Abstract
We aimed to investigate the utility of the isoFSE sequence, one of the variable flip angle 3D fast-spin echo sequences, on 3T-MR for displaying vessel walls and diagnosing vertebrobasilar artery dissection (VAD). We retrospectively evaluated 12 initial and 28 follow-up images from 12 patients diagnosed with either intracranial VAD or carotid artery dissection. The image quality for displaying the vessel wall was scored using a five-point scale (1 poor, 5 excellent) on initial T1-weighted isoFSE images for each region of the arteries. The intracranial artery dissection findings assessed at time points after onset were evaluated on initial and follow-up T1/T2-weighted isoFSE images. For small arteries, including the anterior/posterior inferior cerebellar artery, similar high scores were obtained on both unenhanced and contrast-enhanced T1-weighted isoFSE images (average: 4.7–5.0, p > 0.2). On unenhanced images, dissected vertebral arteries showed significantly lower scores than non-dissected vertebral arteries for both readers (p = 0.017 and 0.015, respectively), but the scores were high (3.9 and 4.0, respectively). Definitive findings of VAD were observed on the initial images except in one case. For all cases, definitive findings were seen on at least one of the initial or follow-up images. Temporal changes in the findings could be observed for all cases. In conclusion, we showed favorable wall visualization on T1-weighted isoFSE images and the utility of follow-up imaging using unenhanced-T1/T2-weighted and contrast-enhanced T1-weighted isoFSE sequences with acceptable scan times, which could promote the regular use of 3D black-blood vessel wall imaging.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Vertebrobasilar artery dissection (VAD) is a relatively important cause of stroke, especially among young patients [1,2,3,4]. Many patients with VAD are reported to be asymptomatic or only exhibit minor symptoms, so VAD may be much more frequent than previously thought [5]. CT angiography and MR angiography (MRA) have been considered to be useful non-invasive techniques [1, 2]. However, these bright-blood imaging methods are inadequate for the evaluation of wall enhancement or intraluminal lesions, such as an atherosclerotic plaque, intramural hematoma, or flap [1,2,3,4, 6,7,8,9]. Recently, the utility of black-blood vessel wall imaging using a variable flip angle three-dimensional (3D) fast-spin echo sequence has been reported for the diagnosis of intracranial dissection and evaluations of plaques. Currently, the following sequences are used for these evaluations: volume isotropic turbo-spin echo acquisition (VISTA, Phillips Inc., The Netherlands), sampling perfection with application optimized contrast using different angle evolutions (SPACE, Siemens Inc., Germany), and CUBE (GE Healthcare, USA) [2,3,4, 6,7,8]. Additionally, a variable flip angle 3D fast-spin echo sequence (isoFSE) has recently been released on a Hitachi 3-T MR scanner (TRILLIUM OVAL, Hitachi, Tokyo, Japan). All of these sequences are based on a similar principle, but the actual images appear to be different among the variable flip angle 3D fast-spin echo sequences because of differences in scan parameters, including flip angle design and scan options. A previous study [9] reported differences in images and scan time between SPACE and VISTA.
In our experience, the isoFSE sequence appears to have some advantages in depicting the vessel walls and diagnosing VAD. However, to the best of our knowledge, no study has reported its utility to date. Therefore, this study aimed to evaluate the utility of T1-weighted (T1W) isoFSE images for displaying the vessel wall and diagnosing VAD. Furthermore, we followed VAD patients using isoFSE sequences and reviewed temporal changes in the findings.
2 Materials and methods
2.1 Patients
This retrospective study was approved by the institutional review boards of our hospital and written informed consent from patients was waived. The privacy of the patients was completely protected. We selected patients following a search of the medical records filed at our institution between January 2015 and February 2017. Eligibility criteria for entry were as follows: (1) adult patients, (2) final diagnosis of intracranial VAD or carotid artery dissection by MR imaging and/or CT angiography and clinical symptoms, and (3) examination with an MR protocol using the isoFSE sequence on a Hitachi 3-T MR scanner within 3 weeks after onset. The exclusion criteria were as follows: (1) age younger than 18 years, (2) contraindications to MR (incompatible metal implants or pacemakers), and (3) diagnostically unacceptable artifacts (such as motion or susceptibility artifacts) that appeared in the images. We evaluated initial isoFSE images. For follow-up isoFSE images, we used images until a time point beyond 2 months after onset because a previous study reported that intramural hematoma of intracranial vertebral artery dissection was optimally revealed in the subacute to early chronic stage and then disappeared after 2 months [5, 10]. Consequently, 12 initial and 28 follow-up isoFSE images in 12 patients [9 men and 3 women aged 32–64 years (median, 52)] were fully analyzable. The symptoms of these patients were headache (n = 8), vertigo (n = 3), nausea (n = 2), Wallenberg syndrome (n = 4), and right-leg paralysis (n = 1).
2.2 MR examination
All brain MR images were obtained on a TRILLIUM OVAL with a 15-channel head matrix coil. Our isoFSE protocol includes T1W/T2W isoFSE images, diffusion-weighted images, 2D T2*-weighted images, 2D FLAIR images, and 3D MRA. In addition to these images, contrast-enhanced (CE) T1W isoFSE images were acquired for seven patients without contraindications to gadolinium-based contrast media. One patient demonstrated a contraindication to the contrast media and the remaining four patients were examined without contrast media upon the decision of attending physicians. T1W/T2W isoFSE sequences were scanned in the coronal oblique plane, while other sequences were scanned in the axial plane.
isoFSE is a type of 3D variable flip angle fast-spin echo sequence. Refocusing flip angles of each radiofrequency (RF) pulse are adjusted so that the signal intensity of each echo changes slowly throughout the echo train. These flip angles are optimized for the visualization of soft tissue or fluid, and we selected scan parameters for soft tissue visualization. T1W isoFSE imaging was performed using the following scan parameters: repetition time, 487 ms; echo time, 16 ms; echo train length, 18; field of view, 170 mm; slice thickness, 0.9 mm; matrix, 224 × 204 (nearly isotropic spatial resolution, 0.76 × 0.83 × 0.90); number of averages, 1; parallel imaging factor, 2.3 (the phase-encoding direction); and scan time, 247 s. A flow reduction pulse was applied to suppress intraluminal blood flow signals. For flow reduction, symmetric gradient pulses of each refocusing RF pulse, consistent with phase encoding and frequency direction, were inserted between respective echoes. We chose to apply fat suppression technique after six patients were scanned, because visualization of hematoma in the third segment of the vertebral artery (VA) surrounded by fat might improve [11]. Fat saturation pulse was used as fat suppression [12]. T2W isoFSE imaging was performed using the following scan parameters: repetition time, 2000 ms; echo time, 238 ms; echo train length, 75; field of view, 170 mm; slice thickness, 0.9 mm; matrix, 224 × 204 (nearly isotropic spatial resolution, 0.76 × 0.83 × 0.90); number of averages, 1; parallel imaging factor, 2 (the phase-encoding direction); and scan time, 282 s.
CE-T1W isoFSE imaging was performed using the same scan parameters in the same plane as in non-enhanced T1W isoFSE imaging after the intravenous injection of 0.1 mmoL/kg (maximum dose: 7.5 mmol) gadodiamide, meglumine gadoterate, gadopentetate dimeglumine, or gadobutrol.
2.3 Imaging analysis
All images were presented in a random order using PACS software (EV Insite R, PSP Corporation, Tokyo, Japan) and 3-megapixel monitors (Totoku, Tokyo, Japan) that allowed for adjustments of the magnification, window, and level settings.
Two radiologists with neuroimaging experience who were blinded to the clinical information independently evaluated the imaging quality of the initial T1W isoFSE images from 12 patients (12 non-enhanced and 7 CE images) that depicted the vessel wall. A five-point scoring system was used to evaluate the image quality of artery wall visualizations, according to a previous study [8] as follows: (1) an unclear wall, intensity of lumen completely higher than that of the outer wall; (2) a part of the wall clearly shown; (3) most of the lumen and wall clearly shown; (4) intensity of the lumen incompletely suppressed, but outer wall clearly shown; and (5) intensity of the lumen completely lower than that of the outer wall. The basilar artery (BA), bilateral intracranial VA, posterior cerebral artery (PCA), anterior inferior cerebellar artery (AICA), and posterior inferior cerebellar artery (PICA) were scored, except for hypoplastic or aplastic arteries. Hypoplastic arteries were defined as those with external diameters less than 1 mm as measured on the T2W isoFSE images for PCA or VA, in accord with a previous study [13]. For AICA or PICA, we defined hypoplastic or aplastic as non-visualized on the T2W isoFSE images.
For diagnoses of intracranial artery dissection, two readers evaluated parts of dissected arteries and the findings of the initial T1/T2W isoFSE images with MRA images. Two weeks later, they evaluated follow-up T1/T2W isoFSE images compared with the initial images using side-by-side comparisons. The other images and clinical data were blinded. Decisions were made by consensus. The definitive findings for evaluations of artery dissection were as follows: flap or double lumen, intramural hematoma on unenhanced T1W isoFSE images; and double lumen (contrast enhancement of false lumen), enhanced flap on CE-T1W isoFSE images [1,2,3,4,5]. Intramural hematoma was recognized as a crescentic high intensity along the wall [1, 10]. The minor findings were as follows: dilatation with a wall irregularity; dilatation on T2W isoFSE images; and intraluminal high intensity corresponding to slow flow or intraluminal hematoma on unenhanced/CE-T1W isoFSE images [2].
2.4 Statistical analysis
Statistical analyses were performed using the Ekuseru-Toukei 2012 (Social Survey Research Information Co., Tokyo, Japan). The Mann–Whitney U test and Kruskal–Wallis test were used to compare scores for unpaired samples. A threshold for significance was set at p < 0.05. For the five-point score, inter-observer agreement was evaluated using weighted “kappa” statistics. We recorded the strength of agreement as either poor (κ = 0.00–0.20), slight (κ = 0.21–0.40), fair (κ = 0.41–0.60), moderate (κ = 0.61–0.80), or excellent or perfect (κ = 0.81–1.00).
3 Results
3.1 Imaging quality of isoFSE images
There was a total of 84 non-dissected arteries: 11 VAs, 10 BAs, 23 PCAs, 21 AICAs, and 19 PICAs. There were 15 dissected arteries, including 13 VAs and 2 BAs. Nine hypoplastic or aplastic arteries (1 PCA, 3 AICAs, and 5 PICAs) were excluded. Table 1 shows scores of the image quality of artery wall visualization on unenhanced T1W isoFSE images. Table 2 shows scores of the image quality of artery wall visualization on CE-T1W isoFSE images. For small arteries, including AICA/PICA, high scores were seen on unenhanced and CE-T1W isoFSE images. There were no significant differences among non-dissected arteries on unenhanced T1W isoFSE images for both readers (p = 0.27 and 0.95, respectively) and among all arteries on CE-T1W isoFSE images (p = 0.39 and 0.79, respectively). On unenhanced T1W isoFSE images, dissected VA showed significantly lower scores than non-dissected VA for both readers (p = 0.017 and 0.015, respectively), but the scores were still high with excellent inter-observer agreement. On unenhanced and CE-T1W isoFSE images, inter-observer agreement between the two readers was either excellent or perfect for arteries, except AICA showed moderate agreement and dissected VA.
3.2 Diagnosis of artery dissection
On unenhanced T1W isoFSE images, 5 arteries in 5 patients (right VA in 2, left VA in 2, and BA to right VA in 1) were diagnosed as dissection. On CE-T1W isoFSE images, 9 arteries in 7 patients (right VA in 5, left VA in 3, and BA to left VA in 1) were diagnosed as dissection. The parts and findings of artery dissection on each image and the duration after onset are summarized in Table 3. Definitive findings for evaluations of artery dissection are highlighted in bold, and were observed on all initial images except in one case. For all cases, the definitive findings were seen on at least one of initial or follow-up images. Temporal changes in the findings were observed for all cases. The size of the intramural hematoma or false lumen or the degree of dilatation changed temporally for most cases. Representative cases are shown in Figs. 1 and 2.
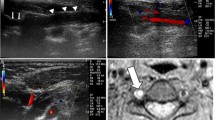
A 44-year-old man with right vertebral artery dissection. Dilatation with a wall irregularity was seen on a 5-mm-thick multiplanar reconstruction of a T2W isoFSE image (a). The T1W isoFSE image revealed intramural hematoma at 8 days after onset (c), but this was not observed at 3 days after onset (b). Aneurysmal dilatation was apparent at 64 days after onset (d)
A 53-year-old man with left vertebral artery dissection. At 15 days after onset, a T1W isoFSE image showed a small intramural hematoma (a). Contrast enhancement of the false lumen could be observed on CE-T1W isoFSE images (b). At 21 days after onset, the size of the intramural hematoma had increased (c, d arrowhead). The contrast enhancement of the false lumen disappeared on CE-T1W isoFSE images (d arrow)
4 Discussion
We evaluated the image quality of artery wall visualization on T1W isoFSE images and the clinical utility for diagnoses of VAD on initial and follow-up T1/T2W isoFSE images. Regarding the image quality of non-dissected artery wall visualization, high scores were seen on unenhanced and CE-T1W isoFSE images, even for small arteries with excellent inter-observer agreement except for AICA. Inter-observer agreement for AICA was lower than for other arteries, which may indicate difficulty in visualizing small artery walls. Nevertheless, moderate agreement and high scores—equal to those for other arteries—were observed. Dissected VA showed lower, but still high scores compared with non-dissected VA. The isoFSE sequence is a 3D black-blood vessel wall imaging technique that uses a variable flip angle fast-spin echo sequence with isotropic high spatial resolution and allows for multiplanar reformations. Furthermore, this is a non-cardiac-gated technique that allows for a shorter scanning time. The presence of different intravoxel velocities in blood vessels causes phase dispersion and results in signal loss, a phenomenon that is amplified with the use of long echo trains and low refocusing flip angles [6]. Therefore, the black-blood effect of this sequence is influenced by flow void, and very slow flow in a dissected or small artery may not be suppressed. By contrast, the conventional 2D black-blood T1W sequence has a high capacity to suppress the flow signal using electrocardiographic-gated double inversion recovery, but has a problem with poor resolution over a long scan time [2]. Several studies have reported the advantages of T1 VISTA and CUBE compared with 2D black-blood T1W sequences [2,3,4, 6, 8, 9]. The motion-sensitized driven-equilibrium (MSDE) sequence obtained a favorable black-blood effect for the 3D sequences [14, 15]. With MSDE preparation, the flow-sensitizing gradient pair introduces phase dispersion among moving spins, resulting in the favorable suppression of flow signals [14]. However, T2 relaxation or diffusion effects caused signal loss of the hematoma on T1W images [15]. isoFSE sequence has a feature where flow signals in the artery tend to be not refocused because of its refocusing RF flip angle pattern. Furthermore, the use of flow reduction gradient pulses helps to minimize flow signal without MSDE preparation. Similar to a previous study of CUBE sequence [8], unenhanced and CE T1W isoFSE on a 3-T MR scanner revealed high-quality wall visualization according to the favorable black-blood effect, even for dissected or small artery walls with intraluminal slow flow. A previous study [3] reported two cases of PICA dissection that were diagnosed by VISTA. Therefore, isoFSE may also be useful for diagnosing dissection of small arteries, such as PICA/AICA. Further studies of patients with PICA/AICA dissection are warranted. In this present study, the scan time of isoFSE was acceptable, at 4–5 min, which was almost equal to or shorter than those of SPACE, CUBE, and VISTA in previous reports [4, 6, 7, 9, 16].
We also demonstrated the clinical utility for the diagnosis of VAD on isoFSE images. Definitive findings of VAD were seen on all initial images except in one case. For all cases, the definitive findings were observed on at least one of the initial or follow-up images. In a previous study, positive rate of intramural hematoma was reported to be 32% [5]. Intramural hematoma of VAD was best revealed in the subacute to early chronic stage, and could not be obtained when the duration between the onset and examination was either within a week or greater than a month [5, 10]. In our present study, intramural hematoma was observed and the sizes of intramural hematomas changed temporally on follow-up images. Such observations of these changes may help in the diagnosis of VAD. We used T2W isoFSE to evaluate dilatation and/or irregularity of the external diameter of the artery within the cistern as a substitute for BPAS, which was reported to be useful for evaluating dilatation and temporal changes but may not visualize PICA [3, 4]. T2W isoFSE may provide more detailed information on the surface appearance of intracranial arteries; notably, Madokoro et al. [17] reported on its utility for the diagnosis of PICA dissection. Performing follow-up MR is recommended to temporal changes in these findings and ensure the diagnosis of VAD with minimal invasiveness [3,4,5, 10]. The utility of CE-T1 VISTA imaging was reported: abnormal vessel enhancement was recognized in 100% of cases [2]. In our present study, findings of VAD were only observed on CE-T1W isoFSE images for two follow-up images. Consequently, the diagnostic utility of follow-up MR using unenhanced T1W and T2W isoFSE and CE-T1W isoFSE sequences was demonstrated. The utility of each sequence was reported in previous studies, but few of these studies fully evaluated all 3D black-blood sequences for VAD follow-up imaging.
Our study has several limitations. First, there were less number of patients, especially for evaluating CE-T1W isoFSE images. We had no consensus regarding whether CE images should be acquired. Further studies with greater numbers of patients, following the guidelines of 3D black-blood CE/unenhanced imaging for VAD diagnosis, are warranted. Second, pathological confirmation and images taken with other modalities or conventional sequences were not obtained. In previous studies [2, 11], VISTA was compared with conventional 2D black-blood sequences, CT angiography, or digital subtraction angiography. Nevertheless, 2D black-blood sequences and CT angiography are inferior to 3D black-blood imaging as discussed above, and might not be useful. In our hospital, 2D black-blood sequences are not included in the VAD protocol and CTA is not always performed, as in the previous studies [8, 9, 18]. Furthermore, findings of VAD may change dramatically and imposing an interval between assessments by MR and other modalities may be controversial. isoFSE showed high scores of artery wall visualization, as in the previous CUBE sequence study [8], and temporal changes of the findings, including definitive VAD findings, ensured the reliability of our diagnosis [3,4,5, 10]. Comparison with other 3D black-blood sequences is necessary. Third, the fat suppression technique was not applied for all T1W isoFSE sequences. This might not influence the results of our study in terms of the evaluation of intracranial arteries that were not surrounded by fat. However, further studies involving the evaluation of fat-suppressed T1W black-blood images are necessary.
In conclusion, we demonstrated favorable wall visualization on T1W isoFSE images and the utility of follow-up imaging totally using unenhanced-T1/T2W and CE-T1W isoFSE sequences. 3D black-blood vessel wall imaging using a variable flip angle fast-spin echo sequence on a 3-T MR scanner could be used by more vendors with acceptable scan times, so the daily use of 3D black-blood imaging could be promoted in more institutions.
References
Lum C, Chakraborty S, Schlossmacher M, Santos M, Mohan R, Sinclair J, et al. Vertebral artery dissection with a normal-appearing lumen at multisection CT angiography: the importance of identifying wall hematoma. AJNR Am J Neuroradiol. 2009;30(4):787 – 92.
Sakurai K, Miura T, Sagisaka T, Hattori M, Matsukawa N, Mase M, et al. Evaluation of luminal and vessel wall abnormalities in subacute and other stages of intracranial vertebrobasilar artery dissections using the volume isotropic turbo-spin-echo acquisition (VISTA) sequence: a preliminary study. J Neuroradiol. 2013;40(1):19–28.
Ishitsuka K, Sakaki Y, Sakai S, Uwatoko T, Aibe H, Ago T, et al. Diagnosis and follow-up of posterior inferior cerebellar artery dissection complicated with ischemic stroke assisted by T1-VISTA: a report of two cases. BMC Neurol. 2016;16:121.
Takemoto K, Takano K, Abe H, Okawa M, Iwaasa M, Higashi T, et al. The new MRI modalities “BPAS and VISTA” for the diagnosis of VA dissection. Acta Neurochir Suppl. 2011;112:59–65.
Hosoya T, Adachi M, Yamaguchi K, Haku T, Kayama T, Kato T. Clinical and neuroradiological features of intracranial vertebrobasilar artery dissection. Stroke. 1999;30(5):1083–90.
Edjlali M, Roca P, Rabrait C, Naggara O, Oppenheim C. 3D fast spin-echo T1 black-blood imaging for the diagnosis of cervical artery dissection. AJNR Am J Neuroradiol. 2013;34(9):E103-6.
Koga H, Oishi T. 3.0T MR Imaging of the Cranial Arterial Wall for the Strategy of Stroke Prevention. Neuroradiol J. 2011;24(1):101 – 14.
Li ML, Xu YY, Hou B, Sun ZY, Zhou HL, Jin ZY, et al. High-resolution intracranial vessel wall imaging using 3D CUBE T1 weighted sequence. Eur J Radiol. 2016;85(4):803–7.
Zhang L, Zhang N, Wu J, Zhang L, Huang Y, Liu X, et al. High resolution three dimensional intracranial arterial wall imaging at 3 T using T1 weighted SPACE. Magn Reson Imaging. 2015;33(9):1026–34.
Kitanaka C, Tanaka J, Kuwahara M, Teraoka A. Magnetic resonance imaging study of intracranial vertebrobasilar artery dissections. Stroke. 1994;25(3):571–5.
Takano K, Yamashita S, Takemoto K, Inoue T, Kuwabara Y, Yoshimitsu K. MRI of intracranial vertebral artery dissection: evaluation of intramural haematoma using a black blood, variable-flip-angle 3D turbo spin-echo sequence. Neuroradiology. 2013;55(7):845 – 51.
Abe T. Fast fat suppression RF pulse train with insensitivity to B1 inhomogeneity for body imaging. Magn Reson Med. 2012;67(2):464–9.
Hoksbergen AW, Fulesdi B, Legemate DA, Csiba L. Collateral configuration of the circle of Willis: transcranial color-coded duplex ultrasonography and comparison with postmortem anatomy. Stroke. 2000;31(6):1346–51.
Nagao E, Yoshiura T, Hiwatashi A, Obara M, Yamashita K, Kamano H, et al. 3D turbo spin-echo sequence with motion-sensitized driven-equilibrium preparation for detection of brain metastases on 3T MR imaging. AJNR Am J Neuroradiol. 2011;32(4):664 – 70.
Wang J, Yarnykh VL, Yuan C. Enhanced image quality in black-blood MRI using the improved motion-sensitized driven-equilibrium (iMSDE) sequence. J Magn Reson Imaging. 2010;31(5):1256–63.
Qiao Y, Zeiler SR, Mirbagheri S, Leigh R, Urrutia V, Wityk R, et al. Intracranial plaque enhancement in patients with cerebrovascular events on high-spatial-resolution MR images. Radiology. 2014;271(2):534 – 42.
Madokoro Y, Sakurai K, Kato D, Kondo Y, Oomura M, Matsukawa N. Utility of T1- and T2-weighted high-resolution vessel wall imaging for the diagnosis and follow up of isolated posterior inferior cerebellar artery dissection with ischemic stroke: report of 4 cases and review of the literature. J Stroke Cerebrovasc Dis. 2017;26(11):2645–51.
Luo Y, Guo ZN, Niu PP, Liu Y, Zhou HW, Jin H, et al. 3D T1-weighted black blood sequence at 3.0 T for the diagnosis of cervical artery dissection. Stroke Vasc Neurol. 2016;1(3):140–6.
Funding
No outside source of funding was received for this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical statement
All procedures in this study were performed in accordance with the ethical standards of our institutional research board and with the 1964 Declaration of Helsinki.
This study did not contain experiments on human or animal participants.
Informed consent
Informed consent was waived in this retrospective study.
About this article
Cite this article
Ogawa, M., Omata, S., Kan, H. et al. Utility of the variable flip angle 3D fast-spin echo (isoFSE) sequence on 3T MR for diagnosing vertebrobasilar artery dissection. Radiol Phys Technol 11, 228–234 (2018). https://doi.org/10.1007/s12194-018-0460-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12194-018-0460-7