Abstract
Several compounds of the phthalate family are widely applied as additives for polymers as polyvinyl chloride (PVC) and polyethylene terephthalate (PET). These compounds are not part of the polymer chains, and therefore, they can be released easily from products and migrate into beverages that come into direct contact causing environmental and human health impacts. Because of this, certain phthalates (PAEs) have been identified as priority pollutants by the European Union (EU), US Environmental Protection Agency (EPA) and other international organizations. Due to that the concentration of these compounds in beverages is found at very low level, a pretreatment step prior to their analysis is necessary; thus, several sample preparation methods have been described, such as liquid–liquid extraction (LLE), solid-phase extraction (SPE), solid-phase microextraction (SPME), and liquid-phase microextraction (LPME). Chromatographic techniques such as gas chromatography (GC) coupled to mass spectrometry (MS) or liquid chromatography (LC) with UV detector, diode array detector (DAD), and MS have been used to analyze PAEs. Additionally, non-chromatographic techniques such as electrochemical sensors and immunoassay-based techniques have been described for PAE analysis in beverages. This review provides an overview of the different analytical techniques for PAE quantification in beverages and their plastic containers, focused in the last 10 years published works, covering the sample preparation and determination, as well as the legislation and the evaluation of main factors that could promote the migration of these plasticizers from polymers into beverages.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Studies related to the environmental destination of some contaminants, principally the so-called emerging contaminants (ECs), have drawn more and more attention. These pollutants are gaining social conscience due to their potential environmental and human health impacts. Nevertheless, these groups of compounds do not have normative status (Magdouli et al. 2013; Álvarez et al. 2015). Among the ECs, the phthalic acid esters or phthalates (PAEs) are considered to be one of their main representatives, due to their large production volume and their multiple applications. PAEs are extensively used as additives for polymers in plastic, particularly in polyvinyl chloride (PVC) and polyethylene terephthalate (PET); but they are also applied in rubber and cellulose and in the production of styrene. PAEs help to improve the flexibility, transparence, and durability of articles manufactured with polymeric matrixes (Khosravi and Price 2015; Silva et al. 2004; Peijnenburg and Struijs 2006; Yang et al. 2015). Different plasticizers exhibit different plasticization effects, depending on the strength of the plasticizer–polymer and plasticizer–plasticizer interactions (Wilkes et al. 2005).
The effect of the PAE levels on the polymer structure properties is related to the decrease of Young’s modulus, tensile strength, hardness, density, melt viscosity, glass transition temperature, electrostatic chargeability, and volume resistivity of polymers (Graham 1973; Rahman and Brazel 2004). PAEs are widely used in many consumable and household products, such as industrial plastics and personal care products.
PAEs of low molecular mass (esters with side chains of 1 to 4 carbons), including the dimethyl phthalate (DMP), diethyl phthalate (DEP), and di-n-butyl phthalate (DBP), are primarily used in personal care products, certain dietary supplements, medications, printing inks, lacquers, and adhesives. High molecular mass PAEs (esters with side chains of 5 or more carbons) including butyl benzyl phthalate (BBP), di(n-octyl)phthalate (DNOP), and di(2-n-ethylhexyl) phthalate (DEHP) are mainly found in flexible PVC used in consumer products like food packaging, floorings, home furnishings, building materials, and medical equipments (Serodio and Nogueira 2006; Sailas et al. 2015; Li et al. 2013; Sakhi et al. 2014). PAEs were first introduced in the 1920s and have been widely applied for more than 90 years in industry (Chang et al. 2015; Otero et al. 2015). Currently, approximately 80% of annual word production of PAEs is used as plasticizers (Yang et al. 2015).
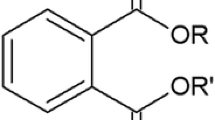
PAEs are a class of organic xenobiotic compounds (Sailas et al. 2015) produced by the esterification of phthalic acid with different alcohols, and consist mainly of one benzene ring and two aliphatic ester groups attached to the benzene ring in an ortho configuration (Fig. 1) (Yang et al. 2015; Farajzadeh et al. 2015). The physicochemical properties of PAEs vary considerably depending on their molecular mass (MM) (Jianlong et al. 2004). Table 1 summarizes the properties of some of these PAEs, which are most commonly found in beverages.
Potential pathways of exposure to PAEs are by ingestion, inhalation, and absorption through the skin (Guo et al. 2011). Human exposure can take place during the production, distribution, and end use of products produced with PET, PVC, and other polymers such as polyurethane, polystyrene, polybutadiene, among others (Fromme et al. 2007).
The mechanical properties of plastics are influenced by plasticizer level (parts per hundred of resin (phr)) as well as the chemical class of plasticizer. The consumption of plasticizers DEHP and BBP stands at 100 phr in flexible PVC, polystyrene commercial products typically range from 25 to 100 phr of DMP, DBP, and DNOP, and low-density polyethylene plastics contain 30 phr of DNOP. The consumption of PAEs (DMP, DEP, DBP, DNOP, BBP, DEHP) ranges from 1 to 5 wt% in PET (Wilkes et al. 2005; Wypych 2012; Graham 1973).
PAEs are lipophilic compounds and can bioaccumulate in fats. Different studies have revealed high toxicity to the human health and to the ecosystem functioning (Gao et al. 2015). The larger molecular weight PAEs (DEHP and DBP) are suspected carcinogens, as well as toxic to the liver, kidneys, and reproductive organs (S. Keresztes et al. 2013). The main concerns related to the exposure to PAEs in humans and wildlife are the effects on reproduction (Net et al. 2015; Zhao et al. 2015), endocrine damage, and their carcinogenic effects (Otero et al. 2015; Heudorf et al. 2007; Fierens et al. 2012; Liu et al. 2015). Beverages might be contaminated during production and bottle process. Nevertheless, migration of plasticizers from plastics into food is the major source of PAE contamination. An overview of the studies related to the PAEs most commonly found in beverages and polymers (Table 1) including sample preparation and detection methods is presented in Tables 2, 3, and 4. This review focuses on the literature available in the last 10 years regarding the screening of PAEs in carbonated and non-carbonated beverages and plastic beverage containers, emphasizing on analytical methods and legislation. Additionally, sample preparation including extraction and preconcentration steps and quantification techniques described for PAEs in beverages and plastic matrices is covered.
Reported Concentrations in Beverages and Plastics
Due to their risk to human health and the environment, certain PAEs have been identified as priority hazardous substances. These compounds have been classified in category 1 (clear evidence for endocrine-disrupting effects in an intact organism) by the European Union (EU) controlling their use as plasticizers in products that may come into contact with food (Dominguez-Morueco et al. 2014). The EU has also set a specific migration limit (SML) values of 1.5 and 0.3 g/kg for DBP and DEHP, respectively. In addition, tolerable daily intakes (TDI) set by the European Food Safety Authority are 0.2 0.5, 0.5, 0.01, and 0.05 mg/kg body weight/day for DEP, BBP, DBP, and DEHP, respectively (Fan et al. 2014; Mihucz and Záray 2015; Ustun et al. 2014). The EPA included the DMP, DEP, DBP, and DEHP in its list of priority pollutants published in 2014, setting a maximum limit for DEHP in drinking water of 6 μg/L. In China, some PAEs (DMP, DBP, and DEHP) have also been identified as priority toxic pollutants (Gao et al. 2014a; Pérez-Feás et al. 2011; Gao et al. 2014b).
Because PAE plasticizer molecules are not attached to polymer chains by primary bonds (Rahman and Brazel 2004), these compounds can easily migrate from the plastic packaging toward the food, beverages, and water (Hammad Khan and Jung 2008; Fierens et al. 2012; S. Keresztes et al. 2013; Liu et al. 2015). Their potential migration (leaching) is a function of several physicochemical factors such as temperature, radiation, solubility, diffusion coefficients, pressure, and presence of solvents and additives (Zaater et al. 2014). PAEs are ubiquitous in nature; consequently, they can be found in groundwater, river water, drinking water, and oceans (Jianlong et al. 2004; Rahman and Brazel 2004; Ustun et al. 2014). Information regarding PAE concentration found in beverages and polymers described in the literature is summarized in Tables 2, 3, and 4. Jia et al. (2014) made an analysis of PAEs in milk and yogurt reporting concentrations in milk of 13 and 57 μg/L for DEP and DEHP and 13 and 43 μg/L for DEP and DEHP, in yogurt. Otero et al. (2015) determined PAE content in three commercial brands of water and plastic bottles, reporting concentrations of 0.061 μg/L for DBP and 1.19 μg/L in water and 0.076 μg/g for DBP and 1.499 μg/g for DEHP in plastic bottles. Liou et al. (2014) found concentrations of 0.006 μg/L for DMP, 0.009 μg/L for DEP, 0.104 μg/L for DBP, and 0.3 μg/L for DEHP in bottled water. Therefore, it is very important to analyze the concentration of these compounds, not only in the products consumed by the human, but also in the materials used to pack these products (Bonini et al. 2008). PAEs can be also found in water supplies. Wu et al. (2013) determined PAE levels in river and seawater samples from seven districts of China. The results showed that concentrations varied from 11 to 61 μg/L for DBP and from 19 to 25 μg/L for DEHP.
The use of PAEs in food packaging materials has now been banned by the European Commission regulation No. 10/2011, but there are still food and drink packages that contain PAEs (S. Keresztes et al. 2013; Jeddi et al. 2015). Ingestion is an important pathway of human exposure to PAEs; thus, it is important to monitor the level of PAEs in beverages and provide data for human exposure assessment.
Sample Preparation for PAE Determination in Beverages
Due to that PAEs are present at very low concentrations in beverages, the development of highly sensitive analytical methods for their quantification is needed. Sample pretreatment is required to extract, preconcentrate, and improve analytical sensitivity during analytical determination of PAEs. This stage should be as fast and inexpensive as possible. A wide variety of sample preparation approaches have been reported in literature for PAEs, such as liquid–liquid extraction (LLE) (Otero et al. 2015; Zaater et al. 2014; S. Keresztes et al. 2013), solid-phase extraction (SPE) (G. Zhiyong et al. 2010; Dominguez-Morueco et al. 2014; Bach et al. 2013), solid-phase microextraction (SPME) (Psillakis and Kalogerakis 2003; Banitaba et al. 2013), and liquid-phase microextraction (LPME) (Xu et al. 2007). However, some of these methods still have some limitations.
LLE is the most frequently used method for extraction of PAEs from beverages (Sun et al. 2012; Otero et al. 2015; Zaater et al. 2014; S. Keresztes et al. 2013; Leitz et al. 2009; Amiridou and Voutsa 2011; Sakhi et al. 2014; Ustun et al. 2014). It has been proven to be a reliable and efficient method. Parameters such as selection of the extraction solvent, the ratio of extraction solvent volume/sample volume, or the number of extraction repetitions had to be optimized during its implementation (Leitz et al. 2009). An organic solvent (50–500 mL) is added into the aqueous sample (500–1000 mL), the content is shaken, and PAEs are collected in organic phase after decantation. In the literature, many different extraction solvents, such as dichloromethane, n-hexane, acetone, and 1,1,2-trichlorotrifluoroethane, have been suggested for the extraction of PAEs from beverages, allowing recovery values ranging between 60 and 114% and preconcentration factors from 20 to 1666 (Amiridou and Voutsa 2011; Otero et al. 2015; Zaater et al. 2014; S. Keresztes et al. 2013). For example, Amiridou and Voutsa (2011) extracted PAEs (DMP, DEP, DBP, BBP, DEHP, and di-n-octyl phthalate (DNOP)) from 1 L of bottled water using 150 mL of dicloromethane as extractant and then concentrated in an evaporator under a stream of nitrogen, obtaining recovery percentages from 70 to 94% (Amiridou and Voutsa 2011). A similar LLE method using dicloromethane was developed by Otero et al. (2015) with a higher ratio of solvent: sample (60:200 mL). They reached recovery percentages between 84 and 91% of PAEs (DBEP, DEHP, BBP, DBP, DEP, DHP, DMP, DNOP, and DINP) from bottled water (Otero et al. 2015), which were better than those previously reported by Amiridou and Voutsa (2011).
The LLE method is relatively easy to implement, but has some disadvantages, such as the use of large volumes of toxic organic solvents and formation of emulsions (Net et al. 2015). In addition, large volumes of solvents involve great contamination problems, that is, neither practical nor environmentally friendly (Fan et al. 2014; Sha et al. 2011; Farahani et al. 2008). The LLE method is a time-consuming procedure integrated by multiple stages, rising high levels of PAE concentration in blanks; finally, it is not easy to automate and is very sensitive to operating conditions (Komjarova and Blust 2006; Farajzadeh et al. 2015).
On the other hand, SPE has received the greatest attention due to its simplicity. In SPE, PAEs are transferred from the water sample (200–1000 mL) to a sorbent and are recovered by elution with organic solvent.
Polymeric reversed-phase sorbents like C18 (Salazar-Beltran et al. 2017), poly(divinylbenzene-co-N-vinylpyrrolidone) (Dominguez-Morueco et al. 2014; Bach et al. 2013), or anionic exchange cartridges (G. Zhiyong et al. 2010) have been proved to be efficient for PAEs. DMP, DEP, DBP, BBP, DEHP, and DNOP were extracted from orange juice by SPE using two kinds of anionic exchange cartridges and acetonitrile as eluent, obtaining recovery percentages from 76 to 112%. The authors concluded that the method was particularly effective for the analysis of low-polarity organic compounds such as DEHP and DNOP due to the characteristics of the selected cartridge (G. Zhiyong et al. 2010). Dominguez-Morueco et al. (2014) applied a poly(divinylbenzene-co-N-vinylpyrrolidone) sorbent as the one used by Zhiyong to extract some PAEs (DMP, DEP, DBP, BBP, and DEHP) from water. However, they described the use of non-polar solvents (dichloromethane, hexane, and acetone) as eluents reaching lower recoveries ranging between 77 and 94% (Dominguez-Morueco et al. 2014).
This extraction method has presented several advantages compared with LLE such as better extraction recoveries, less extraction time, less volume of solvents (2–30 mL), more reproducible results can be expected, and capability to more efficiently remove interfering compounds, and use of polar solvents such as acetonitrile or methanol which are less harmful to the environment (G. Zhiyong et al. 2010). Although some authors have reported the use of non-polar solvents such as dichloromethane, hexane, acetone, and ethyl acetate in smaller amounts (2–10 mL) in comparison to volumes used in LLE (Dominguez-Morueco et al. 2014; Bach et al. 2013). Preconcentration factors between 500 and 1800 have been described (Bach et al. 2013; Dominguez-Morueco et al. 2014; G. Zhiyong et al. 2010), which are higher than in LLE procedures.
SPE can also be used online, directly connected to liquid chromatography (LC) allowing its full automation (Salazar-Beltran et al. 2017; Valsecchi et al. 2015). For example, Salazar-Beltran et al. (2017) extracted PAEs (DMP, DEP, and DBP) from drinking bottled water by online SPE using C18 membranes and acetonitrile as eluent, reaching recovery percentages between 80 and 115% (Salazar-Beltran et al. 2017).
LLE and SPE are widely applied in PAE analysis in different environmental matrices. The EPA published the analytical procedure for the determination of certain PAEs in municipal and industrial wastewater, sediments, and soils using LLE and detection by gas chromatography with electron capture detection (GC/ECD) (US-EPA 1996, 2001).
Recently, new microextraction methods have been developed for the extraction of PAEs, based on SPME and LPME. Some of their advantages are not only the use of small sample volumes (microliter range or smaller), but also a simple sample preparation avoiding the secondary contamination risk that may occur during the pretreatment step and also minimal exposure to toxic organic solvents by the operator, and all the extracted analytes are transferred to the analytical instrument. Nevertheless, these are non-exhaustive procedures. They also provide lack of robustness and poor reproducibility, obtaining relative standard deviation (RSD) values between 0.1 and 28% and preconcentration factors from 5 to 1500 (Farajzadeh et al. 2015).
Xu et al. (2007) applied LPME to extract PAEs (DMP, DEP, and DBP) contained in mineral water. They use n-hexane as solvent, obtaining recovery percentages from 95 to 97% (Xu et al. 2007).
DMP and DEP were extracted from energy drinks using hollow-fiber membrane liquid-phase microextraction (HF-LPME) by Yamini et al. (2015). The target analytes were extracted online and eluted inside the lumen of the HF membrane using n-dodecane as extraction solvent and acetonitrile as acceptor solvent. The recovery percentages reached were between 90 and 92%.
Psillakis and Kalogerakis (2003) applied dynamic SPME to extract some PAEs (DEP, DBP, and DEHP) from mineral water reaching RSD values from 4 to 11%. They use a polydimethylsiloxane/divinylbenzene fiber during 20 min (Psillakis and Kalogerakis 2003). In the same way Banitaba et al. (2013) applied dynamic SPME during 20 min. Nevertheless, they use a poly(3,4-ethylenedioxythiophene)-TiO2 fiber, obtaining recoveries from 86 to 107% and similar RSD values, from 6 to 11% (Banitaba et al. 2013).
Information of PAE analysis in beverages is very limited. The available results are shown in Table 2. It can be due to the challenges in detections or high blank levels caused as the result of laboratory contamination.
The ubiquitous presence of PAEs as a contaminant in laboratory plastic wares, reagents, and sample preparation devices is a potential problem for their quantitative determination. Thus, these major drawbacks during sample preparation cause high blank levels increasing its limits of detection. To avoid PAE contamination, all glassware used should be washed with organic solvents and ultrapure water prior to use. Additionally, the contact of reagents and solutions with plastic ware must be minimized (Ustun et al. 2014; S. Keresztes et al. 2013; Shen 2005; Zia et al. 2013).
Extraction of PAEs from Polymer Materials
The extraction is the crucial step to analyze plasticizers in polymers before their analysis (Gawlik-Jędrysiak 2013). During this stage, PAEs must be separated from the polymer and isolated from other plasticizers to minimize interferences. Several approaches for extracting these organic compounds from plastics have been developed, including Soxhlet extraction (Gawlik-Jędrysiak 2013; Bonini et al. 2008; Bernard et al. 2015) and ultrasound-assisted extraction (UAE) (Fierens et al. 2012; Ni et al. 2016; Gawlik-Jędrysiak 2013; Li et al. 2004; Shen 2005; Otero et al. 2015), which are the most commonly used methods (Cano et al. 2002). Some applications of PAE extraction from plastics are reviewed and summarized in Table 3.
Soxhlet extraction is a traditional method to extract PAEs from solid samples. It is simple in operation and requires minimal training. This procedure can extract more sample mass than most of the other extraction procedures. However, the major disadvantages compared to other procedures are that it requires long extraction times (4–16 h) and large amount of solvents is wasted (100–500 mL), which is not only expensive, but also unfriendly to the environment. Soxhlet extraction is limited by the extractant, because it does not have any type of agitation, so the contact between the matrix and solvent is deficient. Due to the non-polar nature of PAEs, solvents such as dichloromethane, ethyl acetate, and n-hexane have been commonly described for the extraction of these compounds (Luque de Castro and Priego-Capote 2010; Punin Crespo and Lage Yusty 2005; Gawlik-Jędrysiak 2013; Bonini et al. 2008; Kim et al. 2016). Gawlik-Jędrysiak (2013) reported the extraction of DEHP from 1 g of PVC using a Soxhlet apparatus with 100 mL of dichloromethane as solvent. The extraction time was 16 h reaching a recovery percentage of 94% (Gawlik-Jędrysiak 2013). Bernard et al. (2015) also applied Soxhlet to extract DEHP in a smaller amount of PVC (0.1 g), during a greater extraction time (24 h) using 250 mL of ethyl acetate. The recovery percentage obtained was 96% (Bernard et al. 2015).
Nonetheless, other extraction methods have been developed, not only to reduce the use of solvents and extraction times, but also to improve recovery percentages (Marin et al. 1998; Sporring et al. 2005; Punin Crespo and Lage Yusty 2005). UAE is a quick (10–60 min) and efficient sample preparation procedure for plastic materials with recoveries ranging between 47 and 118%. This method uses high frequency to produce vapor bubbles in the liquid and undergo implosive collapse after reaching a specific pressure. It results in a quick increasing in temperature and pressure and causing better penetration of the solvent into the solid matrix. Ultrasounds produce a reactive medium, which attack the sample by passing the analytes from the solid phase to the solvent (Luque-Garcia and de Luque 2003). UAE is a fast and profitable method, due to that it provides an efficient contact between the sample and the solvent. Additionally, it is economic, requires low solvent consumption (4–50 mL), and has low instrumental requirements. The UAE has been applied to a great variety of plastics such as PVC (Gawlik-Jędrysiak 2013; Dong et al. 2013), PET (Li et al. 2004; Otero et al. 2015) and polystyrene (Shen 2005). However, this procedure has two main drawbacks: low reproducibility and repeatability, due to the lack of uniformity of ultrasound energy, and lots of the energy supplied to the bath is wasted (Li et al. 2004; Luque-Garcia and de Luque 2003). Large diversity of organic solvents such as n-hexane, dichloromethane, and methanol has been reported as efficient for PAE extraction from plastics by UAE (Net et al. 2015). For example, Fierens et al. (2012) extracted some PAEs (DMP, DEP, DBP, BBP, and DEHP) from milk bags by UAE. They use 40 mL of n-hexane during 60 min, obtaining recovery percentages from 82 to 99% (Fierens et al. 2012). Gawlik-Jędrysiak (2013) applied UAE to extract DEHP from granulated PVC. The use of smaller solvent volumes (10 mL of methanol) during 15 min was reported in this study allowing a poor recovery percentage (21%) (Gawlik-Jędrysiak 2013).
Chromatographic Techniques for PAE Determination
Several chromatographic methods have been reported for the determination of various PAE esters in beverages and polymers using LC coupled to different detectors such as mass spectrometer (MS), diode array detector (DAD), and UV detector. Also, gas chromatography (GC) with MS and flame ionization detector (FID) has been reported. The chromatographic conditions are summarized in Table 4. Although GC-MS has been the detection technique proposed by the EPA for PAE determination in municipal and industrial wastewater, sediments, and soils (Method 606) (US-EPA 2001), an alternative technique to GC for PAE determination is LC due to its inherent ability to separate these compounds (Cano et al. 2002; Chang et al. 2015; Li et al. 2004; Ranjbari and Hadjmohammadi 2012; Xu et al. 2007; Farahani et al. 2008; Zaater et al. 2014).
The GC has been commonly carried out using non-polar gas chromatographic columns and He as mobile phase. The most common detector used has been MS. It measures the mass-to-charge ratio of the ions produced by the sample. GC-MS has many advantages such as short analysis times, providing high resolution, and sensitivity (Otero et al. 2015; S. Keresztes et al. 2013; Ustun et al. 2014; Farahani et al. 2008). However, this technique has also disadvantages with respect to sample characteristics: the analysis cost is relatively high and this is a destructive technique (Ni et al. 2016).
Keresztes et al. (2013) determined some PAEs (DBP, BBP, and DEHP) in non-carbonated mineral water by GC-MS. They reached limits of quantification (LOQs) between 0.1 and 1.7 μg/L (S. Keresztes et al. 2013). A similar method was proposed by Ustun et al. (2014). They analyzed some PAEs (DMP, DEP, DBP, BBP, DEHP, and DNOP) in bottled lemonade by GC-MS using a fused silica capillary column. The LOQs reached were greater to those published by Keresztes (6 and 21 μg/L) (Ustun et al. 2014).
Reversed-phase LC has been described as an alternative technique to GC for PAE determination (Gao et al. 2014b). Hydrophobic stationary phase bound to a silica support (C18, C8) and mixture of polar solvents (methanol, acetonitrile, or water) as mobile phase have been described in the analysis of different types of beverages. The advantages of LC are that dissolved analytes can be easily recovered and can be fully automated as well as being easy to operate. However, disadvantages of LC are that typically, it has a lower efficiency than GC, can occur a co-elution when compounds being separated are nearly identical in chemical form and functionality, and suffers from high solvent consumption (Jia et al. 2014; Liu et al. 2012). The LCs using UV or DAD detectors are more affordable techniques that ensure good performance. LC also can be coupled to mass spectrometry (LC-MS/MS); this is an advantageous alternative compared to the GC-MS, due to that the sample preparation is easier and no derivatization step is required (Khedr 2013).
Zaater et al. (2014) determined PAEs (DMP, DEP, DBP, BBP, DEHP, and DNOP) in mineral water by LC-UV. They use a C8 column and a mixture of aqueous acetonitrile containing 1% methanol as mobile phase with gradient elution. The limits of detection (LODs) reached were between 0.12 and 0.50 μg/L (Zaater et al. 2014). Fan et al. (2014) applied also LC with DAD to determine some PAEs (DBP, BBP, DEHP) in red wine. They used a C18 column and a mixture of methanol/water as mobile phase with gradient elution allowing LODs from 2.0 to 2.2 μg/L (Fan et al. 2014). Additionally, an analysis of DEHP was done in carbonated cola by LC-MS/MS, using a XDB-C8 column and a mixture of water/acetic acid (99.5:0.5, v/v)/methanol/water (90:10, v/v) as mobile phase with gradient elution. The LOD allowed was 0.013 μg/L (Khedr 2013).
As can be seen in Table 4, the LC-MS methods showed comparable LODs than those performed by GC-MS reaching values between 0.12 and 13 μg/L for LC and between 0.02 and 52 μg/L for GC.
Non-chromatographic Techniques for PAE Determination
Analytical methods that use LC and GC have been commonly reported for PAE determination in beverages (Li et al. 2015; Qiu et al. 2013). However, these methods have the disadvantages of high blank values and high cost of instrumentation (Zhang et al. 2013; Sun and Zhuang 2015). Therefore, it is very important to develop simple and rapid methods to detect PAEs (Zhang et al. 2006; Chen et al. 2014).
Molecular imprinting technology is a newly developed technology, which has become a powerful tool for the preparation of polymeric materials showing highly specific recognition performance toward the template molecule (Zhang et al. 2013; Yongfeng et al. 2012; Li et al. 2015). For example, Zhang et al. (2013) developed a magnetic molecularly imprinted polymer (MMIP) sensor combined with magnetic molecularly imprinted solid-phase extraction (MMISPE) for the determination of DBP in soybean milk and milk samples. Although DBP was not detected in milk samples, the recovery results were between 98 and 102%. MMISPE coupled with MMIP sensing system showed good reproducibility (2.2–2.5% RSD) and satisfactory stability. The LOD of the MMIP sensor coupled with the MMIP was 0.052 ng/L (Zhang et al. 2013). In the same way, Li et al. (2015) synthesized molecular imprinted polymers (MIP) using magnetic graphene oxide and gold nanoparticles and applied as a molecular recognition element to construct DBP electrochemical sensor. The DBP electrochemical sensor showed a LOD of 222.6 ng/L, which is greater than that published by Zhang et al. (2006), exhibiting excellent repeatability (RSD, 2.5%). The applicability of the sensor was demonstrated by the analysis of DBP in wine drinks reaching recovery percentages between 97 and 104% (Li et al. 2015).
In the other hand, immunoassay-based techniques have been developed for the determination of these kinds of plasticizers. The advantages of immunochemical techniques include their low cost, speed of analysis, ease of use, and portability. For example, Zhang et al. (2006) developed a fluorescence immunoassay for the quantitative determination of DBP in water samples. They used an antibody-coated plate format. Each plate was read using an automatic detection microplate reader at λ excitation = 485 nm and λ emission = 528 nm. The assay had a LOD of 20 ng/L. Other similar PAE compounds do not interfere significantly in the analysis using this technique (<10%). The method was applied to analyze tap water, river water, and leachate from plastic drinking water bottles reaching recovery percentages between 91 and 109% (Zhang et al. 2006). Sun and Zhuang (2015) established a biotin-streptavidin enzyme-linked immunosorbent assay (BA-ELISA) using a rabbit polyclonal anti-DBP antibody (pAb-DBP) for the determination of DBP in beverages and drinking water. The LOD was 5 ng/L and the BA-ELISA was highly selective showing lower cross-reactivity values with DBP analogues (<4%). Satisfactory recoveries were obtained in the analysis of real samples (89.5 to 109.5%) with variation coefficient values (6.0 to 8.7%). The concentrations of DBP in beverages and drinking water by this method ranged from 0.45 to 7.06 μg/L (Sun and Zhuang 2015).
The inherent advantages of MIP compared to immunoassay-based techniques include robustness and storage endurance. However, MIP exhibits certain drawbacks, such as complicated preparation process that takes long time, low binding capacity, and poor site accessibility (Zhang et al. 2013; Yongfeng et al. 2012; Li et al. 2015; Zhang et al. 2006; Sun and Zhuang 2015).
Non-chromatographic techniques have shown potential application in the determination of PAEs in beverages; however, these techniques are in development. Therefore, chromatographic techniques show greater advantages such as selectivity and sensibility. Additionally, multiple compounds can be analyzed.
Migration of PAEs from Polymers into Beverages
Since PAEs are physically rather than chemically incorporated into polymeric matrix, these compounds can easily migrate from plastic packaging to beverages and subsequently ingested by humans (S. Keresztes et al. 2013; Ustun et al. 2014; G. Zhiyong et al. 2010). Physicochemical factors such as temperature, pressure, presence of solvents, and radiation could affect the migration rate of PAEs (Serodio and Nogueira 2006; Ni et al. 2016), whereby several methods have been proposed for determining the migration degree of these plasticizers (Jeddi et al. 2015; S. Keresztes et al. 2013; Fasano et al. 2012; Xu et al. 2010; Ustun et al. 2014). Migration studies are commonly conducted with food simulants, providing uniform contact of the packaging with the food.
Additionally, the EU 82/711/EEC and 85/572/EEC directives describe the basic rules necessary for testing migration of the constituents of plastic materials and articles intended to come into contact with foodstuffs, specifying the use of simulants, the contact time, and temperature of exposure. These regulations also establish that allowable leaching of all plasticizers of plastic material entering in contact with food cannot exceed 10 mg per dm2 surface area of the packaging material (Union E 1985, 1982).
Fasano et al. (2012) determined the potential migration of PAEs and adipates from wide range of food packaging materials including plastic wine tops. For this study, samples (31 cm2) were introduced in 100 mL of 15% (v/v) ethanol and incubated at 40 °C for 10 days. The plastic wine tops showed the highest level of migration of PAEs (DMP, DBP, BBP, DEHP) in concentrations ranging from 0.2 to 14.1 μg/L. The authors concluded that the main factor affecting the migration rate of these plasticizers was the use of specific simulants depending on the food product (Fasano et al. 2012).
Keresztes et al. (2013) reported the migration of PAEs (DBP, BBP, and DEHP) from PET bottles at concentrations between 0.1 and 1.2 μg/L. They evaluate the migration rate from PET containers into water when they were stored at 22 °C during 1283 days. The authors concluded that factors such as pH and temperature affect the migration rate (S. Keresztes et al. 2013). In another study, a migration test from plastic containers to mineral water was performed under different storage temperatures, contact times, and storage states (static and dynamic state). The authors concluded that the migration rate of PAEs into beverages depended on not only to the lipophilic characteristic of the beverages, but also to the molecular structure of the PAEs, and it was more significant at higher temperature, longer contact time, and higher dynamic frequency. The concentrations of migrated PAEs (DMP, DEP, DBP, DEHP, BBP, DNOP) from containers to water samples stored for 2 months at 20 °C were between 7.5 and 28 μg/L (Xu et al. 2010). Jeddi et al. (2015) performed a migration test on 500-mL PET bottles, stored at 40 °C during 50 days, finding concentrations from 0.125 to 1.25 μg/L for the analyzed PAEs (DBP, DEHP, and BBP). They concluded that the migration rate was mainly affected by the temperature (Jeddi et al. 2015).
Conclusions and Remarks
In the last decade, several analytical methods for PAE quantification in beverages and plastics have been proposed; in this review, the main stages required to quantify these species, emphasizing the treatment procedures for the extraction of PAEs from beverages and polymers and their determination, are summarized. Studies concerning the determination of PAEs in these matrices addressed several problems during their analysis associated to high blank levels because of the ubiquitous nature of these compounds.
In general, PAEs can be determined in beverages by common analytical techniques such as GC-MS, LC-UV, LC-DAD, and LC-MS prior to their extraction (LLE, SPE, SPME, USE, Soxhlet, SFE). Some of these chromatographic methods can be easily developed and implemented. However, more environmentally friendly analytical methods reducing not only the consumption of solvents and reagents, but also analysis times and costs are preferred. Recently, non-chromatographic techniques based on MIP and immunoassay-based techniques have also been described for PAE analysis in beverages. However, these methods present multiple weaknesses such as poor selectivity, long time, and complicated preparation process.
Many methods for extraction and analysis can be applied to the quantification of PAEs, but the selected procedure depends on the capabilities of each laboratory. Thus, (a) the development of automated sample preparation procedures that reduce not only contamination blank level during analysis but also the analysis time and consumption of sample and reagents, as well as minimize the interaction of the analyst with the samples, and (b) the application of microwave extraction procedures for PAEs in polymeric materials that could reduce not only the extraction time but also the volume used of harmless solvent constitute challenging tasks for the analysis of PAEs in beverages and plastic polymers, respectively.
Since it has been proved that PAEs could easily migrate from polymers as PET and PVC into beverages, it is necessary to apply standardized method for determining their migration degree as described by EU. As a consequence, it is also necessary to establish regulations regarding concentrations of PAEs in drinks and in the polymers used for the production of containers and allowable migration level in order to evaluate the potential risk to human health and environment.
References
Álvarez MS, Esperança JM, Deive FJ, Sanromán MA, Rodríguez A (2015) A biocompatible stepping stone for the removal of emerging contaminants. Sep Purif Technol 153:91–98
Amiridou D, Voutsa D (2011) Alkylphenols and phthalates in bottled waters. J Hazard Mater 185:281–287
Bach C, Dauchy X, Severin I, Munoz JF, Etienne S, Chagnon MC (2013) Effect of temperature on the release of intentionally and non-intentionally added substances from polyethylene terephthalate (PET) bottles into water: chemical analysis and potential toxicity. Food Chem 139:672–680
Banitaba MH, Davarani SS, Pourahadi A (2013) Solid-phase microextraction of phthalate esters from aqueous media by electrophoretically deposited TiO2 nanoparticles on a stainless steel fiber. J Chromatogr A 1283:1–8
Bernard L, Cueff R, Bourdeaux D, Breysse C, Sautou V (2015) Analysis of plasticizers in poly(vinyl chloride) medical devices for infusion and artificial nutrition: comparison and optimization of the extraction procedures, a pre-migration test step. Anal Bioanal Chem 407:1651–1659
Bonini M, Errani E, Zerbinati G, Ferri E, Girotti S (2008) Extraction and gas chromatographic evaluation of plasticizers content in food packaging films. Microchem J 90:31–36
Cano JM, Marın ML, Sanchez A, Hernandis V (2002) Determination of adipate plasticizers in poly(vinyl chloride) by microwave-assisted extraction. J Chromatogr 963:401–409
Chang L, Bi P, Li X, Wei Y (2015) Study of solvent sublation for concentration of trace phthalate esters in plastic beverage packaging and analysis by gas chromatography-mass spectrometry. Food Chem 177:127–133
Chen G, Hu H, Wu T, Tong P, Liu B, Zhu B, Du Y (2014) Rapid and sensitive determination of plasticizer diethylhexyl phthalate in drink by diffuse reflectance UV spectroscopy coupled with membrane filtration. Food Control 35:218–222
Dominguez-Morueco N, Gonzalez-Alonso S, Valcarcel Y (2014) Phthalate occurrence in rivers and tap water from central Spain. Sci Total Environ 500-501:139–146
Dong C, Liu Y, Yang W, Sun X, Wang G (2013) Simultaneous determination of phthalate plasticizers in PVC packaging materials using homogeneous-ultrasonic extraction-GC-MS assisted with continuous wavelet transform. Anal Methods 5:4513–4517
Fan Y, Liu S, Xie Q (2014) Rapid determination of phthalate esters in alcoholic beverages by conventional ionic liquid dispersive liquid-liquid microextraction coupled with high performance liquid chromatography. Talanta 119:291–298
Farahani H, Ganjali MR, Dinarvand R, Norouzi P (2008) Screening method for phthalate esters in water using liquid-phase microextraction based on the solidification of a floating organic microdrop combined with gas chromatography-mass spectrometry. Talanta 76:718–723
Farajzadeh MA, Sorouraddin SM, Afshar-Mogaddam MR (2015) Microextraction methods for the determination of phthalate esters in liquid samples: a review. J Sep Sci 38:2470–2557
Fasano E, Bono-Blay F, Cirillo T, Montuori P, Lacorte S (2012) Migration of phthalates, alkylphenols, bisphenol A and di(2-ethylhexyl)adipate from food packaging. Food Control 27:132–138
Fierens T, Servaes K, Van HM, Geerts L, De HS, Sioen I, Vanermen G (2012) Analysis of phthalates in food products and packaging materials sold on the Belgian market. Food Chem Toxicol 50:2575–2583
Fromme H, Gruber L, Schlummer M, Wolz G, Bohmer S, Angerer J, Mayer R, Liebl B, Bolte G (2007) Intake of phthalates and di(2-ethylhexyl)adipate: results of the integrated exposure assessment survey based on duplicate diet samples and biomonitoring data. Environ Int 33:1012–1020
Gao D, Li Z, Wen Z, Ren N (2014a) Occurrence and fate of phthalate esters in full-scale domestic wastewater treatment plants and their impact on receiving waters along the Songhua river in China. Chemosphere 95:24–32
Gao X, Yang B, Tang Z, Luo X, Wang F, Xu H, Cai X (2014b) Determination of phthalates released from paper packaging materials by solid-phase extraction-high-performance liquid chromatography. J Chromatogr Sci 52:383–392
Gao Y, An T, Ji Y, Li G, Zhao C (2015) Eco-toxicity and human estrogenic exposure risks from OH-initiated photochemical transformation of four phthalates in water: a computational study. Environ Pollut 206:510–517
Gawlik-Jędrysiak M (2013) Determination of phthalate esters content in plastic articles: comparison of extraction methods. J Anal Chem 68:959–960
Graham PR (1973) Phthalate ester plasticizers-why and how they are used. Environ Health Persp 3–12
Guo Y, Wu Q, Kannan K (2011) Phthalate metabolites in urine from China, and implications for human exposures. Environ Int 37:893–898
Hammad Khan M, Jung JY (2008) Ozonation catalyzed by homogeneous and heterogeneous catalysts for degradation of DEHP in aqueous phase. Chemosphere 72:690–696
Heudorf U, Mersch-Sundermann V, Angerer J (2007) Phthalates: toxicology and exposure. Int J Hyg Environ Health 210:623–634
Jeddi M, Rastkari N, Ahmadkhaniha R, Yunesian M (2015) Concentrations of phthalates in bottled water under common storage conditions: do they pose a health risk to children? Food Res Int 69:256–265
Jia W, Chu X, Ling Y, Huang J, Chang J (2014) Analysis of phthalates in milk and milk products by liquid chromatography coupled to quadrupole orbitrap high-resolution mass spectrometry. J Chromatogr A 1362:110–118
Jianlong W, Xuan Z, Weizhong W (2004) Biodegradation of phthalic acid esters (PAEs) in soil bioaugmented with acclimated activated sludge. Process Biochem 39:1837–1841
Keresztes S, Tatar E, Czegeny Z, Zaray G, Mihucz VG (2013) Study on the leaching of phthalates from polyethylene terephthalate bottles into mineral water. Sci Total Environ 458-460:451–458
Khedr A (2013) Optimized extraction method for LC-MS determination of bisphenol A, melamine and di(2-ethylhexyl) phthalate in selected soft drinks, syringes, and milk powder. J Chromatogr B Analyt Technol Biomed Life Sci 930:98–103
Khosravi K, Price GW (2015) Determination of phthalates in soils and biosolids using accelerated solvent extraction coupled with SPE cleanup and GC–MS quantification. Microchem J 121:205–212
Kim JW, Kim YM, Moon HM, Hosaka A, Watanabe C, Teramae N, Choe EK, Myung SW (2016) Comparative study of thermal desorption and solvent extraction-gas chromatography–mass spectrometric analysis for the quantification of phthalates in polymers. J Chromatogr A 1451:33–40
Komjarova I, Blust R (2006) Comparison of liquid-liquid extraction, solid-phase extraction and co-precipitation preconcentration methods for the determination of cadmium, copper, nickel, lead and zinc in seawater. Anal Chim Acta 576:221–229
Leitz J, Kuballa T, Rehm J, Lachenmeier D (2009) Chemical analysis and risk assessment of diethyl phthalate in alcoholic beverages with special regard to unrecorded alcohol. PLoS One 4:1–7
Li X, Zeng Z, Chen Y, Xu Y (2004) Determination of phthalate acid esters plasticizers in plastic by ultrasonic solvent extraction combined with solid-phase microextraction using calix[4]arene fiber. Talanta 63:1013–1022
Li J, Su Q, Li KY, Sun CF, Zhang WB (2013) Rapid analysis of phthalates in beverage and alcoholic samples by multi-walled carbon nanotubes/silica reinforced hollow fibre-solid phase microextraction. Food Chem 141:3714–3720
Li X, Wang X, Li L, Duan H, Luo C (2015) Electrochemical sensor based on magnetic graphene oxide@gold nanoparticles-molecular imprinted polymers for determination of dibutyl phthalate. Talanta 131:354–360
Liou SH, Yang GC, Wang CL, Chiu YH (2014) Monitoring of PAEMs and beta-agonists in urine for a small group of experimental subjects and PAEs and beta-agonists in drinking water consumed by the same subjects. J Hazard Mater 277:169–179
Liu X, Feng W, Bao C, Jia Q (2012) Determination of carbamate pesticides and phthalates in vegetables by a cloud point extraction process using tergitol 15-s-7 and high performance liquid chromatography. Anal Lett 45:2663–2674
Liu X, Sun Z, Chen G, Zhang W, Cai Y, Kong R, Wang X, Suo Y, You J (2015) Determination of phthalate esters in environmental water by magnetic zeolitic imidazolate framework-8 solid-phase extraction coupled with high-performance liquid chromatography. J Chromatogr A 1409:46–52
Luque de Castro MD, Priego-Capote F (2010) Soxhlet extraction: past and present panacea. J Chromatogr A 1217:2383–2392
Luque-Garcia JL, Luque de Castro MD (2003) Ultrasound: a powerful tool for leaching. Trac Trend Anal Chem 22:41–47
Magdouli S, Daghrir R, Brar SK, Drogui P, Tyagi RD (2013) Di 2-ethylhexylphtalate in the aquatic and terrestrial environment: a critical review. J Environ Manag 127:36–49
Marin ML, Jiménez A, Berenguer V, López J (1998) Optimization of variables on the supercritical fluid extraction of phthalate plasticizers. J Supercrit Fluids 12:271–277
Mihucz VG, Záray G (2015) Occurrence of antimony and phthalate esters in polyethylene terephthalate bottled drinking water. Appl Spectrosc Rev 51:183–209
Net S, Delmont A, Sempere R, Paluselli A, Ouddane B (2015) Reliable quantification of phthalates in environmental matrices (air, water, sludge, sediment and soil): a review. Sci Total Environ 515-516:162–180
Ni X, Xing X, Cao Y, Cao G (2016) Determination of phthalates in food packing materials by electrokinetic chromatography with polymeric pseudostationary phase. Food Chem 190:386–391
Otero P, Saha SK, Moane S, Barron J, Clancy G, Murray P (2015) Improved method for rapid detection of phthalates in bottled water by gas chromatography-mass spectrometry. J Chromatogr B 997:229–235
Peijnenburg WJ, Struijs J (2006) Occurrence of phthalate esters in the environment of the Netherlands. Ecotoxicol Environ Saf 63:204–215
Pérez-Feás C, Barciela-Alonso MC, Bermejo-Barrera P (2011) Presence of phthalates in contact lens and cleaning solutions. Microchem J 99:108–113
Psillakis E, Kalogerakis N (2003) Hollow-fibre liquid-phase microextraction of phthalate esters from water. J Chromatogr A 999:145–153
Punin Crespo MO, Lage Yusty MA (2005) Comparison of supercritical fluid extraction and Soxhlet extraction for the determination of PCBs in seaweed samples. Chemosphere 59:1407–1420
Qiu H, Fan L, Li X, Li L, Sun M, Luo C (2013) A microflow chemiluminescence sensor for indirect determination of dibutyl phthalate by hydrolyzing based on biological recognition materials. J Pharm Biomed Anal 75:123–132
Rahman M, Brazel C (2004) The plasticizer market: an assessment of traditional plasticizers and research trends to meet new challenges. Prog Polym Sci 29:1223–1248
Ranjbari E, Hadjmohammadi MR (2012) Magnetic stirring-assisted dispersive liquid-liquid microextraction followed by high performance liquid chromatography for determination of phthalate esters in drinking and environmental water samples. Talanta 100:447–453
Sailas B, Selvanesan P, Moolakkariyil SJ, Sunil K, Eiji M (2015) A monograph on the remediation of hazardous phthalates. J Hazard Mater 298:58–72
Sakhi AK, Lillegaard IT, Voorspoels S, Carlsen MH, Loken EB, Brantsaeter AL, Haugen M, Meltzer HM, Thomsen C (2014) Concentrations of phthalates and bisphenol A in Norwegian foods and beverages and estimated dietary exposure in adults. Environ Int 73:259–269
Salazar-Beltran D, Hinojosa-Reyes L, Ruiz-Ruiz E, Hernandez-Ramirez A, Luis Guzman-Mar J (2017) Determination of phthalates in bottled water by automated on-line solid phase extraction coupled to liquid chromatography with UV detection. Talanta 168:291–297
Serodio P, Nogueira JM (2006) Considerations on ultra-trace analysis of phthalates in drinking water. Water Res 40:2572–2582
Sha C, Yi-Sheng Z, Shui-Yuan C, Tian Q, Hao S (2011) Development of an ionic liquid-based dispersive liquid-liquid micro-extraction method for the determination of phthalate esters in water samples. J Sep Sci 34:1503–1510
Shen HY (2005) Simultaneous screening and determination eight phthalates in plastic products for food use by sonication-assisted extraction/GC-MS methods. Talanta 66:734–739
Silva MJ, Slakman AR, Reidy JA, Preau JL, Herbert AR, Samandar E, Needham LL, Calafat AM (2004) Analysis of human urine for fifteen phthalate metabolites using automated solid-phase extraction. J Chromatogr B 805:161–167
Sporring S, Bøwadt S, Svensmark B, Björklund E (2005) Comprehensive comparison of classic Soxhlet extraction with Soxtec extraction, ultrasonication extraction, supercritical fluid extraction, microwave assisted extraction and accelerated solvent extraction for the determination of polychlorinated biphenyls in soil. J Chromatogr A 1090:1–9
Sun R, Zhuang H (2015) Biotin-streptavidin enzyme-linked immunosorbent assay for the determination of dibutyl phthalate in beverages and drinking water using a specific polyclonal antibody. Food Anal Methods 8:1990–1999
Sun H, Yang Y, Li H, Zhang J, Sun N (2012) Development of multiresidue analysis for twenty phthalate esters in edible vegetable oils by microwave-assisted extraction-gel permeation chromatography-solid phase extraction-gas chromatography-tandem mass spectrometry. J Agric Food Chem 60:5532–5539
Sun M, Tang R, Wu Q, Wang C, Wang Z (2013) Graphene reinforced hollow fiber liquid-phase microextraction for the determination of phthalates in water, juice and milk samples by HPLC. Anal Methods 5:5694–5700
Union E (1982) Council Directive 82/711/EEC laying down the basic rules necessary for testing migration of the constituents of plastic materials and articles intended to come into contact with foodstuffs. In: Community EE (ed). p^pp 1–10, Official Journal of the European Union
Union E (1985) Council Directive 85/572/EEC laying down the list of simulants to be used for testing migration of constituents of plastic materials and articles intended to come into contact with foodstuffs. In: Community EE (ed). p^pp 14–21, Official Journal of the European Union
US-EPA (2001) Methods for Organic Chemical Analysis of Municipal and Industrial Wastewater, Method 606: Phthalate Esters. Code of Federal Regulations 40 CFR 141.136 (Appendix A), Washington, DC.
US-EPA (1996) Method 8061a: phthalate esters by gas chromatography with electron capture detection (GC/ECD). In. p^pp 1–18
Ustun I, Sungur S, Okur R, Sumbul A, Oktar S, Yilmaz N, Gokce C (2014) Determination of phthalates migrating from plastic containers into beverages. Food Anal Methods 8:222–228
Valsecchi S, Polesello S, Mazzoni M, Rusconi M, Petrovic M (2015) On-line sample extraction and purification for the LC–MS determination of emerging contaminants in environmental samples. Trends Environ Analy Chem 8:27–37
Wilkes C, Summers J, Daniels C (2005) Plasticizers. In: HANSER (ed) PVC handbook, p^pp 172–193
Wu X, Hong H, Liu X, Guan W, Meng L, Ye Y, Ma Y (2013) Graphene-dispersive solid-phase extraction of phthalate acid esters from environmental water. Sci Total Environ 444:224–230
Wypych G (2012) Plasticizers use and selection for specific polymers. In: Handbook of plasticizers. vol 11, Second edn. ChemTec Publishing, Toronto, p^pp 273–379
Xu J, Liang P, Zhang T (2007) Dynamic liquid-phase microextraction of three phthalate esters from water samples and determination by gas chromatography. Anal Chim Acta 597:1–5
Xu Q, Yin X, Wang M, Wang H, Zhang N, Shen Y, Xu S, Zhang L, Gu Z (2010) Analysis of phthalate migration from plastic containers to packaged cooking oil and mineral water. J Agric Food Chem 58:11311–11318
Yamini Y, Esrafili A, Ghambarian M (2015) Online injection-based hollow fiber liquid-phase microextraction–high-performance liquid chromatography as a fully automatic sample processing for phthalate esters analysis. Food Anal Methods 9(3):729–737
Yang J, Li Y, Wang Y, Ruan J, Zhang J, Sun C (2015) Recent advances in analysis of phthalate esters in foods. Trac-Trend Anal Chem. 72:10–26
Yongfeng K, Wuping D, Yan L, Junxia K & Jing X (2012) Molecularly imprinted polymers of allyl-β-cyclodextrin and methacrylic acid for the solid-phase extraction of phthalate. Carbohyd Polym 459–464
Zaater MF, Tahboub YR, Al Sayyed AN (2014) Determination of phthalates in Jordanian bottled water using GC-MS and HPLC-UV: environmental study. J Chromatogr Sci 52:447–452
Zhang MC, Wang QE, Zhuang HS (2006) A novel competitive fluorescence immunoassay for the determination of dibutyl phthalate. Anal Bioanal Chem 386:1401–1407
Zhang Z, Luo L, Cai R, Chen H (2013) A sensitive and selective molecularly imprinted sensor combined with magnetic molecularly imprinted solid phase extraction for determination of dibutyl phthalate. Biosens Bioelectron 49:367–373
Zhao HM, Du H, Xiang L, Chen YL, Lu LA, Li YW, Li H, Cai QY, Mo CH (2015) Variations in phthalate ester (PAE) accumulation and their formation mechanism in Chinese flowering cabbage (Brassica parachinensis L.) cultivars grown on PAE-contaminated soils. Environ Pollut 206:95–103
Zhiyong G, Danyi W, Meili W, Sui W (2010) Determination of six phthalic acid esters in orange juice packaged by PVC bottle using SPE and HPLC–UV: application to the migration study. J Chromatogr Sci 48:760–765
Zia AI, Abdul Rahman MS, Mukhopadhyay SC, Yu P, Al-Bahadly IH, Gooneratne CP, Kosel J, Liao T (2013) Technique for rapid detection of phthalates in water and beverages. J Food Eng 116:515–523
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The financial support from the Facultad de Ciencias Químicas of the Universidad Autónoma de Nuevo León and the National Council of Science and Technology of México (CONACyT Ciencia Básica, project number 177990) is hereby acknowledged. D. Salazar-Beltrán is grateful for his PhD scholarship granted by CONACyT.
Conflict of Interest
The authors declare no conflict of interest.
Ethical Approval
This article does not contain any studies with human or animal subjects.
Rights and permissions
About this article
Cite this article
Salazar-Beltrán, D., Hinojosa-Reyes, L., Ruiz-Ruiz, E. et al. Phthalates in Beverages and Plastic Bottles: Sample Preparation and Determination. Food Anal. Methods 11, 48–61 (2018). https://doi.org/10.1007/s12161-017-0961-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-017-0961-8