezi in the equatorial Amazon region, analyzing the effects of six different levels of salinity on the survival and inter-molt period in the larvae of this species in the laboratory. The findings are compared with those of samples of the zooplankton taken in the estuary along the salinity gradient. The findings are discussed and compared with those of previous studies.
Materials and methods
Study area
The Marapanim estuary is located on the northeastern coast of the Brazilian state of Pará, in the region known as the “Salgado” (00°32′–00°52′S, 47°28′–47°45′W), which encompasses a number of estuaries connected to the Atlantic Ocean. The Marapanim estuary is located on the Amazon Macrotidal Mangrove Coast, which corresponds to 10% of the Brazilian coastline but encompasses more than 56% of the country’s mangrove forests, and is thus considered to be a high priority area for conservation (Souza-Filho, 2005; Berrêdo et al., 2008).
The region’s climate is influenced primarily by the Intertropical Convergence Zone, which is responsible for the trade winds and rainfall cycle, which determine the local climatic and hydrological seasons (Berrêdo et al., 2008). Total precipitation in the region of the Marapanim estuary was 2277.2 mm between August 2006 and July 2007 (the study period), with monthly totals ranging from 10.4 mm in August 2006 (dry season) to 760.6 mm in February 2007 (rainy season), with mean salinity of 13.25 ± 6.06 and 24.59 ± 9.62, respectively (Silva & Martinelli-Lemos, 2012).
Collection of ovigerous females
Three ovigerous female specimens of Upogebia vasquezi were collected from the midlittoral zone of the Marapanim estuary (site A1), Pará (0°38′S, 47º32′W), during the low tide on September 1st, 2012 (Fig. 1). The tunnels occupied by the specimens were located in a rocky outcrop, formed by rocks, sand, and mud, with salinity of 30 (measured in situ using an Atago optical refractometer). The specimens were retrieved manually and transferred to polyethylene containers with seawater and local substrate for transportation to the laboratory under constant aeration at ambient temperature.
In the laboratory, the females were maintained individually in glass aquaria with treated seawater at a salinity of 30 (the same as that recorded in the natural environment) under constant aeration, but without provisioning, until the eggs had hatched. The larvae of one female hatched after 2 days, whereas in the other two, they hatched after 6 days. The total brood of each female was not quantified.
Experimental procedures
Once hatched, the most active larvae from each brood were chosen and pooled with those hatched on the same day. The larvae were assigned randomly to eight different treatments (T) with salinity ranging from 0 to 35, in steps of 5—T0, T5, T10, T15, T20, T25, T30, and T35—simulating the saline gradient between the headwaters of the estuary (T0) and the open seas (T35). Two replicates, R1 and R2, each with 60 larvae, were considered for each treatment, the first being derived from the larvae of the first brood, hatched on September 3, 2012, and the other from the larvae of the two broods that hatched on September 7, 2012. As no significant differences were found between the two replicates, the data were pooled for analysis, with 60 larvae per replicate, resulting in a total of 120 larvae per treatment.
The seawater used to rear the larvae was filtered (Eheim and Diatom Filter, 1 μm) and sterilized (ultraviolet filter: Gehaka), and stored under constant aeration. The stock of seawater used in the laboratory had a salinity of 35, and the water for each treatment was obtained by diluting this seawater with freshwater (salinity = 0).
The larvae passed through an acclimatization period of one and a half hours for each five units of salinity (Faleiro et al., 2012). Once the water reached the specific salinity for each treatment, the larvae were distributed in 12 transparent plastic recipients each with 10 individuals (120 larvae per treatment). As the larvae assigned to T0 and T5 did not survive the acclimatization period (100% mortality), these treatments were not included in this experiment.
The U. vasquezi larvae were fed daily on recently hatched Artemia sp. nauplii. The containers were kept at a more or less constant temperature (27.5 ± 1.36°C) and standard photoperiod (12 h dark, 12 h light). The water was changed every 2 days. The treatments continued until the larvae reached the first juvenile stage, and were ended as soon as the last individuals reached the second juvenile instar or died.
Collection of data in the field
The U. vasquezi larvae were collected from their natural habitat using horizontal surface trawls with a zooplankton net (200 μm mesh) at six sites within the Marapanim estuary, A1, B1, A2, B2, A3, and B3 (Fig. 1), representing three different levels of the salinity gradient: (A1 + B1), nearest to the open sea, with a mean salinity of 22; (A2 + B2), intermediate, with a mean of salinity 20; and (A3 + B3), the innermost portion of the estuary with lowest salinity, 15, on average. The distance between each site was approximately 8 km, with A1 and A3 being separated by a distance of 16 km. The A sites (A1, A2, A3) were located on the western margin of the Marapanim River, adjacent to the town of Marapanim and the Araticum, Aracumirim, and Alegria fisher communities. There are no settlements on the eastern margin of the river (sites B1, B2, B3). Adult U. vasquezi were found at all sites except A3.
The samples were collected during the day on the ebb tide once a month over a 1-year period (August 2006 through July 2007), providing a total of 144 samples (6 areas × 2 replicates × 12 months), with two replicates per site. Each trawl had a duration of 3 min, at a speed of 1–1.5 knots. A calibrated Hydrobios flowmeter was attached to the mouth of the net to calculate the volume of water filtered through the trawl. The samples were fixed in buffered 4% formaldehyde. The temperature (°C), hydrogen-ionic potential (pH), and salinity of the water were estimated using a YSI multiparameter analyzer during the collection of the larvae.
In the laboratory, the zooplankton samples (1 l) were divided into smaller aliquots using a Folsom plankton splitter, and a subsample of 250 mL was used for the analysis of the U. vasquezi larvae. The larvae were observed under a Zeiss optical stereomicroscope and a Leica optical microscope with a micrometric reticle. The larvae and different developmental stages were identified based on Oliveira et al. (2014).
Ovigerous females and larvae of Upogebia vasquezi (both cultured and collected from plankton) representing the zoea I, II, III, IV, megalopa, and juvenile I stages were deposited at the Goeldi Museum in Belém, under catalog numbers MPEG 1133, 1135, 1136, 1137, 1138, 1139, and 1140, respectively.
Data analysis
Differences among treatments in the survival rates (%) of the U. vasquezi zoeae up to the last larval stage (megalopa) were analyzed using contingency tables (R × C) for Chi square tests. The survival rate (%) of the respective zoeal stages was calculated from the number of larvae surviving from the previous stage. The duration of the larval development (in days) from hatching to the megalopa stage was also compared among treatments. The normality and homoscedasticity of the variances of the data were checked a priori using the Kolmogorov–Smirnov test and Cochran’s C-test, respectively. As some of the data were not distributed normally or had heterogeneous variance, even after statistical transformations, a nonparametric one-way ANOVA (Kruskal–Wallis’s H-test) was employed for the analysis of the duration of larval development.
The data on the density of the U. vasquezi larvae in the natural environment were log (x + 1) transformed and analyzed for normality using the Shapiro–Wilk test. As the assumptions for a parametric test were satisfied, a one-way ANOVA was then conducted to verify the significance of the differences in the larval densities found along the salinity gradient within the Marapanim estuary. A 95% significance level was adopted for all analyses, which were run in BioEstat 5.0 (Ayres et al., 2007) and Statistica 7.0 (Statsoft, 2004).
Results
Rearing experiment
None of the U. vasquezi larvae survived the lowest salinities (T0 and T5), with 100% mortality being recorded in both cases during the acclimatization period. Survival rates from hatching to the final larval stage varied significantly (χ 2 = 85.03, d.f. = 5, P < 0.0001) among the other treatments, T10–T35 (Fig. 2). At salinity 10, no larvae reached the megalopa stage, although the zoeae were able to complete their development at salinities of 15 and above, with a significant tendency for higher rates of survival at higher salinities (A = 56, χ 2 = 15.66, d.f. = 1, P < 0.0001), ranging from 10% at T15 to 40% at T35 (Fig. 2).
The zoea I larvae were generally present during only the first day of this experiment in most of the treatments (H = 4.40, P = 0.49), although in T10 and T15, some of the zoea I larvae persisted into the second day of this experiment (Fig. 3A). The zoea II larvae had the shortest mean duration in T10 (1 day), and the longest in T15 (5–6 days) with similar values, of 4 or 5 days, being recorded in the other treatments (Fig. 3B), with no significant variation among treatments being found overall (H = 7.56, P = 0.18).
No zoea III larvae were observed in T10, due to the absolute mortality of the zoea II stage. The mean duration of this stage was 4–5 days in most treatments (H = 7.55, P = 0.18), except for T15, where it was just 2 days (Fig. 3C). The duration of the zoea IV larvae (Fig. 3D) and megalopae (Fig. 3E) was similar in treatments T20 to T35, at approximately 8–10 days, with no significant variation in either the larvae (H = 9.42, P = 0.09) or the megalopae (H = 9.20, P = 0.10). Juveniles survived only in salinities of 20 and above (Fig. 3F), with the lowest mean duration in T20 (1 day) and a higher duration (approximately 3 days) in T30 and T35 (H = 9.55, P = 0.09).
Only a very small portion (1%) of the newly hatched larvae (zoea I) reached the second stage (zoea II) in salinity 10 (T10). However, all these individuals had died by the third day of this experiment (Fig. 4A). In T15, 7% of the larvae had completed development to the megalopa stage by the 10th day of this experiment, but none of these individuals reached the juvenile I stage (Fig. 4B). At a salinity of 20, 21% of the larvae had reached the megalopa stage by the 13th day of this experiment, and 3% of these individuals successfully progressed to the juvenile I stage, by the 18th day (Fig. 4C).
The highest percentage of individuals (33%) reaching the megalopa stage was recorded in T25, by the 13th day (Fig. 4D), while the highest percentage of individuals reaching the juvenile I stage (8% by the 16th day) was recorded in T30. The shortest larval cycle was also recorded in T30, with the first megalopae appearing by the 7th day of this experiment (Fig. 4E). The larvae in T35 had the highest cumulative survival rate in all the larval developmental stages (Fig. 4F).
Density of U. vasquezi larvae in the Marapanim estuary
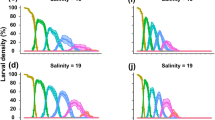
Salinity varied significantly among the sampling points located within the Marapanim estuary (F = 2.43, P = 0.04), with mean values of approximately 15.5 being recorded in the most internal part of the estuary (sites A3 and B3), 20.0 in the intermediate zone (A2 and B2), and 22.0 nearest its mouth (A1 and B1), with recorded values ranging from a 3 to 35 during the study period (Fig. 5A).
Only zoea I, II, and III U. vasquezi larvae were found in the zooplankton samples collected in the estuary, with the first stage being the most abundant overall. Zoea I larvae were collected in all three zones, whereas the other stages were only found in the intermediate zone (A2 and B2) and at the mouth of the estuary (A1 and B1). The mean density of zoea I larvae varied significantly (F = 3.72, P = 0.003) within the estuary, with the highest density being recorded at B2 and no larvae being collected at A3 (Fig. 5B). The zoea II and III larvae were much less abundant overall (Fig. 5C, D), and were found only in the intermediate zone and the mouth of the estuary, i.e., B1, A2, and B2, with significant differences being found among zones (zoea II: F = 0.93, P = 0.46; zoea III: F = 0.80, P = 0.55).
Discussion
The results of the present study indicate clearly that larval stages of U. vasquezi are unable to survive at low salinity, with absolute mortality being recorded in the zoea I larvae in the lowest salinities (T0 and T5), and in the zoea II larvae at T10. The larvae only completed their development at salinities of 15 or higher, even though survival in T15 was only 7%. The highest rates of survival (from hatching to the final larval stage) and reaching the juvenile I stage were recorded at the highest salinities, of 20 and above, indicating that optimal larval development in this species occurs at intermediate to high salinity. The larvae of Upogebia africana and Upogebia pusilla reach the megalopa stage at salinities of at least 25 and 35, respectively, with an optimal interval of 25–35 for U. africana (Paula et al., 2001; Faleiro et al., 2012).
The more euryhaline condition of the U. vasquezi larvae in comparison with other Upogebia species appears to favor the dispersal of the larvae on the Amazon coast, given the low to intermediate salinity found over hundreds of kilometers of coastline, characterized by extensive estuarine habitats (Diele & Simith, 2006). The euryhaline and osmoconformer characteristics of the adult Upogebia, which are amply distributed in estuarine ecosystems (Thompson & Pritchard, 1969), contrast with the more stenohaline larval stages (e.g., Paula et al., 2001; Faleiro et al., 2012). A similar pattern was also recorded in U. vasquezi on the Amazon coast, in the Marapanim estuary, where the adults were significantly more abundant during the rainy season, when mean salinity was 13.5 ± 3.7, whereas the larvae were found in the estuary primarily during the dry season, when mean salinity was much higher, at 28.5 ± 4.3 (Oliveira et al., 2012).
As in U. vasquezi, the larvae of the estuarine crab Ucides cordatus are unable to reach the megalopa stage in salinities of less than 10, but only at levels above 15, although the zoea I larvae are able to tolerate the osmotic stress of low salinities during a number of days (Diele & Simith, 2006; Simith & Diele, 2008). Even though only small numbers of competent megalopae survived at a salinity of 15, this is extremely important for the maintenance of the parental stocks, given that this species reproduces during the rainy season in the Amazon region, when the salinity of coastal waters is greatly reduced (Simith & Diele, 2008). A similar pattern has been recorded in Uca vocator, which reaches the megalopa stage when salinity is at least 10 (Simith et al., 2012). In the case of Uca rapax, complete larval development occurs only when salinity is at least 25, which indicates the more ample dispersal of the larvae to saline, oceanic waters, in comparison with the region’s other estuary-dwelling crabs (Simith et al., 2014).
The duration of the developmental stages of U. vasquezi did not vary significantly among treatments, despite the marked prolongation of the zoea I stage in salinities of 10 and 15, of the zoea II larvae at 15 and the megalopae at 35. In U. pusilla, only temperature had a significant effect on the duration of the development of the larvae (Faleiro et al., 2012). The increase in the duration of the early larval stages and the consequent delay in the transition to the subsequent stages reflects the intolerance of the larvae to osmotic stress (Anger, 2003), even though zoea I stage larvae may tolerate an ampler variation in salinity, as observed in U. africana (Paula et al., 2001). At salinities of 15–20, the final larval stage of U. vasquezi was of a shorter duration, which may reflect the acceleration of the developmental process at the moment the larvae return to the parental habitat, where they are once again exposed to the reduced salinity of the estuarine environment. This would guarantee the survival of the highest possible number of competent megalopae for recruitment (Paula et al., 2001).
On the Amazon coast, as in most other tropical littorals, temperatures are more or less constant, and decapod larval development is thus influenced primarily by salinity (e.g., Oliveira et al., 2012; Silva & Martinelli-Lemos, 2012; Nóbrega et al., 2013), which is itself influenced by the seasonal fluctuations in precipitation levels, with the first 6 months of the year accounting for approximately 70% of the annual total (Moraes et al., 2005). The rainfall cycle associated with the local trade winds determines the seasonal variation in the climate and the hydrological cycle of the local rivers, reflected ultimately in the seasonal variation in the salinity of the estuary waters (Berrêdo et al., 2008). In the specific case of the Marapanim estuary, monthly precipitation ranged from 10.4 mm in the dry season to 760.6 mm in the rainy season, with mean salinity of 28.5 ± 4.3 and 13.5 ± 3.7, respectively (Oliveira et al., 2012; Silva & Martinelli-Lemos, 2012).
The results of the laboratory experiment, which indicated significantly higher survival rates of the U. vasquezi at higher salinity, were similar to the data obtained from the species’ natural environment in the Marapanim estuary. Only the first larval stage (zoea I), which is more euryhaline, was found in the most internal areas of the estuary, where mean salinity was approximately 15.5. Zoeal stages II and III were much less abundant overall and were found only in the areas with relatively high salinity. No zoeae IV or megalopae were collected. The predominance of the intermediate stages (zoea II and III) only in the areas nearest to the coast indicates that early larval stages of U. vasquezi possibly undertake ontogenetic migrations toward adjacent coastal waters, a hypothesis reinforced by the absence of zoea IV larvae in the samples collected from the estuary. The abundance of zoea I larvae in the mouth of the estuary, adjacent to the coast, also indicates the exportation of larvae, as observed in U. pusilla by Faleiro et al. (2012).
Even though U. vasquezi breeds continuously throughout the year on the Amazon coast, peaks in breeding were observed during the rainy season (January, June, and July) when the salinity of the estuary was significantly lower than during other periods, with a mean of 13.5 ± 3.7 (Oliveira et al., 2012). In this scenario, the adoption of mechanisms of larval exportation and dispersal would be essential for the survival of these developmental stages and the ultimate maintenance of the parental population, as observed previously in other decapod populations on the Amazon coast (Diele & Simith, 2006; Simith et al., 2014).
The abundance and distribution of decapod larvae in natural environments have also elucidated the patterns of larval migration (exportation or retention) during the reproductive cycle of a number of other species (e.g., Anger et al., 1994; Christy & Morgan, 1998; Yannicelli et al., 2006; Santos et al., 2008; Pires et al., 2013). Upogebia africana has an obligate marine larval phase, supported by the exportation of the zoea I larvae to estuarine waters during the nocturnal ebb tide and the re-invasion of the megalopae during the flood tide (Wooldridge & Loubser, 1996), with optimal larval survival in the laboratory being recorded at salinities of between 25 and 35 (Paula et al., 2001).
In the Marapanim estuary, the porcelain crab Petrolisthes armatus reproduces continuously and is abundant in rocky outcrops. The larval cycle of porcellanid species includes two zoeal stages and a megalopa (Boschi, 1981). In contrast with U. vasquezi, ovigerous females are less abundant during the rainy season, and all the larval stages are found in the estuary, with a significant increase in the abundance of zoea I and II larvae in the estuary during the dry season (Oliveira et al., 2013). Based on these findings, these authors concluded that the larvae of this species are retained in the estuary, where they remain throughout their life cycle, which may be confirmed through experimental studies, similar to that presented here, which analyze systematically the effects of different salinity levels on larval development.
The results of the present study—from both the laboratory and the field—indicate that the larvae of the U. vasquezi population of the Marapanim estuary develop better at middle to high salinities than low ones. These results support the hypothesis that U. vasquezi undergo development on the continental shelf and may embark on ontogenetic migrations to adjacent coastal areas during the early larval stages, as observed in other mud shrimp species around the world (see Johnson & Gonor, 1982; Thessalou-Legaki, 1990; Paula et al., 2001; Faleiro et al., 2012). The capacity of these larvae to complete their development in water of intermediate salinity may be critical for their dispersal on the extensive continental shelf of the Amazon coast, which is characterized by marked seasonal variation in salinity, controlled by the precipitation cycle. Further studies of the dispersal of the larvae of this species between the Marapanim estuary and adjacent oceanic waters, via the freshwater plume off the mouth of the Amazon River, will be required to better elucidate dispersal patterns in this highly complex coastal region.
References
Anger, K., 1991. Effects of temperature and salinity on the larval development of the Chinese mitten crab Eriocheir sinensis (Decapoda: Grapsidae). Marine Ecology Progress Series 72: 103–110.
Anger, K., 1996. Salinity tolerance of the larvae and first juveniles of a semiterrestrial grapsid crab, Armases miersii (Rathbun). Journal of Experimental Marine Biology and Ecology 202: 205–223.
Anger, K., 2001. The biology of decapod crustacean larvae, Vol. 14., Crustacean Issues A. A. Balkema Publishers, Lisse.
Anger, K., 2003. Salinity as a key parameter in the larval biology of decapods crustaceans. Invertebrate Reproduction and Development 43(1): 29–45.
Anger, K., E. Spivak, C. Bas, D. Ismael & T. Luppi, 1994. Hatching rhythms and dispersion of decapod crustacean larvae in a brackish coastal lagoon in Argentina. Helgolander Meeresuntersuchungen 48: 445–466.
Anger, K., E. Spivak, T. Luppi, C. Bas & D. Ismael, 2008. Larval salinity tolerance of the South American salt-marsh crab, Neohelice (Chasmagnathus) granulata: physiological constraints to estuarine retention, export and reimmigration. Helgoland Marine Research 62: 93–102.
Ayres, M., M. Ayres Jr, D. L. Ayres & A. S. Santos, 2007. BioEstat 5.0 Aplicações estatísticas nas áreas das ciências biológicas e médicas. Instituto do desenvolvimento sustentável Mamirauá – IDSM/MCT/CNPq, Pará.
Berrêdo, J. F., M. L. Costa & M. P. S. Progene, 2008. Efeitos das variações sazonais do clima tropical úmido sobre as águas e sedimentos de manguezais do estuário do rio Marapanim, costa nordeste do Estado do Pará. Acta Amazonica 38(3): 473–482.
Boschi, E. E., 1981. Larvas de Crustacea Decapoda. In Boltovskoy, D. (ed.), Atlas del zooplancton del Atlántico sudoccidental y métodos de trabajo con el zooplancton marino. Inedep, Mar del Plata.
Charmantier, G., 1998. Ontogeny of osmoregulation in crustaceans: a review. Invertebrate Reproduction and Development 33: 177–190.
Charmantier, G., L. Giménez, M. Charmantier-Daures & K. Anger, 2002. Ontogeny of osmoregulation, physiological plasticity and larval export strategy in the grapsid crab Chasmagnathus granulata (Crustacea, Decapoda). Marine Ecology Progress Series 229: 185–194.
Christy, J. H. & S. G. Morgan, 1998. Estuarine immigration by crab postlarvae: mechanisms, reliability and adaptive significance. Marine Ecology Progress Series 174: 51–65.
Costlow, J. D., C. G. Bookhout & R. Monroe, 1960. The effect of salinity and temperature on larval development of Sesarma cinereum (Bosc) reared in the laboratory. Biological Bulletin 118(2): 183–202.
Diele, K. & D. J. B. Simith, 2006. Salinity tolerance of northern Brazilian mangrove crab larvae, Ucides cordatus (Ocypodidae): necessity for larval export? Estuarine, Coastal and Shelf Science 68: 600–608.
Dworschak, P. C., 1988. The biology of Upogebia pusilla (Petagna) (Decapoda, Thalassinidea) III. Growth and production. Marine Ecology 9(1): 51–77.
Dworschak, P. C., 2005. Global diversity in the Thalassinidea (Decapoda): an update (1998–2004). Nauplius 13(1): 57–63.
Dworschak, P. C., D. L. Felder & C. C. Tudge, 2012. Infraorders Axiidea De Saint Laurent, 1979 and Gebiidea De Saint Laurent, 1979 (formerly known collectively as Thalassinidea). In Schran, F. R. & J. C. Vaupel Klein (eds), The Crustacea - Treatise on Zoology: anatomy, taxonomy, biology. Koninklijke Brill NV, Boston: 109–219.
Esser, L. J. & N. Cumberlidge, 2011. Evidence that salt water may not be a barrier to the dispersal of Asian freshwater crabs (Decapoda: Brachyura: Gecarcinucidae and Potamidae). Raffles Bulletin of Zoology 59(2): 259–268.
Faleiro, F., J. Paula & L. Narciso, 2012. Hot and salty: the temperature and salinity preferences of a temperate estuarine shrimp larva, Upogebia pusilla (Decapoda: Thalassinidea). Hydrobiologia 691: 89–95.
Fowler, A. E., N. V. Gerner & M. A. Sewell, 2011. Temperature and salinity tolerances of Stage 1 zoeae predict possible range expansion of an introduced portunid crab, Charybdis japonica, in New Zealand. Biological Invasions 13: 691–699.
Johnson, G. E. & J. J. Gonor, 1982. The tidal exchange of Callianassa californiensis (Crustacea, Decapoda) larvae between the Ocean and the Salmon River estuary, Oregon. Estuarine, Coastal and Shelf Science 14: 501–516.
Melo Jr, M., R. Schwamborn, S. Neumann-Leitão & M. Paranaguá, 2012. Abundance and instantaneous transport of Petrolisthes armatus (Gibbes, 1850) planktonic larvae in the Catuama inlet, Northeast Brazil. Anais da Academia Brasileira de Ciências 84(1): 95–102.
Moraes, B. C., J. M. N. Costa, A. C. L. Costa & M. H. Costa, 2005. Variação espacial e temporal da precipitação no estado do Pará. Acta Amazonica 35(2): 207–214.
Nóbrega, P. S. V., B. Bentes & J. M. Martinelli-Lemos, 2013. Composition of shrimp populations (Crustacea: Decapoda) in non-vegetated areas of two river islands in a Brazilian Amazon estuary. Zoologia 30(6): 652–660.
O’Connor, N. J. & C. E. Epifanio, 1985. The effect of salinity on the dispersal and recruitment of fiddler Crab Larvae. Journal of Crustacean Biology 5(1): 137–145.
Oliveira, D. B., D. C. Silva & J. M. Martinelli-Lemos, 2012. Density of larval and adult forms of the burrowing crustaceans Lepidophthalmus siriboia (Callianassidae) and Upogebia vasquezi (Upogebiidae) in an Amazon estuary, northern Brazil. Journal of the Marine Biological Association of the United Kingdom 92(2): 295–303.
Oliveira, D. B., D. C. Silva & J. M. Martinelli-Lemos, 2013. Larval and adult density of the porcellanid crab Petrolisthes armatus (Anomura: Porcellanidae) in an Amazon estuary, northern Brazil. Zoologia 30(6): 592–600.
Oliveira, D. B., J. M. Martinelli-Lemos & F. A. Abrunhosa, 2014. The complete larval development of the mud shrimp Upogebia vasquezi (Gebiidea: Upogebiidae) reared in the laboratory. Zootaxa 3826(3): 517–543.
Paula, J., R. N. Mendes, S. Paci, P. McLaughlin, F. Gherardi & W. Emmerson, 2001. Combined effects of temperature and salinity on the larval development of the estuarine mud prawn Upogebia africana (Crustacea, Thalassinidea). Hydrobiologia 449: 141–148.
Pires, R. F. T., M. Pan, M. P. Santos, A. Peliz, D. Boutov & A. Santos, 2013. Modelling the variation in larval dispersal of estuarine and coastal ghost shrimp: Upogebia congeners in the Gulf of Cadiz. Marine Ecology Progress Series 492: 153–168.
Sakai, K. & M. Turkay, 2014. A review of the collections of the infraorders Thalassinidea Latreille, 1831 and Callianassidea Dana, 1852 (Decapoda, Pleocyemata) lodged in three German museums, with revised keys to the genera and species. Crustaceana 87(2): 129–211.
Santos, A., A. M. P. Santos, D. V. P. Conwa, C. Bartilotti, P. Lourenço & H. Queiroga, 2008. Diel vertical migration of decapod larvae in the Portuguese coastal upwelling ecosystem: implications for offshore transport. Marine Ecology Progress Series 359: 171–183.
Silva, D. C. & J. M. Martinelli-Lemos, 2012. Species composition and abundance of the benthic community of Axiidea and Gebiidea (Crustacea: Decapoda) in the Marapanim Bay, Amazon estuary, northern Brazil. Zoologia 29(2): 144–158.
Simith, D. J. B. & K. Diele, 2008. O efeito da salinidade no desenvolvimento larval do caranguejo-uçá, Ucides cordatus (Linnaeus, 1763) (Decapoda: Ocypodidae) no Norte do Brasil. Acta Amazonica 38(2): 345–350.
Simith, D. J. B., A. S. Souza, C. R. Maciel, F. A. Abrunhosa & K. Diele, 2012. Influence of salinity on the larval development of the fiddler crab Uca vocator (Ocypodidae) as an indicator of ontogenetic migration towards offshore waters. Helgoland Marine Research 66: 77–85.
Simith, D. J. B., M. A. B. Pires, F. A. Abrunhosa, C. R. Maciel & K. Diele, 2014. Is larval dispersal a necessity for decapod crabs from the Amazon mangroves? Response of Uca rapax zoeae to different salinities and comparison with sympatric species. Journal of Experimental Marine Biology and Ecology 457: 22–30.
Souza Filho, P. W. M., 2005. Costa de manguezais de macromaré da Amazônia: cenários morfológicos, mapeamento e quantificação de áreas usando dados de sensores remotos. Revista Brasileira de Geofísica 23(4): 427–435.
Statsoft Inc., 2004. STATISTICA data analysis software system, version 7, www.statsoft.com.
Thessalou-Legaki, M., 1990. Advanced larval development of Callianassa tyrrhena (Decapoda: Thalassinidea) and the effect of environmental factors. Journal of Crustacean Biology 10(4): 659–666.
Thompson, L. C. & A. W. Pritchard, 1969. Osmoregulatory capacities of Callianassa and Upogebia (Crustacea: Thalassinidea). Biological Bulletin 136(1): 114–129.
Torres, G., L. Giménez & K. Anger, 2011. Growth, tolerance to low salinity, and osmoregulation in decapod crustacean larvae. Aquatic Biology 12: 249–260.
Vuichard, G. S., N. Farías & T. Luppi, 2013. Hatching and larval export of the intertidal crab Neohelice granulata in Mar Chiquita coastal lagoon, Argentina. Iheringia Série Zoologia 103(2): 124–133.
Wooldridge, T. H. & H. Loubser, 1996. Larval release rhythms and tidal exchange in the estuarine mudprawn, Upogebia africana. Hydrobiologia 337: 113–121.
Yannicelli, B., L. R. Castro, A. Valle-Levinson, L. Atkinson & D. Figueroa, 2006. Vertical distribution of decapod larvae in the entrance of an equatorward facing bay of central Chile: implications for transport. Journal of Plankton Research 28(1): 19–37.
Acknowledgments
We thank our colleagues Danielle Arruda, Darlan Simith, Luiz Paulo Melo, Orlando Galli, Rubem Pessoa, and Wellington Trindade for the collection of seawater samples in the field, and Rory Oliveira for helping to collect ovigerous females. We also appreciate the valuable suggestions of Dr. Cristiana Maciel, Dr. Eduardo Martinelli, Dr. Marcelo Petracco, Dr. Ralf Schwamborn, and Dr. Sandra Shumway, which greatly improved the paper. This study is part of the Ph.D thesis of the first author (DBO) and was funded by the Brazilian National Research Council (CNPq) through the CT-AMAZÔNIA project (Grant 553106/2005-8 to JMML), the Brazilian Higher Education Training Program (CAPES), and the Brazilian Carcinology Society (Grant SBC 01/2012). We also thank PROPESP/FADESP (PAPQ Program) for sponsoring the translation of the original manuscript by Stephen Ferrari. All experiments conducted in this study complied with current Brazilian federal legislation (environmental authorization MMA/ICMBIO/SISBIO Number 29434-5) and Pará state laws.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: Vasilis Valavanis
Rights and permissions
About this article
Cite this article
de Oliveira, D.B., Martinelli-Lemos, J.M., de Souza, A.S. et al. Does retention or exportation occur in the larvae of the mud shrimp Upogebia vasquezi (Decapoda, Gebiidea)? Implications for the reproductive strategy of the species on the Amazon coast. Hydrobiologia 773, 241–252 (2016). https://doi.org/10.1007/s10750-016-2708-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-016-2708-8