Abstract
In this work, a simple and selective method based on matrix solid phase dispersion (MSPD) using molecularly imprinted polymers (MIPs) as sorbents was successfully developed for the determination of melamine (MEL) in milk powder. The MIPs have been prepared by surface imprinted polymerization method using carbon nanotube (CNT) as support, MEL as template molecule, γ-aminopropyltriethoxysilane as functional monomer, and tetraethyl orthosilicate as cross-linking agent. The MIPs were characterized by Fourier transform infrared spectrometer and scanning electron microscopy. At the same time, the adsorption isotherm, dynamic adsorption, and selective adsorption of MIPs to MEL were also studied. The prepared MIPs were used as dispersant of MSPD to extraction and separate MEL in milk powder. We optimized the extraction conditions as follows: the ratio of MIPs to sample was 2:3; the dispersion time was 10 min; washing solvent was 2.0 mL acetonitrile; and elution solvent was 4.0 mL ammonium methanol (5:95, v/v). Once the MSPD process was completed, the MEL extracted from milk powder was determined by LC-MS/MS. Under the optimal conditions, the limit of detection of MEL was 0.2 ng g−1. The relative standard deviations of intra-day and inter-day were obtained in the range of 0.4∼5.1 %. The satisfied recoveries of MEL were 92.5∼97.2 %. The MIP-MSPD method has some advantages, such as higher selectivity, lower cost of preparation, less time consuming and organic solvent consumption, simpler sample pretreatment procedure, and higher extraction recovery compared with those existing methods which were used for determination of MEL.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Melamine (MEL; 1,3,5-triazine-2,4,6-triamine) belongs to nitrogen-rich chemical compounds containing cyanamide as the basic unit (Liu et al. 2015). In plastic manufacturing, MEL–formaldehyde resins are necessary for making surface coatings, laminates, adhesives, and flame retardants since the 1950s (Wu et al. 2012). Owing to their high nitrogen content (66 % by mass) and low price, MEL was deliberately added to a number of different types of animal and human food sources to show a false artificial enhancement in protein concentration. In 2007, MEL was found in pet food products and led to kidney toxicity in dogs and cats in the USA (Baynes et al. 2008; Cianciolo et al. 2008). In September 2008, the occurrence of kidney stones in thousands of infants across China captured the attention of the world (Filigenzi et al. 2008; Lin et al. 2008). Investigation of MEL at levels above the safety limit (2.5 ppm in the USA and European Union; 1.0 ppm for infant formula milk powder in China) may cause kidney failure and even death (Langman et al. 2009; Lam et al. 2009). Therefore, the determination of MEL has been of great concern in food safety.
Some modern instrument analytical methods have been employed for the determination of MEL, such as gas chromatography (Yokley et al. 2000), liquid chromatography (Filigenzi et al. 2008), gas chromatography–mass spectrometry (Zhu et al. 2009), liquid chromatography–mass spectrometry (Andersen et al. 2008), high performance liquid chromatography/mass spectrometry (Ehling et al. 2007), surface enhanced Raman spectroscopy (Lin et al. 2008), nuclear magnetic resonance spectroscopy (Lachenmeier et al. 2009), capillary zone electrophoresis/mass spectrum (Cook et al. 2005; Klampfl et al. 2009), electrochemiluminescence (Guo et al. 2011), and chemiluminescence (Wang et al. 2009). In recent years, methods based on hydrophilic interaction liquid chromatography (HILIC) have been developed. HILIC is a consolidated analytical tool for the separation of polar compounds, which is poorly retained by reversed-phase liquid chromatography (Zheng et al. 2012). The use of HILIC separations has significantly grown, especially because HILIC can improve the resolution of MEL (Bernal et al. 2011). However, complicated pre-concentration process and time-consuming steps limit the application of these methods although they are mostly accurate and sensitive. Therefore, there is still an increasing demand for fast, simple, convenient, and sensitive method to solve these problems.
Matrix solid phase dispersion (MSPD), which includes sample homogenization, transferring the blend, seamlessly compressing, eluting, and purifying in a simple column (Ruiz et al. 2005; Bogialli and Di Corcia 2007), is one of the most promising techniques for simultaneous dispersion, extraction, and cleanup of solid, semi-solid, and highly viscous samples (Rodríguez-González et al. 2015; Ferrer et al. 2005). It has been considerably reduced the analytical time, the sample size, and the solvent consumption (Oniszczuk et al. 2013; Rodrigues et al. 2010). Molecularly imprinted polymers (MIPs) have been used as a new kind of selective sorbent in SPE (Masque et al. 2001; Khanahmadzadeh and Tarigh 2014) and MSPD (Tan et al. 2014; Yan et al. 2012) for extracting organic compounds from complex samples because of its high selectivity and stability. The MIPs can remove the interference compounds from the matrix to achieve the purpose of selective enrichment and cleanup, which would lead to a higher accuracy and a lower detection limit in the subsequent analysis (Li et al. 2010a; Nezhadali et al. 2015; Niu et al. 2016). Carbon nanotubes (CNTs) show great characteristics of small size and immense surface area. The particular properties of CNTs make them ideal supports to deposit several materials on them. The incorporation of MIPs onto CNTs allows the development of highly selective extraction procedures due to the specific affinity of the cavities for the template, in preference to other closely related compounds (Du et al. 2016).
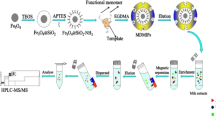
In this work, the MIPs were synthesized with CNTs as carrier, MEL as template, γ-aminopropyltriethoxysilane as functional monomer, and tetraethoxysilane as cross-linker. Adsorption experiments including Scatchard analysis, kinetics absorption, and recognition specificity were carried out. Then, the CNT-based MIPs were used as dispersant in matrix solid-phase dispersion process for the extraction of MEL from milk powder samples, followed by LC-MS/MS analysis with a HILIC Silica column. This method not only had selective adsorption of molecularly imprinting but also had the fast separation of MSPD. It achieved the purpose of rapid selective adsorption for MEL in milk powder samples. Under the optimal conditions, good extraction recoveries and precision were obtained.
Experimental
Reagents
The CNTs used in this study were purchased from Nanoport (Shenzhen, China). Nitric acid (65–68 wt.%), tetraethyl orthosilicate (TEOS), γ-aminopropyltriethoxysilane (KH-550), cetyl trimethyl ammonium bromide (CTAB), methanol, ethanol, N,N-dimethylformamide (DMF), sulfoxide chloride, hydrochloric acid, and acetic acid were of analytical grade and obtained from kermel (Tianjin, China). MEL, cyanuric acid, cyromazine, and atrazine were of analytical standards and obtained from Aladdin (Shanghai, China). Chromatographic grade acetonitrile and ammonium acetate were obtained from Sigma-Aldrich (St, Louis, MO). High purity water was obtained from a Milli-Q Water System (Millipore, Billerica, MA, USA). All other reagents were of analytical grade.
Instruments
Chromatographic analysis was performed by LC-MS/MS (Waters, MA, USA). A Waters Atlantis HILIC Silica column (150 mm × 4.6 mm I.D., 3 μm) was used as an analytical column (Waters, MA, USA). A KQ5200E ultrasonic apparatus (Kunshan Instrument, Kunshan, China), a DZKW-C thermostatic bath (Shanghai, China), a TG 16-WS centrifuge (Xiangyi, Changsha, China), a SHB-B shaking table (Shengtang, Jintan, China), an FTIR 360 Fourier transform infrared spectrometry (Nicolet, Madison, WI, USA), and a scanning electron microscope (SEM) (JSM-6700F, JEOL, Tokyo, Japan) were used.
Preparation of Milk Powder Samples and Standard Solution
For the development and validation of the method, milk powder was randomly bought from markets in Harbin of China. One milk powder sample was checked to be free of MEL according to the method reported in National Standard of China (Ministry of Health of the People’s Republic of China 2008), and it was used as blank sample for calibration and validation purposes. The spiked milk powder samples were prepared by adding MEL standard solutions into product samples.
The standard stock solution of MEL was prepared by dissolving 100.0 mg of MEL into 100 mL deionized water. The solution was stored in a sealed volumetric flask at 4 °C. Working standard solutions were freshly prepared by diluting suitable amounts of the above solution.
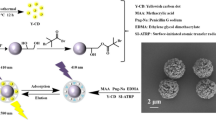
MIP Preparation
First was the processing of CNTs. The impurities such as amorphous carbon and metallic catalyst in the CNTs were removed by using HNO3 (100 mL) solution at 80 °C for 6 h. Then, the CNTs were washed with water. The activated CNTs were mixed with sulfoxide chloride (100 mL) in a round bottom flask at 70 °C for 4 h. The sulfoxide chloride in the mixture was removed at 90 °C by distillation. After being washed with N,N-dimethylformamide and water, the purified solid was dried at 60 °C under vacuum overnight.
Second was the preparation of A and B liquid. The preparation of A liquid is as follows: MEL (0.15 g) was dissolved in mixture composed by 15 mL of ethanol and 10 mL of deionized water and then stir pre-aggregated for 2 h after adding 1.76-mL KH-550 under mechanical agitation. The preparation of B liquid is as follows: the surfactant CTAB (0.17 g) and 2.0 g CNTs were dissolved in 20 mL deionized water and then ultrasonic oscillation for 1 h to make them disperse evenly.
The last was the synthesis of MEL–CNTs–MIPs. B was mixed with A and then mechanical agitation for 30 min after ultrasonic oscillation for 5 min. TEOS (6.7 mL) was added into the mixture slowly under mechanical agitation. The mixture was stood for the night after constant stirring reaction for 4 h. Then, CTAB was removed by using 150 mL 2.0 mol L−1 hydrochloric acid ethanol solution backflow under 80 °C for 24 h. To extract the template, the polymers were subjected to Soxhlet extraction with methanol/acetic acid (8:2, v/v), until no MEL could be detected by UV. Finally, the polymers were dried in vacuum overnight, and stored at room temperature.
Non-imprinted polymers (NIPs) were prepared and processed similarly as above, except that the template molecule was not added.
Binding Experiment
The isothermal adsorption experiments were carried out by adding 20 mg MIPs or NIPs in a 10-mL centrifuge tube containing 8.0 mL of MEL standard solution with 11 concentrations varying from 0.1 to 200 μg mL−1. Then, the mixture was stirred for 24 h on the shaking table at room temperature. Finally, the sorbent was separated, and the MEL in supernatant was detected by UV with detection wavelength of 240 nm.
To investigate the adsorption kinetics of the sorbent, 20 mg of MIPs or NIPs was added into 8 mL of 200 μg mL−1 MEL standard solution and was stirred at room temperature for 1–90 min. The sorbent was separated, and the MEL in supernatant was detected by UV.
The rebinding selectivity experiments were carried out by using cyromazine, cyanuric acid, and atrazine as the structural analog. The MIPs or NIPs (20 mg) were added in 8 mL of 100 μg mL−1 of MEL, cyromazine, cyanuric acid, and atrazine standard solution. The mixture was stirred at room temperature for 24 h. The sorbent was separated, and the MEL in supernatant was detected by UV.
MIP–MSPD Procedure
The milk powder sample (0.3 g) was placed into a glass mortar and gently blended with 0.2 g of MIPs for 10 min using a pestle to obtain homogeneous mixture. The homogenized sample was loaded into a cartridge. The cartridge was rinsed with 2.0 mL acetonitrile and then the analyte was eluted with 4.0 mL ammonium methanol (5:95, v/v) at the flow rate of 1.0 mL min−1. The eluent was combined and evaporated to dryness under nitrogen gas at 40 °C, and the residue was reconstituted with 0.5 mL LC mobile phase for further analysis by LC–MS/MS.
The Standard Method Used in China for Determination of MEL in Milk Powder
In order to verify the reliability and practicability of the method, the Chinese national standards GB/T22388-2008 (Ministry of Health of the People’s Republic of China 2008) for detection of MEL method was compared. Milk powder samples (2.0 g) were put in a 50 mL of centrifuge tube containing 15 mL trichloroacetic acid solution and 5.0 mL of acetonitrile. The mixture was extracted by ultrasonic for 10 min, oscillation for 10 min, and centrifuged at 5000 r/min for 10 min. The supernatant was filtered and transferred to 25-mL volumetric flask and then constant volume with trichloroacetic acid solution. The mixture that was mixed with 5.0 mL of filtrate and 5.0 mL of deionized water was transferred to the solid phase extraction column with cation exchange adsorbent. The column was swab-off after being washed by 3.0 mL of deionized water and 3.0 mL methanol and then eluted with 6.0 mL of 5 % ammonium methanol. The elution speed was less than 1 mL min−1 in the entire process of solid phase extraction. The eluent was combined and evaporated to dryness under nitrogen gas at 50 °C. The residue was reconstituted with 1.0 mL of acetonitrile and then shook for 1.0 min on vortex mixer. The resulted solution was filtrated with microporous membrane filter for further analysis by LC-MS/MS.
LC–MS/MS Analysis
UPLC–MS/MS instruments with electrospray ionization (ESI) source were performed in positive ion mode. The source-dependent parameters of UPLC-MS/MS with ESI source were as follows: capillary voltage, 3.0 kv; desolvation temperature, 500 °C; desolvation gas flow, 600 L h−1; cone voltage, 50 V. The data acquisition was performed in the multiple reaction monitoring (MRM) mode. The collision energy used for ion transitions m/z 127.2 → 68.2 and 127.2 → 85.2 were 20 and 15 eV, respectively. The ion transition m/z 127.2 → 85.2 was used for quantification of MEL. The mobile phase for separation consisted of (A) ammonium acetate (10 mmol L−1) and (B) acetonitrile. The gradient elution was carried out from 10 % A, held for 1 min, then rose to 60 % A in 4 min, held for 1 min, and then back to 10 % A in 1 min, held for 3 min to equilibrate the column. The mobile phase delivered at a flow rate of 0.4 mL min−1. The injection volume was 1.0 μL.
Result and Discussion
Characterizations of the MIPs
The MIPs were characterized by SEM to know the surface morphological image. Figure 1a shows that CNTs were well combined with silicon and MIPs were coated on CNTs successfully without destroying the tubular structure of CNTs. Figure 1b presents the FTIR spectrum of MIPs. The major peaks for MIPs can be assigned as follows: the adsorption band around 3432.03 cm−1, revealing the O–H group. The band around 1734.83 cm−1 is attributed to the characteristic peak of C=O stretching vibration. The peak at 1628.78 cm−1 is attributed to the existence of C=C stretching vibration. The peaks at 1486.25 and 1400.00 cm−1 are attributed to the existence of C–H bending vibration. The peak at 1078.10 cm−1 is attributed to the characteristic peak of S–O stretching vibration.
Adsorption Isotherm
The adsorption isotherm of MIPs and NIPs is shown in Fig. 2a. The equilibrium adsorption of MIPs and NIPs to MEL was increased when the concentration of MEL was increased, and the adsorption amount of MIPs was greater than the adsorption amount of NIPs.
In this experiment, a commonly used Scatchard equation fitting model has been selected for the study about isothermal adsorption data of the MIPs and NIPs (Ma and Chen 2014). The equation can be expressed as follows:
where Q eq is the amount of MEL bound to the polymers at equilibrium (mg g−1); C eq is the free MEL concentration at equilibrium (μg mL−1); K is the dissociation constant (mg L−1); and Q max is the apparent maximum binding amount (mg g−1). The values of K and Q max can be calculated from the slope and intercept of the linear line plotted in Q eq /C eq versus Q eq . The Scatchard analysis curve of MIPs and NIPs was shown in Fig. 2b, c, and each parameter value is shown in Table 1.
The Scatchard plot for MIPs was not a single linear curve and consisted of two linear parts with different slopes (Fig. 2b). It suggests that there exist two classes of heterogeneous binding sites in respect to the affinity for MEL in MIPs. The corresponding equations of the linear parts were respectively as follows:
The binding of MEL to the NIPs was also analyzed by Scatchard method (Fig. 2c). The corresponding equation of the linear part was as follows:
The recognition sites of the molecular imprinting can be improved with CNT as carrier, which increased the adsorption capacity of MIPs. The total binging amount (49.79 mg g−1) of MIPs used CNT as matrix in this work was higher than that of other MIPs without CNT prepared by bulk polymerization [0.345 mg g−1 (Cheng et al. 2013)], surface imprinting using SiO2 [8.64 mg g−1 (Yang et al. 2009)], or mesoporous silica [9.545 mg g−1 (Yu et al. 2015)] as carrier.
Adsorption Kinetics
Adsorption is a physic and chemical process that involves mass transfer of a solute from liquid phase to the adsorbent surface. Kinetics study provided important information about the adsorption mechanism of MEL adsorption onto MIPs and NIPs. The study of kinetics was necessary to depict the adsorption rate of adsorbate and control the residual time of the whole adsorption process. The curve of adsorption amount onto MIPs and NIPs toward MEL over time is shown in Fig. 3a. It can be seen that the binding capacity of MIPs toward MEL increased rapidly at the initial 20 min and then slowed down, as a platform.
In this experiment, two of the most widely used kinetic models, i.e., pseudo-first-order equation and pseudo-second-order equation (Akar et al. 2013), were used to research the adsorption kinetic behavior of MEL onto MIPs. The pseudo-first-order equation and pseudo-second-order equation were respectively as follows:
where Q eq and Q t refer to the amount of MEL adsorbed (mg g−1) at equilibrium and at any time, t (min), respectively. Q e is the theoretic adsorption capacity of the pseudo-first-order kinetic model and the pseudo-second-order kinetic model (mg g−1). K 1 and K 2 are the equilibrium rate constant of pseudo-first-order sorption and pseudo-second-order sorption (min−1), respectively. The values of K 1 and Q e can be calculated from the slope and intercept of the linear line plotted in ln(Q eq − Q t ) versus t, and the slope and intercept of the plot of t/Q t versus t were used to calculate K 2 and Q e .
The two kinetic models analysis curves of MIPs and NIPs are shown in Fig. 3b, c, and each parameter value is shown in Table 2 and shown in Table 3, respectively. It can be seen that both the fitting R 2 of pseudo-second-order showed better than that of pseudo-first-order equation, and the fitting result (20.325 mg/g) of pseudo-second-order was closer to the real experimental data (21.284 mg/g). So, the experimental data of MEL adsorption onto MIPs and NIPs conformed to pseudo-second-order kinetic model. It indicated that the rate of chemical adsorption is limiting step. And, the results also shown that the adsorption of MEL onto NIPs conformed to pseudo-second-order kinetic model.
Adsorption Selectivity
Besides MEL, three structurally related compounds, cyromazine, cyanuric acid, and atrazine (Fig. 4) were employed to research the selection of the MEL adsorption of MIPs in this experiment. The results are shown in Fig. 5. The adsorption amounts of MEL, cyromazine, and cyanuric acid bound to MIPs were all higher than those bound to NIPs. There was no obvious difference between the MIPs and NIPs to adsorb atrazine. Thus, MIPs have selective adsorption to MEL, cyromazine, and cyanuric acid. However, the selection for MEL on MIPs was higher than that for cyromazine and cyanuric acid because MEL was used as imprinting molecule in the process of the synthesis of MIPs and it was combined in the mesh structure. The imprinting holes and specific binding sites were formed after MEL was removed by Soxhlet extraction. There are no holes or sites in NIPs because MEL was not added during the composition.
Optimization of Extraction Conditions
The MIPs were used as dispersant for selective extraction of MEL from milk powder. We have discussed the specificity of the MIPs and have improved that MIPs were selective to MEL in the previous experiments. Other parameters affecting the performance of the MSPD, such as the ratio of MIPs to sample, dispersion time, washing solvent, and elution solvent, were investigated. When one parameter was optimized, the other parameters were fixed at their optimized values.
-
1.
The ratio of MIPs sorbent to sample
The ratio of MIPs sorbent to milk powder sample was selected to make MEL completely adsorbed on the surface of MIPs. In this experiment, the ratio of MIPs sorbent to sample (MIPs/S) for 1/2, 2/3, 1/1, and 2/1 (g/g) was assayed to assess the sorbent effect on the recovery of the MSPD process. The result in Fig. 6a showed that the recovery of MEL was increased when the proportion is from 1/2 to 2/3, and it was basically unchanged when the proportion is from 2/3 to 2/1. So, the satisfied recovery of MEL was obtained with MIP/S of 2/3, 1/1, and 2/1. Otherwise, the sample could not be extracted completely with lower MIP/S. In this work, the suitable MIP/S (2:3) was selected with saving polymers.
-
2.
Dispersion time
The dispersion time was a crucial means of evaluating the efficiency of the assay. So, the effects of different dispersion times (2, 5, 10, 15, and 30 min) on the recovery were examined. As shown in Fig. 6b, the results of experiment indicated that the dispersion with 10 min showed a higher recovery for MEL contained in the milk powder samples. No significant increase in the extraction was observed when the dispersion time increased further.
-
3.
Washing solvent
In this work, different solvents (methanol, acetonitrile, 50 % methanol–water, and 50 % acetonitrile–water) were selected as the washing solvents. As shown in Fig. 6c, acetonitrile can remove impurities cleanly but not for MEL elution. In the following experiment, different volumes of acetonitrile from 1 to 6 mL were studied. The result indicated that the recovery was best with the volume of 2 mL. Therefore, 2 mL of acetonitrile was employed.
-
4.
Elution solvent
Five different solvents (methanol, 2 % ammonium methanol, 2 % acetic acid–methanol, 5 % ammonium methanol and 5 % acetic acid–methanol) were used as elution solvents to investigate their impact on the extraction. As can be seen in Fig. 6d, the most satisfactory result was achieved when ammonium methanol (5:95, v/v) was used.
To investigate the volume of elution solvent, milk powder samples were prepared. During extraction of the milk powder samples, 6 mL fractions were collected and analyzed. The effect of the elution volume on the recovery is shown in Fig. 6e. The result showed that the suitable volume of elution solvent was 4 mL. No significant increase in recovery was observed when the volume of elution solvent increased from 4 to 6 mL. The volume of elution solvent was set at 4 mL in further experiments.
Method Validation
Linear range and limit of detection (LOD) were studied using LC–MS/MS. The calibration curves for quantitative was carried out with matrix-matched standards which were obtained by spiking MEL into blank milk powder in the range of 1–100 ng g−1. The correlation coefficient obtained was 0.9999. The LOD that was defined as the concentration providing a signal-to-noise of three was the minimum concentration of analyte that could be confidently identified by the method (Niska et al. 2009). The LOD of MEL was 0.2 ng g−1. The maximum residue limits (MRLs) of MEL were 1 mg kg−1 in infant formula and 2.5 mg kg−1 in other foods established by China (Chinese Administration of Quality Supervision 2011). Compared with MRLs of MEL, the value of LOD proved that the method was sensitive.
Precision was evaluated by measuring intra-day and inter-day relative standard deviations (RSDs) at three different fortified concentrations of 1.0, 10.0, and 50.0 ng g−1. The intra-day precision was carried out with six times samples analysis in the same day, and the inter-day precision was carried out by analyzing this sample once a day during six consecutive days. The resultant samples were then analyzed by this method. Intra-day and inter-day precisions obtained were in the range of 0.4–2.7 and 2.3–5.1 %, respectively. In all three fortified levels, the recovery of MEL ranged from 92.5 to 97.2 %.
In order to further investigate the performance of MIPs, different analytical methods (Cheng et al. 2013; Zhu et al. 2015; Li et al. 2010b; Hu et al. 2015; He et al. 2014) for determination of MEL were compared and summarized briefly in Table 4. In the aspects of liner range, the recovery, LOD, and RSD, the proposed method was comparable or superior to other methods. Due to the molecularly imprinting technique, this material had selective molecular cognition sites, which can adsorb MEL selectively. MSPD was used in this work, in which extraction and cleanup were performed at the same time. Thus, the sample preparation time and consumption of solvent were decreased in our method. The comparison proved that the method in this study was convenient, rapid, and efficient for analyzing MEL in milk powder.
Application to Aquatic Products Samples
In order to evaluate the feasibility and applicability of the proposed method, it was applied to determine MEL in five milk powder samples collected from different market located in Harbin (China). MEL was detectable with the content of 1.3 ng g−1 in one sample. But, the amount of MEL found in this sample was far lower than the MRLs of MEL. The recoveries of MEL were studied in the real samples by analyzing the spiked samples (2.0 ng g−1) under the optimized conditions. The results showed that MIP–MSPD had good recoveries (94.1 % ± 3.1 %) for the determination of MEL in different milk powder samples.
Conclusion
In this work, MIPs which have high selectivity and extraction efficiency were prepared and applied for the extraction of MEL from milk powder samples successfully. Due to much specific surface area, MIPs with CNT as matrix were more effective for the adsorption of MEL. The LC-MS/MS was used for the separation, identification and quantification of MEL. In order to evaluate the rebinding properties of the MIPs, the isothermal adsorption experiment, kinetics adsorption experiment, and selectivity were studied, respectively. The binding capacity of MIPs to the template is higher than that of NIPs. It also could be found that pseudo-second-order model was more valid to describe the adsorption behavior of MEL onto MIPs and NIPs. The selectivity of MIPs for the original template over related MEL was also demonstrated. Under optimized conditions, the high recoveries of 92.5–97.2 % were obtained with the RSD less than 5.1 % and the LOD obtained was 0.2 ng g−1, which is suitable for the determination of MEL in the low concentration level. All the results indicated that the methods proposed in this work using CNT-based MIPs to extraction and separate MEL in milk powder decreased analysis time and the cost of organic solvents and significantly improved the selectivity and efficiency. Because of these advantages mentioned above, it can be considered that this method is promising and may be a good alternative to the traditional techniques for the analysis of some other complex food samples.
References
Akar E, Altinişik A, Seki Y (2013) Using of activated carbon produced from spent tea leaves for the removal of malachite green from aqueous solution. Ecol Eng 52:19–27
Andersen WC, Turnipseed SB, Karbiwnyk CM, Clark SB, Madson MR, Gieseker CM, Miller RA, Rummel NG, Reimschuessel R (2008) Determination and confirmation of melamine residues in catfish, trout, tilapia, salmon, and shrimp by liquid chromatography with tandem mass spectrometry. J. Agr. Food Chem. 56:4340–4347
Baynes RE, Smith G, Mason SE, Barrett E, Barlow BM, Riviere JE (2008) Pharmacokinetics of melamine in pigs following intravenous administration. Food Chem Toxicol 46:1196–1200
Bernal J, Ares A, Pól J, Wiedmer S (2011) Hydrophilic interaction liquid chromatography in food analysis. J. Chromatogr A 1218:7438–7452
Bogialli S, Di Corcia A (2007) Matrix solid-phase dispersion as a valuable tool for extracting contaminants from foodstuffs. J Biochem Bioph Meth 70:163–179
Cheng WJ, Liu ZJ, Wang Y (2013) Preparation and application of surface molecularly imprinted silica gel for selective extraction of melamine from milk samples. Talanta 116:396–402
Chinese Administration of Quality Supervision (2011) Notice on the limited value of melamine in food. http://www.moh.gov.cn/sps/s7891/201104/9f1311e1e97649f3a26a6b7f7b3d7ae3.shtml.Accessed 6 April 2011
Cianciolo RE, Bischoff K, Ebel JG, Van Winkle TJ, Goldstein RE, Serfilippi LM (2008) Clinicopathologic, histologic, and toxicologic findings in 70 cats inadvertently exposed to pet food contaminated with melamine and cyanuric acid. J Am Vet Med Assoc 233:729–737
Cook HA, Klampfl CW, Buchberger W (2005) Analysis of melamine resins by capillary zone electrophoresis with electrospray ionization-mass spectrometric detection. Electrophoresis 26:1576–1583
Du J, Gao R, Mu H (2016) A novel molecularly imprinted polymer based on carbon nanotubes for selective determination of dioctyl phthalate from beverage samples coupled with GC/MS. Food Anal. Meth. 9:2026–2035
Ehling S, Tefera S, Ho IP (2007) High-performance liquid chromatographic method for the simultaneous detection of the adulteration of cereal flours with melamine and related triazine by-products ammeline, ammelide, and cyanuric acid. Food Addit Contam 24:1319–1325
Ferrer C, Gómez MJ, García-Reyes JF, Ferrer I, Thurman EM, Fernández-Alba AR (2005) Determination of pesticide residues in olives and olive oil by matrix solid-phase dispersion followed by gas chromatography/mass spectrometry and liquid chromatography/tandem mass spectrometry. J Chromatogr A 1069:183–194
Filigenzi MS, Puschner B, Aston LS, Poppenga RH (2008) Diagnostic determination of melamine and related compounds in kidney tissue by liquid chromatography/tandem mass spectrometry. J. Agr. Food Chem. 56:7593–7599
Guo Z, Gai P, Hao T, Wang S, Wei D, Gan N (2011) Determination of melamine in dairy products by an electrochemiluminescent method combined with solid-phase extraction. Talanta 83:1736–1741
He D, Zhang XP, Gao B (2014) Preparation of magnetic molecularly imprinted polymer for the extraction of melamine from milk followed by liquid chromatography-tandem mass spectrometry. Food Control 36:36–41
Hu YX, Feng SL, Gao F (2015) Detection of melamine in milk using molecularly imprinted polymers–surface enhanced Raman spectroscopy. Food Chem 176:123–129
Khanahmadzadeh S, Tarigh A (2014) Ultrasound-assisted combined with nano-sized molecularly imprinted polymer for selective extraction and pre-concentration of amitriptyline in human plasma with gas chromatography-flame detection. J Chromatogr B 972:6–13
Klampfl CW, Andersen L, Haunschmidt M, Himmelsbach M, Buchberger W (2009) Analysis of melamine in milk powder by CZE using UV detection and hyphenation with ESI quadrupole/TOF MS detection. Electrophoresis 30:1743–1746
Lachenmeier DW, Humpfer E, Fang F, Schütz B, Dvortsak P, Sproll C, Spraul M (2009) NMR-spectroscopy for nontargeted screening and simultaneous quantification of health-relevant compounds in foods: the example of melamine. J. Agr. Food Chem. 57:7194–7199
Lam CW, Lan L, Che X, Tam S, Wong SSY, Chen Y, Jin J, Tao SH, Tang XM, Yuen KY, Tam PKH (2009) Diagnosis and spectrum of melamine-related renal disease: plausible mechanism of stone formation in humans. Clin Chim Acta 402:150–155
Langman CB, Alon U, Ingelfinger J, Englund M, Saland JM, Somers MJG, Stapleton FB, Sibu NO, Cochat P, Wong W, Eke FU, Satlin L, Salusky I (2009) A position statement on kidney disease from powdered infant formula-based melamine exposure in Chinese infants. Pediatr Nephrol 24:1263–1266
Li L, Li B, Cheng D, Mao L (2010a) Visual detection of melamine in raw milk using gold nanoparticles as colorimetric probe. Food Chem 122:895–900
Li M, Zhang LY, Meng ZH (2010b) Molecularly-imprinted microspheres for selective extraction and determination of melamine in milk and feed using gas chromatography–mass spectrometry. J Chromatogr B 878:2333–2338
Lin M, He L, Awika J, Yang L, Ledoux DR, Li H, Mustapha A (2008) Detection of melamine in gluten, chicken feed, and processed foods using surface enhanced Raman spectroscopy and HPLC. J Food Sci 73:T129–T134
Liu J, Song H, Liu J, Liu Y, Li L, Tang H, Li YC (2015) Preparation of molecularly imprinted polymer with double templates for rapid simultaneous determination of melamine and dicyandiamide in dairy products. Talanta 134:761–767
Ma GF, Chen LG (2014) Development of magnetic molecularly imprinted polymers based on carbon nanotubes—application for trace analysis of pyrethroids in fruit matrices. J Chromatogr A 1329:1–9
Masque N, Marce RM, Borrull F (2001) Molecularly imprinted polymers: new tailor-made materials for selective solid-phase extraction. Trend Anal Chem 20:477–486
Ministry of Health of the People’s Republic of China (2008) National standards of the People’s Republic of China GB/T 22388-2008
Nezhadali A, Feizy J, Beheshti HR (2015) A molecularly imprinted polymer for the selective extraction and determination of fenvalerate from food samples using high-performance liquid chromatography. Food Anal. Meth. 8:1225–1237
Niska K, Korkea-aho T, Lindfors E, Kiuru T, Tuomainen M, Taskinen J, Peltonen K (2009) Disappearance of malachite green residues in fry of rainbow trout (Oncorhynchus mykiss) after treatment of eggs at the hatching stage. Aquaculture 297:25–30
Niu Y, Yang J, Ma M, Gong Y, Wang Y, Gong B (2016) Preparation of tetracycline surface molecularly imprinted material for the selective recognition of tetracycline in milk. Food Anal Meth 9:2342–2351
Oniszczuk A, Waksmundzka-Hajnos M, Skalicka-Woźniak K, Głowniak K (2013) Comparison of matrix-solid phase dispersion and liquid–solid extraction connected with solid-phase extraction in the quantification of selected furanocoumarins from fruits of Heracleum leskowii by high performance liquid chromatography. Ind Crop Prod 50:131–136
Rodríguez-González N, González-Castro MJ, Beceiro-González E, Muniategui-Lorenzo S (2015) Development of a matrix solid phase dispersion methodology for the determination of triazine herbicides in mussels. Food Chem 173:391–396
Rodrigues SA, Caldas SS, Primel EG (2010) A simple; efficient and environmentally friendly method for the extraction of pesticides from onion by matrix solid-phase dispersion with liquid chromatography–tandem mass spectrometric detection. Anal Chim Acta 678:82–89
Ruiz MJ, Cameán AM, Moreno IM, Picó Y (2005) Determination of microcystins in biological samples by matrix solid-phase dispersion and liquid chromatography–mass spectrometry. J Chromatogr A 1073:257–262
Tan L, Li W, Li H, Tang Y (2014) Development of surface imprinted core–shell nanoparticles and their application in a solid-phase dispersion extraction matrix for methyl parathion. J Chromatogr A 1336:59–66
Wang ZM, Chen DH, Gao X, Song ZH (2009) Subpicogram determination of melamine in milk products using a luminol–myoglobin chemiluminescence system. J. Agr. Food Chem. 57:3464–3469
Wu BW, Wang ZH, Zhao DX, Lu XQ (2012) A novel molecularly imprinted impedimetric sensor for melamine determination. Talanta 101:374–381
Yan H, Cheng X, Sun N, Cai T, Wu R, Han K (2012) Rapid and selective screening of melamine in bovine milk using molecularly imprinted matrix solid-phase dispersion coupled with liquid chromatography-ultraviolet detection. J Chromatogr B 908:137–142
Yang HH, Zhou WH, Guo XC, Chen FR, Zhao HQ, Lin LM, Wang XR (2009) Molecularly imprinted polymer as SPE sorbent for selective extraction of melamine in dairy products. Talanta 80:821–825
Yokley RA, Mayer LC, Rezaaiyan R, Manuli ME, Cheung MW (2000) Analytical method for the determination of cyromazine and melamine residues in soil using LC-UV and GC-MSD. J. Agr. Food Chem. 48:3352–3358
Yu D, Hu X, Wei S, Wang Q, He C, Liu S (2015) Dummy molecularly imprinted mesoporous silica prepared by hybrid imprinting method for solid-phase extraction of bisphenol A. J Chromatogr A 1396:17–24
Zheng X, Yu B, Li K, Dai Y (2012) Determination of melamine in dairy products by HILIC-UV with NH2 column. Food Control 23:245–250
Zhu L, Xu GH, Wei FD (2015) Determination of melamine in powdered milk by molecularly imprinted stir bar sorptive extraction coupled with HPLC. J Colloid Interf Sci 454:8–13
Zhu X, Wang S, Liu Q, Xu Q, Xu S, Chen H (2009) Determination of residues of cyromazine and its metabolite, melamine, in animal-derived food by gas chromatography–mass spectrometry with derivatization. J Agr Food Chem 57:11075–11080
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was funded by the Heilongjiang Province Science Foundation for Youths (no. QC2014C005).
Conflict of Interest
Yu Wang declares that he has no conflict of interest. Lei Gao declares that he has no conflict of interest. Dongli Qin declares that he has no conflict of interest. Ligang Chen declares that he has no conflict of interest.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed Consent
Not applicable.
Rights and permissions
About this article
Cite this article
Wang, Y., Gao, L., Qin, D. et al. Analysis of Melamine in Milk Powder by CNT-MIP with Matrix Solid Phase Dispersion and LC-MS/MS. Food Anal. Methods 10, 1386–1396 (2017). https://doi.org/10.1007/s12161-016-0705-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-016-0705-1