Abstract
Human exposure to plasticizers is widespread because these compounds are commonly found in the environment. Nevertheless, studies on human dietary exposure to plasticizers are limited. This research deals with the evaluation of 27 plasticizer residues in tea infusion samples brewed with and without bag. Levels were determined by solid phase extraction–gas chromatography–mass spectrometry (SPE–GC–MS). Only a small number of plasticizer were detected: di(2-ethylhexyl) phthalate (DEHP), di-n-butyl phthalate (DBP) and DEP were found in all analyzed samples, while di-methyl phthalate (DMP), bis-(2-ethylhexyl) adipate (DEHA) and DiNP in 80, 70 and 10 % of them. Flavored teas showed higher DMP, DEHA and DEHP residues, probably due to the use of essential oils where plasticizers are accumulated. In addition, decaffeinated black tea showed DBP levels 18 times higher compared with the average of the other samples. The plasticizer contents in samples infused with bag was almost always higher than those infused without bag. The evaluation of contribution of infusion teas to phthalate exposure showed that these do not constitute a risk for the consumers.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The tea plant, member of Camellia sinensis, Camellia assamica and Cambodiensis, is a perennial evergreen from China. Tea plants grow in tropical and subtropical areas with adequate rainfall, acid soils and good drainage. The economic and social interest in tea is easily understood from the fact that its infusion is the most frequently consumed beverage next to water, with about 18–20 billion cups consumed daily [1]. Tea is made from the processed leaves of the plant, and it is distinguished into four types (black tea, green tea, oolong tea and brick tea) based on their respective manufacturing methods. Black tea is fermented; green tea is dried and roasted but not fermented; oolong tea is the result of a partial fermentation; and brick tea is obtained from dry whole leaves pressed into molds. The chemical composition of tea is very complex. It consists of proteins (20 % dry weight), amino acids (1–4 % dry weight), carbohydrates (5–7 % dry weight), minerals and trace elements (5 % dry weight). The other minor components are sterols, lipids, pigments as chlorophyll and carotenoids, vitamins (B, C, and E) and volatile compounds [2]. Tea consumption contributes to the overall daily fluid intake, and the amount of calories is insignificant. In addition, caffeine intake is lower than in coffee or cola-soft drinks, and tea contributes to the dietary intake of antioxidant compounds. Large amount of polyphenols (about 30 % of the dry substance), in fact, is contained in tea; among these, flavonoids and flavanols are considered the most important [3]. Total polyphenol content is similar in different types of tea, although these components vary according to the plant variety and to the manufacturing process [4]. Many studies have stressed the beneficial effects on health, including the prevention of cardiovascular diseases, skin cancer, coronary artery disease, Parkinson’s disease and myocardial infarction [5]. Unfortunately, like other plants, tea may also contain different classes of organic and inorganic contaminants. In this regard, some studies demonstrated that the presence of trace elements like metals with a high atomic mass in tea is related to acid soils, whereas trace elements are potentially more bioavailable for root uptake [1, 4, 6–8]. Additionally, the transformation of tea leaves can influence the amount of metals, as reported by Karak and Bhagat [9].
The cultivation of tea plants in soils contaminated by PCBs, fertilizers and organochlorine and organophosphorous pesticides can be reflected in fresh and dry tea. Furthermore, polycyclic aromatic hydrocarbons (PAHs) may accumulate in tea plants [10]. During the production steps, tea can show plasticizer residues due to the machines or to the paper coatings [7]. Furthermore, several classes of plasticizers, like adipates, sebacates and phthalates, are currently used in food packaging [11] and have been found in different matrices [12–16].
Among plasticizers, phthalates gained a dominant position. In particular, di(2-ethylhexyl) phthalate (DEHP), di-isobutyl phthalate (DiBP), benzilbutyl phthalate (BBP) and di-n-butyl phthalate (DBP) are the most used in polymer industry [17]. Several phthalates were included in the list of toxic chemical by the EPA (Environmental Protection Agency) since they can contribute to the insurgence cancer, allergies, endocrine disorders and alterations in the reproductive system [18].
Tea bags are commonly made of filter paper, silk or nylon. The bag contains the tea leaves while the tea is steeped, making it easier to dispose of the leaves, and performs the same function as a tea infuser.
An important source of possible contamination from plasticizers in tea can be the paper coatings. In fact, in the making of tea bag paper, there are a number of critical factors not present in other types of plastic products. Because the paper used is highly porous, it tends to draw the plastic materials due to the capillary attraction and to the high temperature of the water with which the tea infusion is made.
The belief that plasticizes are ubiquitous environmental pollutants has led us to investigate the presence of 27 plasticizers in infusion tea made with and without bags, to survey the daily dietary intake of plasticizers based on tea ingestion. The work focuses on tea samples with paper cover bags because of the large commercial distribution and, for this reason, the impact for the population on the plasticizers intake by tea consumption.
Materials and methods
Samples
30 samples of 10 tea types were analyzed: three samples of black tea; three of robust black tea; three of black tea bergamot flavored; three of black tea with natural lemon flavoring; three of black tea with orange and lemon peel and citrus flavoring; three of decaffeinated black tea; three of 100 % natural green tea; three of black tea with vanilla flavoring; three of green tea flavored with peppermint; and three of organic green tea. All the samples were collected in December 2012 from the local market. Each package was composed of cardboard and contained in its interior between 20 and 25 packets. Each packet was composed of a paper envelope which contained a filter. The filter contained approximately 2 g of tea.
Chemicals
The methanol and water used in this study were purchased as residue analysis grade from Sigma-Aldrich (Steinheim, Germany). Standards of di-methyl adipate (DMA), di-ethyl adipate (DEA), di-methyl phthalate (DMP), di-ethyl phthalate (DEP), di-isobutyl adipate (DiBA), di-n-butyl adipate (DBA), di-propyl phthalate (DPrP), benzyl benzoate (BB), methyl glycol adipate (MGA), di-isobutyl phthalate (DiBP), di-butyl-phthalate (DBP), methyl glycol phthalate (MGP), di-pentyl phthalate (DPP), bis-phenol A (BisPhA), benzyl butyl phthalate (BBP), bis-(2-ethylhexyl) adipate (DEHA), di-isoheptyl phthalate (DiHepP), butyl glycol phthalate (BGP), di-cyclohexyl phthalate (DcHexP), bis-(2-ethylhexyl) phthalate (DEHP), di-phenyl phthalate (DPhP), di-octyl phthalate (DOP), bis-(2-ethylhexyl) terephthalate (DEHT), bis-(2-ethylhexyl) sebacate (DEHS), di-isononyl phthalate (DiNP), di-isodecyl phthalate (DiDP), di-nonyl phthalate (DNP), for a total of 27 plasticizers, all of certified purity >99 %, were purchased from Aldrich Chemical (Chicago, IL, USA). DBP-d4 and DEHP-d4 (100 ng µL−1) in nonane, used as internal standards, were bought from Cambridge Isotope Laboratories Inc. (Andover, MA, USA). Five standard solutions at different concentrations containing mixture of all the plasticizers in analysis were prepared both in methanol and in tea extract using volumetric flasks. DBP-d4 and DEPH-d4 solution (10 mg L−1) was prepared in a similar manner. Calibration standard solutions were spiked with DBP-d4 and DEHP-d4 at a concentration level of 1 mg L−1. All solutions were stored in a refrigerator at 4 °C. The solvents were monitored for the presence of the substances in issue to control their back contamination. Oasis HLB glass (5 cc; 0.2 g) cartridges were purchased from Waters Inc. (Milford, USA). Laboratory glassware was heated at 400 °C for at least 4 h and was covered with aluminum foils prior to use. Laboratory gloves were not used during sample preparation and analysis.
Preparation of samples
In laboratory, a first set of tea samples was prepared taking the tea bags and infusing with 25 mL of 100 °C ultrapure water for 5 min. The same teas were analyzed after preparation of samples by infusion of the leaves without bag using the same procedure. All samples, prepared in triplicate, after cooling down to room temperature, were transferred into pre-cleaned 25-mL volumetric flasks, spiked with DBP-d4 and DEHP-d4 at a concentration level of 1 mg L−1 and subjected to pre-treatment procedures after 30 min.
All samples were solid phase extracted with cartridges, which were conditioned by passing 6 mL of methanol and 6 mL of water. The samples were loaded at a flow rate of 1.0 mL/min. After pre-concentration, the sorbent was rinsed with water (2 × 20 mL) and vacuum dried. Trapped compounds were desorbed using 6 mL of methanol. After rotavaporization to near dryness, the extracts were reconstituted with methanol to a final volume of 1 mL. A blank prepared by SPE of 25 mL of water was included with each sample set.
HRGC–MS analysis
The separation and quantification of plasticizers were conducted with a GC–MS Shimadzu QP2010 equipped with a capillary column Supelco SPB-5MS (5 % diphenyl, 95 % dimethyl polysiloxane, 30 m × 0.25 mm, film 0.25 μm). The injector temperature was 250 °C, and the splitless injection mode was used. The injection volume was 1 µl with a split ratio of 1:15 for 1 min, and the oven temperature was from 60 to 190 °C (5 min hold) at a rate of 8 °C/min; to 240 °C (5 min hold) at a rate of 8 °C/min; and to 315 °C at a rate of 8 °C/min. The carrier gas was helium at a constant flow rate of 36 cm/sec with an interface temperature of 280 °C. The data acquisition was performed in the SIM mode with an ionization energy and emission current of 70 eV and 250 mA, respectively. The 27 analyzed plasticizers were separated by a 40 min chromatographic run. All analytes were identified using the GC–MS data and the characteristic mass fragments for each plasticizer reported in Table 1.
Statistical methods
All statistical calculations were made by IBM SPSS 21 software package for Windows (SPSS Inc., Chicago, IL). When concentrations were below the LOQ only in few samples, these were replaced with the LOD/2 value [19]. To evaluate the difference in plasticizer concentrations, the independent-samples T test on data loge-transformed was applied.
Results and discussion
Method validation
Linearity was assessed by the determination of R 2 coefficient and the linear concentration range for calibration curves (n = 5) constructed with standard solutions prepared in methanol and also in matrix extract. The coefficient of determination was higher than 0.9874 for all plasticizers under analysis (Table 2). The solutions were injected three times, and all peak areas of target ions were normalized against the peak area of characteristic fragment (m/z 153) of DBP-d4 (for plasticizers with retention time between 10.1 and 27.3 min) and DEHP-d4 (for plasticizers with retention time between 30.2 and 39.7 min).
To verify any significant difference of the slopes in each calibration curve obtained in standard solution with the corresponding built with the matrix extract, a t test was carried out. The t-calculated value was obtained as follows:
where \(es_{{(b_{1} - b_{2} )}}\) is the standard error of the difference between two slopes b 1 and b 2. The t-calculated values were compared with the t-tabulated of 2.477, value reported for a confidence level of 95 % (α = 0.05) and six degrees of freedom. The test showed that the values were not significantly different, so it is acceptable to assume that the calibration curves did not differ significantly and that there was not matrix effect. Due to this, the quantifications were carried out by the use of the calibration curves derived from the standard solutions.
Limits of detection (LOD) and of quantifications (LOQ) are shown in Table 2 and were calculated from the RSD % of six replicate injections at the lowest detectable concentration (with a signal-to-noise ratio <3) for each plasticizer, as follows: LOD (mg L−1) = 3 × RSD % × concentration (mg L−1) and LOQ (mg L−1) = 10 × RSD % × concentration (mg L−1), as reported in Pizzutti et al. [20]. Among all plasticizers under analysis, DiDP had the highest detection and quantification limits, 3.752 and 12.502 mg L−1, respectively. For repeatability, relative standard deviations (RSD %) of peak area measurements, carried out six times, were determined at the lowest detectable concentration of each plasticizer. The RSD % values obtained were lower than 7.4 % for all the cases, except for DiNP (10.6 %) (Table 2).
For the accuracy evaluation, appropriate known amounts of plasticizer standards were added in tea samples. The fortified samples were subjected to the pre-treatment procedures previously described after 24 h. Accuracy, calculated on the average of three replicate analyses, was between 82.0 and 110.8 % (found for DiBA and DEHA, respectively). The precision, expressed as RSD %, were very satisfactory: always lower than 7.0 %.
Plasticizer residues in tea samples
The residues of plasticizers found in tea samples are shown in Table 3. DEHP, DBP and DEP residues were found in all analyzed samples, while DMP and DEHA in 80 and 70 % of these, and DiNP only in samples of green peppermint flavored tea; all other plasticizers were lower than their LOQ in all samples. Generally, DEHP or DEHA was the most abundant plasticizer in all samples, except for decaffeinated black tea, which showed DBP value 18 times higher than the average of the other samples and for green peppermint flavored tea, which showed DiNP residues. None of the samples of green tea showed DEHA residues but, compared with black tea samples, the former appears more contaminated by DEHP. Flavored teas showed higher DMP, DEHA and DEHP residues. Probably, the use of essential oils, where plasticizers are notoriously accumulated [12], induces an increase of these contaminants. In fact, plasticizers presented in machinery used to extract and/or decant the oils are transferred to the essential oils during the production cycle [21]. Only black bergamot-flavored tea showed lower residual values of DEP, DBP and DEHP.
There are few available reference data on plasticizer residues in tea. Mohamed et al. [7] reported no detectable values for DMP, DEP, DBP, BBP, DEHA and DEHP in black tea samples collected in glass cups. According to this, no detectable residues of BBP were found; conversely other plasticizer residues, in our study, were determined. Regarding the green tea samples, according to Guo et al. [22], no detectable residues of DcHexP and DOP were found; conversely in our research, concentrations below the quantification limit of BiBP and BBP were found, while Guo et al. [22] reported values of 0.107 mg L−1 and 0.0036 mg L−1, respectively. Our concentrations for DEP and DMP were c.a. 20–25 times higher, while these for DEHP and DBP were c.a. 2–4 times lower.
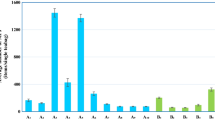
Statistical analysis showed that the amounts of plasticizers found in tea samples analyzed with bags were significantly (p < 0.05) higher than those in samples without bags; particularly, it was found that DEHA, DBP, DEP, DEHP and DMP showed significantly higher amounts in 100, 80, 70, 60 and 40 % of samples (in average +130, +110, +77, +164 and 77 %, respectively). No significant differences were observed only in 30 % of cases (Fig. 1). The increased phthalate amounts in teas infused with bag can be linked with the transfer from the packages, since bags are usually composed of a filter paper coated with plastic materials [23].
Dietary exposure to plasticizer by teas
Risk assessments of plasticizers in Europe were performed by different expert panels. In 1992, the Scientific Committee on Food (SCF) of the European Commission set TDI for DEHP (0.025 mg/kg bw/day) and temporary TDI for DEP (0.2 mg/kg bw/day), DBP (0.05 mg/kg bw/day) and DINP (0.03 mg/kg bw/day) [24]. In 1994, TDI for DEHP was revised establishing a TDI of 0.05 mg/kg bw/day [25]. In 1998, the Scientific Committee on Toxicity, Ecotoxicity and the Environment (CSTEE) of the European Commission proposed further TDI values: 0.10 mg/kg bw/day for DBP, 0.037 mg/kg bw/day for DEHP and 0.15 mg/kg bw/day for DiNP [26]. In 2005, the Scientific Panel on Food Additives, Flavourings, Processing Aids and Materials in Contact with Food (AFC) of the European Food Safety Authority revised TDIs and established the value of 0.01 mg/kg bw/day for DBP, of 0.05 mg/kg bw/day for DEHP and of 0.15 mg/kg bw/day for DiNP [27–29]. The TDI for DEP was set in 2003 by the World Health Organisation (WHO), which proposed a TDI of 5.00 mg/kg bw/day [30]. The SCF has established for DEHA a TDI of 0.30 mg/kg bw/day in 1994 and has confirmed in 2000 [25, 31].
According to the results of our study, the intake of plasticizers was obtained assuming a consumption of one cup of tea (200 mL) a day [32] and considering an adult weighing 60 kg and a 100 % gastrointestinal uptake as: {1 × plasticizer amount in a single cap (µg) × 1}/bw. The EDI values and hazard index (HI) values, calculated as HI = EDI/TDI, for DMP, DEP, DBP, DEHA, DEHP and DiNP from teas, calculated based on mean, lowest and highest amounts, are shown in Table 4. As can be seen from the reported values, no dangerous amounts of these contaminants are assumed through tea drinking.
Conclusions
In conclusion, only six out of twenty-seven plasticizers were found in the analyzed tea samples. DEP was the most abundant plasticizer in decaffeinated black tea samples and DiNP in green tea flavored with peppermint samples. DEHA was the highest in samples of robust black tea and black tea with citrus fruit flavor of bergamot, while DEHP was dominant in all other samples. The results showed that flavored teas were more contaminated by DMP, DEHA and DEHP, probably due to the use of essential oils. It was observed that major amounts of plasticizers were found when the samples were infused with bag. This can be linked with the transfer from the packages composed with plastic materials. However, the results support the conclusion that plasticizers intake through tea does not constitute a risk to the consumers.
References
Shen FM, Chen HW (2008) Element composition of tea leaves and tea infusions and its impact on health. Bull Environ Contam Toxicol 80:300–304
Cabrera C, Artacho R, Giménez R (2006) Beneficial effects of green tea—a review. J Am Coll Nutr 25:79–99
Deka A, Vita JA (2011) Tea and cardiovascular disease. Pharmacol Res 64:136–145
Vinson JA, Dabbagh YA (1998) Tea phenols: antioxidant effectiveness of teas, tea components, tea fractions and their binding with lipoproteins. Nutrition Research 18:1067–1075
Qin F, Chen W (2007) Lead and copper levels in tea samples marketed in Beijing, China. Bull Environ Contam Toxicol 79:247–250
Dulloo AG, Duret C, Rohre D, Girardier L, Mensi N, Fathi M, Chantre P, Vandermander J (1999) Efficacy of a green tea extract rich in catechin polyphenols and caffeine in increasing 24-h energy expenditure and fat oxidation in humans. Am J Clin Nutr 70:1040–1045
Mohamed MA, Ammar AS (2008) Quantitative analysis of phthalates plasticizers in traditional egyptian foods (koushary and foul medams), black tea, instant coffee and bottled waters by solid phase extraction–capillary gas chromatography–mass spectroscopy. Am J Food Technol 3:341–346
Sofuoglu SC, Kavcar P (2008) An exposure and risk assessment for fluoride and trace metals in black tea. J Hazard Mater 158:392–400
Karak T, Bhagat RM (2010) Trace elements in tea leaves, made tea and tea infusion: a review. Food Res Int 43:2234–2252
Lin D, Zhu L, He W, Tu Y (2006) Tea plant uptake and translocation of polycyclic aromatic hydrocarbons from water and around air. J Agric Food Chem 54:3658–3662
Rahman M, Brazel CS (2004) The plasticizer market: an assessment of traditional plasticizers and research trends to meet new challenges. Prog Polym Sci 29:1223–1248
Di Bella G, Saitta M, La Pera L, Alfa M, Dugo G (2004) Pesticide and plasticizer residues in bergamot essential oils from Calabria (Italy). Chemosphere 56:777–782
Di Bella G, Condoleo C, Bruzzese A, Salvo F, Cappello A, Dugo G (2004) Residual plasticizers in vegetable oils. Note I. Rivista Italiana delle Sostanze Grasse 81:207–210
Dugo G, Di Bella G, Saitta M, Condoleo C, Lo Turco V (2004) Residual plasticizers in vegetable oils. Note II. Rivista Italiana delle Sostanze Grasse 81:273–276
Dugo G, Fotia V, Lo Turco V, Maisano R, Potortì AG, Salvo F, Di Bella G (2011) Phthalate, adipate and sebacate residues by HRGC–MS in olive oils from Sicily and Molise (Italy). Food Control 22:982–988
Di Bella G, Potortì AG, Lo Turco V, Saitta M, Dugo G (2014) Plasticizer residues by HRGC–MS in espresso coffees from capsules, pods and moka pots. Food Control 41:185–192
Deanin RD (1978) In: Seymour RB (ed) Additives for plastics. Academic Press, New York
Crinnion WJ (2010) Toxic effects of the easily avoidable phthalates and parabens. Altern Med Rev 15:190–196
Škrbić B, Szyrwińska K, Ðurišić-Mladenović N, Nowicki P, Lulek J (2010) Principal component analysis of indicator PCB profiles in breast milk from Poland. Environ Int 36:862–872
Pizzutti IR, de Kok A, Dickow Cardoso C, Reichert B, de Kroon M, Wind W, Weber Righi L, Caiel da Silva R (2012) A multi-residue method for pesticides analysis in green coffee beans using gas chromatography—negative chemical ionization mass spectrometry in selective ion monitoring mode. J Chromatogr A 1251:16–26
Di Bella G, Saitta M, Lo Curto S, Salvo F, Licandro G, Dugo G (2001) Production process contamination of citrus essential oils by plastic materials. J Agric Food Chem 49:3705–3708
Guo Y, Zhang Z, Liu L, Li Y, Ren N, Kannan K (2012) Occurrence and profiles of phthalates in Foodstuffs from China and their implications for human exposure. J Agric Food Chem 60:6913–6919
Bennet AJ (1957) Tea bag. US Patent 2793120 A
Scientific Committee for food (1995) First report of the Scientific Committee for Food on certain additives used in the manufacture of plastic materials intended to come into contact with foodstuffs. Opinions expressed until 3 May 1992. Reports of the Scientific Committee for Food (33rd Series)
Scientific Committee for Food (1997) Opinions of the Scientific Committee for Food on: caustic sulphite caramel, ammonia caramel, carnauba wax, coumarin, di-2-ethylhexylphthalate, di-2-ethylhexyladipate, 3-monochloro-propane-1,2-diol (3-mcpd), polyethylenglycol 6000, neutron scanning (interrogation) devices, iso-ascorbic acid, acetylated oxidized starch, cadmium. Reports of the Scientific Committee for Food (36th Series)
Scientific Committee on toxicity, ecotoxicity and the environment (1998) Opinion on phthalate migration from soft PVC toys and child-care articles-Date made available since the 16th of June 1998, opinion expressed at the Sixth SCTEE Plenary Meeting, Brussels, 26/27 Nov 1998
European Food Safety Authority (2005) Opinion of the Scientific panel on food additives, flavourings, processing aids and material in contact with food (AFC) on a request from the Commission related to di-butylphthalate (DBP) for use in food contact materials. EFSA J 242:1–17
European Food Safety Authority (2005) Opinion of the Scientific panel on food additives, flavourings, processing aids and materials in contact with food (AFC) on a request from the Commission related to bis(2-ethylhexyl) phthalate (DEHP) for use in food contact materials. EFSA J 243:1–20
European Food Safety Authority (2005) Opinion of the Scientific panel on food additives, flavourings, processing aids and materials in contact with food (AFC) on a request from the Commission related to di-isononylphthalate (DINP) for use in food contact materials. EFSA J 244:1–18
World Health Organization (2003) Diethyl phthalate. Concise International Chemical Assessment Document 52; Geneva. http://www.who.int/ipcs/publications/cicad/en/cicad52.pdf
Scientific Committee for Food (2000) Opinion of the Scientific Committee on Food on a survey on dietary intake of the food contact material di-2-(ethylhexyl) adipate (DEHA). Report No SCF/CS/PM/3276 final/31920. http://ec.europa.eu/food/fs/sc/scf/out77_en.pdf
FAOSTAT (2009) Food and Agriculture Organization statistic database (FAOSTAT). http://faostat3.fao.org/faostat-gateway/go/to/home/E
Acknowledgments
Authors want to thank Prof. Eugenio Cianflone for reading and editing the English version of this paper.
Conflict of interest
None.
Compliance with Ethics Requirements
This article does not contain any studies with human or animal subject.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lo Turco, V., Di Bella, G., Potortì, A.G. et al. Determination of plasticizer residues in tea by solid phase extraction–gas chromatography–mass spectrometry. Eur Food Res Technol 240, 451–458 (2015). https://doi.org/10.1007/s00217-014-2344-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-014-2344-3