Abstract
The determination of phthalates in beverages (soda, lemonade, cola, mineral water) sold in Turkish markets was carried out using gas chromatography-mass spectrometry (GC-MS). The mean phthalate concentrations were determined to be between 0.095 and 0.633 mg/L in soda, 0.018 and 1.219 mg/L in lemonade, 0.019 and 1.123 mg/L in cola, and 0.085 and 0.312 mg/L in mineral water. bis(2-Ethylhexyl) phthalate (DEHP) showed the highest level of migration into beverages. Furthermore, the influence of the type of preservative (sodium benzoate, potassium sorbate, sodium benzoate + potassium sorbate) and storage time were determined.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Phthalates are well-known compounds in the form of dialkyl or alkyl aryl esters of 1,2-benzenedicarboxylic acid, the so-called phthalate esters (PAEs). The unique properties of higher molecular weight PAEs, which primarily include stability, fluidity, and low volatility, make them highly suitable as plasticizers (Chen 2004). This is why they can be found in a broad array of commercial products, including plastics, cosmetics, nail polishes, hair sprays, perfumes, fishing lures, children’s toys, medical devices, food packaging, medications, building materials, home furnishings, transportation, clothing, caulk, dope, paints, adhesives, and lubricants made with polyvinyl chloride plastics.
Phthalates are considered as an endocrine disrupter because they display a variety of toxic effects in animal studies including decreased fertility in females (Biscardi et al. 2003), fetal defect and reduced survival of offspring (Gray et al. 2000), altered hormone levels (Thompson et al. 2004), uterine damage (Seidlova-Wuttke et al. 2004), and male reproduction abnormalities such as reduced sperm production and mobility (Sharpe et al. 1995), Sertoli cell damage (Heindel and Powell 1992), and cryptorchidism and hypospadias (Skakkebaek et al. 2001). The effects of human exposure to phthalates have not been fully studied (Montuori et al. 2008).
Regulations governing the use of plasticizers in food contact applications vary from country to country. In the UK, the acceptable concentrations have been set for bis(2-ethylhexyl) phthalate (DEHP) at 0.05 mg/kg body weight/day, butylbenzyl phthalate (BBP) at 0.1 mg/kg body weight/day, dibutyl phthalate (DBP) at 0.05 mg/kg body weight/day, and diethyl phthalate (DEP) at 0.2 mg/kg body weight/day (MAFF 1996). In Europe, the total tolerable daily intake of total phthalates per person has been estimated to be 0.3 mg/kg body weight (Balafas et al. 1999). In the Turkish Food Codex, the acceptable limits of the phthalates in food are summarized as below:
-
DBP, do not exceed 0.3 mg/kg food
-
BBP, do not exceed 30 mg/kg food
-
DEHP, do not exceed 1.5 mg/kg food
-
Diisononyl phthalate (DINP), do not exceed 9 mg/kg food.
Phthalates are not bound chemically in the plastics and can consequently penetrate these materials and migrate into food that comes into contact. The presence of phthalates in packaging materials and their migration into packaged foods have been confirmed by a number of authors (Balafas et al. 1999; Bosnir et al. 2007; Cavaliere et al. 2008; Ceretti et al. 2010; Holadová et al. 2007; Nanni et al. 2011; Ostrovský et al. 2011; Sannino 2009). The aim of the study is to determine the phthalate levels in beverages (soda, lemonade, cola, mineral water) sold in Turkish markets.
Materials and Methods
Reagents and Standards
Dimethyl phthalate (DMP), DEP, DBP, dipropyl phthalate (DPP), BBP, DEHP, dioctyl phthalate (DOP), and DINP were obtained from Fluka (Buchs, Switzerland). All the phthalate esters were more than 99 % pure.
A stock standard solution of 1,000 mg/L of each compound was prepared in acetonitrile. Working standard solutions of 100 mg/L were prepared weekly in acetonitrile. Stock and working standards were stored at 4 °C in the refrigerator.
Calibration standards were prepared in the range of 0.01–2 and 2–10 mg/L. In all cases, the correlation coefficients of linear functions were >0.995. The calibration curves were created from six calibration standards.
Collection of Samples
Ten different brands from each item beverages (soda, lemonade, cola, mineral water, and orange-flavored soda) were taken from different local markets in Turkey.
Sample Preparation
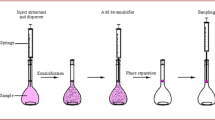
After the beverage had been degassed in an ultrasonic bath for 5 min, an aliquot of either 20 mL was extracted twice with 10 mL of dichloromethane in a separating funnel. A sodium sulfate column was prepared by packing sodium sulfate (1 g) in turn into a glass column fitted with fritted glass disks at the bottom (15 mm × 110 mm). The column was washed with dichloromethane (5 mL) before use. The extract was passed through the column and evaporated to a small volume of approximately 1–2 mL at 40 °C under vacuum. Then, it was transferred to a vial and was analyzed by gas chromatography-mass spectrometry (GC-MS) (Keith et al. 2000).
GC-MS Analysis
GC-MS analysis was performed on a Hewlett-Packard 6890 instrument. A Hewlett-Packard HP-5MS fused silica capillary column (cross-linked 5 % methyl silicone of 30 m × 0.25 mm I.D. and phase thickness of 0.25 μm) was used. The GC temperature program was as follows: initial temperature of 50 °C, hold for 5 min; increased to 90 °C at 2 °C/min, hold for 3 min; and then to 200 °C at 10 °C/min, hold for 10 min. The total run time was 49 min. Helium was used as carrier gas at a flow rate of 1.2 mL/min. The samples were injected in the splitless mode. The sample volume in the direct injection mode was 1 μL. The MS conditions were the following:
-
Instrumentation, HP 6890/5972 mass spectrometer
-
Transfer line temperature, 280 °C
-
Source temperature, 250 °C
-
Electron energy, 70 eV
-
Constant flow, 1 mL/min
-
Pressure, 22.39 psi
Quality Control and Quality Assurance
The amounts of phthalates were determined by comparing their peak areas with those of standards in GC-MS. All analyses were repeated three times for each sample. A blank version of the test performed on the beverages was conducted every day using ultrapure water. All of the blank values were averaged, and the average value was subtracted from the detected phthalate values. The limit of detection (LOD) was determined to be three times the standard deviation of the blank test values. The limit of quantification (LOQ) was taken as three times the LOD. The values of retention time, correlation coefficient, LOD, and LOQ of examined phthalates are listed in Table 1.
The laboratory blank is very important since the contamination is a major problem in the analysis of phthalates, especially from unclean plastic-containing glassware, organic solvents, and many items in laboratory settings (Mohamed and Ammar 2008). In this study, special care was taken to avoid the contact of reagents and solutions with plastic materials. Laboratory glassware was washed prior to use with ultrapure water and dried at 250 °C.
Results
Under the applied GC-MS conditions, the retention times of DMP, DEP, DBP, DPP, BBP, DEHP, DOP, and DINP were 34.51, 36.63, 41.24, 38.98, 40.76, 43.47, 43.95, and 43.53 min, respectively. The chromatogram of phthalate standards was given in Fig. 1.
The phthalate levels of the examined samples were presented in Table 2. The mean phthalate concentrations were determined to be between 0.095 and 0.633 mg/L in soda, 0.018 and 1.219 mg/L in lemonade, 0.019 and 1.123 mg/L in cola, 0.085 and 0.312 mg/L in mineral water, and 0.018 and 0.218 mg/L in soda (orange flavored). DEHP showed the highest level of migration into beverages. A very low level of DOP was detected in two group drinks. The chromatogram of mineral water was given in Fig. 2.
The influence of the type of preservative (sodium benzoate, potassium sorbate, sodium benzoate + potassium sorbate) used in the product manufacture was investigated. The highest phthalate levels were measured in soda samples with sodium benzoate and potassium sorbate used as preservative. The phthalate concentrations in the soda samples preserved with potassium sorbate were found to be similar to the phthalate concentrations in the soda samples preserved with sodium benzoate (Table 3).
The influence of storage time to the phthalate migration was investigated. Related table are shown in Table 4. These data indicate that the phthalate concentrations were increased depending on storage time.
Discussion
US Environmental Protection Agency (EPA)’s maximum contaminant level for DEHP in the bottled water is 0.006 mg/L (EPA 2009). The EU and the World Health Organization (WHO) accepted a permissible limit for drinking waters as 0.008 mg/L of DEHP (European Union Council 2001; WHO 2008). On the other hand, the Japanese authority redefined the permissible DEHP limit in drinking water in 2001 as 0.100 mg/L (Hirose et al. 2004). Bottled water was not examined in our study; however, the concentration of DEHP in soft drinks was 0.248–1.123 mg/L. These concentrations are lower than the permissible limits. BBP concentration was 0.018 to 0.917 mg/L (Table 2). In a previous study, the highest value of BBP was found in bottled water stored at 4 °C (4.592 ± 3.081 μg/L) (Al-Saleh et al. 2010). US EPA has suggested a maximum contaminant level of 0.100 mg/L for BBP in drinking water (US EPA, http://www.masterwater.com/main/epa_regs.asp). BBP concentrations in lemonade, cola, soda, and orange-flavored soda are still much higher than these high limits. Al-Saleh et al. (2010) found that the maximum DEP value in bottled waters is 0.610–1.778 μg/L. Our value (0.092–0.270 mg/L) was higher than the maximum values reported by Al-Saleh et al. (2010). The DEP levels in the current study were also higher than the DEP mean of 0.17 μg/L reported by Montuori et al. (2008). The study did not include tap water and bottled water, but soft drink was tested. The level of phthalate of soft drink which contains a large number of additives should be different from that of water. The total phthalate concentration also increased as the duration of contamination in soft drinks was longer (from 0.880 to 2.898 mg/L; from 1 to 11 months, for cola drink).
DEHP level in water samples contained in polyethylene terephthalate (PET) bottles increased from a level of 400 ng/L during storage of 1 to 8 months to a high level of 3,200 ng/L in the same water sample after storage of 9 to 12 months (Biscardi et al. 2003). Another researchers did not find a difference in the concentrations of phthalates (DEP, DBP, and DEHP) in water from PET bottles exposed to different storage conditions (15 or 30 days, 15–40 °C) (Amiridou and Voutsa 2011). In our study, the level of phthalate increased as the duration of exposure in all beverages (0.880 mg/L in the 1st month and 2.898 mg/L in the 11th month for cola). Exposure time is short in the study of Amiridou and Voutsa (2011), while the duration of phthalate exposure is long in the study of Biscardi et al. (2003) and our studies. The main reason of phthalate migration may be exposure time rather than other factors such as temperature, humidity, light, pH, and additives.
The nearest phthalate levels to our study in the literature were displayed by Bosnir et al. (2007). They compared the concentrations of total and various phthalates in PET-bottled soft drinks preserved with sodium benzoate and/or potassium sorbate. They reported that total phthalate concentration detected in the soft drinks was 0.116–0.819 mg/L. In our study, the levels of total phthalates were 0.739, 0.442, and 1.697 mg/L (soda, cola, and orange-flavored soda, respectively). There was no difference between preservatives such as sodium benzoate and potassium sorbate; however, soft drinks with the use of both preservatives have higher phthalate migration. Researchers found that the highest phthalate levels were measured in soft drinks with potassium sorbate used as preservative (0.819 mg/L), followed by 1.5 times lower levels in drinks preserved with a combination of sodium benzoate and potassium sorbate (0.542 mg/L) and 7 times lower levels in drinks preserved with sodium benzoate (0.117 mg/L). They reported that the phthalate level found in mineral water samples free from preservatives was as low as 0.020 mg/L (Bosnir et al. 2007). Very high levels of phthalates were found in mineral water. The reason for this can be within the preservatives (potassium sorbate). The Food and Drug Administration lists sodium benzoate and potassium sorbate as substances that are generally recognized as safe with a maximum permitted concentration of 0.1 % in accordance with good manufacturing practices (FDA, http://www.fda.gov/Food/FoodIngredientsPackaging/FoodAdditives/ucm191033.htm). Similarly, sodium benzoate and potassium sorbate levels in foods are regulated in Europe by the European Commission Health and Consumer Protection Directorate with a limit of 150 mg/L for sodium benzoate and 300 mg/L for potassium sorbate in nonalcoholic drinks (EC, http://ec.europa.eu/food/fs/sfp/addit_flavor/flav11_en.pdf). Can et al. (2011) found the amount of sodium benzoate to be 4–170 mg/L and the amount of potassium sorbate to be 7–144 mg/L in soft drinks sold markets in Turkey. Values do not exceed the legal limits, but the values are very close to the limit. The reason of the conflict between our study and that of Bosnir et al. (2007) may be the difference in the amount of preservatives in soft drinks.
Phthalate-contaminated food products in Taiwan in 2011 were a major event (Wu et al. 2012). The excessive amount of phthalates was added to foods as a substitute emulsifier. Sports drinks, fruit beverages, tea drinks, fruit jam or jelly, and health food or supplements in tablet or powder form were contaminated with di-(2-ethylhexyl) phthalate and/or diisononyl phthalate. A total of 4,076 retail violations were found in inspections of 49,652 store in Taiwan (Kang et al. 2012). Approximately 900 different food products were affected from contamination. According to data from a small sample, DEHP and DINP concentrations ranged from 9.1 to 34.1 ppm and from 5.2 to 7.9 ppm in tainted sports drinks, and DEHP ranged from 2.4 to 14.6 ppm in fruit beverages, respectively. Taiwan government enacted a rule of phthalate-free certification for all food products in the country. The legal concentrations of DEHP, DIDP, DNOP, DINP, DBP, and BBP were considered below 1 ppm. Our total phthalate levels were 0.442–1.697 mg/L in soft drinks. The phthalate amounts of beverages sold in Turkish markets was much lower than those sold in Taiwan.
In a person weighing 70 kg and drinking daily 1,000 mL of lemonade with 0.018 mg/L of BBP, 0.198 mg/L of DBP, 2.060 mg/L of DEHP, 0.153 mg/L of DEP, and 4.302 mg/L of total phthalates corresponding to the maximum concentration values of these phthalates investigated in the lemonade samples in this study, its estimated intake of BBP (7 mg/day), DBP (3.5 mg/day), DEHP (3.5 mg/day), DEP (14 mg/day) would not exceed the MAFF values currently set for phthalates.
As a conclusion, we can say that phthalate is not thought to create a risk factor for human health in Turkey at the present time.
References
Al-Saleh I, Shinwari N, Alsabbaheen A (2010) Phthalates residues in plastic bottled waters. J Toxicol Sci 36:469–478
Amiridou D, Voutsa D (2011) Alkylphenols and phthalates in bottled waters. J Hazard Mater 185:281–286
Balafas D, Shaw KJ, Whitfield FB (1999) Phthalate and adipate esters in Australian packaging materials. Food Chem 65:279–287
Biscardi D, Monarca S, De Fusco R, Senatore F, Poli P, Buschini A, Rossi C, Zani C (2003) Evaluation of the migration of mutagens/carcinogens from PET bottles into mineral water by Tradescantia/micronuclei test, Comet assay on leukocytes and GC/MS. Sci Total Environ 302:101–108
Bosnir J, Puntaric D, Smit Z, Klaric M, Grgic M, Kosanovic LM (2007) Migration of phthalates from plastic containers into soft drinks and mineral water. Food Technol Biotechnol 45:91–95
Can NO, Arli G, Lafci Y (2011) A novel RP-HPLC method for simultaneous determination of potassium sorbate and sodium benzoate in soft drinks using C(18)-bonded monolithic silica column. J Sep Sci 34:2214–2222
Cavaliere B, Macchione B, Sindona G, Tagarelli A (2008) Tandem mass spectrometry in food safety assessment: the determination of phthalates in olive oil. J Chromatogr A 1205:137–143
Ceretti E, Zani C, Zerbini I, Guzzella L, Scaglia M, Berna V, Donato F, Monarca S, Feretti D (2010) Comparative assessment of genotoxicity of mineral water packed in polyethylene terephthalate (PET) and glass bottles. Water Res 44:1462–1470
Chen CY (2004) Biosynthesis of di-(2-ethylhexyl) phthalate (DEHP) and di-n-butyl phthalate (DBP) from red alga—Bangia atropurpurea. Water Res 38:1014–1018
EPA (2009) (http://water.epa.gov/drink/contaminants/index.cfm#Organic)
European Union Council (2001) Decision No. 2455/2001/EC establishing the list of priority substances in the field of water policy amending Directive 2000/60/EC. Off. J. Eur. Commun. L 3311
Gray JLE, Ostby J, Furr J, Price M, Veeramachaneni DN, Parks L (2000) Perinatal exposure to the phthalates DEHP, BBP and DINP, but not DEP, DMP OR DOTP, alters sexual differentiation of the male rat. Toxicol Sci 58:350–365
Heindel JJ, Powell CJ (1992) Phthalate ester effects on rat Sertoli cell function in vitro effects of phthalate side chain and age of animal. Toxicol Appl Pharmacol 115:116–123
Hirose A, Hasegawa R, Nishikawa A, Takahashi M, Ema M (2004) Revision and establishment of Japanese drinking water quality guidelines for di(2-ethylhexyl) phthalate, toluene and vinyl chloride—differences from the latest WHO guideline drafts. J Toxicol Sci 29:535–539
Holadová K, Prokůpková G, Hajslová J, Poustka J (2007) Headspace solid-phase microextraction of phthalic acid esters from vegetable oil employing solvent based matrix modification. Anal Chim Acta 582:24–33
Kang JJ, Chu SF, Wu ZZ, Chou SW, Tsai SJ, Chiu WT (2012) Crisis management turns Taiwan’s plasticizer nightmare into progressive policy. J Formos Med Assoc 111:409–411
Keith LH, Jones-Lepp TL, Needham LL (eds) (2000) Analysis of environmental endocrine disruptors. American Chemical Society, Blacksburg, Virginia
MAFF (Ministry of Agriculture, Fisheries and Food) (1996) Phthalates in infant formulae. Food Surveillance pp., 83. London: HMSO
Mohamed MA, Ammar AS (2008) Quantitative analysis of phthalates plasticizers in traditional Egyptian foods (koushary and foul medams), black tea, instant coffee and bottled waters by solid phase extraction-capillary gas chromatography-mass spectroscopy. Am J Food Technol 3(5):341–346
Montuori, P.; Jover, E.; Morgantini, M.; Bayona, J.M.; Triassi, M (2008) Assessing human exposure to phthalic acid and phthalate esters from mineral water stored in polyethylene terephthalate and glass bottles. Food Addit. Contam. Part A: Chem. Anal. Control. Expo. Risk. Assess. 25, 511-518
Nanni N, Fiselier K, Grob K, Di Pasquale M, Fabrizi L, Aureli P, Coni E (2011) Contamination of vegetable oils marketed in Italy by phthalic acid esters. Food Control 22:209–214
Ostrovský I, Čabala R, Kubinec R, Górová R, Blaško J, Kubincová J, Řimnáčová L, Lorenz W (2011) Determination of phthalate sum in fatty food by gas chromatography. Food Chem 124:392–395
Sannino A (2009) Survey of phthalate levels in Italian oily foods contained in glass jars with PVC gaskets. Food Addit Contam B 2:166–170
Seidlova-Wuttke D, Jarry H, Wuttke W (2004) Pure estrogenic effect of benzophenone-2 (BP2) but not of bisphenol A (BPA) and dibutylphthalate (DBP) in uterus, vagina and bone. Toxicology 205:103–112
Sharpe RM, Fisher JS, Millar MM, Jobling S, Sumpter JP (1995) Gestational and lactational exposure of rats to xenoestrogens results in reduced testicular size and sperm production. Environ Health Perspect 103:1136–1143
Skakkebaek NE, Rajpert-De Meyts E, Main KM (2001) Testicular dysgenesis syndrome: an increasingly common developmental disorder with environmental aspects. Hum Reprod 16:972–978
Thompson JC, Ross SM, Gaido KW (2004) Di(n-butyl)phthalate impairs cholesterol transport and steroidogenesis in the fetal rat testis through a rapid and reversible mechanism. Endocrinology 145:1227–1237
WHO (2008) Chapter 8: chemical aspects, guidelines for drinking water quality (3rd edn), incorporating the first and second addenda, Recommendations. World Health Organization, Geneva
Wu MT, Wu CF, Wu JR, Chen BH, Chen EK, Chao MC, Liu CK, Ho CK (2012) The public health threat of phthalate-tainted foodstuffs in Taiwan: the policies the government implemented and the lessons we learned. Environ Int 44:75–79
Acknowledgments
This research (111S307) was supported by the Scientific and Technological Research Council of Turkey. The authors would like to thank the Scientific and Technological Research Council of Turkey for financial support.
Conflict of Interest
Sana Sungur declares that she has no conflict of interest. İhsan Ustun declares that he has no conflict of interest. Ramazan Okur declares that he has no conflict of interest. Ahmet Taner Sumbul declares that he has no conflict of interest. Suleyman Oktar declares that he has no conflict of interest. Nigar Yılmaz declares that she has no conflict of interest. Cumali Gökçe declares that he has no conflict of interest. This article does not contain any studies with human or animal subjects.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ustun, I., Sungur, S., Okur, R. et al. Determination of Phthalates Migrating from Plastic Containers into Beverages. Food Anal. Methods 8, 222–228 (2015). https://doi.org/10.1007/s12161-014-9896-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-014-9896-5