Abstract
The purpose of this study was to evaluate the role of color Doppler sonography (CDS) in the diagnosis of extracranial vertebral artery dissections (EVADs). One hundred and fifty consecutive patients (age range 21–51 years, mean 44 years) with a clinical suspicion of vertebral artery dissection (VAD) were included in this study. All patients underwent CDS of vertebral arteries as the first-line imaging modality. Cervical T1-weighted fat-saturated axial MR images served as the gold standard. Of the 150 patients with a clinical suspicion of VAD, 27 patients were ultimately diagnosed with EVADs based on fat-saturated T1-weighted MR imaging. MR imaging was considered positive when crescentic hyperintensity (methemoglobin signal) was demonstrated at the wall of the vertebral artery. CDS was positive in 21 of these 27 patients and revealed either intramural hematoma or a dissecting membrane with two lumina. The most frequent site of involvement was the V1 to proximal V2 segment. The sensitivity, specificity, and positive and negative predictive values of CDS in the diagnosis of EVADs were 77.8, 98.4, 91.3, and 95.3%, respectively. CDS is a reliable diagnostic tool in the diagnosis of EVADs.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Vertebral artery dissection (VAD) is one of the most frequent causes of stroke in patients under 45 years of age. The most common symptoms are dizziness, vertigo, headache, and neck pain. Stroke is also common, especially in extracranial vertebral artery dissections (EVADs) [1]. Prompt diagnosis is essential to avoid serious neurologic complications and to initiate proper treatment. Although conventional angiography is considered the gold standard, the invasive nature of angiography precludes its use as a primary diagnostic tool [2].
Studies that examined the presence of EVADs by color Doppler sonography (CDS) are limited [4, 5]. The aim of this study was to evaluate the role of CDS in the diagnosis of EVADs.
Materials and methods
One hundred and fifty consecutive patients with a clinical suspicion of VAD were included in this study. Twenty-seven (14 men and 13 women, aged 21–51 years, mean age 39.5 ± 4.6 years) of these 150 patients were ultimately diagnosed with EVADs based on fat-saturated T1-weighted cervical magnetic resonance imaging (MRI). Patients with MRI findings compatible with intracranial VAD were not included. Data were collected within a period of 7 years. Informed consent was obtained from each patient. Patients with a clinical suspicion of VAD were referred from the neurologic ward or emergency department. All patients with EVADs underwent both MRI and CDS. Examination of the cervical vertebral artery (VA) was performed according to the method described by Lu et al. [3]. The segment between C5 and C6 was first identified. Then, the artery was followed to its origin and to the level of the atlas loop. CDS was performed with Mindray DC-7 (Medical International Limited, Shenzhen, China) and GE Healthcare (Milwaukee, WI) LOGIQ 5 units equipped with broadband transducers (5–12 MHz). Hypoechoic thickening of the VA wall (intramural hematoma) with or without focal increase in the diameter of the VA and presence of a dissection flap were the criteria used to establish the diagnosis of EVADs. Although not routinely performed, measurements of peak systolic VA velocity (cm/s), VA resistance index (peak systolic velocity − end diastolic velocity/peak systolic velocity), and VA diameter (mm) were obtained in some patients. Color flow and power Doppler imaging were also performed to assess vessel patency, to discriminate between wall thickening and the patent arterial lumen, and to identify the false and true lumina in case of a dissection flap. All CDS examinations were done by a radiologist (C.Y.) with 20 years of experience in CDS. According to our study design, all patients underwent CDS before MRI. The mean time between CDS and MRI was 3.7 h (1–7 h). The time between onset of symptoms and admission was 2.4 days. MRI was performed on a 1.5T Philips Intera Gyroscan scanner (Philips Medical Systems, The Netherlands) or on a 1.5T Siemens Magnetom Avanto Syngo (Erlangen, Germany) unit using a phased-array coil. Thin-section T1-weighted fat-saturated axial cervical MRI was performed in each patient. Demonstration of crescentic hyperintensity (methemoglobin signal) within the wall of the extracranial VA was considered to be diagnostic of EVADs [6]. In addition to T1-weighted fat-saturated axial cervical MRI, DWI was performed in 87 patients, whereas cranial MRI was available in 72 patients. Cervical MR angiography was performed in 13 patients. MRI examinations were evaluated by five radiologists (F.F.G., F.Y.O., M.O., M.A., and A.S.K.) with at least 10 years of experience in MRI.
Statistical analysis
Statistical analyses were performed using SPSS, version 20.0, (SPSS Inc. Chicago, Illinois). Data are expressed as mean ± SD for continuous variables and percentage for categorical variables. Sensitivity, specificity, and positive and negative predictive values of CDS were assessed. T1-weighted fat-saturated axial MRI was used as the reference standard.
Results
Of the 150 patients with a clinical suspicion of VADs, 27 patients were ultimately diagnosed with EVADs based on fat-saturated T1-weighted thin-section cervical MRI. In 27 patients, MRI revealed crescentic hyperintensity in the wall of the extracranial VA indicating dissection. CDS revealed specific signs of dissection in 21 (77.8%) of these 27 patients. Intramural hematoma was seen in 13 patients, whereas a dissecting membrane was identified in eight patients (Table 1). The VA was dissected at the origin of the V0 or proximal V1 segment in 4/27 (15%) patients (Fig. 1). In 16/27 (59%) patients, the VA was dissected between distal V1 and proximal V2 segments (Fig. 2), whereas in 5/27 (18%) patients, the site of dissection was the distal V2 segment. In 2/27 (8%) patients, the V3 segment was dissected. In six patients, MRI revealed EVADs, whereas CDS failed to show specific signs of EVADs (Table 2). Four of these six dissections were located at the distal V2, and two dissections involved the V3 segment (Fig. 3). In two patients, sonography revealed either a wall hematoma or a dissection flap, whereas MRI was normal. The sensitivity, specificity, and positive and negative predictive values of CDS in the diagnosis of EVADs were 77.8%, 98.4%, 91.3%, and 95.3%, respectively (Table 2).
A 41-year-old man presented with posterior circulation stroke and vertigo. Gray-scale US image (a) of the right VA shows focal dilatation of the V1 segment (arrowheads) with an abrupt decrease in diameter of the VA as it enters the transverse process of the sixth cervical vertebra. Longitudinal (b) and transverse (c) CDS images reveal residual color flow and depict wall thickening to a better extent. Transverse fat-saturated T1-weighted MR image (d) shows crescentic hyperintensity consistent with the characteristic methemoglobin signal of VA dissection. Also noted is increased intraluminal signal intensity (arrow) possibly related to slow flow or thrombosis
A 48-year-old man presented to the emergency department with dizziness and vertigo 2 days after a sudden fall in the bathroom. Gray-scale image (a) of the right VA shows a dissecting flap originating from the prevertebral segment just before it passes through the C6 transverse process (arrow). Power Doppler image (b) reveals flow within the channel close to the transducer, whereas no flow is detected in the false lumen. Contrast-enhanced coronal oblique MR angiogram (c) reveals diffuse narrowing and wall irregularities of the right VA (arrows). The left VA appears normal (arrowheads). T1-weighted fat-saturated MR image (d) shows typical crescentic hyperintensity at the wall of the right VA consistent with intramural hematoma (arrow)
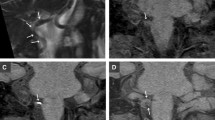
A 41-year-old woman presented with neck pain and dizziness. Her history was positive for a recent chiropractic manipulation. Color Doppler (a) and power Doppler (b) images demonstrate dissection of the left VA in a spiral fashion (arrows). Arrowheads indicate the dissecting membrane. No flow is identified in the false lumen possibly due to intramural hematoma. T1-weighted fat-saturated MR image shows a typical hyperintense signal within the wall of the left VA compatible with dissection (not shown)
No patient was diagnosed with bilateral EVADs. In 21/27 (77%) patients, the dissection was related to minor trauma such as sporting injuries, chiropractic manipulation, abrupt head turning, direct neck impact, massage, and falls. Eighteen (67%) of 27 patients had dizziness, 15/27 (55%) patients had vertigo, 14/27 (50%) patients had headache, and 10/27 (37%) patients had neck pain.
Discussion
In our study, two important findings related to the development of ECVDs were identified: (1) EVAD incidence was 18% in our study population. (2) The sensitivity, specificity, and positive and negative predictive values of CDS in the diagnosis of EVADs were 77.8%, 98.4%, 91.3%, and 95.3%, respectively.
Although CDS is an established diagnostic tool in the evaluation of VAD [4, 7,8,9], findings at CDS are often nonspecific [3, 7, 8]. In studies including both intra- and extracranial VAD, intramural hematoma and visualization of an intimal flap were seen only in a minority of patients [3, 8]. As these studies included both extra- and intracranial dissections, CDS did not provide a specific diagnosis in the majority of patients [8]. In a previous study conducted by Pugliese et al. [8], CDS revealed 10 out of 15 VAD with a sensitivity of 66% and specificity of 60%. Among the true positive cases, in eight, the diagnosis was based on indirect signs, such as high-resistance flow, whereas a specific finding (a dissecting membrane with visualization of the true and false lumen) was seen in only two patients. Lu et al. [3] all collected data on eight patients with nine intracranial and six extracranial dissections. A specific finding (intramural hematoma) was noted in only one artery. Similarly, in a previous study conducted by Wessels et al. [9], sonographic findings typical for dissection were found in 10/40 VADs, including an intimal flap in the V1 segment, in one (2.5%), a double lumen in two (5%) patients, and wall hematoma in seven (18%) patients.
To the best of our knowledge, only several studies and some case reports specifically assessed the role of CDS in the evaluation of EVADs [4, 5, 10, 11]. In a study conducted by Bartels et al., EVADs were primarily detected with CDS in 15/20 (75%) patients. The characteristic findings of hypoechoic wall thickening or a dissection flap were the most frequently encountered findings. In the 15 patients, the diagnosis was temporally related to minor or significant trauma. The most frequent site of dissection was the region between V1 and V2 segments, where the artery is exposed to greatest mechanical injury [4]. They concluded that EVADs could be diagnosed noninvasively with duplex color flow imaging. Hypoechoic wall thickening with or without a localized increase in vessel diameter or a dissecting membrane was the most frequently encountered finding of dissection in that study. Our results are in keeping with this study. In our study, dissection of the VA was temporally linked to minor or moderate trauma in 21/27 (77%) patients. Sonographic findings typical for dissection were found in 21/27 (77%) VAD. Hypoechoic focal wall thickening with or without focal dilatation of the artery was observed in 13/21 (62%) vessels, while a dissecting membrane was seen in 8/21 (38%) arteries. The most frequent site of involvement was the V1-to-proximal V2 segment, which was seen in 16/21 (76%) patients, similar to the findings of Bartels and Flügel [4]. This region is especially vulnerable to trauma. Given the fact that most of our patients had a history of recent minor to moderate trauma, this finding is not surprising. The V3 segment was rarely involved in the study of Bartels and Flügel. Similar to their findings, we found only two dissections at the V3 segment and five dissections in the distal V2. The distal V2 and V3 segments are relatively difficult to examine sonographically [4]. Indeed, CDS failed to show specific signs of dissection in all but one distal V2 and V3 dissection in our study. A short neck and poor patient cooperation hamper the evaluation of these segments. Identification of a high-resistance pattern at duplex imaging may aid in the diagnosis of V2–V3 dissections, albeit this finding is nonspecific, and can also be seen in distal VA steno-occlusions [12].
Digital subtraction angiography (DSA) has long been regarded as the gold standard for diagnosis of dissection. However, DSA is invasive and cannot demonstrate wall hematomas. For this reason, MRI is recommended as the first-line imaging screening tool [13]. In our study, cervical MRI was used to establish the diagnosis of EVADs. In a recent study by Yang and Ran [5], patients with EVADs were divided into three groups: intramural hematoma, occlusion, and double lumen appearance. In the 37 patients with EVADs in their study, intramural hematoma was reported in 30 (81%), occlusion in six (16%), and double lumen in one (3%). There are some differences between our study and that study. We distinguished two groups of patients with intramural hematoma and intimal flaps. None of the patients had total occlusion. Intramural hematoma and intimal flaps were observed in 13 (62%) patients and eight (38%) patients, respectively. In our study, the percentage of patients with intramural hematoma was lower and the percentage of patients with intimal flap was significantly higher than the study conducted by Yang and Ran.
Limitations
Our study has several limitations. First, although T1-weighted MRI with fat suppression is considered the gold standard in the diagnosis of VA dissections, it has some inherent limitations [12, 14]. Inadequate fat suppression and slow flow may simulate the hyperintense mural hematoma signal of dissection. On the other hand, a false-negative diagnosis of dissection on MRI is likely to occur when patients are scanned at the very early stage of dissection. A second limitation is that CDS was performed by an expert radiologist. In addition, sonographic diagnosis of EVAD was based on qualitative findings such as hypoechoic wall thickening and identification of a dissection flap. Thus, our results may not apply to an operator with relatively less experience in CDS of the cervical arteries. The sensitivity and specificity of these qualitative parameters should be validated in further studies, where CDS is performed by relatively less experienced operators than in our study. Finally, only patients with a clinical suspicion of VAD were included in the study, which may inevitably lead to bias.
Conclusion
Our study shows that CDS is a reliable method in the diagnosis of EVADs. The most frequent site of involvement is the region between the V1 and proximal V2 segments that can be easily visualized with CDS. However, it is user-dependent, and evaluation of the distal parts of the VA (distal V2 and V3 segments) may be difficult. Specific sonographic signs of dissection (intramural hematoma of the vessel or a dissection flap) are seen in the majority of EVADs.
References
Gottesman RF, Sharma P, Robinson KA, et al. Clinical characteristics of symptomatic vertebral artery dissection. A Systematic Review. Neurologist. 2012;18:245–54.
Vertinsky AT, Schwartz NE, Fischbein NJ, et al. Comparasion of multidetector CT angiography and MR imaging of cervical artery dissection. Am J Neuroradiol. 2008;29:1753–60.
Lu JC, Sun Y, Jeng JS, et al. Imaging in the diagnosis and follow-up evaluation of vertebral artery dissection. J Ultrasound Med. 2000;19:263–70.
Bartels E, Flügel KA. Evaluation of extracranial vertebral artery dissection with duplex color-flow imaging. Stroke. 1996;27:290–5.
Yang L, Ran H. Extracranial vertebral artery dissection: findings and advantages of ultrasonography. Medicine (Baltimore). 2018;97:e0067.
Rodallec MH, Marteau V, Gerber S, et al. Craniocervical arterial dissection: spectrum of imaging findings and differential diagnosis. RadioGraphics. 2008;28:1711–28.
Flis CM, Jäger HR, Sidhu PS. Carotid and vertebral artery dissections: clinical aspects, imaging features and endovascular treatment. Eur Radiol. 2007;17:820–34.
Pugliese F, Crusco F, Cardaioli G, et al. CT ahgiography versus colour-Doppler US in acute dissection of the vertebral artery. Radiol. Med. 2007;112:435–43.
Wessels T, Mosso M, Krings T, et al. Extracranial and intracranial vertebral artery dissection: long term clinical and duplex sonographic follow-up. J Clin Ultrasound. 2008;36:472–9.
Gottesman RF, Sharma P, Robinson KA, et al. Imaging characteristics of symptomatic vertebral artery dissection. Neurologist. 2012;18:255–60.
Siepmann T, Borchert M, Barlinn K. Vertebral artery dissection with compelling evidence on duplex ultrasound presenting only with neck pain. Neuropsychiatr Dis Treat. 2016;1:2839–41.
Gottesman RF, Sharma P, Robinson KA, et al. Imaging characteristics of symptomatic vertebral artery dissection. Neurologist. 2012;18:255–60.
Provenzale JM. MRI and MRA for evaluation of dissection of craniocerebral arteries: lessons from the medical literature. Emerg Radiol. 2009;16:185–93.
Siepmann T, Borchert M, Barlinn K. Vertebral artery dissection with compelling evidence on duplex ultrasound presenting only with neck pain. Neuropsychiatr Dis Treat. 2016;1:2839–41.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests or sources of funding.
Ethical statement
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008.
Informed consent
Informed consent was obtained from patients for being included in the study.
About this article
Cite this article
Yılmaz, C., Gorgulu, F.F., Oksuzler, F.Y. et al. Color Doppler ultrasonography is a reliable diagnostic tool in the diagnosis of extracranial vertebral artery dissections. J Med Ultrasonics 46, 153–158 (2019). https://doi.org/10.1007/s10396-018-0901-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10396-018-0901-2