Abstract
Background
Diabetes mellitus (DM) is one of the most common diseases in the worldwide. Type 1 diabetes mellitus (T1DM) is characterized by insulin deficiency and beta cells apoptosis. Tropisetron as a 5-HT3 receptor antagonist has positive effects on the inflammation, apoptosis and glucose lowering. The aim of this study was to investigate the effect of tropisetron on β-cells apoptosis and its possible pathways.
Methods
Animals were divided into five equal groups: the control, tropisetron, diabetes, tropisetron-DM and glibenclamide-DM (seven in each group). Tropisetron and glibenclamide were administrated for 2 weeks after type 1 diabetes induction. Real-time PCR, western blot analysis and TUNEL assay were performed.
Results
We found that tropisetron decreased blood glucose and increased insulin secretion. Protein expression of NF-κB was downregulated, while protein expression of SIRT1 upregulated after tropisetron treatment. Moreover, Bax/Bcl2 ratio decreased in tropisetron-DM group and finally, apoptosis improved in pancreas tissue.
Conclusions
It seems that tropisetron administration improves STZ-induced apoptosis and diabetes in the animals. This effect might be resulted from involvement in NF-κB/ SIRT1 pathway.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diabetes mellitus (DM) is a serious chronic metabolic disease, the global prevalence of which is increasing. It has been estimated that it will increase to 629 million people by 2045 [1]. Type 1 diabetes mellitus (T1DM) or insulin-dependent diabetes is an autoimmune disorder, characterized by high glucose level, insulin deficiency and excessive beta cell dysfunction and destruction [2]. Apoptosis is the major pathway in pancreatic cells loss. In fact, increased levels of blood glucose lead to progressive β-cells injuries, pancreatic apoptosis and, eventually, lack of insulin [3,4]. Hyperglycemia, ROS, inflammatory cytokine’s overproduction and T-lymphocytes activation induce β-cells apoptosis [5,6]. Among this, nuclear factor-kappa B (NF-κB) as a key transcription factor plays a critical role in the regulation of immune responses and apoptosis [7]. It has been revealed that hyperglycemia and inflammatory processes activate NF-κB, then it is translocated into the nucleus and induce DNA degradation [6,8]. One of the inhibition factors for NF-κB is silent information regulator-two 1 (SIRT1). Studies have demonstrated that SIRT1 acts as an anti-inflammatory factor by inhibiting NF-κB signaling pathway [9]. Indeed, there are a crosstalk between NF-κB and SIRT1 in the T1DM. SIRT1 increases insulin levels by repressing the action of UCP2 and suppressing the apoptosis [12,13]. Since β cell apoptosis is the major complication of T1DM, a large number of studies are being conducted on many pharmacologic and natural compounds.
Previously, involvement of serotonergic system in the pathophysiology of many diseases has been well known, which included anxiety, non-alcoholic fatty liver disease, gastric ulcer, metabolism and so on [14,15]. Recently, Mao et al. reported blocking 5-HT3 synthesis restored adiposity, high-fat diet-induced obesity and glucose intolerance in ZnT8-deficient mice [16]. Tropisetron is a 5-HT3 receptor antagonist that is used as a safe and well-tolerated drug to neutralize chemotherapy and surgery-induced emesis [17,18]. Positive effects of tropisetron on plasma glucose have been shown, and treatment with tropisetron has decreased blood glucose and improved diabetic nephropathy in rats [19]. Several studies have also reported tropisetron effects on the inflammation, cytokines secretion and apoptosis suppression [20,21], these properties are independent from 5-HT3 receptor pathway [22,23]. One of the targets of inhibitory effect of tropisetron is calcineurin, a Ca/2 + calmodulin-dependent phosphatase, which could mediate T-cell stimulation contributing to its anti-inflammatory properties [23]. Moreover, tropisetron inhibits high glucose-induced apoptosis via the decreased expression of Bax and caspase-3 [24].
According to previous findings, this study was designed to assess the effects of tropisetron on the β-cell pancreatic apoptosis in the STZ-diabetic rats and involvement of SIRT1/NF-κB pathway.
Materials and methods
Reagents: tropisetron (Cayman Chemical Co, USA), streptozotocin (Sigma, St. Louis, Mo, USA)), insulin kit (E-EL-R2466Elabscience, USA), glibenclamide (Sigma, India), TUNEL kit (In Situ Cell Death Detection Kit, POD Version 13.0:Cat. No. 11 684 817 910, Roch).
Experimental procedures
Thirty-five male wistar rats (250 ± 20 g) were used in this study. Animals were housed in a temperature-controlled room at a 12:12 light/dark cycle with free access to the food and tap water. All procedures were according to the Guidelines for Animal Care and Use at the Qom and Urmia Universities of Medical Sciences (IR.MUQ.REC.1397.087 and IR.UMSU.REC.1396.359). Animals randomly divided into five equal groups (N = 7). Control group: animals that received saline for 2 weeks intraperitoneally (ip). Tropisetron group: received 3 mg/kg tropisetron for 2 weeks ip [25]. Diabetes group (DM): animals that received 50 mg/kg of streptozotocin (STZ) ip to induce type 1 diabetes (freshly dissolved in the saline) [26]. Tropisetron-treated diabetes group (Tropisetron-DM): diabetic animals that received tropisetron for 2 weeks ip after diabetes induction and glibenclamide-treated diabetes group (Glibenclamide-DM): diabetic rats that received 1 mg/kg/day glibenclamide for two weeks ip after diabetes induction [26]. Forty-eight hours after the STZ injection, hyperglycemia was determined by measuring the tail vein blood glucose content using by glucometer (Elegance, Model: no: CT-X10 Germany). Rats with blood glucose of higher than 300 mg/dl were considered diabetic. At the end of the experiment, the animals were anaesthetized with sodium pentobarbital (35 mg/kg, ip. The abdomen was opened via midline incision; the pancreas was immediately removed and washed in ice-cold physiological saline. Blood samples were directly collected from the heart. A part of the pancreas was dissected and fixed in 10% buffered formaldehyde solution and other part kept at − 70 °C.
Real-time RT-PCR
Total RNA was extracted from frozen tissue samples using the RNX-plus Kit (Cinnagen, Tehran, Iran) according to the manufacturer’s instructions. The quantity and purity of the RNA samples were measured by Nanodrop spectrophotometer (Thermo scientific, USA). Complementary DNAs (cDNA) were made from mRNA templates for qRT-PCR using the Pocket Script RT perMix (BioNeer, Alameda, CA, USA). The synthesized cDNAs were used as templates for real-time PCR. Real-time PCR analysis was performed with Accu Power 2 × Green star qPCR Master Mix (Biofact). The quantitation of data was performed using the comparative 2−ΔΔCt method. Glyceraldehyde-3-pPhosphate dehydrogenase (GAPDH) was used as an internal control [27]. Primer for studied gene was designed by primer3 software (v. 0.4.0) (https://primer3.ut.ee), and based on sequences found in the Ensemble. Primer sequence homology and total gene specificity were confirmed with BLAST analysis (www.ncbi.nlm.nih.gov/blast). The primer sequence was as follows:
GAPDH(F)GACAACTTTGGCATCGTGGA (R)ATGCAGGGATGATGTTCTGG
Bax(F)CGGCGAATTGGAGATGAACTGG (R)CTAGCAAAGTAGAAGAGGGCAACC
Bcl2 (F)TGTGGATGACTGACTACCTGAACC (R)CAGCCAGGAGAAATCAAACAGAGG
Western blot
NF-κB-p65-1and SIRT1 expressions in the pancreas tissue were determined by Western immunoblotting. Briefly, the protein concentration of the supernatant was measured by the Bradford assay kit (Sigma Aldrich, USA) and then, a 20 µg protein was loaded in each well after mixing with a 2X sample loading buffer. The proteins were separated in 10% SDS-gels and transferred to PVDF membranes in an hour for all the loaded samples. Subsequently, the blocking of the membranes was performed in a 5% skim milk buffer containing 0.1% Tween-20 for 1.5 h and then probed with primary antibodies against NF-κB-p65-1(ab16502), SIRT1(sc-15404) and β-actin (sc-130657) overnight at 4 °C in a shaker incubator. After 4 × 5 min washing with a Tris buffered saline solution containing 0.1% Tween-20, the HRP-conjugated secondary antibody (1:7000, cell signaling) was added to the membranes. After 1 h of incubation in the shaker, the membranes were bathed in the wash buffer and washed at least 3 × 5 min. Then, the membranes were incubated with the enhanced chemiluminescence (ECL, Amersham) reagents in dark room. This was followed by exposing of the membrane to an X-ray film and visualization of the chemiluminescence of the binding by means of a visualizing machine. The intensity of the bands was determined using Image J software (IJ 1.46r version, NIH, USA) and normalized to the bands of the internal control (beta-actin).
TUNEL assay
Paraffin sections of pancreas tissues rehydrated and stained by the terminal deoxynucleotidyl transferase (TdT)-mediated dUTP nick end labeling (TUNEL) kit (Roche in Situ Cell Death Detection Kit, POD Version 13.0, Germany) according to the manufacturer's instructions and cell apoptosis was observed under light microscope.
Statistical analysis
Data were expressed as mean ± SEM. First, data normality was checked by the Kolmogorov–Smirnov test. To find the differences between groups and data comparison (diabetes group and treatment groups), statistical analysis was performed by two-way analysis of variances (ANOVA) and Tukey’s post hoc test using SPSS. P < 0.05 considered to be statistically significant.
Results
Tropisetron decreased blood glucose level and increased plasma insulin secretion in the STZ-induced diabetic rats
Two-way ANOVA analysis on blood glucose level showed the significant effect of tropisetron (F1,10 = 165.31; p < 0.001). Post hoc comparisons revealed that in the Tropisetron-DM and Glibenclamide-DM groups, blood glucose markedly decreased compared with the diabetes group (Fig. 1a). Contrarily, insulin plasma level significantly increased (F1,10 = 68.25; p < 0.001). More analysis demonstrated that administration of tropisetron and glibenclamide markedly increased insulin level compared with the diabetic group (Fig. 1b).
a Blood glucose concentration in different experimental groups. (Mean ± SEM, N = 7), *p < 0.001 compared with the control group, #p < 0.001 compared with the diabetes group (two-way ANOVA followed by Tukey’s post hoc test). b Plasma levels of insulin in different experimental groups. (Mean ± SEM, N = 7), *p < 0.001 compared with the control group, #p < 0.001 compared with the diabetes group (two-way ANOVA followed by Tukey’s post hoc test)
Tropisetron increased SIRT1 and decreased NF-κB protein expressions in the pancreas of STZ-induced diabetic rats
To gain insight into role of NF-κB and SIRT1, we studied the effects of tropisetron on the NF-κB-p65-1 and SIRT1 proteins expression in the STZ-induced animals. Two-way ANOVA showed the decrement effect of protein expression of NF-κB-p65-1 (F1,10 = 25.97; p < 0.001). Post hoc comparison revealed that 2 weeks’ treatment with tropisetron and glibenclamide markedly decreased NF-κB-p65-1 protein expression compared with the diabetes group (Fig. 2a). We also found the marked effect of SIRT1 protein expression in STZ-induced mice (F1,10 = 53.31; p < 0.001). More analysis showed that administration of tropisetron and glibenclamide strikingly upregulated protein expression of SIRT1 compared with the diabetic group compared with the control animals (Fig. 2b).
a Protein expression of NF-κB-p65-1 in different experimental groups. (Mean ± SEM, N = 7), *p < 0.001 compared with the control group, #p < 0.001 compared with the diabetes group (two-way ANOVA followed by Tukey’s post hoc test). b Protein expression of SIRT1in different experimental groups. (Mean ± SEM, N = 7), *p < 0.001 compared with the control group, #p < 0.001 compared with the diabetes group (two-way ANOVA followed by Tukey’s post hoc test)
Tropisetron increased Bax/Bcl2 ratio gene expression in the pancreas of STZ-induced diabetic rats
As shown in Fig. 3, two-way ANOVA analysis demonstrated the significant decrease of gene expression of Bax/Bcl2 in the diabetic rats (F1,10 = 141.32; p < 0.001). Post hoc comparison showed that in the Tropisetron-DM and Glibenclamide-DM groups, expression of Bax/Bcl2 markedly lowered compared with the STZ-diabetic group.
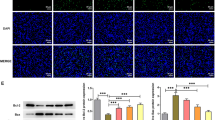
Tropisetron attenuated apoptotic cells in the pancreas of STZ-induced diabetic rats
Obtained results of two-way ANOVA analysis revealed the significant effect of apoptotic cells in the pancreas of STZ-induced rats (F1,10 = 919.14; p < 0.001). More analysis displayed that after tropisetron and glibenclamide treatment, pancreas apoptotic cells markedly decreased compared with the STZ-diabetic group (Fig. 4).
Discussion
The major findings of this study revealed that tropisetron, an antagonist of 5HT3 receptor, increased insulin plasma level and decreased blood glucose in diabetic rats. Tropisetron treatment also led to a decrease in pancreatic apoptotic cells, which was revealed via decreased Bax and increased Bcl2 gene expressions. Therefore, ratio of Bax/Bcl2 was decreased in the STZ-treated group compared to the diabetic group. Further molecular analysis revealed that administration of tropisetron increased SIRT1 and decreased NF-κB protein expressions.
Nowadays, tropisetron is used to treat the chemotherapy-induced emesis; however, many pieces of evidence have proposed new aspects for immune-modulatory and anti-inflammatory effects of tropisetron on diabetes, fatty liver, brain damage and so on [20,21].
Streptozotocin (STZ) is usually utilized for diabetes induction in the animal models; it is associated with pancreas tissue destruction, insulin deficiency and blood glucose enhancement. Our results showed that tropisetron could increase insulin levels and decrease blood sugar among diabetic rats. Many reports are compatible with these data and confirm the positive effects of tropisetron on the blood glucose decrement and insulin increment in diabetic situations [19,28].
It is well known that β-cell apoptosis is the major and important reason for insulin loss and T1DM induction. Indeed, activation of helper T-cells and release of inflammatory cytokines and chemokines from the pancreas tissue launch apoptosis cascade, destroy pancreas tissue and lead to insulin loss [29].
For this purpose, in the present study, the gene expression of Bax and Bcl2 was evaluated. The data revealed that the 2-week treatment with tropisetron led to a decrease in Bax and an increase in Bcl2 protein expressions, thereby decreasing the Bax/Bcl2 ratio in the diabetic pancreas. However, the number of apoptotic cells by TUNEL assay also concomitantly increased.
Several documents have shown attenuating effects of tropisetron on the apoptosis in the liver and brain injuries [20,25,30]. Bax, as a proapoptotic factor, and Bcl2, as an antiapoptotic agent, have important roles in the apoptosis process in the T1DM [31]. Exposure to chronic high glucose disturbs pro- and anti-apoptotic balance in the β-cells, leading to apoptosis [32,33]. Federici et al. demonstrated that high glucose medium exposed human islets could lead to the down-regulation of Bcl2 and up-regulation of Bax [32]. Activation of pro-apoptotic protein Bax triggers the release of cytochrome c from mitochondria and contributes to apoptosis. An increased ratio of Bax/Bcl2 may activate caspase-3 as a pivotal mediator of apoptosis and, thus, play an important role in programmed cell death or apoptosis process [22,23].
Activation of NF-κB as a transcriptional regulator has a critical role in promoting β-cell apoptosis in T1DM [34]. Several studies have reported that phospho-NF-kB p65 protein elevation has been detected in the diabetic pancreas tissue, contributing to the increased expression of inflammatory cytokines, COX-2 and iNOS [6,8,20], which in turn activates signaling pathways linked to the apoptosis process [35]. Both hyperglycemia and increased level of IL1β as an inflammatory cytokine activate NF-κB and start apoptosis process via different intermediates [35]. Accordingly, chronic treatment of pancreatic β cells with IL-1β results in iNOS production and, then, affects electron transfer. Thus, they decrease ATP synthesis in mitochondria and increase the expression of proinflammatory genes as well as ER stress, which are known as the main mechanisms causing beta cell dysfunction and apoptosis [36]. These processes can promote the activation of downstream effector caspases, which plays a potential role in this cascade [37]. Moreover, Bcl2 expression is downregulated following NF-κB activation via the involvement of 4-Hydroxynonenal (HNE) [38].
Interestingly, in the present study, tropisetron administration lowered increased protein expression of NF-κB in the diabetic rats compared to the control group. Similarly, Rahimian et al. showed that tropisetron decreased NF-κB and caspase 3 expressions in a rat model of Alzheimer’s disease [20]. Moreover, it was reported that tropisetron down-regulated NF-κB expression and inhibited human T-cell activation in in vitro [32].
NF-kB activation is an important modulator that impacts both the DNA binding ability and transcriptional activity of the protein [22,23]. Recently, it has been suggested that there is an interaction between NF-κB and SIRT1 in diabetes. SIRT1 is the NAD-dependent deacetylase, which controls many cellular processes, including insulin secretion, oxidative stress and inflammation in the pancreas tissue [39].
In turn, SIRT1 deacetylates NF-κB, leading to increased Ikβα association or lack of transactivation potential of the protein and reduction in NF-κB transcription activity, thereby reducing production of proinflammatory cytokines and anti-apoptotic molecules [22,23,32].
It has been indicated that increased insulin secretion is due to blocking the apoptosis process, thus promoting pancreatic beta-cell survival in SIRT1 overexpression status [34]. Prud’homme et al. reported anti-apoptotic effects of SIRT1 in the pancreatic islet cells [13]. Another study showed that activation of SIRT1 attenuated high glucose-induced neuronal apoptosis via deacetylating p53 [40]. Lee et al. exhibited that SIRT1 exerted a cytoprotective effect on pancreatic beta cells through inhibiting NF-κB, thereby increasing insulin synthesis and mitigating insulin resistance [41]. It has been found that SIRT1 suppresses Bax expression through P53 down-regulation and mitochondria-dependent apoptosis in MC3T3-E1 cells [42].
In the present study, a significant increase was found in SIRT1 protein expression after tropisetron treatment in the pancreas of diabetic animals. In line with these results, Artesunate could improve pancreatic beta cells apoptosis via inhibiting IL1β and NF-κB by the SIRT1 activation [43]. More importantly, tropisetron attenuated d-galactose-induced brain aging via SIRT1 signaling activation [30].
Since the activation of NF-κB has been attributed to the apoptosis of β cells, SIRT1 activation may declare an important supporting signaling against inflammatory reactions. To the knowledge of the present authors, this is the first study, which could provide evidence for the protective effect of tropisetron against pancreatic apoptosis in part via the up-regulation of SIRT1 and down-regulation of NF-κB protein expressions. The effect of tropisetron in our study was similar to that of glibenclamide.
Conclusion
This study, for the first time, demonstrated that treatment with tropisetron could improve STZ-induced pancreatic apoptosis in the animals through increase of SIRT1 expression and, thus, decrease of NF-κB expression, Bax/Bcl2 ratio decrement and, finally, apoptosis attenuation. Therefore, tropisetron could be considered as a potential candidate for Langerhans islands restoring and diabetes treatment. Future studies can help confirm this hypothesis.
References
Cho NH, Shaw JE, Karuranga S, Huang Y, da Rocha Fernandes JD, et al. IDF Diabetes Atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract. 2018;138:271–81.
Bhattacharya P, Fan J, Haddad C, Essani A, Gopisetty A, Elshabrawy HA, et al. A novel pancreatic β-cell targeting bispecific-antibody (BsAb) can prevent the development of type 1 diabetes in NOD mice. Clin Immunol. 2014;153(1):187–98.
Kim WH, Lee JW, Suh YH, Hong SH, Choi JS, Lim JH, et al. Exposure to chronic high glucose induces beta-cell apoptosis through decreased interaction of glucokinase with mitochondria: downregulation of glucokinase in pancreatic beta-cells. Diabetes. 2005;54(9):2602–11.
Cnop M, Welsh N, Jonas JC, Jörns A, Lenzen S, Eizirik DL. Mechanisms of pancreatic beta-cell death in type 1 and type 2 diabetes: many differences, few similarities. Diabetes. 2005;54:S97–107.
Melloul D. Role of NF-kappaB in beta-cell death. Biochem Soc Trans. 2008;36(Pt 3):334–9.
Zhao Y, Krishnamurthy B, Mollah ZU, Kay TW, Thomas HE. NF-κB in type 1 diabetes. Inflamm Allergy Drug Targets. 2011;10(3):208–17.
Kuryłowicz A, Nauman J. The role of nuclear factor-kappaB in the development of autoimmune diseases: a link between genes and environment. Acta Biochim Pol. 2008;55(4):629–47.
Ide Y, Matsui T, Ishibashi Y, Takeuchi M, Yamagishi S. Pigment epithelium-derived factor inhibits advanced glycation end product-elicited mesangial cell damage by blocking NF-kappaB activation. Microvasc Res. 2010;80(2):227–32.
Yang H, Zhang W, Pan H, Feldser HG, Lainez E, Miller C, et al. SIRT1 activators suppress inflammatory responses through promotion of p65 deacetylation and inhibition of NF-κB activity. PLoS ONE. 2012;7(9):e46364.
Kauppinen A, Suuronen T, Ojala J, Kaarniranta K, Salminen A. Antagonistic crosstalk between NF-κB and SIRT1 in the regulation of inflammation and metabolic disorders. Cell Signal. 2013;25(10):1939–48.
Yuan Q, Zhang D, Liu C, Zhang C, Yuan D. Chikusetsusaponin V inhibits LPS-activated inflammatory responses via SIRT1/NF-κB signaling pathway in RAW264.7 cells. Inflammation. 2018;41(6):2149–59.
Bordone L, Motta MC, Picard F, Robinson A, Jhala US, Apfeld J, et al. Sirt1 regulates insulin secretion by repressing UCP2 in pancreatic β Cells. PLoS Biol. 2015;13(12):e1002346.
Prud'homme GJ, Glinka Y, Udovyk O, Hasilo C, Paraskevas S, Wang Q. GABA protects pancreatic beta cells against apoptosis by increasing SIRT1 expression and activity. Biochem Biophys Res Commun. 2014;452(3):649–54.
Kwon YJ, Hong KW, Park BJ, Jung DH. Serotonin receptor 3B polymorphisms are associated with type 2 diabetes: The Korean genome and epidemiology study. Diabetes Res Clin Pract. 2019;153:76–85.
Tharmaraja T, Stahl D, Hopkins CWP, Persaud SJ, Jones PM, Ismail K, et al. The association between selective serotonin reuptake inhibitors and glycemia: a systematic review and meta-analysis of randomized controlled trials. Psychosom Med. 2019;81(7):570–83.
Mao Z, Lin H, Su W, Li J, Zhou M, Li Z, et al. Deficiency of ZnT8 promotes adiposity and metabolic dysfunction by increasing peripheral serotonin production. Diabetes. 2019;68(6):1197–209.
He XH, Shi YK, Yang JL, Zhang CG, Liu P, Zhou AP, et al. Tropisetron in attenuation of nausea and vomiting in patients undergoing high-dose chemo-radiotherapy supported by autologous peripheral blood stem cell transplantation. Ai Zheng. 2004;23(4):456–60.
Alon E, Kocian R, Nett PC, Koechli OR, Baettig U, Grimaudo V. Tropisetron for the prevention of postoperative nausea and vomiting in women undergoing gynecologic surgery. Anesth Analg. 1996;82(2):338–41.
Barzegar-Fallah A, Alimoradi H, Asadi F, Dehpour AR, Asgari M, Shafiei M. Tropisetron ameliorates early diabetic nephropathy in streptozotocin-induced diabetic rats. Clin Exp Pharmacol Physiol. 2015;42(4):361–8.
Rahimian R, Fakhfouri G, Ejtemaei Mehr S, Ghia JE, Genazzani AA, Payandemehr B, et al. Tropisetron attenuates amyloid-beta-induced inflammatory and apoptotic responses in rats. Eur J Clin Invest. 2013;43(10):1039–51.
Yu Y, Zhu W, Liang Q, Liu J, Yang X, Sun G. Tropisetron attenuates lipopolysaccharide induced neuroinflammation by inhibiting NF-κB and SP/NK1R signaling pathway. J Neuroimmunol. 2018;320:80–6.
Barzegar-Fallah A, Alimoradi H, Razmi A, Dehpour AR, Asgari M, Shafiei M. Inhibition of calcineurin/NFAT pathway plays an essential role in renoprotective effect of tropisetron in early stage of diabetic nephropathy. Eur J Pharmacol. 2015;767:152–9.
Barzegar-Fallah A, Alimoradi H, Mehrzadi S, Barzegar-Fallah N, Zendedel A, Abbasi A, et al. The neuroprotective effect of tropisetron on vincristine-induced neurotoxicity. Neurotoxicology. 2014;41:1–8.
Aminzadeh A. Protective effect of tropisetron on high glucose induced apoptosis and oxidative stress in PC12 cells: roles of JNK, P38 MAPKs, and mitochondria pathway. Metab Brain Dis. 2017;32(3):819–26.
Gholizadeh-Ghaleh Aziz S, Naderi R, Mahmodian N. Ameliorative effects of tropisetron on liver injury in streptozotocin-induced diabetic rats. Arch Physiol Biochem. 2019;15:1–6.
García-Galicia MC, Burgueño-Tapia E, Romero-Rojas A, García-Zebadúa JC, Cornejo-Garrido J, Ordaz-Pichardo C. Anti-hyperglycemic effect, inhibition of inflammatory cytokines expression, and histopathology profile in streptozotocin-induced diabetic rats treated with Arracacia tolucensis aerial-parts extracts. J Ethnopharmacol. 2014;152(1):91–8.
Komeili Movahhed T, Moslehi A, Golchoob M, Ababzadeh S. Allantoin improves methionine-choline deficient diet-induced nonalcoholic steatohepatitis in mice through involvement in endoplasmic reticulum stress and hepatocytes apoptosis-related genes expressions. Iran J Basic Med Sci. 2019;22(7):736–44.
Asadi F, Razmi A, Dehpour AR, Shafiei M. Tropisetron inhibits high glucose-induced calcineurin/NFAT hypertrophic pathway in H9c2 myocardial cells. J Pharm Pharmacol. 2016;68(4):485–93.
Tomita T. Apoptosis of pancreatic β-cells in type 1 diabetes. Bosn J Basic Med Sci. 2017;17(3):183–93.
Mirshafa A, Mohammadi H, Shokrzadeh M, Mohammadi E, Talebpour Amiri F, Shaki F. Tropisetron protects against brain aging via attenuating oxidative stress, apoptosis and inflammation: The role of SIRT1 signaling. Life Sci. 2020;248:117452.
Edlich F. BCL-2 proteins and apoptosis: recent insights and unknowns. Biochem Biophys Res Commun. 2018;500(1):26–34.
Federici M, Hribal M, Perego L, Ranalli M, Caradonna Z, Perego C, et al. High glucose causes apoptosis in cultured human pancreatic islets of Langerhans: a potential role for regulation of specific Bcl family genes toward an apoptotic cell death program. Diabetes. 2001;50(6):1290–301.
Kooptiwut S, Kaewin S, Semprasert N, Sujjitjoon J, Junking M, Suksri K, et al. Estradiol prevents high glucose-induced β-cell apoptosis by decreased BTG2 expression. Sci Rep. 2018;8(1):12256.
Mandrup-Poulsen T. Apoptotic signal transduction pathways in diabetes. Biochem Pharmacol. 2003;66(8):1433–40.
Kang Z, Zeng J, Zhang T, Lin S, Gao J, Jiang C, et al. Hyperglycemia induces NF-κB activation and MCP-1 expression via downregulating GLP-1R expression in rat mesangial cells: inhibition by metformin. Cell Biol Int. 2019;43(8):940–53.
Khodabandehloo H, Gorgani-Firuzjaee S, Panahi G, Meshkani R. Molecular and cellular mechanisms linking inflammation to insulin resistance and β-cell dysfunction. Transl Res. 2016;167(1):228–56.
Chandirasegaran G, Elanchezhiyan C, Ghosh K, Sethupathy S. Berberine chloride ameliorates oxidative stress, inflammation and apoptosis in the pancreas of Streptozotocin induced diabetic rats. Biomed Pharmacother. 2017;95:175–85.
Timucin AC, Basaga H. Pro-apoptotic effects of lipid oxidation products: HNE at the crossroads of NF-κB pathway and anti-apoptotic Bcl-2. Free Radic Biol Med. 2017;111:209–18.
Wang W, Sun W, Cheng Y, Xu Z, Cai L. Role of sirtuin-1 in diabetic nephropathy. J Mol Med (Berl). 2019;97(3):291–309.
Shi X, Pi L, Zhou S, Li X, Min F, Wang S, Liu Z, Wu J. Activation of Sirtuin 1 attenuates high glucose-induced neuronal apoptosis by deacetylating p53. Front Endocrinol (Lausanne). 2018;9:274.
Lee JH, Song MY, Song EK, Kim EK, Moon WS, Han MK, et al. Overexpression of SIRT1 protects pancreatic beta-cells against cytokine toxicity by suppressing the nuclear factor-kappaB signaling pathway. Diabetes. 2009;58(2):344–51.
Gu X, Wang Z, Gao J, Han D, Zhang L, Chen P, et al. SIRT1 suppresses p53-dependent apoptosis by modulation of p21 in osteoblast-like MC3T3-E1 cells exposed to fluoride. Toxicol In Vitro. 2019;57:28–38.
Yu L, Chen JF, Shuai X, Xu Y, Ding Y, Zhang J, et al. Artesunate protects pancreatic beta cells against cytokine-induced damage via SIRT1 inhibiting NF-κB activation. J Endocrinol Invest. 2016;39(1):83–91.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Naderi, R., Shirpoor, A., Samadi, M. et al. Tropisetron attenuates pancreas apoptosis in the STZ-induced diabetic rats: involvement of SIRT1/NF-κB signaling. Pharmacol. Rep 72, 1657–1665 (2020). https://doi.org/10.1007/s43440-020-00146-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43440-020-00146-7