Abstract
To obtain enzymatic preparations with higher laccase activity levels from Funalia floccosa LPSC 232, available for use in several applications, co-cultures with six filamentous microfungi were tested. A laccase non-producing soil fungus, identified as Penicillium commune GHAIE86, showed an outstanding ability to increase laccase activity (3-fold as compared to that for monoculture) when inoculated in 6-day-old F. floccosa cultures. Maximum laccase production with the F. floccosa and P. commune co-culture reached 60 U/mL, or twice that induced by chemical treatments alone. Our study demonstrated that co-culture with soil fungi might be a promising method for improving laccase production in F. floccosa. Although the enhancement of laccase activity was a function of P. commune inoculation time, two laccase isoenzymes produced by F. floccosa remained unchanged when strains were co-cultured. These data are compatible with the potential of F. floccosa in agricultural applications in soil, whose enzyme machinery could be activated by soil fungi such as P. commune.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Funalia floccosa LPSC 232 (formerly known as Coriolopsis rigida) is a model white-rot fungus which produces extracellular laccases (E.C. 1.10.3.2), with promising enzymes for industrial applications such as the paper, textile, and food industries as well as for detoxification treatments and biosensor development (Saparrat et al. 2014). This fungus is therefore an environmentally friendly candidate to produce large amounts of these enzymes. In addition, this fungus has potential for promoting the growth and development of several plants of economic importance such as blueberries, tomato, and eucalyptus (Almonacid et al. 2015; Arriagada et al. 2012, 2014) in co-inoculation with mycorrhizal fungi. It could also be used in the degradation of polycyclic aromatic hydrocarbons (Gómez et al. 2006), the decolouration of industrial anthraquinone dyes (Sánchez-López et al. 2008), the transformation and detoxification of the phenolic content of olive mill waste (Sampedro et al. 2004), and the bioremediation of soil contaminated by crude oil (Colombo et al. 1996).
Culture systems reported to produce laccases from F. floccosa include inoculation in liquid media and on lignocellulosic-rich solid matrices (Alcántara et al. 2007). To our knowledge, enzyme levels obtained using these procedures are low and need to be supplemented with high-cost chemical inductors as compounds related to lignin and its derivatives, phenolic and other aromatic compounds, copper ions, and industrial waste water (Saparrat 2004; Saparrat et al. 2014). However, chemical inducers are expensive and, in some cases, toxic and ineffective, with their possible practical applications being limited due to their availability or high cost (Saparrat et al. 2010, 2014). Currently, the search for economical and safe methods for the production of laccase is therefore one of the most interesting areas of enzyme research (Saparrat et al. 2014). In this regard, its production on a large scale is hampered by several technical constraints, including inoculum formulations and their mode of application (Baldrian 2008). One of the new sustainable strategies to obtain laccases from white-rot fungi is co-cultivation with other fungi (Ma and Ruan 2015). The combination of ligninolytic fungi has dramatic dynamic effects on the production of lignocellulose-active enzyme, which may lead to divergent degradative processes of dead wood and forest litter (Mali et al. 2017), antibiotic (Gao et al. 2018), and decolorization of industrial dye (Kumari and Narian 2016). Other studies have shown that co-cultivation of white-rot fungi, such as Lentinula edodes, Pleurotus ostreatus, Trametes versicolor, and Phanerochaete chrysosporium, with filamentous fungi, such as Trichoderma spp. and Paecilomyces carneus, increases laccase production (Chan-Cupul et al. 2014; Flores et al. 2009, 2010; Mata et al. 2005; Savoie et al. 2001). Although the mechanisms involved in this laccase induction have not been fully elucidated, they relate to their role in biological interactions or stress defense (Crowe and Olsson 2001). The production of laccase by white-rot fungi in co-culture with filamentous fungi other than Trichoderma sp. has been poorly documented and, to date, there are no studies of F. floccosa laccase production in co-culture. This study was therefore undertaken in order to (a) evaluate the production of laccase by the white-rot fungus F. floccosa in co-culture with six micro fungi strains in order to make high levels of this activity available for use in various applications; (b) determine whether, by establishing co-cultures, the inoculation time of the microfungi affects the laccase activity of F. flocossa; and (c) characterize the natural isoenzymes secreted in the interaction.
Material and methods
Fungal strains
Funalia floccosa LPSC 232 (Spegazzini Institute Culture Collection) isolated from decaying wood collected from a subtropical rain forest in Argentina (Ibañez 1998); Penicillium GHAIE86 isolated from coffee plantation soil in Veracruz, México; Penicillium chrysogenum EEZ 10, Penicillium brevicompactum EEZ32, and Fusarium graminearum BAFC 122 isolated from soil from Castañar de Ibor, Granada (Spain) and Buenos Aires (Argentina), respectively; Mucor racemosus EEZ113 isolated from the dry residue of olive oil industry; and Paecilomyces farinosus BAFC F8846 isolated from Funneliformis mosseae sporocarps were obtained from the collection of the Estación Experimental del Zaidín (EEZ), CSIC (Granada, Spain) and the culture collection of the Faculty of Exact and Natural Sciences, University of Buenos Aires (BAFC) (Argentina). All strains were maintained at 4 °C on malt extract agar plates.
Co-cultures of F. floccosa and microfungi for laccase activity evaluation
All fungi were grown separately at 28 °C in 250-mL Erlenmeyer flasks containing 70 mL of a glucose-yeast medium on a rotary shaker at 80 rpm (Evans and Niven 1951). The flasks were inoculated with 2 cm2 agar plugs covered by 7-day-old mycelia. After 7 days, the mycelia were collected and homogenized with distilled water (1:1 v/v, mycelia:water). One milliliter of the suspension was used as inoculums (mycelia, 500 mg).
Laccase production by F. floccosa grown in monoculture and in co-cultures, with microfungi inoculated at the same time, was studied in 250-mL Erlenmeyer flasks containing 70 mL of medium and incubated on static cultures for 21 days as described by Guillén et al. (1992). Each experiment was performed in triplicate.
Laccase activity was assayed by the oxidation of 5 mmol/L 2,6-dimethoxyphenol (DMP) to coerulignone in 100 mmol/L sodium acetate buffer (pH 5.0) (Muñoz et al. 1997).
Morpho-physiological characteristics of isolate Penicillium GHAIE86
Morphological identification was carried out according to the method described by Pitt (2000). The isolate was inoculated as 3-point cultures in Czapek yeast extract agar (CYA, 25 °C and 37 °C), malt extract agar (MEA, 25 °C), and 25% glycerol nitrate agar (G25N, 25 °C) and incubated for 7 days in darkness.
Texture, pigmentation, and colony diameter as well as sporulation were recorded in each medium. The structures differentiated, such as conidiophores, phialides, and conidia, in these cultures were examined using an Olympus microscope Model CX41 UC-MAD3.
Molecular identification of Penicillium GHAIE86
Genomic DNA from the fungus was isolated from 7-day glucose-yeast medium mycelium using a Genomix DNA extraction kit (Talent, Italy) according to the manufacturer’s instructions. The eluted DNA was stored at − 20 °C and used as a template for PCR amplifications. The ITS region was amplified by PCR using the primers and protocol described elsewhere (White et al. 1990). The PCR-amplified fragment was purified using the UltraCleanTM PCR Clean-Up TM kit (MoBio Laboratories Inc., USA) according to the manufacturer’s instructions and was sent for direct sequencing to STABVIDA®. Automated sequencing of both strands was performed using the BigDye Terminator Kit from Applied Biosystems and the 96-capillary 3730xL DNA Analyzer from Applied Biosystems. The sequence was corrected using Chromas version 1.43 (Griffith University, Brisbane, Australia) and was analyzed and edited using Bioedit Sequence Alignment Editor version 7.0.9.0 (Hall 1999). The sequence was deposited in the GenBank database under accession number KY174328.
Optimization of co-culture F. floccose-P. commune for laccase production
Laccase production by static F. floccosa cultures grown in monoculture and in co-culture with P. commune inoculated at the same time or after 3 and 6 days of F. floccosa inoculation and its characterization was carried out in 250-mL Erlenmeyer flasks containing 70 mL of medium as described previously. The inoculum of both fungi was performed as indicated previously. All the cultures were maintained for 21 days at 28 °C under static conditions. Three replicate cultures of each treatment were tested. Samples were taken after 12, 18, and 21 days from the cultures and the supernatants, separated from mycelia by centrifugation at 8000×g for 10 min, were analyzed for laccase activity.
Laccase purification
A crude enzyme preparation with laccase activity was obtained both from 21-day-old cultures of F. floccosa grown alone and in co-culture with P. commune added after 6 days of incubation. The culture liquid was separated from mycelia by centrifugation at 20,000×g for 20 min, dialyzed against 10 mmol/L sodium acetate (pH 5.0) through a 12–14-kDa cutoff membrane filtration (Spectrum) and was then concentrated by ultrafiltration (Pall Filtron, 3-kDa cutoff membrane).
One-milliliter samples of the crude enzyme preparation were applied to a Superdex 200 column (Amersham Pharmacia Biotech HR 16/60) equilibrated with a 50 mmol/L phosphate buffer (pH 7.1) containing 150 mmol/L NaCl at a flow rate of 0.4 mL/min. A laccase peak was pooled, concentrated (Filtron Microsep, 3-kDa cutoff), and dialyzed against 10 mmol/L sodium acetate (pH 5.0) by using a PD-10 desalting column (Amersham Biosciences). Then, 1-mL samples were applied to a Mono-Q anion-exchange column (Pharmacia HR 5/50) equilibrated with the same buffer. The laccase isoenzymes were eluted with a linear NaCl gradient from 0 to 250 mmol/L for 50 min and from 250 mmol/L to 1 mol/L for 7.5 min at a flow rate of 0.4 mL/min. Fractions of 2 mL were collected and the laccase peaks were pooled, concentrated, and stored with 10% (w/v) glycerol at − 4 °C.
Proteins were determined according to the Bradford method, using bovine albumin as standard and the BioRad kit assay (Bradford 1976).
Isoelectric focusing, zymogram, and molecular mass of laccases
A preliminary characterization of laccase activity from a crude preparation of 21-day-old static F. floccosa cultures grown in monoculture and in co-culture with P. commune inoculated after 6 days of incubation was carried out using isoelectric focusing (IEF) and by estimating molecular mass.
The isoelectric point of the laccases was determined by zymograms on 5% polyacrylamide gels with a thickness of 1 mm and a pH range from 3 to 10 (Amersham Pharmacia). The sample (20 μL) contained 5–10 mU of laccase activity. The anode and cathode solutions were 1 mol/L phosphoric acid and 1 mol/L sodium hydroxide, respectively. The pH gradient was measured on the gel by means of a contact electrode. Protein bands with laccase activity were detected by using 5 mmol/L DMP in 200 mmol/L sodium acetate buffer (pH 5.0) after the gels were washed for 10 min with the same buffer (Díaz et al. 2010).
The molecular mass of isoenzymes was estimated by size-exclusion chromatography. This chromatography was carried out on a Superdex 200 column as described above. The column was calibrated with blue dextran (2000 kDa), albumin (67 kDa), ovoalbumin (43 kDa), chymotrypsinogen A (25 kDa), and ribonuclease A (13.7 kDa).
Statistical analysis
The differences in laccase production among the treatments were assessed using one-way ANOVA with Tukey’s honest significance difference (HSD) post hoc test. Normal distribution and heteroscedasticity of data were tested by the Shapiro–Wilk and Breusch–Pagan tests, respectively.
Results and discussion
Screening of microfungi with ability to enhance laccase production by F. floccose
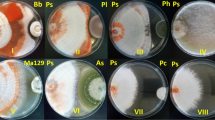
We analyzed the levels of extracellular laccase activity from 21-day-old static F. floccosa cultures grown in monoculture and in co-culture with each of the six microfungi. We found that F. floccosa grown in static cultures produced a similar level of laccase activity to that reported previously for this fungus (Saparrat et al. 2002) (Fig. 1).
Extracellular laccase activity of static F. floccosa LPSC 232 cultures grown in monoculture (F.flo) and dual co-culture with P. commune GHAIE 86 (P.co), P. chrysogenum EEZ 10 (P.ch), P. brevicompactum EEZ 32 (P.br), M. racemosus EEZ 113 (M.ra), F. graminearum BAFC 122 (F.gra), and P. farinosus BAFC F8846 (P.fa) after 21 days of incubation. Values are means of three replicates. Error bars correspond to standard deviation. Bars with the same letter are not significantly different (Tukey’s test, P < 0.01)
Although any of these tested fungal strains produced extracellular laccase activity when grown axenically on the glucose-yeast medium under static conditions (data not shown), most of them were found to increase the laccase activity of F. floccosa (Fig. 1). However, while the co-cultures with M. racemosus and P. farinosus showed similar levels of laccase activity compared to those from the F. floccose monoculture, the highest induction of laccase activity was obtained in the co-cultures of F. floccosa with Penicillium GHAIE86.
Morpho-physiological characteristics and molecular identification of Penicillium GHAIE86
The coloration and characteristics of the colonies developed on the different media used as well as the microscopic morphology of the differentiated conidial system were typical representatives of P. commune according to Pitt’s description (Pitt 2000) included in subgenus Penicillium, section Penicillium.
The nucleotidic ITS sequence of isolate GHAIE86 showed 100% homology with the ITS sequences of P. commune and one sequence from P. camemberti. However, following morphological identification, our strain was classified as P. commune.
For a long time P. commune was considered as a food-borne fungus, frequently associated with cheese and cottonseed meal spoilage (Samson et al. 1996; Wagener et al. 1980). However, in the last decade, different reports have shown that this species proliferates in very distant and varied environments. It has been isolated from soil samples collected in China (Liu et al. 2013), Canada (Out et al. 2016), and the Kingdom of Saudi Arabia (Mohamed et al. 2016). There are also studies that have used P. commune strains isolated from the following: rhizospheric soil samples collected in India (Jain et al. 2013), marine sediments from southern China Sea (Gao et al. 2011; Shang et al. 2012), and leaves and berries of Vitis vinifera from Portugal (Oliveira et al. 2017). Taking into account this information, it is feasible to assume that P. commune is a cosmopolitan species with a high saprophytic ability to colonize different types of substrates.
Laccase produced by F. floccosa LPSC 232 and P. commune GHAIE86 co-cultures established with simultaneous and delayed inoculation
We also tested laccase activity in combined static F. floccosa and P. commune cultures at different inoculation times. Figure 2 shows that laccase activity from F. floccosa simultaneously inoculated with P. commune was lower than that from F. floccosa monoculture. In soil fungus samples inoculated after 3 days of F. floccosa incubation, we observed an increase in laccase production after 12 days of F. floccosa-P. commune co-culture. The highest production of laccase activity in co-cultures of both fungi was obtained when fungus P. commune was added to a 6-day F. floccosa culture (Fig. 2), which was three times higher than that of the F. floccosa control. Our results suggest that the production of laccase by F. floccosa in interaction with P. commune is dependent on a subsequently longer inoculation time as reported by Chan-Cupul et al. (2014) for the P. carneus-Trametes maxima couple.
Time course of laccase activity in the extracellular fluid of F. floccosa LPSC 232 static cultures grown in monoculture (monoculture, ▲) and in dual co-culture with P. commune GHAIE 86 inoculated at the same time (■) or after 3 (●) and 6 days (x) of F. floccosa inoculation. The results shown are mean values of 3 replicate flasks
Several findings demonstrate that fungal laccases can be involved in physiological processes such as lignin degradation and detoxification reactions and also as a virulence factor in interactions with other organisms (Crowe and Olsson 2001). This latter role in biological interactions is considered to be species-specific and also related to nutritional conditions (Chan-Cupul et al. 2014; Flores et al. 2009). Another possible hypothesis is that laccases are released by basidiomycetes as part of a defensive response to mycelial invasion, such as sequestering of nutrients already occupied by competitive/antagonistic organisms (Baldrian 2004). It should be noted that the presence of P. commune did not affect F. floccosa growth rates, since, in co-cultures, growth of this latter fungus was identical to that estimated for monocultures, as shown by the typical color of the F. floccose mycelium and the presence of clamp connections (Supplementary Fig. 1).
The induction of laccase activity in F. floccosa by phenols from agro-industrial residues or heavy metals such as Cu2+ has been previously reported (Saparrat et al. 2002, 2010). All these supplements have several limitations because they can be highly toxic and adversely affect the laccase-producing fungus as well as by their wastes that can pollute the environment. Therefore, our study shows data about the use of an environmentally safe strategy to improve laccase (sustainable) production in F. floccosa using biological techniques such as the inoculation with P. commune. Although different authors have proposed the use of biological induction to increase laccase activity, as seen in the co-culture of Hypholoma fasciculare with Bacillus subtilis (Griffith et al. 1994), P. ostreatus (Velázquez-Cedeño et al. 2004) and Trametes sp. AH28-2 (Zhang et al. 2006) with Trichoderma, Trametes maxima with P. carneus (Chan-Cupul et al. 2014), and Rhizostonia solani with Pseudomonas fluorescent (Crowe and Olsson 2001), laccase activity levels obtained in these studies were very low. For this reason, our main concern in this study was to find a fungus capable of stimulating the F. floccosa laccase synthesis to a significant degree. Previous studies indicated the induction of different enzymes like endoglucanase and β-glucosidase in co-cultures of Aspergillus niger and Fusarium oxysporum (Hernández et al. 2018). However, these studies also observed that when more than two strains were cultured, the relationships of competition were established and a decrease of the amount of enzymes and the extracellular protein isoforms produced. For this reason, the selection of the appropriate fungus and also the conditions of the co-culture are the important factors to be considered. Our results demonstrate the potential of P. commune for producing high levels of laccase activity in F. floccosa. This is the first study to demonstrate the capacity of P. commune to enhance laccase production in white-rot fungi under co-culture conditions, although further studies need to be carried out to elucidate the physiological mechanisms involved in these responses. Several findings show the degradative effect of F. floccosa on numerous aromatic compounds and complex matrices involving its extracellular laccase activity (Saparrat et al. 2010, 2014). Colombo et al. (1996) have reported the ability of this fungus to degrade the aliphatic and aromatic fractions of a crude oil from an artificially contaminated soil that was also colonized by P. chrysogenum, which showed a low degradative capacity. Therefore, our study suggests the potential role of soil fungi in laccase induction, such those belonging to the genus Penicillium, which might activate the degradation of xenobiotics by F. floccosa in polluted soils. Saparrat et al. (2010) have reported the existence of at least three laccase genes in the genome of this fungus, which showed a differential regulation at the transcriptional level, although just two laccase isoenzymes encoded by the lcc1 gene have been the only ligninolytic enzymatic components found. One explanation for this increased laccase production could be the synergistic relationship between F. floccosa and P. commune. The capacity of P. commune to produce secondary metabolites under competitive conditions is probably related to the induction of laccase activity in F. floccosa. In fact, a defense mechanism in F. floccosa to counteract the deleterious effect of amphotericin B via the induction of laccase has been proposed, which leads to the highest levels of extracellular laccase activity, even higher than copper (Saparrat et al. 2010). Further studies are necessary to determine whether the secondary metabolites from P. commune such as amphotericin B are the agents responsible for laccase induction in F. floccosa. Concomitantly, Svahn et al. (2015) have recently reported the production of amphotericin B by a Penicillium nalgiovense isolate from soil. The remarkable ability of Penicillium species to produce several bioactive molecules as well as the high diversity and environmental distribution of this fungal genus (more than 200 species are currently listed in the Index Fungorum database) are well-known. However, their potential use in co-culture systems, as compared to those used with other microfungi belonging to Trichoderma spp., has received little attention.
With regard to inoculation time, our results show that asynchronous inoculation of microfungi in co-cultures with F. floccosa has a significant effect on laccase activity, as has been reported previously by Dwivedi et al. (2011). The interspecific fungal interactions are quite complex and their physiological mechanisms are little understood (Boddy 2000; Iakovlev and Stenlid 2000). Combative mechanisms in fungal competition are closely related to the initial conditions of the resource to be colonized. The lowest laccase activity levels (below those from F. floccosa monocultures) recorded under simultaneous inoculation conditions may be due to the rapid development of the microfungi. These microfungi produced large amounts of spores which germinated quickly and formed masses of mycelium (characterized by a lack of clamp connections), taking advantage of the nutrients and inhibiting the combative mechanisms of F. floccosa. Interspecific fungal interactions, besides playing a key role in community structures and ecological processes, are a promising strategy not only to induce enzymes but also to provide a high diversity of molecules such as pheromones and secondary metabolites with important biotechnological applications (Bertrand et al. 2013).
Purification and characterization of laccase isoenzymes produced in co-cultures F. floccosa LPSC 232 and P. commune GHAIE86
To determine the possible induction of new F. floccosa laccase isoenzymes under co-culture conditions, we purified the laccases produced by F. floccosa when grown in monoculture and those produced in co-cultures with P. commune. In order to design the purification process, we carried out a preliminary characterization of the extracellular proteins with laccase activity from a crude preparation obtained from 21-day-old culture liquid supernatants of F. floccosa grown in monoculture and in co-culture with P. commune inoculated after 6 days of F. floccose inoculation due to its higher laccase activity levels.
Isoelectric focusing of crude enzyme preparations obtained from an extracellular supernatant of F. floccosa monocultures and from the co-culture of F. floccosa and P. commune showed two bands with a pI of 3.4 and 3.6 in all cases (Fig. 3). These pI values are in line with those reported in the literature, as the pI values for fungal laccases, ranging from 2.6 to 6.9, are generally in the acidic range (Baldrian 2006; Saparrat et al. 2008).
Isoelectric focusing of laccase activity in the extracellular fluid of F. floccosa LPSC 232 cultures grown in monoculture (monoculture, lane 1) and in dual co-culture with P. commune GRAIE 86 inoculated after 6 days of F. floccosa inoculation (lane 2) at pH 3.0–8.0. The gel was stained with 5 mmol/L DMP in 200 mmol/L sodium acetate buffer (pH 5.0)
The purification process was carried out using two chromatographic separations involving exclusion molecular chromatography followed by ion-exchange chromatography. The conditions for F. floccosa laccases purification were similar for all the treatments. During the first stage of chromatography (Superdex 200), a major protein peak containing laccase activity was obtained (Figs. 4a and 5a). The chromatographic profile of the F. floccosa laccase from co-cultures with P. commune was identical to the profile of the monoculture grown for this study and to that previously described by Díaz et al. (2010). Using size-exclusion chromatography, we estimated the molecular mass of both laccase produced by F. floccosa and that from co-cultures F. floccosa-P. commune to be 60 kDa. Previous studies carried out with F. floccosa laccase indicated a molecular mass of 66 kDa (Díaz et al. 2010; Saparrat et al. 2002). Several fungal laccases show a molecular mass of 60 and 80 kDa (Guo et al. 2008).
Purification of laccases from 21-day-old static F. floccosa LPSC 232 cultures grown in dual co-culture with P. commune GRAIE 86 inoculated after 6 days of F. floccosa inoculation by chromatography on Superdex 200 (a) and Mono-Q (b) columns. Absorbance at 280 nm (solid line), NaCl gradient (dashed line), and laccase activity (■) are indicated
In the second chromatographic step involving a high-resolution ion-exchange column (Mono-Q), it was necessary to resolve two laccase activity peaks (LacI and LacII). These isoenzymes detected in co-cultures of F. floccosa and P. commune have elution times (48 and 50 min, respectively) and NaCl concentrations (14 and 16%, respectively) similar to those for the F. floccosa monocultures analyzed in this study and previously described by Díaz et al. (2010) (Figs. 4b and 5b). Different systems and culture conditions, such as growth in co-cultures with other organisms, are known to induce the expression of new laccase isoenzymes (Baldrian 2004). One of the main objectives of this study was therefore to identify and compare the enzymes produced by F. floccosa in mono- or co-culture with a second organism such as P. commune. However, our findings provided by size-exclusion and ion-exchange chromatography show laccase isoenzymes (LacI and LacII) produced by F. floccosa, either in monoculture or in co-culture with P. commune (Fig. 5a, b) to be identical.
At the end of the purification process, LacI and LacII were purified 2.9- and 1.7-fold in monoculture conditions and 2.9- and 2.1-fold in co-culture conditions (Table 1), respectively. Although the yield from purification was similar in both types of F. floccosa culture, higher specific activity was detected in laccase isoenzymes produced in co-cultures than in those produced in F. floccosa monocultures. This is probably due to the different culture conditions or to the different catalytic properties among laccases from the same organism, as has been described for Pycnoporus sanguineus (Dantán-González et al. 2008) and Panus tigrinus (Quaratino et al. 2008).
Conclusions
The interaction between F. floccosa LPSC 232 and P. commune GHAIE86 enhances laccase production. The inoculation time of P. commune on F. floccosa static cultures plays an important role in laccase enhancement. The increase in laccases produced by F. floccosa in a co-culture system could be an attractive alternative to those required in a monoculture system using chemical inductors.
References
Alcántara T, Gómez J, Pazos M, Sanromán MA (2007) Enhanced production of laccase in Coriolopsis rigida grown on barley bran in flask or expanded-bed bioreactor. World J Microbiol Biotech 23:1189–1194
Almonacid L, Fuentes A, Ortiz J, Salas C, García-Romera I, Ocampo JA, Arriagada C (2015) Effect of mixing soil saprophytic fungi with organic residues on the response Solanum lycopersicum to arbuscular mycorrhizal fungi. Soil Use Manage 31:155–164
Arriagada C, Manquel D, Cornejo P, Soto J, Sampedro I, Ocampo JA (2012) Effect of the co-inoculation with saprobe and mycorrhizal fungi on Vaccinium corymbosum growth and some soil enzymatic activities. J Soil Sci Plant Nut 12:283–294
Arriagada C, Almonacid L, Cornejo P, García-Romera I, Ocampo JA (2014) Influence of an organic amendment comprising saprophytic and mycorrhizal fungi on soil quality and growth of Eucalyptus globulus in the presence of sewage sludge contaminated with aluminium. Arch Agron Soil Sci 60:1229–1248
Baldrian P (2004) Increase of laccase activity during interspecific interactions of white-rot fungi. FEMS Microbiol Ecol 50:245–253
Baldrian P (2006) Fungal laccases, occurrence and properties. FEMS Microbiol Rev 30:215–242
Baldrian P (2008) Wood-inhabiting ligninolytic basidiomycetes in soils: ecology and constraints for applicability in bioremediation. Fungal Ecol 1:4–12
Bertrand S, Schumpp O, Bohni N, Monod M, Gindro K, Wolfender JL (2013) De novo production of metabolites by fungal co-culture of Trichophyton rubrum and Bionectria ochroleuca. J Nat Prod 76:1157–1165
Boddy L (2000) Interspecific combative interactions between wood-decaying basidiomicetes. FEMS Microbiol Ecol 31:185–194
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Chan-Cupul W, Heredia Abarca G, Martínez Carrera D, Rodríguez Vázquez R (2014) Enhancement of ligninolytic enzyme activities in a Trametes maxima–Paecilomyces carneus co-culture: key factors revealed after screening using a Plackett–Burman experimental design. Electron J Biotechnol 17:114–121
Colombo JC, Cabello M, Arambarri AM (1996) Biodegradation of aliphatic and aromatic hydrocarbons by natural soil microflora and pure culture of imperfect and ligninolitic fungi. Environ Pollut 94:355–362
Crowe JD, Olsson S (2001) Induction of laccase activity in Rhizoctonia solani by antagonistic Pseudomonas fluorescens strains and a range of chemical treatments. Appl Environ Microb 67:2088–2094
Dantán-González E, Vite-Vallejo O, Martínez-Anaya C, Méndez-Sánchez M, González MC, Palomares LA, Folch-Mallol J (2008) Production of two novel laccase isoforms by a thermotolerant strain of Pycnoporus sanguineus isolated from an oil-polluted tropical habitat. Int Microbiol 11:163–169
Díaz R, Saparrat M, Jurado M, García-Romera I, Ocampo JA, Martínez MJ (2010) Biochemical and molecular characterization of Coriolopsis rigida laccases involved in transformation of the water soluble fraction of “alpeorujo”. Appl Microbiol Biotechnol 88:133–142
Dwivedi P, Vivekanand V, Pared N, Sharma A, Singh RP (2011) Co-cultivation of mutant Penicilliym oxalicum SAUE-3.510 and Pleurotus ostreatus for simultaneous biosynthesis or xylanase and laccase under solid-state fermentation. New Biotechnol 28:617–626
Evans JB, Niven CF (1951) Nutrition of the heterofermentative Lactobacilli that cause greening of cured meat products. J Bacteriol 62:599–603
Flores C, Vidal C, Trejo-Hernández MR, Galindo E, Serrano-Carreón L (2009) Selection of Trichoderma strains capable of increasing laccase production by Pleurotus ostreatus and Agaricus bisporus in dual cultures. J Appl Microbiol 106:249–257
Flores C, Casasanero R, Trejo-Hernández MR, Galindo E, Serrano CL (2010) Production of laccase by Pleurotus ostreatus in submerged fermentation in co-culture with Trichoderma viride. J Appl Microbiol 108:810–817
Gao SS, Li XM, Zhang Y, Li CS, Cui CM, Wang BG (2011) Comazaphilones A-F, Azaphilone derivatives from the marine sediment derived fungus Penicillium commune QSD-17. J Nat Prod 74:256–251
Gao N, Liu C-X, Xu Q-M, Cheng J-S, Yuan Y-J (2018) Simultaneous removal of ciprofloxacin, norfloxacin, sulfamethoxazole by co-producing oxidative enzymes system of Phanerochaete chrysosporium and Pycnoporus sanguineus. Chemosphere 195:146–155
Gómez J, Rodríguez D, Pazos M, Sanromán MA (2006) Applicability of Coriolopsis rigida for biodegradation of polycyclic aromatic hydrocarbons. Biotechnol Lett 28:1013–1017
Griffith GS, Rayner ADMR, Wildman HG (1994) Interspecific interactions and mycelial morphogenesis of Hypholoma fasciculare (Agaricaceae). Nova Hedwigia 59:47–75
Guillén F, Martínez AT, Martínez MJ (1992) Substrate specificity and properties of the aryl-alcohol oxidase from the ligninolytic fungus Pleurotus eryngii. Eur J Biochem 209:603–611
Guo M, Lu F, Liu M, Li T, Pu J, Wang N, Liang P, Zhang C (2008) Purification of recombinant laccase from Trametes versicolor in Pichia methanolica and its use for the decolorization of anthraquinone dye. Biotechnol Lett 30:2091–2096
Hall TA (1999) BioEdit: a user friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp Ser 41:95–98
Hernández C, Milagres AMF, Vázquez-Marrufo G, Muñoz-Páez KM, García-Pérez JA, Alarcón E (2018) An ascomycota coculture in batch bioreactor is better than polycultures for cellulase production. Folia Microbiol doi 63:467–478. https://doi.org/10.1007/s12223-018-0588-1
Iakovlev A, Stenlid J (2000) Spatiotemporal patterns of laccase activity in interacting mycelia of wood-decaying basidiomycete fungi. Microbial Ecol 39:236–245
Ibañez CG (1998) Contribución al estudio de hongos xilófagos en la Provincia de Misiones, Argentina (Basidiomycetes, Aphyllophorales). II. Polyporaceae. Bol Soc Argent Bot 33:157–169
Jain N, Bhargava A, Tarafdar CJ, Singh KS, Panwar J (2013) A biomimetic approach towards synthesis of zinc oxide nanoparticles. Appl Microbiol Biotechnol 97:859–869
Kumari S, Narian R (2016) Decolorization of synthetic brilliant green carpet industry dye through fungal co-culture technology. J Environ Manage 180:172–179
Liu FZ, Ren JW, Tang JS, Liu XZ, Che YS, Yao XS (2013) Cyclohexanone derivatives with cytotoxicity from the fungus Penicillium commune. Fitot 87:78–83
Ma K, Ruan Z (2015) Production of a lignocellulolytic enzyme system for simultaneous bio-delignification and saccharification of corn stover employing co-culture of fungi. Bioresour Technol 175:586–593
Mali T, Kuuskeri J, Shah F, Lundell TK (2017) Interactions affects hyphal growth and enzyme profiles in combinations of coniferous wood-decaying fungi of Agaricomycetes. PLoS One 12:e0185171
Mata G, Murrieta Hernández DM, Iglesias Andreu LD (2005) Changes in lignocellulolytic enzyme activities in six Pleurotus spp. strains cultivated on coffee pulp in confrontation with Trichoderma spp. World J Microbiol Biotechnol 21:143–150
Mohamed S, Al-Yami M, Sadik A (2016) Detection of mycophages associated with fungal strains isolated from soil of KSA and identified via 18S rRNA gene. J Pharm Biol Chem Sci 7:1375–1380
Muñoz C, Guillén F, Martínez AT, Martínez MJ (1997) Induction and characterization of laccase in the ligninolytic fungus Pleurotus eryngii. Curr Microbiol 34:1–5
Oliveira M, Arenas M, Lage O, Cunha M, Amorim MI (2017) Epiphytic fungal community in Vitis vinifera of the Portuguese wine regions. Lett App Microbiol 66:93–102
Out B, Boyle S, Cheeptham N (2016) Identification of fungi from soil in the Nakimu caves of Glacier National Park. J Exp Microbiol Immunol 2:26–32
Pitt JI (2000) A laboratory guide to common Penicillium species. 3rd Ed. Food Science. Australia Publishers. CSIRO, Australia, pp 197
Quaratino D, Ciaffi M, Federici E, D’Annibale A (2008) Response surface methodology study of laccase production in Panus tigrinus liquid cultures. Biochem Eng J 39:236–245
Sampedro I, Aranda E, Martín J, García-Garrido JM, García-Romera I, Ocampo JA (2004) Saprobic fungi decrease plant toxicity caused by olive mill residues. Appl Soil Ecol 26:149–156
Samson AR, Hoekstra ES, Frisvad JC, Filtenborg O (1996) Introduction to food-borne fungi. Centraalbureau voor Schimmelcultures (CBS), Baarn, The Netherlands. No. Ed. 5 pp 322
Sánchez-López MI, Vanhulle SF, Mertens V, Guerra G, Figueroa SH, Decock C, Corbisier AM, Penninckx MJ (2008) Autochthonous white rot fungi from the tropical forest: potential of Cuban strains for dyes and textile industrial effluents decolourisation. Afr J Biotech 7:1983–1990
Saparrat MCN (2004) Optimizing production of extracellular laccase from Grammothele subargentea CLPS no. 436 strain. World J Microb Biot 20:583–586
Saparrat MCN, Guillén F, Arambarri AM, Martínez AT, Martínez MJ (2002) Induction, isolation and characterization of two laccases from the white rot basidiomycete Coriolopsis rigida. App Environ Microbiol 68:1534–1540
Saparrat MCN, Mocchiutti P, Liggieri CS, Aulicino MB, Caffini NO, Balatti PA, Martínez MJ (2008) Ligninolytic enzyme ability and potential biotechnology applications of the white-rot fungus Grammothele subargentea LPSC no. 436 strain. Process Biochem 43:368–375
Saparrat MCN, Jurado M, Díaz R, García-Romera I, Martínez MJ (2010) Transformation of the water soluble fraction from “alpeorujo” by Coriolopsis rigida: the role of laccase in the process and its impact on Azospirillum brasiliense survival. Chemosphere 78:72–76
Saparrat MCN, Balatti P, Arambarri AM, Martínez MJ (2014) Coriolopsis rigida, a potential model of white-rot fungi that produce extracellular laccases: a review. J Ind Microbiol Biotechnol 41:607–617
Savoie JM, Mata G, Mamoun M (2001) Variability in brown line formation and extracellular laccase production during interaction between white-rot basidiomycetes and Trichoderma harzianum biotype Th2. Mycologia 93:243–248
Shang Z, Li X, Meng L, Li C, Gao S, Huang C, Wang B (2012) Chemical profile of the secondary metabolites produced by a deep-sea sediment-derived fungus Penicillium commune SD-118. Chin J Oceanol Limnol 30:305–314
Svahn KS, Chryssanthou E, Olsen BR, Bohlin L, Göransson U (2015) Penicillium nalgiovense Laxa isolated from Antarctica is a new source of the antifungal metabolite amphotericin B. Fungal Biol Biotech 2:1
Velázquez-Cedeño MA, Farnet AM, Ferré E, Savoie JM (2004) Variations of lignocellulosic activities in dual cultures of Pleurotus ostreatus and Trichoderma longibrachiatum on unsterilized wheat straw. Mycologia 96:712–719
Wagener ER, Davis DN, Diener LU (1980) Penitrem A and roquefortine production by Penicillium commune. Appl Environ Microbiol 39:882–887
White TJ, Burns T, Lee S, Taylor JW (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ (eds) PCR protocols: a guide to methods and applications. Academic Press, New York, pp 315–320
Zhang H, Hong YZ, Xiao YZ, Yuan J, Tu XM, Zhang XQ (2006) Efficient production of laccases by Trametes sp. AH28-2 in cocultivation with a Trichoderma strain. Appl Microbiol Biotechnol 73:89–94
Acknowledgements
G. Heredia wishes to thank the Agencia Española de Cooperación Internacional (AECI-MAEC Program) for providing financial support for a sabbatical stay at the Department of Microbiology, Estación Experimental del Zaidín. M.C.N. Saparrat is a researcher from CONICET in Argentina. Inmaculada Sampedro wishes to thank MINECO for her “Ramón y Cajal” contract. The English text was corrected by Michael O’Shea.
Funding
This study was supported by Spanish projects AGL2008-00572/AGR and Prolipapel II S-2009AMB-1480 as well as the Agencia Nacional de Promoción Científíca y Tecnológica (PICT 2015-1620 to Saparrat, M.).
Author information
Authors and Affiliations
Contributions
R.D. and G.H. contributed equally to this study.
Corresponding author
Electronic supplementary material
Supplementary Fig. S1
(DOCX 69 kb)
Rights and permissions
About this article
Cite this article
Rodríguez, R.D., Heredia, G., Siles, J.A. et al. Enhancing laccase production by white-rot fungus Funalia floccosa LPSC 232 in co-culture with Penicillium commune GHAIE86. Folia Microbiol 64, 91–99 (2019). https://doi.org/10.1007/s12223-018-0635-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12223-018-0635-y